Abstract
Objectives:
Cushing’s disease (CD) is a rare condition with a prevalence of roughly 39 cases per million in the general population. Healthcare costs are substantial for CD patients with either untreated or inadequately controlled disease. This study assesses the 3-year budget impact of pasireotide on a US managed care health plan following pasireotide (Signifor) availability.
Methods:
Two scenarios were evaluated to understand the differences in costs associated with the introduction of pasireotide. The first scenario evaluates the budget impact of pasireotide from the perspective of an entire health plan (total budget impact) and the second from the perspective of the pharmacy budget (pharmacy budget impact). Both scenarios evaluate the annual incremental budget impact with and without pasireotide. Scenario 1 includes costs for medical procedures, drug therapies, monitoring, surgical complications, comorbidities for patients with controlled or uncontrolled CD, and adverse events. Procedures include transsphenoidal surgery, bilateral adrenalectomy, radiotherapy and radiosurgery. Drugs include pasireotide (indicated for CD), mifepristone (indicated to control hyperglycemia secondary to hypercortisolism in patients with Cushing’s syndrome) as well as several off-label treatments (ketoconazole, cabergoline, mitotane). Scenario 2 considers costs solely from the perspective of a health plan pharmacy. Costs are in $2013.
Results:
The estimated total budget impact is $0.0115 per-member per-month (PMPM) in the first year following FDA approval, $0.0184 in the second year, and $0.0194 in the third year. Introduction of pasireotide is expected to increase the pharmacy budget by $0.0257 PMPM in the first year, $0.0363 in the second year, and $0.0360 in the third year.
Limitations:
Model inputs rely on the small body of literature available for Cushing’s disease.
Conclusions:
Cushing’s disease is severe disease with debilitating comorbidities and substantial healthcare costs when untreated or inadequately controlled. The inclusion of pasireotide in a health plan formulary appears to have only a small impact on the total health plan or pharmacy budget.
Keywords::
Introduction
Cushing’s disease (CD) is a rare disease and a form of Cushing’s syndrome that is a result of long-term exposure to glucocorticoids caused by excessive secretion of adrenocorticotropin hormone (ACTH) by a pituitary corticotroph tumor (pituitary adenoma)Citation1–5. Patients with CD experience a remarkably broad spectrum and high prevalence of comorbidities associated with chronic hypercortisolism, and diagnosis of CD is challenging in part because of the many disparate manifestations of the diseaseCitation2. A claims study conducted in the US found that 44% of patients had a CD-related condition or procedure prior to their first Cushing’s syndrome diagnosis code, which suggests a delayed diagnosis cohort may existCitation6. Mortality studies have been conducted among only a limited set of patients. However, one meta-analysis shows that the overall CD-related mortality among CD patients that are treated primarily with transsphenoidal surgery (TSS) is roughly twice that of the general populationCitation7.
Epidemiological studies of CD are few and provide only very limited information regarding estimates of the incidence and prevalence of this rare disorder in the general populationCitation8–10. A chart review of records from the National Health Service in Vizcaya, Spain found a prevalence of 39.1 CD cases per million inhabitants at the end of 1992 and an average incidence of newly diagnosed cases was 2.4 per million per year. Another epidemiological study using registry data from Denmark found an incidence rate of 1.2–1.7 cases per million per year. A cross-sectional study in the province of Liège, Belgium conducted in 2005 showed a prevalence of 94 cases of pituitary adenomas per 100,000; 5.9% of these cases, or 55 cases per million, were associated with CD. In the US, a retrospective cohort study of CD patients using claims data from 2007–2010 reported an incidence of 7.6 per million per year, with rates varying by age and sexCitation11.
Chronic comorbidities associated with CD place a substantial burden on patients. The many comorbidities associated with CD include ischemic heart disease, obesity, hypertension, impairment of glucose tolerance, dyslipidemia, and thrombotic diathesisCitation3,Citation12–14, which together increase cardiovascular risk. Other comorbidities found among CD patients include infections (urinary tract infections and pneumonia), psychopathologies (anxiety, depression, and cognitive deficit), as well as skeletal problems (fractures and osteoporosis)Citation3,Citation12–14. Patients most often complain of fatigue/weakness (85%), changes in physical appearance (63%), emotional instability (61%), cognitive impairment (49%), depression (32%), and sleeping difficulties (12%); 80% report interference with family life and relations with their partner and 56% with school/work performanceCitation15. Elevated mortality in CD patients may reflect increased frequency or severity of infections as well as elevated cardiovascular and cerebrovascular riskCitation16.
