Abstract
Objectives:
To estimate biologic cost per effectively treated patient with rheumatoid arthritis (RA) using a claims-based algorithm for effectiveness.
Methods:
Patients with RA aged 18–63 years in the IMS PharMetrics Plus database were categorized as effectively treated if they met all six criteria: (1) a medication possession ratio ≥80% (subcutaneous) or at least as many infusions as specified in US labeling (intravenous); (2) no biologic dose increase; (3) no biologic switch; (4) no new non-biologic disease-modifying anti-rheumatic drug; (5) no new or increased oral glucocorticoid; and (6) ≤1 glucocorticoid injection. Biologic cost per effectively treated patient was defined as total cost of the index biologic (drug plus intravenous administration) divided by the number of patients categorized by the algorithm as effectively treated. Similar methods were used for the index biologic in the second year and for a second biologic after a switch.
Results:
Rates that the index biologic was categorized as effective in the first year were 31.0% etanercept (2243/7247), 28.6% adalimumab (1426/4991), 28.6% abatacept (332/1160), 27.2% golimumab (71/261), and 20.2% infliximab (474/2352). Mean biologic cost per effectively treated patient, per the algorithm, was $50,141 etanercept, $53,386 golimumab, $56,942 adalimumab, $73,516 abatacept, and $114,089 infliximab. Biologic cost per effectively treated patient, using this algorithm, was lower for patients who continued the index biologic in the second year and higher after switching.
Conclusions:
When a claims-based algorithm was applied to a large commercial claims database, etanercept was categorized as the most effective and had the lowest estimated 1-year biologic cost per effectively treated patient. This proxy for effectiveness from claims databases was validated against a clinical effectiveness scale, but analyses of the second year or the year after a biologic switch were not included in the validation. Costs of other medications were not included in cost calculations.
Introduction
Guidelines published in 2012 by the American College of Rheumatology (ACR) for the treatment of rheumatoid arthritis (RA) recommend synthetic disease-modifying anti-rheumatic drugs (DMARDs)—such as hydroxychloroquine, leflunomide, methotrexate, and sulfasalazine—for patients with early RA, low disease activity, and an absence of poor prognostic features, as well as for patients with early RA, moderate or high disease activity, and poor prognostic featuresCitation1,Citation2. ACR recommendations also suggest a more aggressive approach to inhibit the progression of joint damage and other complications from RA that may develop soon after diagnosisCitation1,Citation3. This approach may include treatment with biologics, which are designed to inhibit specific components of the immune system that play a role in inflammationCitation3.
Biologics approved by the US Food and Drug Administration (FDA) for first-line treatment of moderate-to-severe RA (in conjunction with or after the use of non-biologic DMARDs) include tumor necrosis factor (TNF) blockers—adalimumab, certolizumab pegol, etanercept, golimumab (in combination with methotrexate), and infliximab (in combination with methotrexate)—as well as the non-TNF blockers abatacept and tocilizumab. Adalimumab, etanercept, and infliximab are the most commonly used biologic therapies for RACitation4,Citation5. After 3 months (or an adequate trial) of first-line treatment, if the patient has moderate or high disease activity related to lack of response or loss of benefit from the treatment, switching to another biologic is recommendedCitation2. Subsequent biologic treatment (for patients who have had an inadequate response to one or more TNF blockers) may involve either another biologic that is approved in first-line treatment or a biologic that is only approved in second-line treatment, such as rituximab (in combination with methotrexate) or anakinra.
The comparative cost-effectiveness of biologics has important implications for patients, clinicians, and payers, but individual studies typically evaluate either cost or effectiveness, not both. Head-to-head randomized clinical studies have been conducted to compare two or three biologics, but these studies did not consider cost and are limited in generalizability to patients who meet eligibility criteria for enrollment in such trialsCitation6–12. Observational head-to-head studies have reported comparable effectiveness between the TNF blockers in clinical practice, but differences in utilization or the total cost of careCitation13,Citation14. Several other cost comparisons of biologics have been done, but they did not evaluate treatment effectivenessCitation4,Citation5,Citation15–24.
Retrospective analysis of commercial claims databases makes it possible to assess and compare numerous available biologics simultaneously. Historically, the primary limitation of these data was the lack of RA disease activity measures—the gold standard for clinical effectiveness in RA—to assess treatment effectiveness. A claims-based effectiveness algorithm was developed and validated in the US veteran population enrolled in the Veterans Affairs Rheumatoid Arthritis (VARA) registryCitation25. A separate study confirmed the utility of the algorithm in a commercial claims database as wellCitation26.
