Abstract
Objectives:
This study examined total healthcare costs and rates of patients with rheumatoid arthritis (RA) who switch biologic disease-modifying anti-rheumatic drug (bDMARD) therapy in a real world setting.
Methods:
A retrospective longitudinal analysis was conducted in patients with RA using IMS PharMetrics Plus database from 1/1/2004 to 3/31/2010. The first-line cohort included patients newly initiated on abatacept or the tumor necrosis factor-alpha inhibitors (anti-TNFs) adalimumab, etanercept, or infliximab, with 12 months of continuous follow-up. The second-line cohort included patients initiating a bDMARD with evidence of a different bDMARD within the previous 2 years and with 12 months of continuous follow-up. Switching was defined as a different bDMARD claim within a 200% gap in days supply from the previous bDMARD claim. Non-switchers stayed on their bDMARD in the follow-up period. Monthly total healthcare costs for switchers and non-switchers and rates of bDMARD switching were examined. Switch rates for each bDMARD were also compared.
Results:
First-line switchers had significantly higher monthly total healthcare costs after the switch than non-switchers ($3759 vs $2343; p < 0.05), as did second-line switchers ($3956 vs $2616; p < 0.05). First-line abatacept (2.1%) had significantly lower rates of switching compared to adalimumab (9.5%), etanercept (9.0%), and infliximab (5.5%). Second-line abatacept (8.0%) had significantly lower rates of switching compared to adalimumab (16.7%), etanercept (14.4%), and infliximab (14.3%).
Limitations:
There are no clinical data available in this database and, therefore, this study did not examine the clinical drivers of healthcare costs and switch rates.
Conclusions:
Monthly total healthcare costs were higher for bDMARD switchers following the switch compared to non-switchers. Patients on abatacept switched less frequently than patients on anti-TNFs. This study highlights the need to identify patients who are likely to switch in order to ensure they receive the appropriate therapy which may improve outcomes and decrease healthcare costs.
Keywords::
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive, and systemic autoimmune disease which affects 1.3 million adults in the USCitation1. Activation of T-cells by an unknown antigen(s) resulting in the proliferation of synoviocytes, endothelial cells, and other pro-inflammatory cells, as well as induction of autoantibody formation and secretion of pro-inflammatory cytokines and proteases are implicated in the pathogenesis of RACitation2. An increased understanding of the pathogenesis of RA has resulted in the development of several therapeutic agents called biologic disease-modifying anti-rheumatic drugs (bDMARDs). bDMARDs consist of therapeutic agents with differing mechanisms of action (MOA) including tumor necrosis factor-alpha inhibitors (anti-TNFs) such as adalimumab, etanercept, and infliximab, and a selective T-cell co-stimulation modulator—abataceptCitation2. Early and aggressive treatment with bDMARDs has been shown to improve disease outcomes such as the inhibition of radiographic progression and structural damageCitation2.
Given the number of bDMARD treatment options available to clinicians and their effectiveness in treating RA, switching between the various bDMARDs is common practice in real-world settingsCitation3,Citation4. Lack of efficacy and side-effects are the primary underlying reasons that RA patients are switched from their initial bDMARD to another bDMARDCitation5,Citation6. Oei et al.Citation5 used clinical data and reasons for switching or stopping bDMARDs captured in a longitudinal registry of RA patients from a VA medical center. The authors found that adverse events and lack of efficacy were the two most prominent reasons for switching or stopping bDMARDsCitation5. There is evidence to suggest that patients who have a lack or loss of efficacy to an anti-TNF agent are less likely to respond to a second anti-TNF agentCitation7,Citation8. This indicates that mechanism of action (MOA) may play an important role in response and, consequently, adherence to the bDMARD that a patient is switched to. There are also some studies which support the theory that switching between anti-TNF agents may be effective; however, the effectiveness of the switch bDMARD may depend on the reason for failure of the previous anti-TNF agentCitation8–11.