The psychiatric effects of CD take a particularly large toll on patients’ health-related quality-of-life (HRQoL) and long-term function. A number of studies have documented long-lasting adverse effects on behavioral and cognitive functions caused by functional and, over time, structural alterations in specific brain target areas due to prolonged, increased endogenous or exogenous exposure to glucocorticoidsCitation17–19. In HRQoL studies among CD patients, the effects of hypercortisolism on HRQoL are seen in the physical, mental, and emotional componentsCitation20–22. This finding was similar among childrenCitation23.
Cushing’s disease is associated with substantial healthcare costsCitation8,Citation13,Citation24. A cross-sectional US study found that the economic burden of CD patients is substantial, with hospitalizations or emergency department (ED) visits observed in >34% of patients, an average of 19.8 office visits per patient, and up to $35,000 in annual total costs, of which $31,395 is for medical costsCitation25. Diagnosis of CD is complicated and associated with frequent physician visits and diagnostic tests and proceduresCitation24. Following diagnosis, patients can undergo multiple surgeries and require long-term monitoring due to a threat of disease recurrence. These factors, along with management of comorbidities, increase healthcare resource utilization and place a heavy economic burden on patients and payers.
The primary treatment for CD is TSS, a procedure in which the corticotroph adenoma is selectively removed, preferably performed by a surgeon with substantial experience with this procedureCitation26. Locating the tumor may require careful sectioning through the pituitary gland, because, while some tumors have an identifiable pseudo-capsule, others do not display a distinct border between the tumor and normal pituitary tissueCitation2. Surgical complications frequently occur and are inversely related to the experience of the neurosurgeonCitation26. The reported initial success rate for pituitary surgery for CD varies between 60–86%. However, up to 25% of these patients with successful treatment suffer from recurrence after apparent remissionCitation27. In such instances, second-line therapeutic options include medical procedures such as repeat pituitary surgery, radiosurgery (RS), radiotherapy (RT), or bilateral adrenalectomy (BLA). Medical therapies in this setting include mifepristone, which is indicated to control hyperglycemia secondary to hypercortisolism in patients with Cushing’s syndrome, or off-label medical therapies such as ketoconazole and cabergolineCitation2,Citation28. Although uncommon, these treatment options may also be used as first line treatmentCitation29.
Pasireotide (Signifor) is a pituitary-directed somatostatin analog approved by the Food and Drug Administration (FDA) on December 14, 2012 for the treatment of adult patients with CD for whom pituitary surgery is not an option or has not been curativeCitation30. The objective of this study was to assess the total and the pharmacy budget impact of adding pasireotide to a health plan formulary of a US managed care health plan for the treatment of this chronic disease with a broad spectrum of comorbidities.
Methods
Model design
This study assesses the budget impact of pasireotide availability in two scenarios. The base case scenario, Scenario 1, evaluates the total budget impact to a US managed care health plan, including medical procedures and drug therapies, treatment-related complications or adverse events (AE), costs associated with managing comorbidities, and costs of monitoring. Assumptions used in Scenario 1 are listed in Supplementary Table 1. A second scenario, from the perspective of the pharmacy budget, includes only drug therapy options and drug costs. All costs were inflated to $2013 using the Consumer Price Index for Medical Care ServicesCitation31. Neither scenario includes discounting as the models were designed per recommendations from the ISPOR Task Force on Good Research Practices—Budget Impact Analysis, which suggests that, as the budget impact analysis presents financial streams over time, it is not necessary to discount these costsCitation32. The models were built in Microsoft Excel 2010.