This study used the validated algorithm to estimate the effectiveness of biologic therapies among commercially insured patients with RA. The study also explored biologic costs overall and biologic costs per effectively treated patient according to the algorithm. Because RA is a chronic condition that requires continuous treatment, comparative effectiveness research is needed not only for first-line biologic treatment, but also when patients continue the initial biologic treatment beyond the first year or switch to a second biologic treatment in the first year. Clinical guidelines for RA treatment do not specify how a second biologic treatment should be selected when the first biologic treatment is considered ineffective. Thus, the study included examinations of the costs and estimated effectiveness separately for the first year of index biologic treatment, continuing a biologic classified as effective in the first year into the second, and switching to a second biologic treatment in the first year.
Patients and methods
Objective
The objective of this study was to implement the claims-based algorithm in a US managed-care claims database to estimate the effectiveness of biologics indicated in the first-line treatment of moderate-to-severe RA, and to assess biologic costs per effectively treated patient according to this algorithm. Secondary objectives were to assess the apparent effectiveness and biologic cost per effectively treated patient among patients who continued their index biologic treatment in the second year and those who switched to a second biologic treatment in the first year.
Data source
Data were obtained from the aggregated IMS PharMetrics Plus Database of adjudicated health plan claims for over 150 million unique enrollees from 2006 to the present. More than 50% of these enrollees have full medical and pharmacy benefit coverage. The PharMetrics Plus population primarily consists of commercially insured individuals across all geographical regions and therapy areas and multiple payers. Patients in PharMetrics Plus have been treated by a majority of providers (90% of hospitals, 80% of physicians) in the US.
Diagnoses and procedures (primary and secondary) in every case setting are captured and coded to US claims standards (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] Current Procedural Terminology [CPT-4] and Healthcare Common Procedure Coding System [HCPCS]). Drug treatments are captured by filled prescriptions (National Drug Code [NDC] and Generic Product Identification [GPI] coded) and administered in a medical setting (HCPCS coded). Financial information includes amounts allowed and paid by health plans. High-level benefit design variables (e.g., health plan type, payer type) are available to address their effect on resource utilization and costs. Patient demographics are reported as recorded on claims and enrollment records. PharMetrics Plus is designed to follow patients over the course of years; health plan enrollment start and stop dates are available and are incorporated in all longitudinal analyses. All data are compliant with the Health Insurance Portability and Accountability Act (HIPAA) to protect patient privacy.
Cohort selection
The population included adults diagnosed with RA who had at least one claim between January 1, 2007, and December 31, 2010, for a biologic that was approved for first-line treatment of RA (abatacept, adalimumab, certolizumab pegol, etanercept, golimumab, or infliximab). To identify patients who were new to biologic treatment, patients were excluded if during the previous 6 months they had received any biologic agent indicated for treatment of RA (abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, or tocilizumab). During the period covered in this analysis, tocilizumab and rituximab were indicated for use in patients with RA only as second-line biologic treatment; more recently, tocilizumab was approved for first-line biologic treatment in RA.
The index pharmacy claim was defined as the first claim for a study medication, and the index date was defined as the date of that claim. Patients were required to be between ages 18–63 years (inclusive) on the index date to ensure that they had at least 1 full year of subsequent coverage in the commercial insurance plan before they turned age 65 and were eligible for Medicare coverage. Patients were required to be continuously enrolled in the commercial insurance plan with medical and pharmacy benefits for the 6-month ‘pre-index’ period and the 12-month ‘post-index’ period. They were required to have at least one diagnosis of RA (ICD-9-CM code 714.0x) in any position on medical claims during the pre-index period or up to 30 days after the index date.
Patients were excluded if in the pre-index period or up to 30 days after the index date they had a diagnosis (ICD-9-CM code) for another condition for which any of these agents was approved: plaque psoriasis (696.1x), psoriatic arthritis (696.0x), ankylosing spondylitis (720.0x), juvenile idiopathic arthritis (714.3x), Crohn’s disease (555.xx), ulcerative colitis (556.xx), non-Hodgkin’s lymphoma (200.xx, 202.xx), or chronic lymphocytic leukemia (204.1x). Patients were excluded if they had a claim for any biologic prior to its FDA approval date in RA, claims for more than one biologic on the index date, a medical claim (with a J-code) for a self-administered subcutaneous injection (because the duration of the claim could not be determined and, thus, treatment adherence could not be evaluated), or a pharmacy claim (with an NDC code) for a physician-administered intravenous (IV) infusion (because the infusion date could not be determined). Certolizumab pegol was originally considered for the analysis, but was not included due to inadequate sample size (n = 138; <1% of patients who satisfied all other criteria for inclusion in the analysis; see ).
Table 1. Attrition of study sample.
Patient age was derived from year of birth and year of index date. Sex, payer type, and plan type were obtained directly from the database. The Dartmouth-Manitoba adaptationCitation27 of the Charlson Comorbidity Index was used, measured in the 6-month pre-index period. RA-related costs in the 6-month pre-index period included the sum of all allowed amounts on claim services where an RA diagnosis was present.