Given the prevalence of switching bDMARDs in real world settings, it is important to examine the association between switching and healthcare utilization and costs at a population level since this practice may have implications for the economics of RA management. To date, there have been no studies in the literature which have examined the real world impact of switching bDMARDs. This study examined the association between switching and healthcare costs in patients on both first and second-line bDMARDs. In addition, this study also examined the rates of switching in patients treated with bDMARDs.
Methods
A retrospective longitudinal analysis was conducted using IMS PharMetrics Plus, a database of commercially insured US patients. The database contains de-identified insurance claims data for more than 70 million members covered by over 100 commercial health plans across the US. The IMS PharMetrics Plus database is geographically representative of the US population and includes a variety of demographic measures. The database also includes medical claims from both the inpatient and outpatient setting, diagnosis and procedure codes, and pharmacy claims.
Data were available from January 1, 2004 to March 31, 2010. Patients who initiated a bDMARD (intravenous abatacept, adalimumab, etanercept, or infliximab) from July 1, 2004 to March 31, 2009 were identified; rituximab, which was commercially available during this time period, was not included since it does not have a first-line indication in RA. Date of first bDMARD claim served as the index date. Patients were required to have 6 months pre-index and 12 months post-index continuous eligibility in the database. Patients were included if they had a diagnosis of RA (ICD-9 714.0, 714.2) during the 6-month pre-index period and if they were ≥18 years of age at index. Patients were excluded if they had a diagnosis of Crohn’s disease, psoriasis, psoriatic arthritis, ulcerative colitis, ankylosing spondylitis, regional enteritis, or anal fistula during the pre-index period. Patients were also excluded if they had <7 days supply of adalimumab or etanercept, or if they had claims for more than one bDMARD on the same date. The study population was stratified into first- and second-line cohorts.
First-line cohort
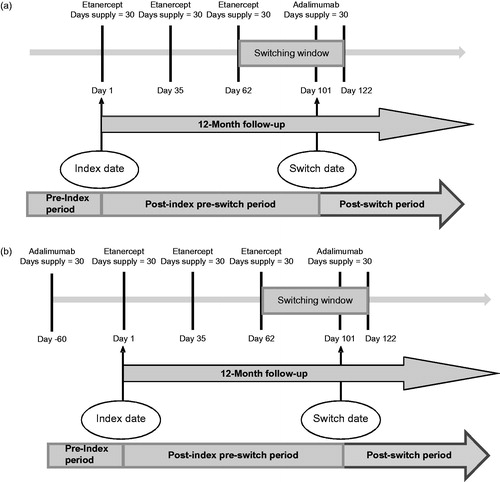
Patients were considered to be on their first bDMARD if there was no evidence of a different bDMARD at any time prior to index date. The date on which a patient had a claim for their first bDMARD (or the index bDMARD) was designated as the index date. Patients were followed for 12 months post-index to determine if they switched bDMARD (those that switched from their first bDMARD to a second bDMARD), i.e., had a claim for a new bDMARD within 200% gap in days supply of the previous index bDMARD claim. For example, a patient who initiated first-line etanercept and who had a subsequent claim for adalimumab within 60 days (i.e., within 200% gap in days supply) of their last etanercept claim would be considered a switcher (). Days supply values were based on the ‘Days Supply’ field in pharmacy claims for SC agents and were imputed based on maximum dose interval from the label for IV agents (i.e., 28 days for abatacept and 56 days for infliximab). Patients who switched or re-started their previous biologic beyond the 200% gap were excluded from the analysis. This definition of switch ensured that the analysis focused on a population that was consistently on a biologic without large gaps in therapy. The non-switcher cohort was comprised of patients who stayed on their index therapy in the post-index period or discontinued without evidence of re-starting a bDMARD during the post-index period.