Patient population
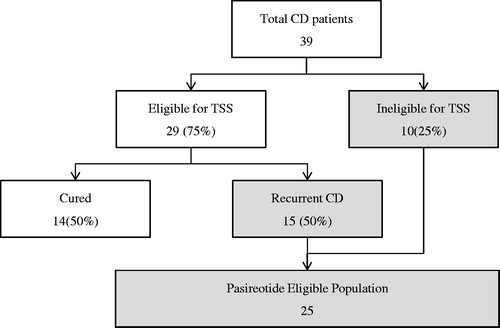
Both scenarios assume a health-plan covered population size of 1 million members in which the plan pays all health costs for those covered, and assume all patients with CD will be treated with some form of treatment. First-line TSS is the standard of care for patients with CD, but pharmacologic treatment may be appropriate for some patients who are poor surgical candidates, including those with undetectable tumors, tumors that are surgically unapproachable, or comorbidities or contraindications that preclude surgical interventionCitation26,Citation33. We assumed 75% of patients with CD receive first-line TSS while the remaining 25% receive drug therapies. Based on Alwani et al.Citation27, which reports that 28 of 79 patients exhibited ‘early relapse’ following TSS while 10 of 79 patients exhibited ‘late relapse’, for a total of 38 out of 79 or 48.1%, we assumed 50% of patients who received first-line TSS initially fail first-line TSS or have recurrent disease after initial success and are now seeking additional treatmentCitation27. Additionally, we assumed in Scenario 2 that 75% of these patients ineligible for first-line TSS or with recurrent CD after first-line TSS receive drug therapies. Prevalence rates and inputs for calculation of the CD patient population in Scenario 1 are shown in . illustrates the patient flow used to estimate the number of patients eligible for treatment in Scenario 1. Eligible patient population inputs and references for Scenario 2 are presented in Supplementary Table 2.
Table 1. Treatment-eligible patient population estimates (Scenario 1).
Treated shares
Treated shares provide a distribution of treatment options in a market with and without pasireotide availability. A treated share is the share of the market for each respective treatment option. Treated shares for mifepristone are assumed to grow over time while shares for all other treatment are assumed to normalize in both scenarios. With the introduction of pasireotide, all annual treated shares are normalized to match expected market uptake of pasireotide. Therefore, the portion of patients who undergo medical procedures relative to those who receive drug therapies is dynamic and fluctuates over time in Scenario 1. However, Scenario 2 assumes that the portion of patients who receive either medical procedures or drug therapies is constant over time, with 75% receiving drug therapies. Both scenarios use the same treated shares, but drug therapy shares are normalized to 100% in Scenario 2. Please see Supplementary Tables 3–6 for more detail.
Treatment duration
Both scenarios incorporate treatment efficacy/failure rates to obtain the duration of treatment for drug therapies. Patients who fail repeat surgical therapy are assigned all costs, which are reflected in costs for the first year. Patients who fail drug therapies were assumed to incur a full year of costs, unless the prescribing information suggests that clinical benefit be monitored and treatment discontinued for non-responders. Clinical benefit in this case is defined to include clinically meaningful reduction in 24-h UFC levels and/or improvement in signs or symptoms of the disease. Based on this definition of clinical benefit, 66.3% of patients responded to pasireotide treatment and continued treatment after 2 months of treatment (see Supplementary Table 1).
Treatment costs
Methods for calculating procedure costs were obtained from a detailed micro-costing analysis conducted from a US payer perspective that estimated treatment costs for TSS, BLA, RS, and RT in 2011Citation34. This analysis was then updated with 2013 values for use in the total budget impact model. TSS and BLA costs are comprised of hospitalization costs and physician fees for the surgery (based on CPT [Current Procedural Terminology] codes). Hospitalization charges, including nursing and room and board, are obtained from the Healthcare Cost and Utilization Project (HCUP) National Inpatient Sample database and converted to mean Medicare reimbursement values using a Medicare cost-to-charge ratioCitation35. Calculations for the cost of RT and RS were taken from a literature poster presentationCitation34. Reimbursement values for each CPT code (50th percentile) for physician services or outpatient services/procedures/labs were obtained from the MAG Mutual Physicians’ Fee and Coding Guide 2012, and were summed separately to generate costs of surgery or radiation therapy.
Costs for drug therapies in both scenarios are calculated based on the mean recommended daily dosage for each treatment (obtained from published literature)Citation2,Citation28,Citation36–38. Daily drug costs are estimated by multiplying mean daily dose (in mg) with the lowest drug cost per mg that was obtained using the wholesale acquisition cost (WAC) from the 2013 Drug Topics RedbookCitation39. Pharmacy dispensing fees were not incorporated.
Surgical complications and adverse events
A literature review was conducted and eight studies identified as the most appropriate clinical studies for inclusion in the total budget impact modelCitation37,Citation38,Citation40–45. Fourteen unique AEs were identified, of which hypopituitarism was associated with TSS, RS, and RT. TSS was associated with the greatest number of AEs or complications (6), followed by mifepristone (3) and BLA (2). One AE or complication was identified for each pasireotide, ketoconazole, cabergoline, RS, and RT.
