Abstract
Objective:
To determine the cost-effectiveness of the treatment of advanced hormone-dependent prostate cancer with degarelix compared to luteinizing hormone-releasing hormone (LHRH) agonists in the UK using the latest available evidence and the model submitted to AWMSG.
Methods:
A cost-effectiveness model was developed from the perspective of the UK National Health Service evaluating monthly injection of degarelix against 3-monthly leuprorelin therapy plus anti-androgen flare cover for the first-line treatment of patients with advanced (locally advanced or metastatic) hormone-dependent prostate cancer. A Markov process model was constructed using the patient population characteristics and efficacy information from the CS21 Phase III clinical trial and associated extension study (CS21A). The intention-to-treat (ITT) population and a high-risk sub-group with a PSA level >20 ng/mL were modeled.
Results:
In the base-case analysis using the patient access scheme (PAS) price, degarelix was dominant compared to leuprorelin with cost savings of £3633 in the ITT population and £4310 in the PSA > 20 ng/mL sub-group. The chance of being cost-effective was 95% in the ITT population and 96% in the PSA > 20 ng/mL sub-group at a threshold of £20,000 per quality-adjusted life-year (QALY). In addition, degarelix remained dominant when PSA progression was assumed equal and only the benefits of preventing testosterone flare were taken into account. Treatment with degarelix also remained dominant in both populations when the list price was used. The additional investment required to treat patients with degarelix could be offset in 19 months for the ITT population and 13 months for the PSA > 20 ng/mL population. The model was most sensitive to the hazard ratio assumed for PSA progression between degarelix and leuprorelin and the quality-of-life (utility) of patients receiving palliative care.
Conclusion:
Degarelix is likely to be cost-effective compared to leuprorelin plus anti-androgen flare cover in the first-line treatment of advanced hormone-dependent prostate cancer.
Introduction
Prostate cancer is the most common cancer in men, accounting for ∼25% of new diagnoses of malignant cancer in England and WalesCitation1. Recently published figures for the UK as a whole indicate that 45,410 men were diagnosed with prostate cancer in 2012Citation2. The incidence of prostate cancer increases with age, and 1% of all men aged >85 years are diagnosed with the condition in England and Wales every yearCitation1.
Prostate cancer is the second most common cause of death in men with any cancer in the UK—second only to lung cancerCitation3. Most of the deaths are estimated to occur in patients with hormone-refractory metastatic prostate cancerCitation4. According to data from the Office for National Statistics for 2006–2011, 92.6% of men in England survived prostate cancer for 1 year and 80.2% for 5 years or more, with the proportions varying considerably with ageCitation5.
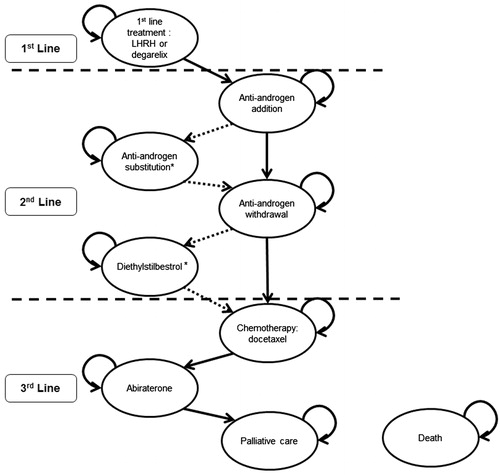
Advanced prostate cancer is defined as locally-advanced or advanced metastatic disease (i.e., where the cancer has spread beyond the prostatic capsule)Citation1. UK treatment patterns for advanced prostate cancer are covered by the National Institute for Health and Care Excellence (NICE) clinical guidance CG58Citation1, which is currently under review. In addition to this, the European Association for Urology (EAU) guidelines published in 2012 provide up-to-date guidance for the treatment of prostate cancer in the UKCitation6. Using details from these two guidelines and information gained from UK clinician expert opinion, the current UK treatment pathway for advanced hormone-dependent prostate cancer is summarized in .
Figure 1. Current treatment pathway for advanced (locally advanced or metastatic) hormone-dependent prostate cancer. This pathway is based on the information derived from the NICE clinical guideline on prostate cancer (CG58)Citation1, the EAU guidelines on prostate cancerCitation6, and expert opinion from UK clinicians [Personal Communication. UK Clinical Experts]. EAU, European Association of Urology; GnRH, gonadotropin-releasing hormone; LHRH, luteinising hormone-releasing hormone; NICE, National Institute for Health and Care Excellence.
![Figure 1. Current treatment pathway for advanced (locally advanced or metastatic) hormone-dependent prostate cancer. This pathway is based on the information derived from the NICE clinical guideline on prostate cancer (CG58)Citation1, the EAU guidelines on prostate cancerCitation6, and expert opinion from UK clinicians [Personal Communication. UK Clinical Experts]. EAU, European Association of Urology; GnRH, gonadotropin-releasing hormone; LHRH, luteinising hormone-releasing hormone; NICE, National Institute for Health and Care Excellence.](/cms/asset/3ef8c6ef-9900-46b7-bdfb-6bb4f610c2e8/ijme_a_893240_f0001_b.jpg)
A patient’s treatment pathway and initial treatment options will depend on the stage of their disease at presentation and diagnosisCitation1. Most men with advanced prostate cancer currently receive hormonal therapy in the form of a luteinizing hormone-releasing hormone (LHRH) agonist such as leuprorelin, goserelin or triptorelin. LHRH agonists are associated with an initial surge in testosterone levels (testosterone flare), which delays achievement of castration and, in advanced disease, can result in clinical symptoms (flare). Potential flare effects include increased bone pain, acute bladder outlet obstruction, obstructive renal failure, spinal cord compression and fatal cardiovascular events due to hypercoagulation statusCitation6–10. LHRH agonists are, therefore, mostly prescribed at first in combination with anti-androgen therapy, such as bicalutamide, to reduce the incidence of flare; however, data documenting the frequency and clinical consequences of a testosterone-induced flare in modern clinical practice are lacking and a recent study of a large patient cohort (n = 1566) treated for metastatic prostate cancer showed no significant differences in known flare complications between those receiving or not receiving anti-androgensCitation7.