Second-line cohort
To maximize the population for the second-line analysis, we defined a new population during the same study period. The second-line population was defined as patients initiating their second bDMARD with evidence of only one different bDMARD up to 2 years prior to the index date. The date on which a patient had a claim for their second bDMARD (or the index bDMARD) was designated as the index date (). The second-line patients were also divided into switchers (those that switched from a second bDMARD to a third bDMARD) and non-switchers based on the same criteria as the first-line population. Patients were followed for the 12 month post-index period after starting their second biologic to determine if they switched bDMARD, i.e., had a claim for a new, third bDMARD, within 200% gap in days supply of the previous index bDMARD claim. Patients who switched or re-started their previous biologic beyond the 200% gap were excluded from the analysis. The non-switcher cohort was comprised of patients who stayed on their index bDMARD therapy or discontinued without evidence of re-starting a bDMARD during the post-index period.
Study outcomes
The study period for switchers and non-switchers was divided into a pre-index period and a post-index period. The post-index period for switchers was further divided into a pre-switch and a post-switch period (). Outcomes of interest included mean monthly total healthcare costs in the pre-index and post-switch periods for switchers and in the pre-index and post-index period for non-switchers. Total healthcare costs were comprised of medical and pharmacy costs (including the cost of bDMARDs). Costs were determined to be RA-related if there was an ICD-9 diagnosis of RA in the primary position on that claim. All costs were based on paid amounts reported in the database.
Switch rates for the index bDMARDs were also examined, for both first-line and second-line cohorts. In addition, a sub-analysis of the anti-TNF agents examined switching within class to another anti-TNF agent.
Statistical analysis
Analyses were run separately for the first-line and second-line cohorts. For baseline characteristics, t-tests were used for continuous variables and chi-square tests for categorical variables to determine if differences existed between switchers and non-switchers. Monthly total healthcare costs of switchers and non-switchers were compared using Wilcoxon signed rank tests. A multivariate cost model was developed utilizing a gamma regression with a log transformation to determine if differences existed between post-switch costs of switchers and post-index costs of non-switchers, while controlling for age, gender, Charlson Comorbidity Index (CCI), and pre-index costs. The proportion of patients who switched within the post-index period was determined for each index bDMARD. Logistic regression was used to determine the odds of switching for the bDMARDs while controlling for age, gender, CCI, pre-index hospitalization, and pre-index costs.
Results
First-line cohort
The initial population included 10,281 patients with RA who were identified as being on a first-line bDMARD (Appendix Table A1a); 524 patients were excluded from the analysis (they either switched beyond the allowable gap or re-started their index bDMARD and later switched). Of the remaining 9757 patients, a total of 7.8% (n = 765) switched their bDMARD therapy within the first year of initiation. Baseline characteristics are presented in . bDMARD switchers tended to be younger (53 years vs 55 years; p < 0.001), have higher pre-index hospitalization rates (9.5% vs 7.2%; p = 0.015), and have higher pre-index monthly total healthcare costs ($1025 vs $796; p < 0.001) compared to non-switchers.
Table 1. Pre-index characteristics of switchers and non-switchers.
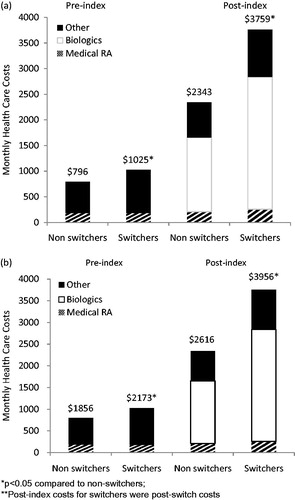
Mean time to switch for the switching cohort was 171 days. Monthly post-switch costs were $1416 higher compared to the post-index costs of non-switchers ($3759 vs $2343; p < 0.001; ). Cost breakdowns by type of service are depicted in Appendix Table A2a. In a Gamma regression for monthly total healthcare cost, post-switch costs increased by 51% (p < 0.001) compared to the post-index costs of non-switchers (Appendix Table A3a).