This model also accounts for only the most common surgical complications and treatment-emergent AEs associated with substantial costs and resource utilization. Costs for stroke, endometrial thickening, meningitis, cerebrospinal fluid (CSF) leak, Nelson’s syndromeCitation46, thrombolytic events, and hepatotoxicity are assumed to be one-time costs and are based on sources from the literature. Costs of each complication/AE and their corresponding prevalence rates can be found in Supplementary Table 7.
Comorbidities
Data on prevalence of comorbidities, cost, and clinical effectiveness of available treatments were obtained from published literatureCitation47,Citation48. Costs were obtained for CD patients with controlled and uncontrolled disease or, when not available in CD, from the general population. A total of 32 comorbidities associated with CD were identified, of which 17 had sufficient data for inclusion in the analysis. The cost of comorbid disease for a patient with controlled or uncontrolled CD is calculated as the product of the cost of the comorbidity and the prevalence rate for that patient population.
Monitoring
In Scenario 1, post-operative lab monitoring is required after BLA at regular intervals. Reimbursement for each lab test is obtained using specific CPT codes for each test in the MAG Mutual Physicians’ Fee and Coding Guide 2012. Costs were inflated to $2013 using the Consumer Price Index for Medical Care Services. Supplementary Table 8 presents the frequency and unit cost of each procedure used in monitoring treatment and disease.
Results
Total cost of treatment
The total annual cost per patient on each treatment option in Scenario 1 is presented in . These costs account for the cost and duration of the treatments themselves, the cost and rate of treatment-associated adverse events or complications, the cost of treating comorbidities, and the cost of monitoring the disease and treatment. Cost components are presented in Supplementary Tables 7 and 8. Mifepristone is the most expensive drug therapy with an annual per patient cost of $207,562, while BLA is the most costly medical procedure at $72,525. Scenario 2 only includes drug costs, as presented in in Supplementary Table 9.
Table 2. Total cost estimates associated with Cushing’s disease treatments (Scenario 1).
The cost of pasireotide is based on a cost of $14,383.56 for 60 ampules or 30 days, for an annual cost of $175,000 per year (365 days) regardless of starting dose, and incorporates a response rate of 66.3% after 2 months of full treatment (see Supplementary Table 1).
Budget impact
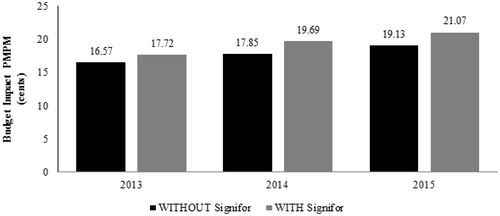
The expected total difference in the budget in Scenario 1 for the entire US managed care health plan is $137,505 in the first year, $219,892 in the second year, and $231,954 in the third year after pasireotide launch, based on a covered population of 1 million members (). On a per-member per-month (PMPM) basis, the estimated budget impact on a health plan with one million covered lives is $0.0115 (1.15 cents) in the first year, $0.0184 (1.84 cents) in the second year, and $0.0194 (1.94 cents) in the third year (). The estimated budget impact of pasireotide in Scenario 2 is $0.0257 (2.57 cents) PMPM in the first year, $0.0363 (3.63 cents) in the second year, and $0.0360 (3.60 cents) in the third year after launch (see Supplementary Table 10).
Table 3. Cushing’s disease budget impact summary (Scenario 1).
Sensitivity analysis
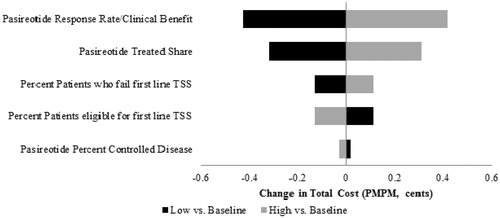
Five model parameters in the total budget impact model are closely examined in a one-way sensitivity analysis: (1) percentage of patients who fail first line TSS, (2) percentage of patients ineligible for first line TSS, (3) pasireotide treated shares, (4) pasireotide response rate/clinical benefit, and (5) pasireotide percentage of controlled disease. By varying parameters by ±10%, which translates into a 10% increase or decrease proportional to the value rather than an absolute 10 percentage point increase or decrease in the probabilities, the most significant impact is caused by the pasireotide response/clinical benefit rate at 2 months after the initiation of treatment and is expected to alter the budget impact of the third year by less than $0.01 (1 cent) PMPM. Varying the parameters up to 25% rather than 10% produces a similarly small effect, altering the budget impact by less than $0.015 (1.5 cents). The range of the budget impact due to a ±10% change in key parameters is presented in a tornado diagram in .