Patients with locally-advanced disease may receive hormonal therapy in combination with radiotherapy. Some patients with localized prostate cancer with a high risk of extracapsular disease (Gleason score ≥8 or prostate-specific antigen (PSA) levels >20 ng/mL) may also be treated according to the pathway for locally-advanced cancer. In rare cases, in which the cancer is already very advanced at presentation, bilateral orchidectomy may be necessaryCitation1.
As a gonadotropin-releasing hormone antagonist, degarelix provides an alternative to LHRH agonists that does not induce the initial testosterone flare. In addition, pooled analysis of data from six prospective randomized controlled trials indicates that the benefits of degarelix are not limited to the suppression of the initial testosterone flare; indeed, degarelix may offer more rapid and prolonged disease control without microsurges (testosterone, follicle-stimulating hormone [FSH] and luteinizing hormone) and lower probability of disease progression compared with LHRH agonistsCitation9,Citation11–13. These findings suggest that degarelix patients may remain on first-line hormonal therapy for longer. Symptoms associated with LHRH agonists—increases in hormone levels in the form of short-term flare surges, medium- to long-term microsurges and poorer long-term FSH control—may contribute to a faster PSA progression when compared to degarelixCitation13–16.
A large number of patients are diagnosed with prostate cancer each year, making the selection of the most appropriate treatments extremely important for local healthcare budgets. Many patients currently receive LHRH agonists—more than 430,000 packs of LHRH agonists were sold in England and Wales in 2012: the equivalent of ∼950,000 monthly treatments (Ferring Pharmaceuticals Ltd. Data on file). In addition, the recent approval of abiraterone for hormone-refractory prostate cancer, which is an expensive treatment, has implications for local budgets and increases the relevance of assessing the cost-effectiveness of existing treatments for hormone-dependent prostate cancer. Degarelix aims to prolong the time to disease progression; leading to improved health-related quality-of-life for patients and reducing their utilization of third-line treatments (). Cost-effectiveness analysis is one tool payers can use to assess and potentially improve the performance of their healthcare systems by indicating which interventions maximize health within the available resource constraints.
Four analyses evaluating the cost-effectiveness of degarelix compared to LHRH agonists have previously been published: a manuscript published in 2012 comparing degarelix with triptorelin for patients with metastatic prostate cancer based solely upon the differences between degarelix and LHRH agonists in terms of the clinical outcomes of testosterone flare, a US analysis of cost-effectiveness based upon the effects of PSA progression (recurrence) on movement through lines of treatment therapy and two posters presented at the International Society For Pharmacoeconomics and Outcomes Research meetings detailing cost-effectiveness analyses submitted to the Scottish Medicines Consortium and All Wales Medicines Strategy Group (AWMSG)Citation17–20. Degarelix was approved for use in both Scotland and Wales with the application of a patient access scheme (PAS) following publication of the manuscript by Lu et al.Citation17, meaning that the analyses used within the submissions were not available to Lu et al. at the time of writingCitation21,Citation22.
The aim of this paper is to provide a full and transparent assessment of the cost-effectiveness of treatment of advanced hormone-dependent prostate cancer with degarelix compared to LHRH agonists in the UK, based upon the latest available evidence. The model detailed within this paper is based upon the health technology assessment submission to the AWMSG in 2012 and current UK clinical practice. The Welsh PAS for degarelix is taken into account, along with clinical evidence regarding the role of PSA in disease progressionCitation23,Citation24. Clinicians confirmed that PSA progression is widely used as a marker for disease progression and to determine movement from first- to second-line treatment (Ferring Pharmaceuticals Ltd. Advisory board report. Data on file).
Patients and methods
A Markov process model was constructed to perform a cost–utility analysis of degarelix as first-line treatment for patients with advanced hormone-dependent prostate cancer compared to standard treatment with LHRH agonists with anti-androgen flare protection.
The population modeled was designed to reflect the participant population of the CS21 Phase III clinical trial (FE 200486 CS21). The CS21 trial assessed the efficacy of degarelix 240/80 mg and 240/160 mg compared to leuprorelin 7.5 mg. This trial, along with the associated 5-year extension study (CS21A), was the source of the main efficacy parameter for the model, the duration of response on first-line treatmentCitation12,Citation13,Citation25,Citation26. Data from trial CS21 and the extension study were used as CS21 is the only long trial that measured PSA progression at the licensed dose. Degarelix data are taken only from the 240/80 mg arm, as this is the globally-licensed dose relevant for the UK. The key model parameters are shown in and more detailed information can be found in the Appendix.
Table 1. Key model parameters applied per cycle (28 days).
From the trial, two populations were defined: the intention-to-treat population (ITT) including all randomized participants and a high-risk sub-group with a PSA level >20 ng/mL. Elevated PSA levels indicate a greater risk of disease progressionCitation27. This population was modeled in isolation in order to examine the specific benefit these patients might be expected to receive from the rapid reduction in testosterone levels with degarelix. Both patient populations were assumed to have a starting age of 72 years, the mean age of trial participants at baseline.
The cost output of the model was the expected service use costs incurred by the National Health Service (NHS) in Wales, with 2011 costs presented as GBP. Cost outcomes were discounted at an annual rate of 3.5%, in line with UK health technology assessment requirementsCitation28.