Figure 2. Mean monthly total healthcare costs of (a) first-line cohort and (b) second-line cohort. *p < 0.05 compared to non-switchers; **Post-index costs for switchers were post-switch costs.
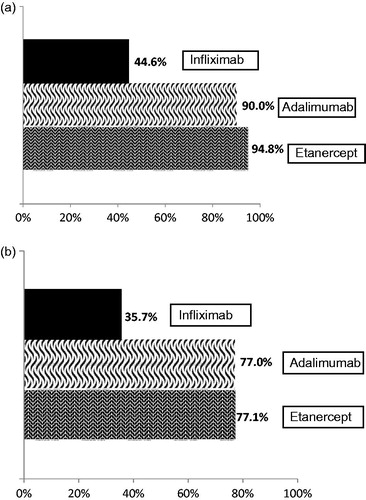
When considering the proportion of switchers for each index bDMARD, 9.5% of adalimumab, 9.0% of etanercept, 5.5% of infliximab, and 2.1% of abatacept patients switched to another bDMARD (). After controlling for potential confounders, abatacept patients had significantly lower odds of switching compared to the anti-TNFs combined (OR = 0.24; 95% confidence interval (CI) = 0.14, 0.44) (Appendix Table A4a). A majority of patients who switched from adalimumab or etanercept within the first year, switched to a second anti-TNF agent (90.0%, 94.8%, respectively); this was less frequent with infliximab initiators (44.6%; ).
Figure 3. bDMARD switch rates in (a) first-line cohort (*p < 0.05 compared to abatacept; abatacept n = 582, adalimumab n = 2526, etanercept n = 4268, infliximab n = 2381) and (b) second-line cohort (*p < 0.05 compared to abatacept; abatacept n = 489, adalimumab n = 807, etanercept n = 487, infliximab n = 391).
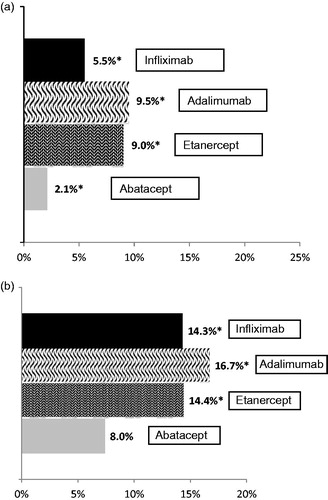
Figure 4. TNF-TNF switch rates in (a) first-line cohort and (b) second-line cohort. TNF-TNF switch is defined as those patients that switch from one TNF to another, i.e., from etanercept, adalimumab, or infliximab to either etanercept, adalimimab, or infliximab. Abatacept was not included considering 100% of Abatacept switches would be to a bDMARD with a different mechanism of action.
Second-line cohort
The initial population included 2354 patients identified as being on a second-line bDMARD, of which 180 were excluded due to switching or re-starting their index medication beyond the allowable gap (Appendix Table A1b). Of the 2174 remaining second-line patients, 300 (13.8%) switched to a third bDMARD. Baseline characteristics are presented in . Second-line switchers had higher pre-index total healthcare costs ($2173 vs $1856; p = 0.002) and pre-index RA-related costs ($1332 vs $1182; p = 0.041) than non-switchers.
Mean time to switch for second-line switchers was 138 days. Monthly post-switch costs were $1340 higher compared to the post-index costs of non-switchers ($3956–$2616; p < 0.001; ). Cost breakdown by type of service is depicted in Appendix Table A2b. In a Gamma regression for monthly total healthcare cost post-switch costs of switchers was 43% higher than post-index costs of non-switchers (p < 0.001, Appendix Table A3b).
Of the second-line bDMARD cohort, 16.7% of adalimumab, 14.4% of etanercept, 14.3% of infliximab, and 8.0% of abatacept patients switched to another bDMARD (). Logistic regression (Appendix Table A4b) determined that second-line abatacept patients had lower odds of switching compared to the anti-TNF patients combined (OR = 0.45; 95% CI = 0.32, 0.65). Approximately 77% of second-line etanercept and adalimumab switchers switched to another anti-TNF, compared to 35.7% of infliximab users ().
