Abstract
Objective:
Asenapine is the first tetracyclic antipsychotic approved in Canada for the treatment of schizophrenia (SCZ). Asenapine has shown a comparable efficacy profile to other atypical antipsychotics and it is associated with a favourable metabolic profile and less weight gain. This study aimed to assess the economic impact of asenapine compared to other atypical antipsychotics in the treatment of SCZ in Canada.
Methods:
A decision tree combined with a Markov model was constructed to assess the cost-utility of asenapine compared with other atypical antipsychotics. The decision tree takes into account the occurrence of extrapyramidal symptoms, the probability of switching to a different antipsychotic, and the probability of gaining weight. The Markov model takes into account long-term metabolic complications including diabetes, hypertension, coronary heart diseases, and stroke. In the base-case analysis, asenapine was compared to olanzapine. Asenapine was also compared with other atypical antipsychotics commonly used in Canada in alternative scenarios. Analyses were conducted from both Canadian Ministry of Health (MoH) and societal perspectives over a 5-year time horizon.
Results:
In the treatment of SCZ, asenapine is a dominant strategy over olanzapine from both MoH and societal perspectives. Compared to quetiapine, asenapine is also a dominant strategy. Furthermore, asenapine has a favorable economic impact compared to ziprasidone and aripiprazole, as these antipsychotics are not cost-effective compared to asenapine from both MoH and societal perspectives.
Conclusion:
Despite the short time horizon, the lack of compliance data and the assumptions made, this economic evaluation demonstrates that asenapine is a cost-effective strategy compared to olanzapine and to most of the atypical antipsychotics frequently used in Canada.
Introduction
Schizophrenia (SCZ) is one of the most debilitating mental disorders. Reports of SCZ prevalence vary considerably between and within countries. In Canada, the SCZ prevalence is estimated at 1%, according to a 2002 report on mental illnessesCitation1. SCZ has a significant economic impact. In Canada, a 2004 prevalence-based cost-of-illness study assessed the economic burden of SCZ. The direct healthcare and non-healthcare costs were estimated at $CAD2.02 billion and productivity loss at $CAD4.83 billion, for a total cost of $CAD6.85 billionCitation2.
A variety of treatment options for SCZ are available, including typical and atypical antipsychotics. Differences between typical and atypical antipsychotics are not well defined, as many clinician experts recognize that antipsychotic drugs differ in their potencies and have a wide range of adverse effect profiles, and that therapy should be tailored to the individualCitation3,Citation4. Generally, typical antipsychotics are effective in treating psychotic symptoms, but often lead to motor side-effects. Atypical antipsychotics, which are associated with fewer and less severe motor side-effects, have gradually replaced typical agentsCitation5–7. In fact, of all antipsychotics, the proportion of atypical antipsychotics use (risperidone, olanzapine, clozapine, and quetiapine) rose from 13% in 1996 to 64% in 2006 in FinlandCitation8. This study has also reported that quetiapine was associated with a higher risk for overall mortality when compared with perphenazine. In addition, some new antipsychotics might be associated with cardiac side-effects, while many of them have been shown to induce metabolic side-effects, including weight gain and higher triglyceride and cholesterol levelsCitation9,Citation10. The prevalence of metabolic syndrome is reported at ∼40% in chronic SCZ, or twice that of the general populationCitation11. Furthermore, weight gain and metabolic effects are risk factors for poor adherence to antipsychotics in SCZ patients, which has been associated with poor clinical outcomes including higher hospitalization and suicide rates and increased risk of relapseCitation12–17.
Few economic evaluations have taken into account the metabolic impact of atypical antipsychoticsCitation18,Citation19. To date, the use of asenapine for the treatment of SCZ has not been evaluated in Canada from an economic standpoint. Therefore, the aim of this study was to assess, from a Canadian perspective, the economic impact of asenapine compared to other atypical antipsychotics in the treatment of SCZ.