Model structure
The model observed a time horizon of 20 years, after which the majority of patients are expected to have died. The cycle length in the model is 28 days, the same length as LHRH agonists and degarelix treatment periods. A number of LHRH agonists were included in the model as potential comparators: leuprorelin, goserelin, and triptorelin. Each LHRH agonist could be administered monthly or 3-monthly, and all were assumed to require anti-androgen drugs for flare protection for the first month of treatment. This assumption was validated by Welsh clinicians (Personal Communication. UK Clinical Experts. 2011). In the base-case analysis, the comparator was 3-monthly leuprorelin 11.25 mg. This was chosen as leuprorelin was used in the CS21 clinical trial (at a 7.5 mg dose, which is not licensed in the UK) and it was the second most commonly prescribed LHRH agonist in the UK in 2010–2011 (Ferring Pharmaceuticals Ltd. Data on file).As this comparator is also cheaper than 3-monthly goserelin 10.8 mg, cost-effectiveness of degarelix vs leuprorelin would also mean cost-effectiveness vs goserelin, assuming equal efficacy between LHRH agonists. Based on published reviews of clinical literatureCitation29,Citation30, this analysis assumes that there are no significant differences in treatment efficacy.
The structure of the model and the health states were based on the current UK treatment pathway in . Additional health states were included based on EAU guidelines, which enables the model to reflect variation in treatment practices across the UK and Europe. The model structure and health states are shown in . In the base-case model, anti-androgen substitution and diethylstilbestrol are not included in the treatment pathway (see ). These two treatments are not part of the current UK treatment pathway and patients skip over these to the next health state in the base case. The effect of including these health states are examined in the sensitivity analysis.
Clinical expert input
Four clinical experts were invited to an advisory board; one clinical pharmacologist, one urologist and two oncologists, with the sole purpose of reviewing, synthesizing and adapting the clinical assumptions on which the model is based to best reflect clinical practice and experience. Consensus was reached in the meeting and written up in a consensus report, approved by the clinical experts. The model was, thereafter, adapted in accordance with the experts’ opinion.
Efficacy inputs
Data from the 240/80 mg degarelix arm and the leuprorelin arm from the CS21 clinical trial were used to estimate the per-28-day cycle risk of patients no longer responding to first-line treatment (disease progression) and moving onto subsequent lines of treatment (). PSA progression was used as the marker for disease progression. It was defined in the CS21 clinical trial as two consecutive increases in PSA of 50% or more above the nadir (the lowest level observed), accompanied by an absolute increase of 5 ng/mL or more on two consecutive occasions at least 2 weeks apart. In the model, PSA progression was used as a marker for disease progression, as directly observed data for disease progression (e.g. tumor size) was not measured in the trial. This assumption was validated and supported by Welsh clinicians.
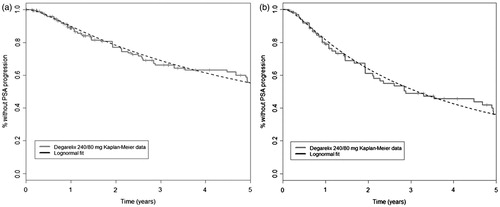
Survival analyses were performed to fit multiple parametric models to the observations of the CS21A extension period for degarelix-treated patients. Using the curve with the best fit, the results of the trial were extrapolated to generate estimates of the long-term efficacy of degarelix. Selection of the optimal curve was based on the Akaike Information Criterion score and the choice was validated by Welsh physicians by visual inspection. Log-normal distributions were determined to have the best fit, and these are shown alongside the Kaplan–Meier data in . The long-term response profile of patients treated with LHRH agonists was estimated by applying a hazard ratio generated from the 1-year comparative trial period and applied to the extrapolated degarelix curve. This approach was used because there were no long-term comparative observations; patients crossed over to the degarelix treatment group at the end of the 1-year comparative study period. No long-term data for PSA progression were identified for LHRH agonists in literature searches. Assumptions around the hazard ratio and the duration over which continued benefit could be assumed were, therefore, analysed in sensitivity analysis.
Figure 3. Log-normal curve fits to Kaplan–Meier data for (a) ITT and (b) PSA > 20 ng/mL populations. ITT, intention to treat; PSA, prostate-specific antigen.
The efficacy of LHRH agonists included in the model was assumed to be equal, regardless of treatment drug, dosage and frequency. This was supported by a literature review designed to identify clinical trials or systematic reviews comparing multiple LHRH agonistsCitation30. Due to the evidence identified by the literature review and included within the summaries of product characteristics, it was concluded that LHRH agonists are equivalent pharmacologically and that no LHRH agonist has shown superior clinical efficacy or tolerability compared to another. In addition, no statistically significant differences have been found in clinical outcomes between different doses or frequencies of injections of LHRH agonistsCitation22,Citation30–36.
The probability of progression for subsequent lines of treatment was estimated from average durations of response published in the EAU prostate cancer guidelines ()Citation6.
Mortality
The background mortality of patients was modeled using age- and gender-specific mortality rates from published UK life expectancy tablesCitation37. Prostate cancer specific mortality is incorporated as the relative survival of prostate cancer patients compared to members of the general population, taken from Scottish registry data. This source was chosen because it is more recent than similar Welsh registry data and age-specific rates that were available. Parametric curves were fit to the relative survival data to extrapolate beyond the reported 5 years. Five curves were fit to the data (Weibull, log-normal, exponential, Gompertz, and log-logistic), of which the log-logistic was the best fit. In the base case, prostate cancer specific mortality is set equal for all health states, assuming no difference in disease stage across different treatments. Differential mortality for treatments was tested in the sensitivity analysis. A multiplier was applied for patients receiving second-line chemotherapy with abiraterone to reflect evidence that there is a reduction in mortality risk for these patients, in line with a report published by NICE in the UKCitation38. The report indicates a mean survival of 825 days for patients treated with abiraterone, compared to 550 days for patients on standard care. Therefore, a relative risk of mortality of 0.67 (550/825) is applied to the mortality risk of patients receiving abiraterone in the model.