Discussion
This retrospective database analysis demonstrates the economic burden associated with switching of bDMARD agents in RA. Switchers have different characteristics than non-switchers at baseline, namely higher co-morbidity scores and higher total healthcare costs, which indicates a population with worse overall health status. However, even when controlling for baseline factors, switchers still cost more in the post-index period. The differences in costs were driven primarily by bDMARD costs; however, RA and non-RA related medical costs were elevated as well. Increased RA-related outpatient costs might be related to increased physician visits needed when switching to a new medication. However, without clinical data it is difficult to know why non-RA inpatient and outpatient costs were increased. This may be related to the overall health status of the switch population or due to adverse events. Further research is warranted to determine the clinical factors that impact the difference in costs of the switcher vs non-switcher populations.
This analysis also determined switch rates of the study bDMARDs and demonstrated a decreased likelihood of switching with abatacept compared with adalimumab, etanercept, and infliximab. There are a few recent studies that looked at switch rates of bDMARDs in the US; however, these focused primarily on anti-TNF agents. Bonafede et al.Citation11 examined treatment patterns with first-line anti-TNF agents in a commercial claims database and found that 13%, 12%, and 13% of etanercept, adalimumab, and infliximab patients, respectively, switched to another bDMARD within 1 year. The authors also found that the majority of the patients who switched went to another anti-TNF agent. Another claims data study by Ogale et al.Citation12 found that for first-line anti-TNF agents (adalimumab, etanercept, and infliximab), the percentage of patients who switched ranged from 12.0% (etanercept) to 15.4% (adalimumab) within 1 year. For subsequent-line anti-TNFs, switching ranged from 22.4% (infliximab) to 38.2% (adalimumab). For the non-anti-TNF therapies, 23.4% switched from abatacept and 17.5% switched from rituximab at some point during the follow-up yearCitation13. Switch rates from both these studies were higher than those reported in the current study. This may be partly due to the fact that the definition of switch used in both these studies was different; switch was defined as any change in bDMARD (regardless of treatment gap) within 1 year. When applying similar criteria to the current study, the switch rates were as follows: abatacept 7.3%, adalimumab 14.6%, etanercept 13.7%, and infliximab 9.5% for first-line cohort, and abatacept 14.1%, adalimumab 24.5%, etanercept 21.0%, and infliximab 19.5% for second-line cohort. Thus, the results for the first-line cohort reported here are more consistent with the Bonafede et al. and Ogale et al. studies. Results for the second-line cohort are still a little lower than what was reported in the Ogale et al. study, however it must be noted that the Ogale et al. study looked at a ‘subsequent-line’ population, who may have already been on a third or fourth biologic, and, therefore, more likely to switch.
The current study identifies a population of patients who switch first or second-line bDMARD within a year 1 period and determines the real world economic implications of switching. Beyond the economic impact of switching, there may also be clinical implications, at least among those that switch within class between the anti-TNF agents. In a study of a large registry of patients with RA, Greenberg et al.Citation13 found that response, remission, and persistence were diminished for patients who switched to their second or third anti-TNF compared to those on their first anti-TNF.
There are a number of limitations to this study. No clinical data are available within claims data to understand the rationale for the bDMARD switch, therefore it cannot be determined if certain reasons for switch (e.g. an adverse event) led to more costs and differential switch rates compared to others. Secondly, this study looked at IV abatacept and three commonly prescribed anti-TNFs but did not consider other bDMARD agents or the subcutaneous (SC) formulation of abatacept. Certolizumab and golimumab are relatively new anti-TNFs and, therefore, had a very small sample size in the claims data used in this analysis. Rituximab and tocilizumab, two non-anti-TNFs, were not indicated for first-line use at the time of analysis and had minimal ‘switch-to’ data available, therefore they were not included in this study. There were no claims data available on SC abatacept at the time of analysis and, therefore, it could not be considered in the current study. Further analysis of more recent claims data would allow for inclusion of the newer agents to determine how their availability as additional treatment options has impacted rates of switching. In addition, no co-payment, socioeconomic (i.e., income), or benefit design variables were available to understand if co-payments or co-insurance relative to income level influenced the switching rates.