Figure 3. Cushing’s disease budget impact model sensitivity analysis (Scenario 1). Input ranges for each parameter are as follows: pasireotide response rate/clinical benefit (60–73%), pasireotide treated share (8–10% in 2013, 14–18% in 2014, 17–21% in 2015), percentage of patients who fail first line TSS (45–55%), patients eligible for first line TSS (68–83%), and pasireotide percentage of controlled disease (50–61%). PMPM, per member per month; TSS, transsphenoidal surgery.
Discussion
Treatment options for CD are few, consisting of medical procedures like TSS and BLA that have substantial complications. Pasireotide is a new alternative drug therapy for patients with CD. This model was developed to make both a comprehensive estimate of the PMPM impact to a health plan’s total budget of including pasireotide on drug formularies and provide an estimate specific to pharmacies. Costs for the total budget impact include medical procedures, drug therapies, complications and AEs, comorbid disease, and monitoring. Only the cost of drug therapies was considered for the pharmacy budget impact. To our knowledge, there are no other studies that estimate the budget impact of treatments for CD. This study is the first to analyze the budget impact of treatments for CD from the perspective of a US health plan.
The budget impact of adding pasireotide to a drug pharmacy and to a health plan is small, which is common for rare diseases. The total budget impact on a PMPM basis is $0.0115 (1.15 cents) PMPM in the first year, $0.0184 (1.84 cents) in the second year, and $0.0194 (1.94 cents) in the third year. The estimated pharmacy budget impact of pasireotide is similarly modest at $0.0257 (2.57 cents) PMPM in the first year, $0.0363 (3.63 cents) in the second year, and $0.0360 (3.60 cents) in the third year after launch.
There are several limitations to the present analysis. Because CD is an orphan condition with limited published data, a number of assumptions were made that may influence the interpretation of results. First, assumptions were made to estimate the expected duration of treatment based on anticipated monitoring for response. Second, AE and complication rates were often taken from single-armed studies with small patient populations. Third, in the analysis of the total cost of CD comorbidities, some comorbidity cost estimates were obtained from the general population due to limited data availability, and only selected comorbidities were included to avoid double counting, limiting the accuracy of the estimate. Fourth, no co-payment structures were incorporated into this budget analysis, therefore the findings of this model may not be generalizable to plans with cost-sharing arrangements. Fifth, since published estimates are not available, the estimate of shares for CD treatments may under- or over-estimate the actual utilization. Finally, these results are relevant for US managed care health plans and not necessarily generalizable to other countries or health systems.
According to the sensitivity analysis, the most substantial cost driver is the pasireotide response rate/clinical benefit, for which a 10% variation in its price changes the PMPM budget impact in the third year by less than $0.005 (0.5 cents). The next largest cost drivers are the pasireotide treated shares and percentage of patients who fail first line TSS. Please see for more detail.
Conclusion
Cushing’s disease is severe disease with debilitating comorbidities and substantial healthcare costs when untreated or inadequately controlled. The inclusion of pasireotide in a health plan formulary appears to have a small impact on the total health plan or pharmacy budget.
Supplementary Appendix
Download PDF (48.1 KB)Transparency
Declaration of funding
This research was sponsored by Novartis Pharmaceuticals Corporation.
Declaration of financial/other relationships
H.L.T. and D.N. are employees of Analysis Group and have disclosed receiving consulting fees from Novartis Pharmaceuticals. M.P.N. and W.H.L. have disclosed that they are employees of Novartis and are stock option share-holders of Novartis Pharmaceuticals. The authors have disclosed that they have received no additional support or contributions from others in the preparation of this manuscript. The authors also declare that they have no competing interests. CMRO Peer Reviewers on this manuscript have received honorarium for their review work, but have no other relevant financial relationships to disclose.
Notes
*Signifor is a registered trademark of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA.