Consistent with HIPAA requirements, identifying information was removed from patient records to maintain confidentiality; thus, approval of an Institutional Review Board was not required.
Effectiveness in the first year, as assessed by claims-based algorithm
Clinical effectiveness was assessed as a dichotomous variable. For the index biologic to be categorized as effective, a patient who received that index biologic was required to meet all of the following six criteria during a 1-year follow-up period:
High adherence to index biologic:
A medication possession ratio (MPR) of 80% or greater for a self-administered biologic (adalimumab, etanercept, or golimumab), where MPR = total days’ supply dispensed ÷ 365.
Sufficient infusions for an intravenously administered biologic corresponding to its expected dosing schedule based on US prescribing information (abatacept ≥14 infusions, or infliximab ≥7 infusions).
No increase in biologic dose compared to the starting dose, as follows:
Abatacept: difference of ≥100 mg between ending and starting dose.
Adalimumab: dose escalation to 40 mg once weekly.
Etanercept: dose escalation to 50 mg twice weekly.
Golimumab: difference of ≥25 mg/week between ending and starting dose.
Infliximab: difference of ≥100 mg between ending and starting dose or >120% of the number of infusions expected.
No switch to a different biologic.
No initiation of a new non-biologic DMARD (hydroxychloroquine, leflunomide, methotrexate, or sulfasalazine) that the patient was not already taking during the 6-month pre-index period.
No new or increased oral glucocorticoid treatment, as follows:
No more than 30-day supply (cumulative) of a new oral glucocorticoid at least 90 days after the index date (for those with no oral glucocorticoid claims during the 6-month pre-index period).
No increase by ≥20% in cumulative oral glucocorticoid dose at months 7–12 relative to the 6-month pre-index period (for those with any oral glucocorticoid claim during the pre-index period); if a patient switched to a different oral glucocorticoid, doses were converted to prednisone equivalents for comparison, using an established conversion chartCitation28.
Parenteral or intra-articular glucocorticoid injections on no more than 1 unique day after the patient had been on biologic treatment for at least 90 days.
The index biologic was categorized as ‘effective’ if a patient met all of the six criteria and ‘not effective’ if a patient did not meet all of the criteria.
Effectiveness in the second year, as assessed by claims-based algorithm
Patients who satisfied the effectiveness algorithm in the first year and had continuous health-plan enrollment for 2 years after the index date were analyzed for effectiveness in the second year. The claims-based algorithm was applied to data from day 366 to day 730 after the index date, with the following changes to the algorithm (criteria 3, 4, and 5b were not changed):
The number of days’ supply remaining from the original post-index period that continued into the new post-index period was included for MPR of subcutaneous biologics in criterion 1a;
The acceptable number of infusions for criterion 1b was 11–16 for abatacept and 5–8 for infliximab, based on the dosing schedule from the US prescribing information;
The first dose from the original post-index period was used to evaluate dose increases for patients receiving IV medications in criterion 2;
Criterion 5a included a new oral glucocorticoid that was initiated at any time during the second year, since the patient had already been taking the index biologic for ≥1 year; and
Criterion 6 included glucocorticoid injections at any time during the second year, since the patient had already been taking the index biologic for ≥1 year.
Effectiveness of a second biologic after a switch, as assessed by claims-based algorithm
Patients who switched to a different biologic in the first year and had at least 1 additional year of continuous health-plan enrollment immediately following the date they switched were analyzed for effectiveness of the second biologic. This analysis used the same algorithm and criteria as the analysis of effectiveness for the index biologic. Algorithm criteria that assessed changes from baseline, such as changes in DMARD or glucocorticoid treatment, were based on comparisons to the index date, not the date of the switch. Tocilizumab was removed from the analysis of second biologic treatment due to small sample size. Rituximab was removed from the analysis of second biologic treatment due to the long infusion interval, the effects of which were not adequately validated in the original algorithm that was developed with data from the Veterans’ Administration (VA).
Cost of biologic therapy
The total 1-year biologic cost was calculated by summing allowed amounts (health plan paid and patient paid) for all medical and pharmacy claims for that biologic during the first year of the post-index period. Costs included both medication costs and professional administration costs, where applicable. Costs of other medications used in that year, such as DMARDs or other biologics, were not included in the cost calculations. Administration costs were based on the allowed amount on a claim with an administration code for an IV biologic (abatacept or infliximab). Subcutaneous agents (adalimumab, etanercept, or golimumab) were self-administered and, thus, had no administration cost. Costs were adjusted using the annual medical care component of the Consumer Price Index (CPI) to reflect inflation between 2007–2011. Similar methods were used to determine 1-year biologic costs in the second year of the post-index period or the first year after switching.
