Abstract
Introduction:
Myelofibrosis is a non-frequent chronic myeloproliferative Philadelphia-negative chromosome neoplasm. It is a heavy incapacitating orphan disease and associated with high morbidity and mortality. In this context, indirect and non-medical costs are expected to be high. The main objective of this project is to estimate the economic burden of this disease in Spain.
Methods:
Thirty-three patients with a diagnosis of myelofibrosis for at least 1 year participated in a questionnaire in three Spanish centers. The study consisted of analyzing in various aspects the cost and impact of the disease; indeed, daily life time limitations with a need of informal care, symtomatology. Additionally, information concerning the clinical management of the disease was collected through a focus group of eight experts.
Results:
The mean age was 65 years. 15 of 33 patients were at their productive stage. Six had difficulties at work and eight have received informal care. Bone and muscular pain were the main symptoms of patients (72%). The estimated global indirect and non-medical costs of the disease were 86,315€ per patient (20% working and 80% informal care), which reached 104,153€ at productive stage patients (45%) and 168,459€ for more symptomatic patients.
Conclusions:
The economic burden of indirect and non-medical costs of myelofibrosis are important (15,142€/annual) as a result, and should be considered in economic evaluation, as well as in preventive plans for patients and caregivers, despite the fact that studies with larger numbers of patients should be done.
Introduction
Myelofibrosis (MF) is an orphan hematologic disease, included in the neoplasm myeloproliferative chronic Philadelphia-negative chromosome group. It is characterized by the reduction or increase of the blood cells count, by leukoerythroblastosis at peripheral blood, by fibrosis at the bone marrow, and by extramedular hematopoiesis. The disease generally results in splenomegaly and the clinical main manifestations include anemia, symptoms derived from the splenomegaly, constitutional symptoms, cachexia, and progressive weakness. The primary myelofibrosis prevalence is ∼2 per 100,000 of the populationCitation1; and estimating that one out of three patients with MF presents secondary myelofibrosis to polycythemia vera and essential thrombocytopenia, this value would reach three cases per each 100,000 inhabitants. Due to this low prevalence and the fact that this medical need is yet uncovered, the Myelofibrosis is categorized as an orphan disease (∼1400 patients in Spain).
A recent international study from Cervantes et al.Citation2, which analyzed 1054 patients with primary myelofibrosis, proved a mean survival of 5.7 years from the diagnosis. Nevertheless, the degenerative and progressive cycle of the disease significantly lowers the overall life expectancy. The progressive incapacitating symptoms of the MF, similar to those of a metastatic cancer, reduce life expectancy, impacting, therefore, negatively the patients daily life and causing absenteeism and working disabilityCitation1. Moreover, a study found that 50% of the patients result in a higher morbidity and mortality derived by splenomegalyCitation3.
Until 2012 no pharmacological treatment or specific indication of MF was approved by the European or American agency (FDA or EMA). Treatments used were not specific for the disease; had an effect only on a limited number of symptoms, proving, therefore, limited efficiency; and were very often related with severe adverse effectsCitation2. The only treatment which could be considered curative was the allotransplant of hematopoietic progenitors. Nevertheless, this treatment was only indicated for a minority of patients and with specific conditions: relatively young patients with a compatible donor, belonging to a higher prognostic risk category, and associated with high morbidity, severe adverse events and premature mortalityCitation4. The first drug specifically indicated for this disease, and which has been approved by the European Medical Agency (EMA) and the FDA, is ruxolitinib. The available information for this drug shows that: (1) There is evidence that the drug provides a significant survival improvement, for patients with 3 years of follow-up (27 vs 41 deaths, hazard ratio = 0.58; 95% confidence interval = 0.36–0.95; p = 0.03)Citation5, (2) The fibrosis grade evolves (EHA 2013)Citation6, and (3) The splenomegaly and the symptoms associated to the disease are significantly and quickly reduced, improving, therefore, the patients’ functional capacity and quality-of-lifeCitation7.
Analyses of socio-economic impact on the MF are rare. Nevertheless, because of the clinical cycle of the disease, this study has measured the repercuted costs, both for the patients and the public health system. A recent north-American study shows that medical and pharmacological costs for patients with MF were ∼5-times higher than for a group of patients without cancerCitation8. In this study the annual costs for the MFP were estimated to 34,690$ (25,972€), which from the medical visits represented the highest part. The objective of the present study is to highlight the use of resources associated with the MF disease in Spain, enhancing the economic impact on the indirect and non-medical costs.