Method
A model-based cost-utility analysis was performed. For the base-case analysis, asenapine was compared to olanzapine because it has been used as the comparator in clinical trialsCitation20. In addition, olanzapine is one of the most commonly prescribed atypical antipsychotic drugs in Canada for SCZ. The patient population presented the characteristics of patients included in clinical trials of asenapine in SCZ (moderate-to-severe SCZ and onset at age 40 years)Citation20 encompass short-term and long-term outcomes and the costs associated with atypical antipsychotic use, this economic evaluation was conducted over a 5-year time horizon. Given the low adherence to antipsychotic medications in SCZ patients, a longer perspective was not consideredCitation12–14.
Model structure
A decision tree combined with a time-dependent Markov model was constructed (). A focus was placed on weight gain and long-term metabolic complications associated with treatments. According to expert cliniciansCitation21, this model structure was clinically meaningful for accurate representation of disease evolution and treatment.
Figure 1. Model structure. Decision tree model (A) and Markov model (B). *Patients may die from any health state of the model.
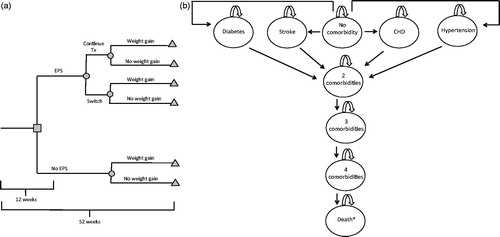
A decision tree with a 1-year time horizon was constructed to take into account the occurrence of EPS-related events, the probability of switching treatment, and the probability of gaining weight. A proportion of patients who experienced EPS discontinued their first treatment and switched to another. The treatment used when a treatment switch occurred was one of the other atypical antipsychotics available in Canada (aripiprazole, ziprasidone, risperidone, and quetiapine). Weight gain was considered according to results of clinical trials, where patients tend to significantly gain weight (≥7%) within the first year of treatmentCitation20.
A Markov model was developed for the subsequent years of treatment. Markov health states included long-term metabolic complications such as diabetes, hypertension, coronary heart diseases (CHDs), and stroke associated with weight gain, and the absorbing health state was death. According to the prevalence of each complication at age 40, as reported for the overall population, a proportion of SCZ patients who entered the Markov model were already suffering from metabolic complications. Thereafter, patients progressed in the model health states with the reported annual incidence rate for each complication, taking into account the elevated risks for patients with weight gain associated with their SCZ treatment. Diabetes and hypertension are chronic diseases and stroke and CHDs are punctual events with chronic consequences. Then, they were included in the model once the condition occurred, and they remained until death.
Clinical data
For the base-case scenario, the incidence rates of EPS-related adverse effects and of significant weight gain (≥7%) for asenapine and olanzapine were taken from the literatureCitation22 (). The proportion of treatment switches due to an EPS-related event was also estimated from clinical trials comparing asenapine and olanzapineCitation20. A ≥7% weight gain was considered significant, according to data reported in asenapine clinical trials. Moreover, this measure is one of the clinical meaningful cuts, used in most SCZ clinical trials, and is recognized by the National Institute for Clinical ExcellenceCitation23–26. In order to appropriately capture weight gain complications, only studies that reported weight gain data for 24 weeks or more were consideredCitation20,Citation22. Because EPS-related events occur early after treatment initiation, all studies, including short duration study, which reported a proportion of patients who experienced an EPS-related event, were considered.
Table 1. Model inputs: clinical parameters.
The efficacy of asenapine and olanzapine in treating SCZ symptoms were considered similar. This assumption was based on a meta-analysis that incorporated asenapine data to the findings based on the network meta-analysis previously publishedCitation27,Citation28. Only studies that included second-generation antipsychotic active controls were considered by the authors to compare asenapine to other atypicals. The differences between asenapine and olanzapine were not statistically significant, according to the change in PANSS total score (effect size of 2.9, 95% CI = 0.1–5.9).