Cost per effectively treated patient
For each analysis, 1-year cost per effectively treated patient was calculated as follows:
Statistical analysis
For categorical measures, the distribution of patients across the categories of each characteristic was described using cross-tabulations, showing the frequency (number of cases) and the proportion of study patients observed in each category. Descriptive statistics (confidence interval, mean, median, standard deviation [SD], minimum, and maximum) were computed for continuous measures. Effectiveness per the algorithm was compared between biologics using binomial tests; etanercept was used as the comparator because it was the most commonly used biologic.
Results
Patient characteristics
There were 16,011 patients available for analysis after applying the inclusion and exclusion criteria (). The number and proportion of patients who received each index biologic were as follows (in decreasing order): etanercept, 7247 (45.3%); adalimumab, 4991 (31.2%); infliximab, 2352 (14.7%); abatacept, 1160 (7.2%); and golimumab, 261 (1.6%).
Patient characteristics and prior healthcare resource utilization are summarized in . Mean (SD) patient age at the index date ranged from 48.9 (9.9) to 51.0 (8.8) years for each index biologic. Overall, 12,280 (76.7%) patients were female, ranging from 73.6% for golimumab to 80.9% for abatacept. Overall, 23.7% of patients had a Charlson Comorbidity Index score >1 in the 6-month pre-index period, ranging from 23.0% for adalimumab to 28.3% for abatacept. Median pre-index RA-related medical cost per patient was $507 overall, ranging from $475 for infliximab to $668 for abatacept.
Table 2. Patient characteristics and pre-index rheumatoid arthritis-related costs.
Overall effectiveness per claims-based algorithm
The number of subjects available for each analysis of effectiveness and the attrition reasons for each analysis are provided in . Effectiveness per the claims-based algorithm is presented in for each analysis (first year of index biologic, second year of index biologic, and first year after a switch).
Table 3. Proportion (and 95% confidence interval) of patients satisfying each algorithm criterion.
Index biologic: first year
When the algorithm was applied to the first year of index biologic treatment, the proportions of patients categorized as effectively treated were 31.0% (2243/7247) for etanercept, 28.6% each for abatacept (332/1160) and adalimumab (1426/4991), 27.2% (71/261) for golimumab, and 20.2% (474/2352) for infliximab. Significantly more patients were categorized as effectively treated with etanercept than with adalimumab (p = 0.004) or infliximab (p < 0.001); the other differences vs etanercept for index biologic treatment were not statistically significant.
Index biologic: second year
Of the 4546 patients who satisfied all effectiveness criteria in the first year, 1999 patients were continuously enrolled in their health plan for at least 2 years after the index date and were available for analysis of effectiveness in the second year (). The proportions of patients categorized as effectively treated by the claims-based algorithm in the second year were 47.4% (294/620) for adalimumab, 47.2% (469/994) for etanercept, 45.4% (104/229) for infliximab, and 44.9% (70/156) for abatacept. In the second year, the differences between etanercept and the other biologic treatments did not reach statistical significance.
Second biologic
A total of 1303 patients who switched to a second biologic in the first year had at least 1 additional year of health-plan enrollment and were available for the analysis of effectiveness after a biologic switch (). The proportions of patients categorized as effectively treated with the second biologic treatment were 25.0% (49/196) for abatacept, 23.3% (14/60) for golimumab, 21.7% (69/318) for etanercept, 20.9% (110/527) for adalimumab, and 10.9% (22/202) for infliximab. Use of etanercept as the second biologic treatment was categorized as effective for significantly more patients than infliximab (p = 0.001); the other differences vs etanercept for the second biologic treatment were not statistically significant.
Achievement of each algorithm criterion
The number and proportions of patients who satisfied each criterion of the algorithm, with 95% confidence intervals, are shown by biologic in and are separated by index biologic treatment, the second year of continued index biologic treatment, and the second biologic treatment after a switch. Every criterion was analyzed in each patient, regardless of whether they failed another criterion during that year or when that failure occurred; thus, a single patient could be counted as failing several different criteria.
Index biologic: first year
In the first year, the criterion of high adherence had the lowest achievement rates of any algorithm criterion (ranging from 37.5–45.5% of patients) except for infliximab, for which slightly more patients achieved the high adherence criterion (58.8%) than the criterion for no increase in biologic dose (53.7% of patients).
Index biologic: second year
Patients who were effectively treated in the first year showed nominally higher achievement rates for individual criteria in the second year of therapy, compared to rates for the same agents in the first year of therapy. In particular, rates of high adherence increased to a range of 61.7–73.4% in the second year.
Second biologic
Achievement rates of individual criteria were nominally lower for the second biologic treatment after a switch than when the same treatment was used as the index biologic. For example, in the first year 78.5–84.7% of patients did not switch to a second biologic, whereas 68.9–81.1% of patients did not switch from a second biologic to a third biologic.