Adverse events
The effects of adverse events related to clinical flare were not included in the base-case model, but were incorporated as part of sensitivity analyses as the increased risk of musculoskeletal events and spinal cord compression associated with testosterone flares following treatment initiation. The risk of spinal cord compression was taken from a study by Oh et al.Citation7, which found that rates of compression or fracture were less than 1% in the first 30 days after beginning LHRH agonist therapy, regardless of anti-androgen use. As spinal cord compression is a rare event, data were not available from the trial. Events were incorporated in the same manner as in Lu et al.Citation17, which used a decision tree to estimate the proportion of patients experiencing mild and severe spinal cord compression as a result of the testosterone flare associated with LHRH agonist treatment. Patients have a risk of suffering from spinal cord compression in the first cycle of the model and those who experience it are treated with rescue therapies. Consultation with Welsh physicians indicated that rescue therapy would consist of surgery (5% of patients) or radiotherapy (95% of patients). The outcomes of treatment are then modeled (no lasting complications, improvement or paraplegia), with the proportion of patients experiencing each outcome taken from Lu et al.Citation17.
The cycle risk of other musculoskeletal events was estimated from curves fit to events recorded as part of the CS21A clinical trial. The other adverse event that was significantly different between the two arms of the CS21 trial was injection site reactionsCitation12. These were not included in the model as the cost and quality-of-life impacts of these events are negligible. The injection-site reactions were mainly mild or moderate in intensity and predominantly occurred with the first dose (33% of initiation dose injections compared to 4% with maintenance doses)Citation12,Citation25.
Quality-of-life
The health-related utility values associated with each health state were obtained from published literature and related prostate cancer AWMSG guidance (). A search identified the publication by Bayoumi et al.Citation39 as the most up-to-date source in the available literature for first- and second-line hormonal treatment and palliative care health states, whereas AWMSG guidance was used to populate utility values for chemotherapy with abirateroneCitation40. Health-related quality-of-life data collected in the CS21 clinical trial were not used in the model; these only captured the quality-of-life of patients pre-progression, and could not be used to model the quality-of-life decline that results from disease progression. In sensitivity analyses, additional utility decrements associated with adverse events were introduced.
Costs
The costs associated with residing in each health state were categorized into drug costs and administration costs, see . Administration costs were calculated separately at treatment initiation (first cycle) and for subsequent cycles. The requirement for hospital visits and service use was modeled to reflect the frequency of drug administration; degarelix (monthly treatment) incurs greater administration costs per cycle compared to 3-monthly leuprorelin. The frequency of resource use across treatments was elicited from clinical experts in Wales or identified from published literature. The cost of degarelix used in the base-case model included a confidential PAS price.
Model outputs
To examine the robustness of the model to key assumptions and data source choices, multiple scenario analyses were performed. The base-case model and the alternative scenarios modeled are described in .
Table 2. Scenarios modeled in sensitivity analyses.
Deterministic sensitivity analysis was performed to examine the impact of variation in individual parameters. This took the form of an analysis of extremes, in which incremental cost-effectiveness ratios (ICERs) were generated for the greatest and smallest credible values for each parameter.
Probabilistic sensitivity analyses were run with 1000 iterations, in which parameter values were randomly selected using Monte-Carlo simulation methods. The proportion of sampled ICERs that were indicative of cost effectiveness at different willingness-to-pay thresholds was used to generate a cost-effectiveness acceptability curve.
As the model is expected to be especially sensitive to the main efficacy parameters (the hazard ratios of response of degarelix compared to LHRH agonists) these were investigated separately in threshold analyses. These analyses determined the parameter values at which the ICER is £30,000, £20,000 and £0 (where the costs of the degarelix and LHRH agonist are equal).
A second threshold analysis was performed to determine the length of time before there was return on the additional investment required to treat patients with degarelix. The time horizon was extended in 1-month increments to determine the point at which the model predicted incremental cost savings with degarelix.
A final threshold analysis was performed to determine the percentage increase in the degarelix list price that would be required to result in an incremental cost of £0 over the duration of the model.
Results
The discounted results of the base-case model, calculated using the PAS price for degarelix, demonstrate that degarelix is dominant compared to 3-monthly leuprorelin 11.25 mg. The model estimates that treatment with degarelix leads to cost savings of £3633 in the ITT population and £4310 in the PSA > 20 ng/mL group and quality-adjusted life year (QALY) gains of 0.20 and 0.24, respectively ().
Table 3. Results of the base-case model.
The breakdown of the costs and QALYs is shown in . These demonstrate that degarelix is associated with increased costs for first-line drug and administration costs and cost-savings for subsequent tiers of treatment.
Table 4. Results breakdown.
Deterministic sensitivity analysis
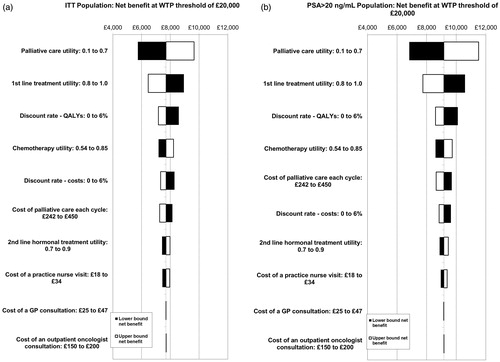
The 10 most influential parameters for each population are shown in the tornado diagrams in . The greatest variation in ICER was seen for the utility of patients receiving palliative care. These graphs do not show the impact of the efficacy hazard ratios, as sensitivity to these parameters are examined separately in threshold analyses.
Figure 4. Tornado diagrams of 10 most influential parameters for the (a) ITT and (b) PSA > 20 ng/mL populations. The impact of the efficacy hazard ratios is not included here. The sensitivity to these parameters is examined separately in threshold analyses. GP, general practitioner; ICER, incremental cost-effectiveness ratio; ITT, intention to treat; QALYs, quality-adjusted life years; PSA, prostate-specific antigen; WTP, willingness to pay.