To avoid possible confounders with SCZ and antipsychotic use, the risks for selected complications in the general population who gained weight were extracted. The risks of developing long-term complications according to metabolic changes were extracted from the literature (). In studies that presented weight gain measures other than a ≥7% increase, the risk corresponding to the most conservative significant weight gain was applied. According to average weight observed in SCZ clinical trialsCitation20,Citation29, an increase of at least 5 kg corresponds to a 7% increase of initial body weight.
Costs data
All costs are expressed in Canadian dollar 2011 values. All costs estimated before 2011 were adjusted to June 2011 levels based on the health component of the Canadian Consumer Price IndexCitation30.
The costs included in the analysis from a Ministry of Health (MoH) perspective were those associated with medications, EPS management, and healthcare resources used in the management of metabolic complications (). The cost of asenapine was provided by Lundbeck Canada Inc. (Montreal, Quebec, Canada), and the costs of the other antipsychotics were taken from the Liste des médicaments (list of medications) provided by the Régie de l’assurance maladie du Québec (RAMQ, Quebec’s health insurance board)Citation31. Costs differ across dosages as well as manufacturers. Therefore, the RAMQ database was used to estimate the mean cost of these treatments, based on their use in a real-life setting. Patients with a diagnosis of SCZ (International Classification of Diseases, ICD-9 295.0–295.9) and who had a valid prescription for any dose and any brand of olanzapine, quetiapine, or risperidone on February 1, 2011 were identified. A mean daily cost for each of these antipsychotics was then estimated. Unit cost for each antipsychotic, including original and generic products, was obtained from the Liste des médicaments, according to the different doses and manufacturers. According to expert clinicians as well as the Canadian guidelines, management of EPS-related symptoms would require one extra physician visitCitation32. The costs associated with metabolic complications were those covered by the MoH, including medical costs (physician, outpatient care, emergency visits, hospitalizations, and intensive care unit) and medications. These costs were estimated using data from pharmaceutical and medical services retrieved from Quebec’s Provincial Health Plan database. More specifically, for each complication, the difference between the median annual costs incurred by patients aged from 40–44 years who had the complication from January 1, 2003 to December 31, 2009 and the median annual costs incurred by patients in the same age range who did not have the complication was calculated.
Table 2. Model inputs: costs.
For the societal perspective analysis, additional costs associated with loss of productivity and informal care due to long-term metabolic complications were considered (). Costs associated with productivity losses were obtained from Canadian public sourcesCitation33–37. The estimated overall productivity loss for a complication was divided by the prevalence of the complication in the overall Canadian population in the estimated year to obtain the cost per patient. In the literature, only patients with stroke have been reported to require significant home careCitation38. In fact, based on a Canadian study, caregiver expenses account for 12% of the total 1-year stroke cost, which amounts to $27,245 for a person younger than 55 yearsCitation39. Therefore, in the present economic evaluation, only informal care associated with stroke was considered.
Utility
Utilities associated with SCZ and disutilities associated with EPS and metabolic complications were taken into account in this analysis ().
Lenert et al.Citation62 found that a moderate state of SCZ was associated with a utility of 0.75. They also found that side-effects related to SCZ medication were associated with a mean reduction in utility of 0.074 for acute EPS events and 0.031 for weight gain. EPS-associated disutility was estimated to last for 3 months, whereas it was permanent in the case of weight gain. Disutilities associated with weight gain and EPS were subtracted from the baseline utility observed in SCZ patients.
Schultz and KopecCitation40 estimated the impact of various self-reported chronic conditions on health-related quality-of-life, as measured by the Health Utilities Index Mark 3. According to their results, the mean disutility for each metabolic complication included in the model was taken into account (). When more than one complication was present concomitantly, the disutility of only the most debilitating complication was considered in the base-case analysis. Taking into account baseline utility for SCZ, these disutility values were used to adjust the number of QALYs according to development of long-term metabolic complications.