Methodology
From March–June 2013, 33 patients from three hospitals were interviewed via a questionnaire specifically designed for the project. The three hospitals considered in the study were the Hospital del Mar in Barcelona (n = 15), the Hospital La Paz in Madrid (n = 7) and the Institut Català d’Oncologia in Barcelona (n = 11). The patients were selected on the basis of their availability during the follow-up period and of their compatibility with the inclusion criteria established in .
Table 1. Inclusion/exclusion criteria.
The minimum required size of the sample was established at 30 patients and was calculated based on the epidemiology of the disease in Spain and on a Canadian model presenting an average of 60,000€Citation9 of indirect costs per patient. Moreover, a population definition of 1400 patients and a level of precision of 36,000€ were considered in the sample size calculation.
The questionnaire was divided in various sections, evaluating therefore various impacts of the disease: (1) Sociodemographic, (2) Clinic (or clinical practice), (3) Symptomatologic (through the standardized questionnaire MFSAF), (4) Subjective evaluation taking a Visual Analogic Scale for the quality-of-life, (5) Limitations for the daily activities, (6) Implications on the working productivity, and (7) Implications on the needs for informal care. The needs for informal care are understood as the care given by the family and as the affective setting of a person whose autonomy is limitedCitation10.
The data analysis of the first sections is only descriptive. Complementarily, the two last sections have been oriented to facilitate calculations and to obtain an estimation of the indirect and non-medical costs associated with the disease. In the case of the ‘working’ section, three factors that could be associated with costs have been considered: the percentage of productivity loss, the cease of professional activity, and the absenteeism. The patients were asked about salary, allowing, therefore, estimation through the human capital methodCitation11. The considered working time horizon (or time span until on working stage) was established on the base of the age at diagnosis until 65 years, the average age of retirement. In the ‘informal care’ section, we directed questions to assess the amount of people consecrating weekly dedication. To estimate the economic burden associated with the disease, the dedication per year was extrapolated to 5.7 years, taking into account a dedicational cost per hour, as well as a time period from the diagnosis until the moment of the interview. This cost per hour was established as 12.7€, based on an officially reported cost for elderly people in informal care in SpainCitation12. The economic cost was also analyzed considering if the patients were ‘working actives’ or if they were highly symptomatic (differentiating in the MFSAF scale, those with an individual sum of punctuations above the mean seen in all the study patients).
The design of this model was validated by the Ethical Committees of the three participating centers and was officially supported by the group of Spanish specialists—Grupo Español de Enfermedades Mieloproliferativas Filadelfia Negativas (GEMFIN).
Additionally, we organized a focus group with eight hematologists specialized in myelofibrosis in Spain. The main objective was to reach a consensus on the clinical management and the use of resources, through a questionnaire based on the patients consulted in the last year.
Results
presents the main sociodemographic and clinical (or clinical practice) results. The average age of the 33 patients who participated in the process is 64.9 years, and 54.6% of these patients are women. The average time from the appearance of the symptoms until the diagnosis was 13.8 months and an average of 51.4 months has been identified between the diagnosis and the date of the interview.
Table 2. Socio-demographic and clinical characteristics of the patients with myelofibrosis.
and present the symptomatology and the quality-of-life declared by the patients with myelofibrosis. The most punctuated symptoms (highest severity) in the MFSAF questionnaireCitation11 were muscular pain (4.2) and inactivity (4.0), followed by pruritus (3.0), early satiety (2.7), and night sweats (2.6). The less punctuated items were abdominal pain (2.6) and under the left-side ribs pain (1.8). The average quality-of-life declared through a visual analogic scale results in 80.3 before the disease and 39.1 in the worst moment of the disease.
Table 3. Symptomatology declared in the economic burden of the myelofibrosis study.
Table 4. Subjective quality-of-life declared in the myelofibrosis economic burden study.