Probabilistic sensitivity analysis
The mean results of the probabilistic sensitivity analysis confirm the deterministic results (). Each of the 1000 sampled iterations is shown in for both patient populations. The cone shape that can be seen in the graphs is caused by the large impact of the hazard ratio for PSA progression on the model. As the hazard ratio for PSA progression tends towards 1, the impact of variation in other parameters, such as utilities, reduces. Conversely, as the hazard ratio reaches its upper bound, these parameters have the potential to cause large variation in outcomes.
Figure 5. Cost-effectiveness plane for (a) ITT and (b) PSA > 20 ng/mL populations. ITT, intention to treat; PSA, prostate-specific antigen; QALYs, quality-adjusted life years.
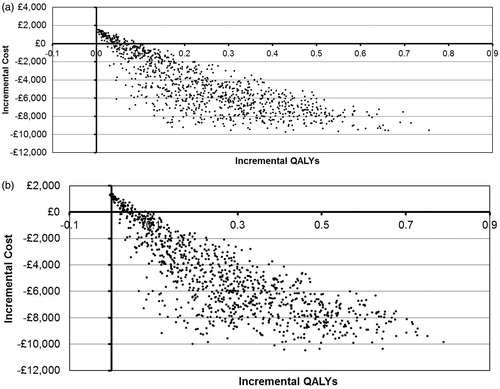
The probability of cost-effectiveness at a willingness-to-pay threshold of £20,000 was 95% for the ITT population and 96% for the PSA > 20 ng/mL population. At a willingness-to-pay threshold of £30,000, the probabilities of cost effectiveness were 96% and 97%, respectively.
Threshold analysis
Efficacy hazard ratios
shows the values of the main efficacy hazard ratios that produce estimates of £30,000, £20,000, and £0 per QALY for each of the patient populations. These indicate that, for the ITT population, the true value of the hazard ratio would have to be 64% of the mean value for degarelix to stop being cost-effective at a willingness-to-pay threshold of £20,000. For the PSA > 20 ng/mL population the value would have to be 61% of the mean value.
Table 5. Hazard ratio threshold analysis results.
Return on investment
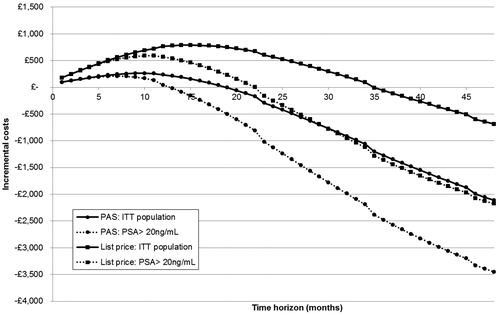
shows the cumulative incremental costs predicted by the model from 1- to 48-month time horizons with and without the degarelix PAS. Based on this analysis, the additional investment required to treat patients with degarelix with the PAS is expected to be mitigated after ∼19 months for the ITT population and 13 months for the PSA > 20 ng/mL population. Without the PAS this rises to ∼35 months for the ITT population and 22 months for the PSA > 20 ng/mL population.
Drug cost of degarelix
An additional threshold analysis indicated that the list price of degarelix would have to increase by 29.6% before the expected incremental savings are lost. With an increase in price of 29.6%, the cost of degarelix starter injections would be £336.91, and the cost of maintenance injections would be £167.64.
Scenario analyses
Summary results for the alternative scenarios modeled are shown in . Degarelix remained dominant for all of the scenarios modeled, but results were most sensitive to the assumptions surrounding the duration of the differential PSA progression rates (Scenarios 1 and 2).
Table 6. Scenario analysis results.
Discussion
The results presented in this analysis indicate that degarelix is likely to be cost-effective for use in the UK with all scenarios tested indicating degarelix is dominant (i.e., less costly and more effective) compared to LHRH agonists. The results obtained from this cost-effectiveness model are based upon statistical analysis of clinical trial data from the relevant clinical trials (CS21 and CS21a) supplemented by literature only where clinical trial data were not available (for utilities and spinal cord compression rates). The model presented is a relatively simple model based upon longer time to PSA progression as demonstrated within the CS21 trial (and, therefore, increased time spent on first-line treatment) and a reduction in musculoskeletal events associated with flareCitation45.
A previous cost-effectiveness analysis of degarelix vs LHRH agonists reported an ICER of £59,000 per QALY gained with degarelix at full list priceCitation17. However, there were significant differences between that study and the evaluation presented here, and these are detailed in . Chief among these was the source of incremental benefit of degarelix. While the base-case analysis in our model incorporated differential PSA progression rates for the two treatments, the model reported by Lu et al.Citation17 assumed no difference in PSA progression, but instead focused on the reduction in testosterone flare and related adverse events. In addition, the model by Lu et al. used an incorrect price for degarelix; the price included VAT at 17.5%, whilst the cost of the comparator drug did not have VAT included.
Table 7. Comparison to economic model reported by Lu et al.Citation17.
The model presented here is well-balanced, including the effects of degarelix on both adverse events and PSA progression using the latest available trial data. Extensive sensitivity analyses have been conducted in order to examine model sensitivity to key assumptions.
The key model limitations derive primarily from the quality of the clinical trial data used to inform the model in terms of population treated and the maturity of the available data. There were many patients with localized disease in the CS21 trial (approximately one third of patients); this high proportion of patients with early stage disease may not be reflective of UK clinical practice where treatment with LHRH agonists is usually in patients with advanced prostate cancer.