Mortality
Survival rates were taken from the most recent Canadian life tables available for men and women in the general populationCitation41. Because SCZ patients present higher suicide rates than the general population, mortality rates of the general population were adjusted by suicide rates reported in SCZ patients. The suicide rate was estimated to be 9.56-times higher in SCZ men and 6.73-times higher in SCZ women compared to the general population ()Citation42. To incorporate the increased risk of suicide associated with SCZ, the estimated mortality due to suicide in the general Canadian population for men and women at 40 years was first subtracted from the mortality observed in the general populationCitation43. The higher risk of suicide in the SCZ population was then added.
The risks of mortality associated with complications of interest were also taken into account (). In the case of several concomitant complications, all mortality risks were included for the base-case scenario (). The risk of mortality caused by fatal stroke or CHD events (mostly MI) were also included. Fatal cases of stroke and CHD events were estimated using the RAMQ database.
Analyses
For the base-case analysis, the incremental cost-utility ratios (ICURs) were calculated as the total cost associated with asenapine minus the total cost associated with olanzapine divided by the number of QALYs associated with asenapine minus the number of QALYs associated with olanzapine. Costs and benefits were discounted at a rate of 5% per year.
A complementary analysis using a 10-year time horizon was performed to assess the impact of metabolic complications over a longer period. In addition, although the base-case model was performed using olanzapine as the comparator, scenarios using other atypical antipsychotics available in Canada (quetiapine, ziprasidone, aripiprazole, and risperidone) were considered. For these comparative treatments, incidence rates of significant weight gain and EPS were obtained by indirect comparisons using the Bucher methodCitation44. Data for the indirect comparisons were taken from pivotal clinical trials of asenapine and from meta-analyses of atypical antipsychoticsCitation20,Citation22,Citation45–47.
Robustness of the results of this analysis was tested by deterministic and probabilistic sensitivity analyses. Confidence intervals were used as lower and upper bounds when available. When confidence intervals were not available, a ±25% variation was applied to the base-case parameters (). Deterministic analyses were performed by varying individually within lower and upper bounds all key parameters. Probabilistic sensitivity analyses were conducted using Monte Carlo simulation by varying simultaneously all key parameters. The probabilistic analysis was undertaken by randomly sampling each parameter distributions and calculating the expected costs and expected number of QALYs for that combination of parameter values for a total of 10,000 replications. Probabilistic sensitivity analysis was performed using Oracle Crystal Ball version 11.1.1.1.00.
Results
Base-case analysis
Over a 5-year time period, asenapine was found to be a dominant strategy over olanzapine in the treatment of SCZ, from both a MoH and a societal perspective. Thus, the costs associated with the use of asenapine are lower than the costs associated with the use of olanzapine, and the number of QALYs obtained with asenapine is higher than the number obtained with olanzapine ().
Table 3. ICURs: base-case scenario and 10-year time horizon scenario/1000 individuals.
Complementary analyses
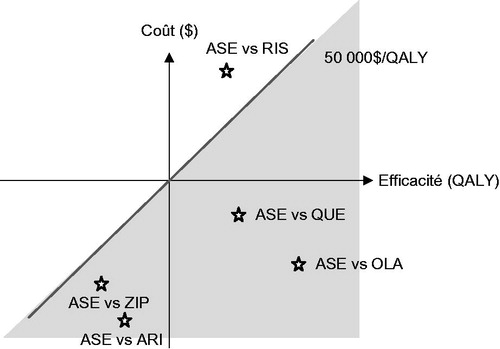
From both MoH and societal perspectives, asenapine remained a dominant alternative over a 10-year time horizon ().