Mean 1-year cost of biologic per patient
Index biologic: first year
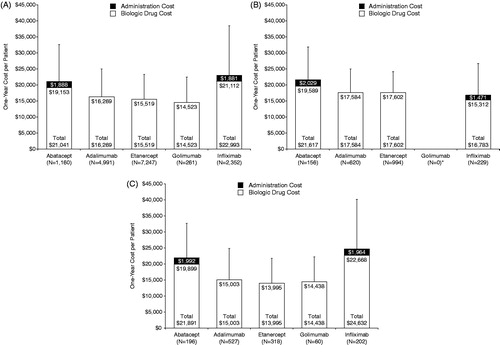
For the index biologic, mean total 1-year cost per patient was $14,523 for golimumab, $15,519 for etanercept, $16,269 for adalimumab, $21,041 for abatacept, and $22,993 for infliximab ().
Figure 1. One-year cost of biologic per patient. (a) Index biologic treatment: Mean (+SD) cost of biologic in the first year after the index date. (b) Continued index biologic treatment: Mean (+SD) cost of biologic in the second year after the index date. *No patient met the criteria for continued index biologic treatment with golimumab. (c) Second biologic treatment: Mean (+SD) cost of biologic in the first year after switch. SD, standard deviation.
Index biologic: second year
For the second year of continued index treatment, mean total 1-year cost per patient was $16,783 for infliximab, $17,584 for adalimumab, $17,602 for etanercept, and $21,617 for abatacept ().
Second biologic
After a switch, mean total 1-year cost of the second biologic treatment per patient was $13,995 for etanercept, $14,438 for golimumab, $15,003 for adalimumab, $21,891 for abatacept, and $24,632 for infliximab ().
One-year cost per effectively treated patient
Index biologic: first year
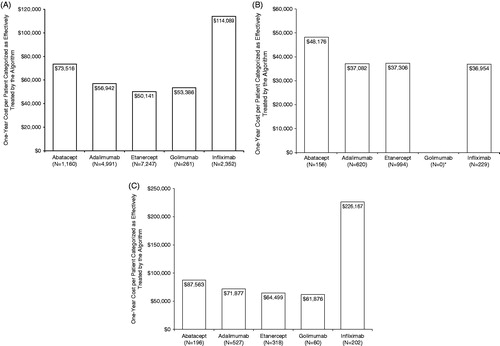
For index biologic treatment, the 1-year costs per effectively treated patient, as categorized by the algorithm, were $50,141 for etanercept, $53,386 for golimumab, $56,942 for adalimumab, $73,516 for abatacept, and $114,089 for infliximab ().
Figure 2. One-year cost per patient categorized as effectively treated by the algorithm. (a) Index biologic treatment: first year after index date. (b) Continued index biologic treatment: second year after index date. * No patient met the criteria for continued index biologic treatment with golimumab. (c) Second biologic treatment: first year after switch.
Index biologic: second year
For the second year of continued index biologic treatment, the 1-year costs per effectively treated patient, as categorized by the algorithm, were $36,954 for infliximab, $37,082 for adalimumab, $37,306 for etanercept, and $48,176 for abatacept ().
Second biologic
After a switch, the 1-year costs per effectively treated patient, as categorized by the algorithm, with the second biologic were $61,876 for golimumab, $64,499 for etanercept, $71,877 for adalimumab, $87,563 for abatacept, and $226,167 for infliximab ().
Discussion
In this analysis of more than 16,000 patients with RA who initiated biologic treatment between 2007–2010, effectiveness results for the first year of therapy are consistent with those from the original validation study in the VARA registryCitation25. Just under 30% of patients with RA who initiated therapy with a biologic in this commercial claims database satisfied all six criteria of the algorithm and were categorized as effectively treated in the first year. Across biologics, ∼40–60% of patients had sufficient adherence, 79–85% did not switch to another biologic, 83–87% did not initiate or increase use of oral glucocorticoids, and 90–94% did not receive multiple glucocorticoid injections during the first year of therapy. The greatest variation in effectiveness criteria was observed for the biologic dose increase criterion, where 88–99% of patients for most biologics completed 12 months of treatment without any dose increase, compared to only 54% of patients receiving infliximab, which in turn led to its lowest rate of effectiveness according to the algorithm.
This analysis extended prior work with the algorithm by considering the cost implications of these variations in effectiveness with respect to biologic treatment. First-year costs for biologic treatment among patients with RA ranged from ∼$15,000 (etanercept, golimumab) to ∼$23,000 (infliximab). However, after factoring in the estimates of effectiveness per the claims-based algorithm for each biologic, cost per effectively treated patient ranged from ∼$50,000 (etanercept, golimumab) to as much as $114,000 (infliximab).