References
- Orth DN. Cushing’s syndrome. N Engl J Med 1995;332:791-803
- Biller BM, Grossman AB, Stewart PM, et al. Treatment of adrenocorticotropin-dependent Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2008;93:2454-62
- Feelders RA, Pulgar SJ, Kempel A, Pereira AM. The burden of Cushing’s disease: clinical and health-related quality of life aspects. Eur J Endocrinol 2012;167:311-26
- Arnaldi G, Boscaro M. New treatment guidelines on Cushing's disease. F1000 Med Rep 2009;1: 64. doi:10.3410/M1-64
- Arnaldi G, Angeli A, Atkinson AB, et al. Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2003;88:5593-602
- Burton T, Rey G, Neary M, et al. Development of an algorithm to identify Cushing's disease patients in a US administrative claims database. Poster presented at: The 13th International Pituitary Congress, San Francisco, CA. June 12–14, 2013
- Clayton RN, Raskauskiene D, Reulen RC, Jones PW. Mortality and morbidity in Cushing’s disease over 50 years in stoke-on-Trent, UK: Audit and meta-analysis of literature. J Clin Endocrinol Metab 2011;96:632-42
- Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease. Clin Endocrinol (Oxf) 1994;40:479-84
- Lindholm J, Juul S, Jorgensen JO, et al. Incidence and late prognosis of Cushing’s syndrome: a population-based study. J Clin Endocrinol Metab 2001;86:117-23
- Daly AF, Rixhon M, Adam C, et al. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 2006;91:4769-75
- Broder M, Neary M, Chang E, et al. Incidence of Cushing’s disease in the United States. Poster presented at: The 95th Annual Meeting of the Endocrine Society, San Francisco, CA. June 15–18, 2013
- Patel D, Stephens J, Wiegand P, et al. Reduction in comorbidities and cost savings associated with biochemical control in patients with Cushing's disease: a literature-based analysis. Poster presented at: The ISPOR 14th Annual European Congress, Madrid, Spain. November 5–8, 2011
- Swearingen B, Wu N, Chen S, et al. Healthcare resource use and costs among patients with Cushing's disease. Endocr Pract 2011;17:681-90
- Feelders R, Forsythe A, Stemmer V, et al. Burden of Cushing's disease – A retrospective chart audit. Poster presented at: The European NeuoEndocrine Association, Vienna, Austria. September 15–17, 2012
- Gotch PM. Cushing's syndrome from the patient's perspective. Endocrinol Metab Clin North Am 1994;23:607-17
- Clayton RN. Mortality in Cushing's Disease. Neuroendocrinology 2010;92(Suppl 1):71-6
- Pereira AM, Tiemensma J, Romijn JA, Biermasz NR. Cognitive impairment and psychopathology in patients with pituitary diseases. Neth J Med 2012;70:255-60
- Brown ES. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann N Y Acad Sci 2009;1179:41-55
- Fietta P, Fietta P, Delsante G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci 2009;63:613-22
- van Aken MO, Pereira AM, Biermasz NR, et al. Quality of life in patients after long-term biochemical cure of Cushing's disease. J Clin Endocrinol Metab 2005;90:3279-86
- Alcalar N, Ozkan S, Kadioglu P, et al. Evaluation of depression, quality of life and body image in patients with Cushing's disease. Pituitary 2012;16:333-40
- Wagenmakers MAEM, Netea-Maier RT, Prins JB, et al. Impaired quality of life in patients in long-term remission of Cushing's syndrome of both adrenal and pituitary origin: a remaining effect of long-standing hypercortisolism? Eur J Endocrinol 2012;167:687-95
- Keil MF, Merke DP, Gandhi R, et al. Quality of life in children and adolescents 1-year after cure of Cushing syndrome: a prospective study. Clin Endocrinol (Oxf) 2009;71:326-33
- Nieman LK, Biller BM, Findling JW, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2008;93:1526-40
- Broder M, Neary M, Chang E, et al. Annual economic burden associated with Cushing's disease in the United States. Poster presented at: The 95th Annual Meeting of the Endocrine Society, San Francisco, CA. 15–18 June, 2013
- Brown RL, Weiss RE. An approach to the evaluation and treatment of Cushing's disease. Expert Rev Anticancer Ther 2006;6(Suppl 9):S37-S46