When compared to quetiapine, asenapine was also a dominant alternative. In addition, the comparison of asenapine with ziprasidone and aripiprazole showed unfavourable cost-effectiveness ratios for both comparators. In fact, the ICURs located in the lower left quadrant of the cost-effectiveness plane and above the unofficial $50,000/QALY threshold indicate that ziprasidone and aripiprazole are not cost-effective compared to asenapine (). More specifically, ziprasidone was associated with higher QALYs and costs compared to asenapine, for an estimated cost-utility ratio of $63,204/QALY from a MoH perspective and $62,432/QALY from a societal perspective. Moreover, aripiprazole was associated with higher costs and QALYs compared to asenapine, with an ICUR of $1,485,625/QALY and $1,485,623/QALY from a MoH and a societal perspective, respectively. Furthermore, comparing asenapine to risperidone from a MoH perspective, the estimated cost-utility ratio was estimated at $72,319/QALY, and from a societal perspective it was estimated at $71,520/QALY.
Sensitivity analysis
Results of the deterministic and probabilistic analyses confirmed the robustness of the base-case results. According to the deterministic analysis results, asenapine remained a dominant strategy from both perspectives. The probabilistic sensitivity analysis also confirmed the robustness of the base-case results. From both a MoH and a societal perspective, asenapine was a dominant alternative over olanzapine in 100% of the Monte Carlo simulations.
Discussion
This study evaluated the economic impact of asenapine in the treatment of SCZ in Canada. Findings of this analysis suggest that, compared with olanzapine, asenapine is a dominant alternative from both a MoH and a societal perspective. In fact, asenapine is associated with lower treatment costs and a lower risk of gaining weight than olanzapine, which leads to a lower risk of developing metabolic complications. Complementary analyses using different atypical antipsychotics also confirmed the cost-effectiveness of asenapine compared to quetiapine, ziprasidone, and aripiprazole. However, although asenapine provides more QALYs than risperidone, the cost-utility ratio is above the $50,000/QALY threshold generally considered for drug decision-making. This is mainly due to the much lower treatment cost of risperidone compared with asenapine.
This is the first Canadian economic evaluation of asenapine in the treatment of SCZ. To date, few studies have assessed the economic impact of atypical antipsychotics with a focus on metabolic changes and their complications on quality-of-life and survival. For example, in a recent study, a semi-Markov model was constructed to evaluate the cost and predicted incidence of long-term complications associated with metabolic changes induced by treatment with atypical antipsychotic agentsCitation18. More recently, Kasteng et al.Citation19 found that treatment with aripiprazole was a dominant strategy over olanzapine, with 0.08 QALYs gained and cost savings of $US4000 per patient over a lifetime horizon. However, this study has some limitations, including the lack of consideration of non-metabolic adverse events and drug switching or discontinuation.
This economic evaluation has several strengths. First, the type of analysis chosen, a cost-utility analysis, allows considering atypical antipsychotic-related metabolic effects on mortality and morbidity. In addition, the analysis accounted for adverse events associated with treatment, including EPS and weight gain as well as treatment switches due to EPS. Furthermore, because weight gain is a progressive adverse effect, the choice of a 1-year period for weight gain development better reflects the reality. Moreover, although clinical trials of asenapine were mostly performed against olanzapine, indirect comparisons with other atypical antipsychotics using a validated method enabled a broader appreciation of the cost-effectiveness of asenapine. Pharmaceutical and medical services were taken from a RAMQ database to estimate the costs of metabolic complications based on real-life settings. The RAMQ database was also used to accurately estimate the cost of antipsychotics used by SCZ patients in real-life settings. Because different doses of antipsychotics are used for different indications, estimates of treatment costs in real-life settings, in a representative Canadian province, and specifically in a SCZ population, constituted the most appropriate method.
However, this economic evaluation has several limitations. First, as for any model-based analysis, many assumptions were made, which may increase the uncertainty of the results. However, a conservative approach was adopted to define each model assumption. For example, the time horizon was limited to 5 years, although the benefits of reducing weight gain can extend beyond that period. The impact of a 10-year time horizon was, though, assessed in the complementary analyses. Given the low adherence to antipsychotic medications in SCZ patients, a longer perspective was not consideredCitation12–14.