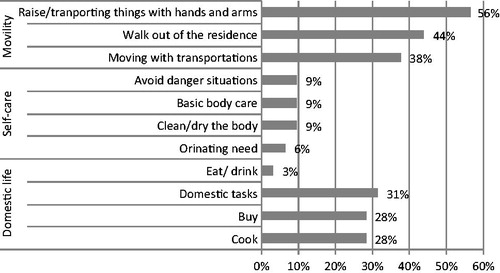
Concerning the daily life limitations associated with the disease, it is highlighted that 56.3% of the patients experienced a decrease in their quality-of-life when transporting objects with hands or arms, 43.8% when walking outside home, and 37.5% both for domestic tasks and moving with general transportations ().
shows the number and percentage of patients with MF, experiencing problems associated with indirect costs. In relation to the calculation of working economic costs, 52% of the interviewed patients were categorized as ‘non active’ (older than 65 years, understood as retired and/or house-keeper) and 18% (6 patients) experienced a negative impact on their work. These six patients represent 37.5% of the ‘active’ patients. Apart from these figures, 24% (8 patients) received informal care.
Table 5. Patients with myelofibrosis with working problems or given care through caregivers by hospital.
Work loss cost is estimated at 15,077€ per patient, whilst the cost corresponding to the informal care represents 71,259€ per patient, reaching, therefore, an overall cost of 86,315€ per patient. This cost, divided by the 5.7 years of expected survival, results in 15,142€ per year. Considering only the actively working patients, the costs ascend to 104,153€, and to 168,459€ considering only the more symptomatic patients. If splitting the costs between patients in a work life cycle (<65 years) and the rest, the costs would be 74.590 and 100.885€ respectively.
shows the indirect and non-medical costs. The costs estimations are presented taking into account three variables:
The total average costs per patient, depending on the working costs and the informal care costs;
The average costs per patient, per year (costs per patient of the prior section divided by the time between the diagnosis and the interview date); and
The costs over the life of the patient (prior values multiplied by 5.7 years, the average survival of the patients).
Table 6. Indirect and non-medical costs (€) of the patients with myelofibrosis.
Concerning the study through the expert panel of the patients’ clinical management, the main results are the following: (1) among the classification generated while considering the patients diagnosed last year, 43.3% had asymptomatic splenomegaly and did not present constitutional symptoms and 66.7% had symptomatic splenomegaly and/or constitutional symptoms, (2) 2.3% of the patients received an allogenic transplantation, (3) the clinicians declared a period of 5.4 months between the first suspicion of the disease and the final diagnosis, and (4) the mean (SD) of annual patients per specialist is 25 (12), representing, therefore, ∼14% of the clinical practice in Spain for this sample ().
Table 7. Characterization of the patient with MF through the experts’ panel on clinical management.
Discussion
The indirect and non-medical costs estimation shows a total cost per patient of 86,315€ (of which 17.5% of these costs is associated with the working area and 72.5% associated with informal care). These costs, multiplied by 1400 patients, imply a total cost of 120 million euros. This result is even more important when being contrasted with other studies’ estimations. For example, in Spain, the informal care costs are estimated to reach between 11,300–23,700 million eurosCitation13. Another Canadian model based on the data from COMFORT-II patients compared ruxolitinib with the best available therapy and estimated indirect costs of productivity loss of, respectively, 52,573€ and 70,581€Citation9.
Regarding the symptoms and daily life limitations associated with the disease, the study reveals some important information to highlight. Almost four of the symptoms considered in the MFSAF scale were observed in 50% or more of the patients, the frequencies of movement limitations were higher for 1/3 of the patients, and the majority of limitations concerning the domestic area were reported on 30% of the patients. In addition, 24.4% of the patients received informal care. This figure is considered as high by the authors, for example, if compared with the constitutional symptoms self-reported in 458 myelofibrosis patients studied by Mesa et al.Citation14. Nevertheless, this study was based on a sample of only 54% symptomatic splenomegaly patients and our study was fully based on splenomegaly patients. In conclusion, these results most certainly demonstrate not only a clinical need for the patients, but also a probable deficit in health and social attention.