The subsequent effectiveness and cost of treatment within two sub-groups of the initial cohort were also explored. These included patients who switched biologics in the first year but were observable for a year following the switch, and patients for whom the initial biologic was categorized as effective in the first year and who had a second year of observable data. Consistent with a recent study that used registry dataCitation29, this analysis found that the second biologic treatment after a switch was categorized by the algorithm as less effective than the first biologic therapy. Success rates for individual criteria were much lower for the second biologic treatment, particularly for biologic switch rates (i.e., switching to a third biologic treatment). Among the sub-group of patients who completed the first year of new biologic treatment, satisfied the effectiveness algorithm, and were continuously enrolled in the health plan for a second year, rates of effectiveness generally were higher in the second year than they had been for the full sample in the first year.
The total 1-year costs of the second biologic treatment among patients who switched and the total 1-year costs in the second year among patients who continued index biologic treatment were similar to the costs in the first year of index biologic treatment. However, the cost implications of differences in effectiveness per the algorithm were profound. In the second year of continued index biologic treatment, the cost was reduced to ∼$37,000 (etanercept, adalimumab, infliximab) to ∼$48,000 (abatacept) per effectively treated patient, per the algorithm. In contrast, 1-year costs for the second biologic treatment after a switch were much higher and ranged from ∼$60,000 (etanercept, golimumab) to ∼$226,000 (infliximab) per effectively treated patient, per the algorithm. However, these uses of the algorithm to categorize biologic treatment as effective were extensions beyond the population in which the algorithm was originally validated.
Dose escalation may have been a major contributor to the large difference in costs between infliximab and the other TNF blockers in the first year of treatment. Dose escalation in patients with RA is not included in etanercept product labeling. The adalimumab labeling notes that some patients with RA not receiving concomitant methotrexate may benefit from doubling the total dose (by giving it every week instead of every 2 weeks). Dose escalation can be particularly costly for infliximab, because the product labeling notes that some patients may benefit from increasing the dose by a factor of 3.3–6.7 (from 3 mg/kg every 8 weeks to 10 mg/kg every 8 weeks or 10 mg/kg every 4 weeks). The substantial increase in infliximab cost with dose escalation, combined with a high proportion of infliximab-treated patients who failed the algorithm by increasing their dose, led to very high estimated costs per effectively treated patient in the first year as compared with the other TNF blockers when infliximab was either the index treatment or the second biologic treatment after a switch. This cost difference was absent among patients who were categorized as effectively treated in the first year (without dose escalation) and, thus, were included in the analysis of the second year of continued treatment. Within this sub-group, the rate of dose escalation for infliximab decreased from 46% in the first year to 25% in the second year, and the total costs of infliximab and the cost per effectively treated patient with infliximab were comparable to those of the other biologic treatments in the second year.
Limitations
The effectiveness algorithm implemented here defines ‘effectiveness’ from pharmacy claims data and visit data that are readily available to a payer. Clinician and patient perspectives of effectiveness may value the algorithm criteria differently. For example, increasing the dose of a biologic to obtain the desired response suggests that the initial dose was not sufficient and decreases its cost-effectiveness from the payer perspective. On the other hand, patients may subsequently achieve a clinical response with a dose increase, which may be highly valued by the clinician and patient. Although this could lower the cost per effectively treated patient according to the algorithm, some of the decrease would be offset by the increased cost associated with increasing the dose of the biologic treatment. Additionally, altering the algorithm to allow for greater dose escalation of some biologics, consistent with their FDA approved labeling, without failing the other algorithm criteria might be reasonable. However, it would require re-validation of the original effectiveness algorithm before it could be applied in studies such as this one in order to confirm that this strategy yielded the same clinical outcome as the benefit achieved by patients who did not need to escalate dose.
Payers may also experience varying levels of cost consequence among patients who succeed or fail individual algorithm criteria. For example, the addition of traditional DMARDs to the treatment regimen counts as ineffectiveness in this algorithm, and may certainly decrease patients’ treatment satisfaction and convenience, but the cost differential between traditional and biologic DMARDs may result in only marginally increased cost to the payer.
Comparisons between biologics were complicated by large differences in sample sizes between the most commonly used TNF blockers (adalimumab, etanercept, and infliximab) and the other biologics. In the analysis of index biologic treatment, a range of values was observed for baseline comorbidity and prior RA-related treatment costs. Patients may have been preferentially selected for one biologic over another as the index treatment based on the severity of disease, but it is not possible to confirm this hypothesis from the claims data.
Because all six of the algorithm criteria were evaluated in every patient, each patient may not have met more than one algorithm criterion for effectiveness. For example, it is likely that many of the patients who switched to another biologic also failed the criterion for insufficient adherence after they switched biologic treatment. One alternative approach to better distinguish failure criteria would be to apply the criteria conditionally. For example, adherence could be measured only until the date on which a patient switched to another biologic, when applicable. This approach, however, would not alter the algorithm’s overall estimates of treatment effectiveness, and prior validation studies did not differentially estimate the predictive value of individual criteria on overall treatment response.