- Alwani RA, de Herder WW, van Aken MO, et al. Biochemical predictors of outcome of pituitary surgery for Cushing's disease. Neuroendocrinology 2010;91:169-78
- Corcept Therapeutics Incorporated. Korlym [package insert]. 2012. Menlo Park, CA
- Broder M, Neary M, Chang E, et al. Real-world treatment patterns in Cushing's disease patients in two large US nationwide databases: application of novel, graphical methodology. Poster presented at: The 95th Annual Meeting of the Endocrine Society, San Francisco, CA. June 15–18, 2013
- Food and Drug Administration (FDA). FDA Approved Drug Products. 2013 [cited 2013 Dec 16]; Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails
- Bureau of Labor Statustics UDoL. Consumer Price Index (CPI) for Medical Care Services (CUSR0000SAM2). 2013 [cited 2013 Jul 16]; Available from: http://www.bls.gov/cpi/
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health 2007;10:336-47
- Feelders RA, Hofland LJ, de Herder WW. Medical treatment of Cushing's syndrome: adrenal-blocking drugs and ketaconazole. Neuroendocrinology 2010;92(Suppl 1):111-5
- Patel DA, Maldonado M, Stephens JM, et al. Cost of second line non-pharmacologic interventions and their related complications in Cushing's disease: a literature-based economic analysis. Poster presented at: The 12th Annual International Pituitary Congress, Boston, MA. June 1–3, 2011
- Agency for Healthcare Research & Quality (AHRQ). Healthcare Cost and Utilization Project (HCUP). [cited 2012 Dec 16]; Available from: http://www.ahrq.gov/research/data/hcup/index.html
- Novartis Pharmaceuticals Corp. Signifor [package insert]. 2012. East Hanover, NJ
- Pivonello R, De Martino MC, Cappabianca P, et al. The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 2009;94:223-30
- Fleseriu M, Biller BMK, Findling JW, et al. Mifepristone, a glucocorticoid receptor antagonist, produces clinical and metabolic benefits in patients with Cushing’s syndrome. J Clin Endocrinol Metab 2012;97:2039-49
- Thomson Reuters. ReadyPrice Online. RedBook 2013 [cited 2013 Jul 11]; Available from: http://www.thomsonhc.com/micromedex2/librarian
- Colao A, Petersenn S, Newell-Price J, et al. A 12-month Phase III study of pasireotide in Cushing’s disease. N Engl J Med 2012;366:914-24
- Lila AR, Gopal RA, Acharya SV, et al. Efficacy of cabergoline in uncured (persistent or recurrent) Cushing disease after pituitary surgical treatment with or without radiotherapy. Endocr Pract 2010;16:968-76
- Moncet D, Morando DJ, Pitoia F, et al. Ketoconazole therapy: an efficacious alternative to achieve eucortisolism in patients with Cushing’s syndrome. Medicina (B Aires) 2007;67:26-31
- Castinetti F, Nagai M, Dufour H, et al. Gamma knife radiosurgery is a successful adjunctive treatment in Cushing’s disease. Eur J Endocrinol 2007;156:91-8
- Ding XF, Li HZ, Yan WG, et al. Role of adrenalectomy in recurrent Cushing’s disease. Chin Med J 2010;123:1658-62
- Rollin GF, Ferreira NP, Czepielewski MAl. Prospective evaluation of transsphenoidal pituitary surgery in 108 patients with Cushing’s disease. Arq Bras Endocrinol Metabol 2007;51:1355-61
- Barber TM, Adams E, Ansorge O, et al. Nelson's syndrome. Eur J Endocrinol 2010;163:495-507
- Truong H, Nellesen D, Neary M, Ludlam W. Economic burden of multiple chronic comorbidities associated with Cushing's Disease, a rare endocrine disorder. Poster presented at: The DIA/NORD US Conference on Rare Diseases and Orphan Products: The New Era in Health Care, Bethesda, MD. October 7–9, 2013
- Truong HL, Nellesen D, Ludlam W, Neary M. Budget Impact of Signifor (Pasireotide) for the Treatment of Cushing’s Disease, a rare endocrine disorder associated with considerable comorbidities. Poster presented at: The AMCP Nexus Conference: Connecting Health Care and Innovation, San Antonio, TX. October 14-18, 2013
- Medi-Span. Medi-Span Price Rx. Medi-Span 2013 [cited 2013 Mar 15]; Available from: https://pricerx.medispan.com/
- First Databank (FDB). First Databank (FDB) 2013 [cited 2013 Mar 13]; Available from: http://www.fdbhealth.com/