Furthermore, this economic evaluation considered that asenapine and olanzapine are similarly effective in treating SCZ symptoms, based on a published meta-analysisCitation28. However, other comparisons have been published, with different conclusions that could be explained by the selection of studiesCitation48,Citation49. It would have been interesting to include the efficacy of SCZ treatments in the model to assess the impact of these different conclusions on the ICERs estimated in the present economic evaluation. In addition, according to the Canadian clinical guidelines in SCZ, clozapine remains the treatment of choice in cases of non-responseCitation32. Therefore, clozapine could have been considered in cases of switch. However, it would have minimal impact on the ICERs, as weight gain and treatment costs considered in cases of switch were a weighted mean of antipsychotics available.
Moreover, the development of complications was limited to only one per cycle, although some patients may develop more than one complication in the same year. Furthermore, the model allows for only one treatment switch, although several switches may be required before obtaining the optimal treatment in terms of efficacy and safety. A further limitation is the assumption that patients remained on their medication continuously for 5 years, even though studies have reported significant non-adherence rates to antipsychotic treatment across SCZ populationsCitation14,Citation50. However, lack of treatment persistence was observed across all atypical antipsychotic agents. Therefore, the predicted clinical and economic benefits with asenapine would apply for the proportion of SCZ patients who would persist with their pharmacological regimen. This approach was also adopted in another Canadian economic evaluation of atypical antipsychotics in SCZCitation18. In addition, this persistence assumption has been applied both to asenapine and comparative treatments. Moreover, the model included increased risks of metabolic complications due to weight gain, but did not take into account the impact of existing comorbidities on the development of new complications. However, the assumption of metabolic complications as independent outcomes was conservative, because the synergistic effect of these complications was not taken into account. Despite these limitations, findings of this analysis are robust according to sensitivity analyses.
Conclusions
This economic evaluation demonstrates that asenapine is a cost-effective strategy compared to olanzapine and most of the atypical antipsychotics and provides an economic argument for using asenapine compared with other atypical antipsychotics in Canada.
Transparency
Declaration of funding:
Financial support for this study was provided by Lundbeck Canada Inc. and Lundbeck SAS.
Declaration of financial/other relationships:
JL, CB, and KM received consulting fees from Lundbeck Canada Inc. DG is an employee of Lundbeck Canada Inc. and MB is an employee of Lundbeck SAS. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Dr Bloom and Dr Iskandar, psychiatrists at the Douglas Institute, Montreal, Quebec, Canada for reviewing the model assumptions from a clinical standpoint.
Previous presentation: Presented at ISPOR 17th Annual International Meeting, June 2–6, 2012, Washington, DC, USA.
References
- Health Canada. A report on mental illnesses in Canada. Ottawa, Canada: Health Canada, 2002
- Goeree R, Farahati F, Burke N, et al. The economic burden of schizophrenia in Canada in 2004. Curr Med Res Opin 2005;21:2017-28
- Tyrer P, Kendall T. The spurious advance of antipsychotic drug therapy. Lancet 2009;373:4-5
- Parks J, Radke A, Parker G, et al. Principles of antipsychotic prescribing for policy makers, circa 2008. Translating knowledge to promote individualized treatment. Schizophrenia Bull 2009;35:931-6
- Bishara D, Taylor D. Asenapine monotherapy in the acute treatment of both schizophrenia and bipolar I disorder. Neuropsychiatr Dis Treat 2009;5:483-90
- Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. Am J Psychiatry 1994;151:825-35