This algorithm is a proxy for effectiveness, but does not measure actual treatment response by indicators accepted within rheumatology, such as patient-reported Health Assessment Questionnaire disability scores or clinician-rated Disease Activity Score-28 (DAS-28). However, the original algorithm was validated against the DAS-28 from a large RA registryCitation25, and was further evaluated in a comparison of claims data and clinical effectiveness data in a commercial databaseCitation26.
Interpretation of results from the second year is limited by several factors. The algorithm was neither designed nor explicitly validated to evaluate effectiveness beyond 1 year, and some of the algorithm criteria needed to be modified to fit the second-year analysis. The categorization of patients for analysis of costs in the second year with continued index biologic treatment was subject to selection bias, because initial biologic treatment needed to be categorized as effective in the first year and the patient needed to have a second year of continuous coverage in the health plan. Additionally, golimumab was not approved for use in RA until April 2009, and no patient who received golimumab could meet all of the criteria for inclusion in the analysis of continued index biologic treatment.
Interpretation of results after a biologic switch is limited by the fact that the baseline for concomitant therapies was not reset at the date of the switch. For example, a patient who received a new DMARD or increased glucocorticoid treatment between the original index date and the date of the switch would fail the algorithm again for the second biologic when the prescription for the concomitant medication was refilled. However, achievement rates for criteria that were not dependent on the baseline, such as adherence to the second biologic, switching to a third biologic, or biologic dose escalation after switching, tended to decrease more (>10% difference) for the second biologic than the criteria that were dependent on the baseline, which suggests that this approach had little influence on the results.
An index biologic with a lower acquisition and administration cost may actually have higher total 1-year costs if patients frequently need to switch to another, more costly, biologic. Budget impact models for 1-year biologic costs have included costs after switching to address this issueCitation4,Citation5. In this analysis, total 1-year biologic costs did not include the costs of biologics after a switch. Instead, the influence of treatment failure on costs was addressed by analyzing costs per effectively treated patient. Future analyses of biologic effectiveness and cost should consider the inclusion of costs of all biologics, not just those of the index biologic.
Conclusion
When a claims-based algorithm for effectiveness in RA was applied to biologic treatment in a large commercial claims database, etanercept was among the most effective according to the algorithm and among those with the lowest cost per effectively treated patient, either as the index treatment (in both the first and second years) or as the second biologic treatment after a switch. Infliximab treatment was characterized by high rates of dose increase, which contributed to lower estimates of effectiveness and higher cost per effectively treated patient according to the claims-based algorithm. Overall, a greater proportion of index biologics that were categorized as effective in the first year continued to be effective in the second year, when compared to biologics that were categorized as not effective in the first year of treatment. Further, many patients who switched from their index biologic to a second biologic treatment subsequently switched to a third biologic. These observations may provide insight to both payers and clinicians who have little evidence-based guidance on extended management of biologic therapy in RA.
Transparency
Declaration of funding
This work was funded by Immunex Corporation, a wholly owned subsidiary of Amgen Inc., and by Wyeth, which was acquired by Pfizer Inc. in October 2009.
Declaration of financial/other relationships
Amgen Inc. participated in study design and data analysis, and assisted in preparation of the manuscript. J. Curtis has received consulting fees or honoraria from Roche/Genentech, UCB, Janssen, CORRONA, Amgen, Pfizer, Bristol-Myers Squibb, Crescendo, and AbbVie. V. Schabert, J. Yeaw, and J. Korn are employees of IMS Health, which received consulting fees for conducting the study from Amgen Inc. C. Quach was a graduate intern of Amgen Inc. at the time of this report. H. Yun has no conflicts of interest to disclose. D. Harrison and D. Collier are employees and stockholders of Amgen Inc. G. Joseph is an employee and stockholder of Sanofi, and a former employee and stockholder of Amgen Inc. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing support was provided by Jonathan Latham (PharmaScribe, LLC, on behalf of Amgen Inc.) and Edward Mancini (Amgen Inc.). Portions of this work were presented at the 2013 Annual Meeting of the International Society for Pharmacoeconomics and Outcomes Research and at the 2013 Annual Meeting of the American College of Rheumatology.