The treatment of early stage patients in CS21 is likely to have biased against degarelix because these patients are less likely to show rapid progression and, therefore, unlikely to experience PSA progression within the trial. The proportions of patients experiencing PSA progression or death within the trial were 14% on the leuprorelin arm and 9% on the degarelix armCitation13. As would be expected, PSA progression occurred more frequently in both treatment groups in patients with high baseline PSA and patients with advanced disease. In patients with metastatic disease, 21.6% of those in the degarelix 240/80 mg group and 36.2% of those in the leuprorelin group experienced PSA progressionCitation13. It is, therefore, likely that, if the trial had been conducted in a group consisting only of patients with advanced prostate cancer, the treatment benefits from degarelix would have been greater. The small sample sizes in the advanced prostate cancer sub-group in the trial unfortunately did not allow modeling to be to be conducted in this sub-group alone (n = 101 for degarelix, n = 99 for leuprorelin in the sub-group), additionally analyzing for this sub-group only would break randomization, potentially resulting in an imbalance in characteristics between the two arms which could bias resultsCitation12.
Another model limitation is the lack of anti-androgen cover provided to most patients within the CS21 clinical trial (89% did not receive concomitant bicalutamide)Citation12. Provision of bicalutamide, however, did not appear to have a large impact on the probability of testosterone flare (81% of patients who did not receive bicalutamide experienced testosterone flare compared to 74% of patients who did receive bicalutamide and 0% of patients receiving degarelix)Citation12. Pooled analysis from CS21 and CS35 degarelix trials showed that the PSA progression-free survival failure rate (adjusted for baseline PSA, prostate cancer stage and Gleason score) was significantly lower with degarelix than with LHRH agonists in combination with anti-androgen flare protection for all patients (HR = 0.490, p = 0.0028). It also showed that patients receiving LHRH agonists in combination with anti-androgen flare protection still experienced testosterone flareCitation51. Similar results have been seen elsewhere in published literatureCitation7.
Other limitations include the lack of head-to-head data for degarelix and goserelin or triptorelin. However, it is assumed that data from the clinical study relating to leuprorelin could be used for the goserelin arm of the economic model. The literature suggestsCitation30 that LHRH agonists may be equally effective, but no formal meta-analysis was conducted, and available published evidence is not conclusive. In addition, significant uncertainty surrounds the QALY gains that can be achieved through the use of degarelix, which is highlighted in the probabilistic sensitivity analysis. There is a lack of utility information available from the clinical trial and a lack of high-quality evidence from published literature. However, in all cases, even assuming large confidence intervals in the utilities modeled, QALY gains (rather than decrements) were shown with degarelixCitation39.
Finally, the benefits of degarelix in terms of cost-saving derive primarily from reduced resource use at latter lines of treatment (i.e., reduced cost of chemotherapy, abiraterone, and palliative care). Whilst these reduced costs could not be observed within the trial period available, UK literature indicates that the costs of the final year of life for patients with prostate cancer are high (over £14,000 per patient)Citation45. The costs currently included in the model for the latter health states such as palliative care are relatively modest in comparison.
Aside from the limitations within the clinical data detailed above, the model presented within this analysis has increased validity compared to previous modeling conducted by Lu et al.Citation17, because inputs are derived from patient-level data from clinical trials rather than published literature. Nevertheless, the model presented includes the same weakness in terms of the scarcity of available data for spinal cord compression rates, but these rates have minimal impact within the model presented here.
Both parameter and structural uncertainty surrounding the source of clinical benefits within our model were examined through extensive sensitivity analysis. The model is sensitive to structural assumptions surrounding the source of clinical benefit (i.e., whether benefit is derived from slowing PSA progression or solely from prevention of testosterone flare and associated flare symptoms). Clinical evidence on the benefits of preventing PSA progression is not yet conclusive; however, there is a growing weight of evidence regarding the long-term effects of PSA on disease progression and mortalityCitation27,Citation52,Citation53.
The model presented in the base case is likely to be conservative as it does not account for benefits related to the difference in rates of cardiovascular events and mortality between LHRH agonists and degarelix and the likely difference in mortality due to reduced PSA progression. Recently-presented evidence shows that, within pooled degarelix data, in comparison with LHRH agonists, degarelix decreased the risk of subsequent serious cardiovascular events and serious cardiovascular events or death over 1 year of treatment in men with a history of cardiovascular disease by more than 50%Citation10. Additionally, Southwest Oncology Group data show that PSA progression predicts overall survival in hormone-sensitive and castration-resistant prostate cancerCitation27. A recently-presented pooled analysis from the degarelix trials shows a significant improvement in overall survival in the degarelix group compared to the LHRH agonist group (p = 0.0329)Citation9,Citation11. When a differential mortality risk is incorporated into the model (Scenario 11), patients treated with degarelix incur higher costs compared to the cost incurred in the base case. However, the results of the scenario analysis indicate that treatment with degarelix remains cost-saving and continues to be the dominant treatment strategy.
The treatment pathway used within our model is easily generalizable to other European settings: sensitivity analysis showed that results did not differ substantially when alternative treatments in the EAU guidelinesCitation6 were included in the treatment sequence. However, differences in local costs and the basis of the decision to move from first-line to second-line treatments (whether this is based upon PSA progression or not) would impact the generalizability of the results.
Conclusions
The economic analysis presented in this paper, which is based on the model utilized in the recent successful submission to the AWMSG, has shown that, due to the increase in time to PSA progression demonstrated with the clinical trials, degarelix is estimated to be less costly over a lifetime of treatment than the current standard treatment pathway using leuprorelin, whether or not the PAS in operation in Wales and Scotland is taken into account. This cost-effectiveness estimate is likely to be conservative; the current model does not incorporate the reduced risk of cardiovascular events experienced by patients treated with degarelix. Future analyses should explore how this additional benefit impacts the cost-effectiveness of treatment with degarelix.
Degarelix is cost-saving with the PAS when the benefits of slowing PSA progression are taken into account and remains cost-saving when only the benefits of preventing testosterone flare are taken into account.
Transparency
Declaration of funding
This paper was sponsored by Ferring Pharmaceuticals, Ltd.