Conclusion
This study demonstrates that patients with RA who subsequently switch their bDMARD have significantly higher total healthcare costs at baseline and significantly higher total healthcare costs after switching compared to patients who do not switch. This is true for both first- and second-line bDMARD populations, with even higher total healthcare costs post-switch in patients that switch from their second to third bDMARD. As a first-line or second-line agent, abatacept treatment resulted in less frequent switching relative to etanercept, adalimumab, and infliximab. This research highlights the need to identify patients who are likely to switch and the reasons to switch to ensure they receive the appropriate therapy which may improve outcomes and decrease healthcare costs.
Supplementary Appendix
Download PDF (148.3 KB)Transparency
Declaration of funding
Funding provided by Bristol-Myers Squibb.
Declaration of financial/other relationships
BM, MY, and LR are employees of Bristol-Myers Squibb. DT is a former employee of Bristol-Myers Squibb. CMRO Peer Reviewers on this manuscript have no relevant financial relationships to disclose.
References
- Helmick CG, Felson DT, Lawrence RC, et al; National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum 2008;58:15-25
- Feely MG, Erickson A, O'Dell JR. Therapeutic options for rheumatoid arthritis. Expert Opin Pharmacother 2009;10:2095-106
- Zhang J, Shan Y, Reed G, et al. Thresholds in disease activity for switching biologics in rheumatoid arthritis patients: experience from a large US cohort. Arth Care Res 2011;63:1672-9
- van Vollenhoven RF. Switching between anti-tumour necrosis factors: trying to get a handle on a complex issue. Ann Rheum Dis 2007;66:849-51
- Oei HB, Hooker RS, Cipher DJ, et al. High rates of stopping or switching biological medications in veterans with rheumatoid arthritis. Clin Exp Rheumatol 2009;27:926-34
- Bartelds GM, Wijbrandts CA, Nurmohamed MT, et al. Anti-infliximab and anti-adalimumab antibodies in relation to response to adalimumab in infliximab switchers and anti-tumour necrosis factor naive patients: a cohort study. Ann Rheum Dis 2010;69:817-21
- Hyrich KL, Lunt M, Watson KD, et al; British Society for Rheumatology Biologics Register. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56:13-20
- Bombardieri S, Ruiz AA, Fardellone P, et al; Research in Active Rheumatoid Arthritis (ReAct) Study Group. Effectiveness of adalimumab for rheumatoid arthritis in patients with a history of TNF-antagonist therapy in clinical practice. Rheumatology (Oxford) 2007;46:1191-9
- Gomez-Reino JJ, Carmona L; BIOBADASER Group. Switching TNF antagonists in patients with chronic arthritis: an observational study of 488 patients over a four-year period. Arthritis Res Ther 2006;8:R29
- Atzeni F, Sarzi-Puttini P, Gorla R, et al. Switching rheumatoid arthritis treatments: an update. Autoimmun Rev 2011;10:397-403
- Bonafede M, Fox KM, Watson C, et al. Treatment patterns in the first year after initiating tumor necrosis factor blockers in real-world settings. Adv Ther 2012;29:664-74
- Ogale S, Hitraya E, Henk HJ. Patterns of biologic agent utilization among patients with rheumatoid arthritis: a retrospective cohort study. BMC Musculoskelet Disord 2011;12:204
- Greenberg JD, Reed G, Decktor D, et al; CORRONA Investigators. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 2012;71:1134-42