On another side, the evaluation of the quality-of-life level during the worst moment from the diagnosis (EVA scale—39.1) was interpreted as a very low value. As a reference, to put this value into context, this value is lower than the one estimated during the worst stage reported for schizophrenia, anxiety, or mental health, and almost equivalent to the value during the worst stage of declared depression in Spain (2013). Finally, it is important to enhance that the average declared time period between the appearances of symptoms and the diagnosis is higher than a year (13.8 months). This result is different for each center and oscillated between 7.8 months (Hospital del Mar), 10.8 months (Institut Català d’Oncologia), and 25.8 months (Hospital La Paz). These results demonstrate that there is an important need to increase the number of diagnoses and early treatments, and that this need must be analyzed seriously and in-depth. Increase in MT diagnosis could indeed improve the prognosis of the patients, as is the case for any similar disease. This point is even more important when we consider that, in the future, some treatments could act directly on the life cycle of the disease.
Taking into account the social aspect in the economic evaluation is becoming more and more important for the studies. It is the case in the new ‘value based pricing’ proposal in the UK, which is expected to have a short-term impact in SpainCitation15. Therefore, we have to consider this additional social value in our study, in order to offer a more complete evaluationCitation16. The literature dealing with indirect costs also points in this direction and flourished a reality: their economic burden can reach high values. For example, in Spain, a highly prevalent disease such as diabetes has 17,630 million € of indirect costs per year associatedCitation17. On the myelofibrosis perspective, this study quantifies a total amount of 15,142€ of annual indirect costs. The comparison with the direct costs estimated in the US for the primary myelofibrosis (25,972€Citation7) shows the importance and the weight of this type of costs, although they are associated with an orphan disease. Finally, these costs must be even more taken into account, if we consider the results for the working population.
The main limitation of this study is the small number of patients enrolled in the study (33). Nevertheless, the quantity alignment with the sample size calculation made and explained, as well as the orphan disease nature of the study, would slightly compensate for this lack of data. Another limitation was the extrapolation of the patient survival data, taking into account the time horizon from the diagnosis to the date of the interview. However, it must be considered that: (1) the costs obtained in relation to the needs of informal care could be higher (progressive deterioration of the disease) and could be lower concerning the working area (age near or above retirement); (2) The described follow-up reaches 51.4 months (4.3 years), which represents 75% of the extrapolated time (5.7 years); and (3) Finally, despite the practical totality of the patients from the centers enrolled in the study through calls from the hospitals (full population; not spontaneous visit population), the Hospital de La Paz data collection presented two biases: (a) some patients were not interviewed because they were considered too elderly to understand the questionnaire, and (b) not all the patients from the center were interviewed, because the required number for the sample had already been reached. This last issue could have created a gender bias in the randomness of the interviews (71% were men, whereas the total percentage uses to be in the center is ∼50%).
Finally, regarding the clinical management analysis, collected through the experts’ panel, a specific result stands out: the variability in the number of days in hospitalizations declared by the clinicians is important. We have to emphasize that the expert’s panel responds to the heterogeneous profile of the disease, thus validating this result. This result brings us to the conclusion that this variability is a factor with an important unitary cost and, therefore, the use of this data could not be suitable for ‘direct resources’ costing. Also, the future existence of drugs such as ruxolitinib could ameliorate the disease progression of the patients and cause additional costs, symptoms, and quality-of-life improvements.
This affirmation invites us to take the results with great and deep interest, but also with a particular caution that needs to be taken into account in the economic estimation. We can specifically observe this while contrasting the results with the North-American direct cost study previously citedCitation7. Indeed, the cost item considered as the most important in this study is the number and duration of visits. Therefore, it is highly probable that the Spanish direct costs estimation would be higher, considering the use of resources defined in , and more specifically considering the high number of hospitalizations that has defined this estimation. Thus, for a future analysis, it could be interesting to establish a direct cost analysis based on medical chart reviews and considering a more significant sample of patients.
Conclusions
The indirect and non-medical costs of the myelofibrosis are not directly imputable to the National Health System, but imply, nevertheless, an important economic burden, and reflect the increasing needs of the patients (due to the chronic nature of the disease). Results like those found in this study for the indirect costs (86,315€/total; 15,142€/annual) reflect the need to work towards these statements and towards the integration of these costs in the economic and social evaluations, remembering that the patients are the center of the health assistance, and that the system must attend their needs and those of their entourage.
Transparency
Declaration of funding
This study was funded by Novartis Farmaceutica, Barcelona, Spain. Novartis participated without conditioning the analysis.