Declaration of financial/other relationships
Dawn Lee, Joshua Porter, Daniel Gladwell and Nic Brereton have disclosed that they are employees of BresMed, a company that was reimbursed by Ferring Pharmaceuticals Ltd as a consultancy for their time on the developing of the model and preparation of the manuscript. Sandy Nielsen is a full time employee of Ferring Pharmaceuticals Ltd. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary Appendix
Download PDF (34.5 KB)Acknowledgments
The authors thank Statistician Anders Malmberg of Ferring Pharmaceuticals Ltd, and Editor Susan Molone of BresMed, for their assistance.
References
- National Collaborating Centre for Cancer. Prostate cancer: diagnosis and treatment (CG58) - full guidance. London, UK: National Institute for Health and Clinical Excellence, 2010 . Available at: http://www.nice.org.uk/nicemedia/pdf/CG58NICEGuideline.pdf
- Ferlay FJ, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 2013;49:1374–403
- Office for National Statistics. Statistical bulletin: cancer incidence and mortality in the United Kingdom, 2008–2010. UK: Office of National Statistics, 2012. Available at: http://www.ons.gov.uk/ons/rel/cancer-unit/cancer-incidence-and-mortality/2008-2010/stb-cancer-incidence-and-mortality-in-the-united-kindom--2008-2010.html
- National Institute for Health and Clinical Excellence. Prostate cancer (hormone refractory) – docetaxel guidance: technology appraisal TA101. London, UK: National Institute for Health and Clinical Excellence, 2006 . Available at: http://www.nice.org.uk/nicemedia/live/11578/33348/33348.pdf
- Office for National Statistics. Statistical bulletin: cancer survival in England: patients diagnosed 2006–2010 and followed up to 2011. UK: Office of National Statistics, 2012. Available at: http://www.ons.gov.uk/ons/rel/cancer-unit/cancer-survival/2006---2010--followed-up-to-2011/stb-cancer-survival.html
- Heidenreich A, Bastian PJ, Bellmunt J, et al. Guidelines on prostate cancer. Arnhem: European Association of Urology, 2012
- Oh WK, Landrum MB, Lamont EB, et al. Does oral antiandrogen use before leuteinizing hormone-releasing hormone therapy in patients with metastatic prostate cancer prevent clinical consequences of a testosterone flare? Urology 2010;75:642–7
- Crawford ED, Shore N, Miller K, et al. Degarelix versus LHRH agonists: differential skeletal and urinary tract outcomes from an analysis of six comparative randomized clinical trials. American Society of Clinical Oncology 2013 Genitourinary Cancers Symposium (ASCO-GU). Orlando, FL; 2013
- Klotz L, Miller K, Crawford D, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. Eur Urol 2014; epub ahead of print
- Albertsen PC, Klotz L, Tombal B, et al. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol 2014;65:565–73
- Miller K, Crawford ED, Shore N, et al. Disease control-related outcomes from an analysis of six comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone (LHRH) agonists. 28th Annual European Association of Urology (EAU) Congress. Milan, Italy; 2013
- Klotz L, Boccon-Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU Int 2008;102:1531–8
- Tombal B, Miller K, Boccon-Gibod L, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. Eur Urol 2010;57:836–42
- Porter AT, Ben-Josef E. Humoral mechanisms in prostate cancer: a role for FSH. Urol Oncol 2001;6:131–8
- Morote J, Orsola A, Planas J, et al. Redefining clinically significant castration levels in patients with prostate cancer receiving continuous androgen deprivation therapy. J Urol 2007;178:1290–5
- Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med 2010;363:1621–30
- Lu L, Peters J, Roome C, et al. Cost-effectiveness analysis of degarelix for advanced hormone-dependent prostate cancer. BJU Int 2012;109:1183–92
- Hind T, Hatoum EDC, Nielsen SK, et al. Cost-effectiveness analysis comparing degarelix with leuprolide in hormonal therapy for patients with locally advanced prostate cancer. Expert Rev Pharmacoecon Outcomes Res 2013;13:261–70
- Lee D, Gladwell D, Nielsen S, et al. PCN70 The cost effectiveness of Degarelix for the treatment of prostate cancer in the UK. Value Health 2012;15:A421
- Fisher D, Brereton N, Tate E. PCN100 a cost-utility analysis of Degarelix in the treatment of advanced hormone-dependent prostate cancer in Scotland. Value Health 2011;14:A452
- Scottish Medicines Consortium. Degarelix 120 mg and 80 mg powder and solvent for solution for injection. SMC No. (560/09). Glasgow, UK: Scottish Medicines Consortium, 2010. Available at: http://www.scottishmedicines.org.uk/files/advice/degarelix_Firmagon_RESUBMISSION_FINAL_DECEMBER_2010.doc_for_website.pdf
- All Wales Medicines Strategy Group Final Appraisal Recommendation 4112: Degarelix (Firmagon®) 80 mg and 120 mg injection November 2012. Wales, UK: All Wales Medicines Strategy Group, 2012. Available at: http://www.awmsg.org/awmsgonline/app/appraisalinfo/755;jsessionid=00f9e76a1513f2188ea8e896a135
- Misra UK, Payne S, Pizzo SV. Ligation of prostate cancer cell surface GRP78 activates a proproliferative and antiapoptotic feedback loop: a role for secreted prostate-specific antigen. J Biol Chem 2011;286:1248–59
- Huang SP, Bao BY, Wu MT, et al. Impact of prostate-specific antigen (PSA) nadir and time to PSA nadir on disease progression in prostate cancer treated with androgen-deprivation therapy. Prostate 2011;71:1189–97
- Crawford ED, Tombal B, Miller K, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. J Urol 2011;186:889–97
- Crawford E, Shore N, Moul J, et al. Long term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a one-arm crossover from leuprolide to degarelix. Eur Urol 2014;[Accepted]