- Bilder RM, Goldman RS, Volavka J, et al. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. Am J Psychiatry 2002;159:1018-28
- Tiihonen J, Lonnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009;374:620-7
- Raschi E, Poluzzi E, Godman B, et al. Torsadogenic risk of antipsychotics: combining adverse event reports with drug utilization data across Europe. PloS One 2013;8:e81208
- van Os J, Kapur S. Schizophrenia. Lancet 2009;374:635-45
- Yevtushenko OO, Cooper SJ, O'Neill R, et al. Influence of 5-HT2C receptor and leptin gene polymorphisms, smoking and drug treatment on metabolic disturbances in patients with schizophrenia. Br J Psychiatry 2008;192:424-8
- Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999;56:241-7
- Robinson DG, Woerner MG, Alvir JM, et al. Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 1999;156:544-9
- Goff DC, Hill M, Freudenreich O. Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry 2010;71(2 Suppl):20-6
- Wirshing DA. Schizophrenia and obesity: impact of antipsychotic medications. J Clin Psychiatry 2004;65(18 Suppl):13-26
- Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. J Clin Psychiatry 2001;62(7 Suppl):32-7
- Wong MM, Chen EY, Lui SS, et al. Medication adherence and subjective weight perception in patients with first-episode psychotic disorder. Clin Schizophr Relat Psychoses 2011;5:135-41
- McIntyre RS, Cragin L, Sorensen S, et al. Comparison of the metabolic and economic consequences of long-term treatment of schizophrenia using ziprasidone, olanzapine, quetiapine and risperidone in Canada: a cost-effectiveness analysis. J Eval Clin Pract 2010;16:744-55
- Kasteng F, Eriksson J, Sennfalt K, Lindgren P. Metabolic effects and cost-effectiveness of aripiprazole versus olanzapine in schizophrenia and bipolar disorder. Acta Psychiatr Scand 2011;124:214–25
- Schoemaker J, Naber D, Vrijland P, et al. Long-term assessment of Asenapine vs. Olanzapine in patients with schizophrenia or schizoaffective disorder. Pharmacopsychiatry 2010;43:138-46
- Bloom D, Iskandar H. Psychiatrists. [Consulted August 2011]. Montreal, Quebec: Douglas Mental Health University Institute
- Citrome L. Asenapine for schizophrenia and bipolar disorder: a review of the efficacy and safety profile for this newly approved sublingually absorbed second-generation antipsychotic. Int J Clin Pract 2009;63:1762-84
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23
- McEvoy JP, Lieberman JA, Perkins DO, et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry 2007;164:1050-60
- Stroup TS, Lieberman JA, McEvoy JP, et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611-22
- National Institute for Health & Clinical Excellence. Schizophrenia: core interventions in the treatment and management of schizophrenia in adult in primary and secondary care (Updated edition). National Clinical Guideline Number 82. London: National Collaborating Centre for Mental Health, 2010
- Leucht S, Arbter D, Engel RR, et al. How effective are second-generation antipsychotic drugs? A meta-analysis of placebo-controlled trials. Mol Psychiatry 2009;14:429-47
- Szegedi A, Verweij P, van Duijnhoven W, et al. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry 2012;73:1533-40
- Kane JM, Mackle M, Snow-Adami L, et al. A randomized placebo-controlled trial of asenapine for the prevention of relapse of schizophrenia after long-term treatment. J Clin Psychiatry 2011;72:349-55
- Statistics Canada. Consumer Price Index, Health Care: CANSIM Table 326-0020. 2011
- Régie de l'assurance maladie du Québec. Liste de médicaments. 33e édition. Quebec, Canada, 2011
- Canadian Psychiatric Association. Clinical practice guidelines. Treatment of schizophrenia. Can J Psychiatry 2005;50(13 Suppl 1):7S-57S
- Public Health Agency of Canada. Tracking heart disease and stroke in Canada. Cat HP32-3/2009E, Ottawa, Canada, 2009
- Public Health Agency of Canada. Report from the Canadian Chronic Disease Surveillance System: Hypertension in Canada, 2010. Chronic Disease Surveillance Division, ed. Ottawa: Public Health Agency of Canada 2010