Declaration of financial/other interests
Emmanuel Gimenez and Juan Diego Gonzalez are employees of Antares-Consulting, a company that received a grant from Novartis for co-ordinating this study. Concepcion Boque, Patricia Velez, Ana Kerguelen, Francisca Ferrer-Marin, and Manuel Perez-Encinas have to disclose that they received sponsorship from Novartis for their role in developing this study. Carlos Besses has disclosed that he has received grants from Novartis, and honoraria from Novartis and Shire for educational purposes. Francisco Cervantes has disclosed that he is a consultant to Novartis, Celgene, and sanofi Aventis; and he is on the Speakers’ bureau of Novartis and Shire. Juan Carlos Hernandez-Boluda and Mercedes Rodriguez have disclosed they have received grants from Novartis. Reyes Calzada is an employee of Novartis Farmaceutica, Barcelona, Spain. JME peer reviewers on this manuscript have no relevant financial relationships to disclose.
References
- Left M, Avellaneda A, Bel Prieto A, et al. Enfermedades raras. un enfoque práctico. Instituto de Investigación de Enfermedades Raras del Instituto de Salud Carlos III. España: Ministerio de Sanidad and Consumo, 2004
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood 2009;113:2895-901
- Cervantes F, Alvarez-Larran A, Talarn C, et al. Myelofibrosis with myeloid metaplasia following essential thrombocythaemia: actuarial probability. Presenting characteristics and evolution in a series of 195 patients. Br J Haematol 2002;118:786-90
- Ballen KK, Shrestha S, Sobocinski KA, et al. Outcome of transplantation for myelofibrosis. Biol Blood Marrow Transpl J Am Soc Blood Marrow Transpl 2010;16:358-67
- Verstovsek S, Mesa RA, Gotlib J, et al. Efficacy. Safety and survival with ruxolitinib treatment in patients with myelofibrosis: results of a meann 2-year follow-up of COMFORT-I. Haematologica 2013; 98:1865-71
- Wilkins BS, Radia D, Woodley C, et al. Resolution of bone marrow fibrosis in a patient receiving JAK1/JAK2 inhibitortreatment with ruxolitinib. Haematologica 2013;98:1872-6
- Mesa RA, Gotlib J, Gupta V, et al. Effect of ruxolitinib therapy on myelofibrosis-related symptoms and other patient-reported outcomes in COMFORT-I: a randomized. double-blind placebo controlled-trial. J Clin Oncol 2013;31:1285-92
- Price GL, Pohl GH, Xie J, et al. A retrospective observational study of annual healthcare costs for patients with forms of myeloproliferative neoplasms (MPN). 53rd ASH Annual Meeting and Exposition (abstract # 2060) American Society of Hematology. San Diego, CA; December 2011
- El Ouagari K, Knight CJ, Mendelson E. Cost-effectiveness of ruxolitinib versus best available therapy for medical treatment of the myelofibrosis: societal perspective. Poster at 54th ASH Annual Meeting and Exposition. Atlanta; December 2012
- Oliva J, Vilaplana C, Osuna R. El valor de los cuidados informales prestados en España a personas en situacion de dependencia. Instituto de Estudios Fiscales, 2011. Available at: http://www.ief.es/documentos/investigacion/seminarios/economia_publica/2011_11Julio.pdf
- Liljas B. How to calculate indirect costs in economic evaluations. Pharmacoeconomics 1998;13:1-7
- IMSERSO. Las personas mayores en España. Datos estadisticos estatales y por Comunidades Autonomas. Informe 2008/Tomo II. Colección Documentos. Serie Documentos Estadísticos. Spain: Ministerio de Sanidad and Politica Social
- Oliva J. Osuna R. Costs of the informal care en Spain. Presupuesto y Gasto Público 2009;56:163-81
- Mesa R, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res 2009;33:1199-203
- Gimenez E, Rovira J, Gonzalez JD, et al. Diez años de umbral coste-efectividad. Farm Hosp 2013;37:85-7
- Claxton K, Sculpher M, Carroll S. Value-based pricing for pharmaceuticals: its role, specification and prospects in a newly devolved NHS. CHE Research Paper 60. York: University of York, 2011
- London School of Economics. Madrid: Work presented at Forum “Diálogo en Diabetes-Madrid 2013”
- Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood 2006;108:1497-503