- Hussain M, Goldman B, Tangen C, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009;27:2450–6
- National Institute for Health and Care Excellence. Guide to methods of technology appraisal. London, UK: National Institute for Health and Clinical Excellence, 2013. Available at: http://www.nice.org.uk/media/D45/1E/GuideToMethodsTechnologyAppraisal2013.pdf
- Heyns CF. Triptorelin in the treatment of prostate cancer. Clinical Efficacy and Tolerability. Am J Cancer 2005;4:169–83
- Seidenfeld J, Samson DJ, Hasselblad V, et al. Single-therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta-analysis. Ann Intern Med 2000;132:566–7
- Herranz Amo F. Comparative analysis of six months formulation of LHRH analogues for prostate cancer treatment. Arch Espan Urol 2010;63:275–81
- Novara G, Galfano A, Secco S, et al. Impact of surgical and medical castration on serum testosterone level in prostate cancer patients. Urol Int 2009;82:249–55
- Akaza H, Usami M, Koiso K, et al. Long-term clinical study on luteinising hormone-releasing hormone agonist depot formulation in the treatment of stage D prostatic cancer. The TAP-144-SR Study Group. Jpn J Clin Oncol 1992;22:177–84
- Ferring Pharmaceuticals Ltd. Gonapeptyl depot 3.75 mg. Summary of product characteristics. Surrey, UK: Datapharm Communications Ltd, 2013. Available at: http://www.medicines.org.uk/emc/medicine/12870/SPC/Gonapeptyl+Depot+3.75+mg/. [Last accessed 26 January 2014]
- AstraZeneca UK Ltd. Zoladex® 3.6 mg Implant. Summary of product characteristics. Surrey, UK: Datapharm Communications Ltd, 2013. Available at: http://www.medicines.org.uk/emc/medicine/7855/SPC/Zoladex+3.6mg+Implant/. [Last accessed 26 January 2014]
- Takeda UK Ltd. Prostap® 3 DCS 11.25 mg. Summary of product characteristics. Surrey, UK: Datapharm Communications Ltd, 2013. Available at: http://www.medicines.org.uk/emc/medicine/24680/SPC/Prostap+3+DCS/. [Last accessed January 26, 2014]
- Office for National Statistics. United Kingdom, Interim Life Tables 2007–09. UK: Office of National Statistics, 2012. Available at: www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-61850. [Last accessed 27 January 2014]
- National institute for Health and Care Excellence. Final appraisal determination: Abiraterone for castration-resistant metastatic prostate cancer previously treated with a docetaxel-containing regimen. London, UK: National Institute for Health and Clinical Excellence, 2012. Available at: www.nice.org.uk/nicemedia/live/13484/59217/59217.pdf. [Last accessed 27 January 2014]
- Bayoumi AM, Brown AD, Garber AM. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst 2000;92:1731–9
- All Wales Medicines Strategy Group. AWMSG Secretariat Assessment Report – Advice no. 0612 Abiraterone (Zytiga®) 250 mg tablets. Wales, UK: All Wales Medicines Strategy Group, 2011. Available at: http://www.awmsg.org/awmsgonline/app/appraisalinfo/3. [Last accessed 27 January 2014]
- British National Formulary 63. London, UK: BMJ Group and Pharmaceutical Press, 2012. Available at: www.bnf.org. [Last accessed 20 March 2012]
- Guest JF, Ruiz FJ, Greener MJ, et al. Palliative care treatment patterns and associated costs of healthcare resource use for specific advanced cancer patients in the UK. Eur J Cancer Care 2006;15:65–73
- Personal Social Services Research Unit. Unit Costs of Health and Social Care 2011. Kent, UK: Personal Social Services Research Unit, 2011. Available at: www.pssru.ac.uk/pdf/uc/uc2011/uc2011.pdf. [Last accessed 27 January 2014]
- Department of Health. NHS Reference Costs 2010-11. UK: Department of Health, 2011. Available at: https://www.gov.uk/government/publications/2010-11-reference-costs-publication. [Last accessed 27 January 2014]
- Gkougkousis E, Oozeerally Z, Allchorne P, et al. 149 The usage of hospital resources by prostate cancer patients in the last year of life. J Urol 2013;189:e61
- British National Formulary 59. London, UK: BMJ Group and Pharmaceutical Press, 2011. Available at: www.bnf.org
- Curtis L. Unit Costs of Health and Social Care 2008. Kent, UK: Personal Social Services Research Unit, 2008. Available at: http://www.pssru.ac.uk/archive/pdf/uc/uc2008/uc2008.pdf
- National Institute for Health and Clinical Excellence. CG75: metastatic spinal cord compression: diagnosis and management of adults at risk of and with metastatic spinal cord compression. London, UK: NICE; 2008
- Bennett C, Matchar D, McCrory G, et al. Cost-effective models for Flutamide for prostate carcinoma patients: are they helpful to policy makers? Cancer 1996;77:1854–61
- Hollingworth W, Gray D, Martin B, et al. Rapid Magnetic Resonance Imaging for diagnosing cancer-related low back pain: a cost-effectiveness analysis. J Gen Intern Med 2003;18:303–12
- Iversen P, Damber JE, Malmberg A, et al. Improved outcomes with degarelix monotherapy compared with luteinizing hormone-releasing hormone (LHRH) agonists plus antiandrogen flare protection in the treatment of men with advanced prostate cancer. 29th Congress of the Scandinavian Association of Urologists. Sandefjord, Norway; 2013
- Halabi S, Vogelzang NJ, Ou SS, et al. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol: Official J Am Soc Clin Oncol 2009;27:2766–71
- Armstrong AJ, Garrett-Mayer E, de Wit R, et al. Prediction of survival following first-line chemotherapy in men with castration-resistant metastatic prostate cancer. Clin Cancer Res: Official J Am Assoc Cancer Res 2010;16:203–11
- Ekman M, Johnell O, Lidgren L. The economic cost of low back pain in sweden in 2001. Acta Orthopaedica 2005;76(2):275-84