- Patra J, Popova S, Rhem J, et al. Economic cost of chronic disease in Canada: 1995–2003. Prepared for the Ontario Chronic Disease Prevention Alliance and the Ontario Public Health Association. 2007
- Heart and Stroke Foundation of Canada. The growing burden of heart disease and stroke in Canada. Vol 1-896242-30-8. Ottawa, Canada: Heart and Stroke Foundation of Canada, 2003
- Public Health Agency of Canada. Diabetes in Canada: highlights from the National Diabetes Surveillance System 2004 – 2005. Ottawa: Public Health Agency of Canada, 2008
- Wilkins K, Park E. Home care in Canada. Health Rep 1998;10:29–37(ENG);31–40(FRE)
- Goeree R, Blackhouse R, Petrovic R, et al. Cost of stroke in Canada: a 1-year prospective study. J Med Econ 2005;8:147-67
- Schultz SE, Kopec JA. Impact of chronic conditions. Health Rep 2003;14:41-53
- Statistics Canada. Life Tables, Canada, provinces and territories, 2000-2002 (Catalogue no. 84-537-XIE). Ottawa: Minister of Industry E, 2006
- Brown S. Excess mortality of schizophrenia. A meta-analysis. Br J Psychiatry 1997;171:502-8
- Statistics Canada. Death and mortality rates, by selected groupes causes, age, group and sex, Table 102-0551 (Catalogue no. 84F0209X). Ottowa: Minister of Industry, 2007
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91
- Komossa K, Rummel-Kluge C, Hunger H, et al. Olanzapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst Rev 2010;(3):CD006654
- Rummel-Kluge C, Komossa K, Schwarz S, et al. Second-generation antipsychotic drugs and extrapyramidal side effects: a systematic review and meta-analysis of head-to-head comparisons. Schizophrenia Bull 2012;38:167-77
- Rummel-Kluge C, Komossa K, Schwarz S, et al. Head-to-head comparisons of metabolic side effects of second generation antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Schizophr Res 2010;123:225-33
- Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013;382:951-62
- Potkin SG, Phiri P, Szegedi A, et al. Long-term effects of asenapine or olanzapine in patients with persistent negative symptoms of schizophrenia: a pooled analysis. Schizophr Res 2013;150:442-9
- Liu-Seifert H, Adams DH, Kinon BJ. Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs. BMC Med 2005;3:21
- Oguma Y, Sesso HD, Paffenbarger RS, Jr., et al. Weight change and risk of developing type 2 diabetes. Obes Res 2005;13:945-51
- Colditz GA, Willett WC, Rotnitzky A, et al. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann Intern Med 1995;122:481-6
- Williams PT. Increases in weight and body size increase the odds for hypertension during 7 years of follow-up. Obesity (Silver Spring) 2008;16:2541-8
- Huang Z, Willett WC, Manson JE, et al. Body weight, weight change, and risk for hypertension in women. Ann Intern Med 1998;128:81-8
- Galanis DJ, Harris T, Sharp DS, et al. Relative weight, weight change, and risk of coronary heart disease in the Honolulu Heart Program. Am J Epidemiol 1998;147:379-86
- Willett WC, Manson JE, Stampfer MJ, et al. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal' weight range. JAMA 1995;273:461-5
- Asplund K, Karvanen J, Giampaoli S, et al. Relative risks for stroke by age, sex, and population based on follow-up of 18 European populations in the MORGAM Project. Stroke 2009;40:2319-26
- Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care 2005;28:1339-45
- Lotufo PA, Gaziano JM, Chae CU, et al. Diabetes and all-cause and coronary heart disease mortality among US male physicians. Arch Intern Med 2001;161:242-7
- Biagini E, Elhendy A, Schinkel AF, et al. Comparison of all-cause mortality in women with known or suspected coronary artery disease referred for dobutamine stress echocardiography with normal versus abnormal test results. Am J Cardiol 2005;95:1072-5
- Bronnum-Hansen H, Davidsen M, Thorvaldsen P. Long-term survival and causes of death after stroke. Stroke 2001;32:2131-6
- Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res 2004;71:155-65
- Régie de l’assurance maladie du Québec. Manuel des médecins spécialistes. Quebec, Canada, 2011