References
- Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008;59:762-84
- Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625-39
- Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Physician 2005;72:1037-47
- Schabert VF, Watson C, Gandra SR, et al. Annual costs of tumor necrosis factor inhibitors using real-world data in a commercially insured population in the United States. J Med Econ 2012;15:264-75
- Bonafede MM, Gandra SR, Watson C, et al. Cost per treated patient for etanercept, adalimumab, and infliximab across adult indications: a claims analysis. Adv Ther 2012;29:234-48
- Estellat C, Ravaud P. Lack of head-to-head trials and fair control arms: randomized controlled trials of biologic treatment for rheumatoid arthritis. Arch Intern Med 2012;172:237-44
- Schiff M, Keiserman M, Codding C, et al. Efficacy and safety of abatacept or infliximab vs placebo in ATTEST: a phase III, multi-centre, randomised, double-blind, placebo-controlled study in patients with rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2008;67:1096-103
- Weinblatt ME, Schiff M, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: findings of a phase IIIb, multinational, prospective, randomized study. Arthritis Rheum 2013;65:28-38
- Kume K, Amano K, Yamada S, et al. Tocilizumab monotherapy reduces arterial stiffness as effectively as etanercept or adalimumab monotherapy in rheumatoid arthritis: an open-label randomized controlled trial. J Rheumatol 2011;38:2169-71
- van Vollenhoven RF, Fleischmann R, Cohen S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 2012;367:508-19
- Greenberg JD, Reed G, Decktor D, et al. A comparative effectiveness study of adalimumab, etanercept and infliximab in biologically naive and switched rheumatoid arthritis patients: results from the US CORRONA registry. Ann Rheum Dis 2012;71:1134-42
- Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 2013;381:1541-50
- Moots RJ, Haraoui B, Matucci-Cerinic M, et al. Differences in biologic dose-escalation, non-biologic and steroid intensification among three anti-TNF agents: evidence from clinical practice. Clin Exp Rheumatol 2011;29:26-34
- Schabert VF, Bruce B, Ferrufino CF, et al. Disability outcomes and dose escalation with etanercept, adalimumab, and infliximab in rheumatoid arthritis patients: a US-based retrospective comparative effectiveness study. Curr Med Res Opin 2012;28:569-80
- Bullano MF, McNeeley BJ, Yu YF, et al. Comparison of costs associated with the use of etanercept, infliximab, and adalimumab for the treatment of rheumatoid arthritis. Manag Care Interface 2006;19:47-53
- Etemad L, Yu EB, Wanke LA. Dose adjustment over time of etanercept and infliximab in patients with rheumatoid arthritis. Manag Care Interface 2005;18:21-7
- Gilbert TD, Jr., Smith D, Ollendorf DA. Patterns of use, dosing, and economic impact of biologic agent use in patients with rheumatoid arthritis: a retrospective cohort study. BMC Musculoskelet Disord 2004;5:36
- Ollendorf DA, Klingman D, Hazard E, et al. Differences in annual medication costs and rates of dosage increase between tumor necrosis factor-antagonist therapies for rheumatoid arthritis in a managed care population. Clin Ther 2009;31:825-35
- Carter CT, Changolkar AK, Scott McKenzie R. Adalimumab, etanercept, and infliximab utilization patterns and drug costs among rheumatoid arthritis patients. J Med Econ 2012;15:332-9
- Harrison DJ, Huang X, Globe D. Dosing patterns and costs of tumor necrosis factor inhibitor use for rheumatoid arthritis. Am J Health Syst Pharm 2010;67:1281-7
- Tang B, Rahman M, Waters HC, et al. Treatment persistence with adalimumab, etanercept, or infliximab in combination with methotrexate and the effects on health care costs in patients with rheumatoid arthritis. Clin Ther 2008;30:1375-84
- Wu E, Chen L, Birnbaum H, et al. Cost of care for patients with rheumatoid arthritis receiving TNF-antagonist therapy using claims data. Curr Med Res Opin 2007;23:1749-59
- Weycker D, Yu EB, Woolley JM, et al. Retrospective study of the costs of care during the first year of therapy with etanercept or infliximab among patients aged > or =65 years with rheumatoid arthritis. Clin Ther 2005;27:646-56
- Zeidler J, Mittendorf T, Muller R, et al. Biologic TNF inhibiting agents for treatment of inflammatory rheumatic diseases: dosing patterns and related costs in Switzerland from a payers perspective. Health Econ Rev 2012;2:20
- Curtis JR, Baddley JW, Yang S, et al. Derivation and preliminary validation of an administrative claims-based algorithm for the effectiveness of medications for rheumatoid arthritis. Arthritis Res Ther 2011;13:R155
- Curtis JR, Chastek B, Becker L, et al. Further evaluation of a claims-based algorithm to determine the effectiveness of biologics for rheumatoid arthritis using commercial claims data. Arthritis Res Ther 2013;15:404
- Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075-9
- Hamilton RJ. Tarascon Pharmacopoeia 2011 Library edn. Philadelphia, PA: Tarascon, 2011
- Hyrich KL, Lunt M, Watson KD, et al. Outcomes after switching from one anti-tumor necrosis factor alpha agent to a second anti-tumor necrosis factor alpha agent in patients with rheumatoid arthritis: results from a large UK national cohort study. Arthritis Rheum 2007;56:13-20

