Abstract
Objective:
Rituximab is part of standard therapy for many non-Hodgkin lymphoma (NHL) patients, and is usually administered as an intravenous (IV) infusion. A formulation for subcutaneous (SC) injection will be available from June 2014. A time and motion study was conducted to investigate the staff time and costs associated with administration of SC and IV rituximab.
Research design and methods:
The time and motion study was conducted in three UK centers alongside a phase III trial of SC rituximab in patients with NHL (ClinicalTrials.gov identifier NCT01461928). Active healthcare professional (HCP) time spent on the preparation and administration of IV and SC rituximab was recorded and used to calculate the associated costs.
Results:
Total active HCP time associated with administration of IV rituximab was 223.3 min (95% CI = 218.0–228.7), vs 48.5 min (95% CI = 45.5–51.6) for SC rituximab, a saving of 174.8 min (95% CI = 172.5–177.1) per session. Patient time in the treatment room was 263.8 min (95% CI = 236.6–294.3) for IV rituximab and 70.0 min (95% CI = 57.1–87.2) for SC rituximab, per session. The SC formulation reduced total mean staff costs by £115.17 (95% CI = 98.95–136.93) per session. Differing monitoring scenarios during infusion consistently showed time and cost savings for SC rituximab.
Limitations:
Study limitations include the non-interventional design and lack of statistical power, and the investigational nature of SC rituximab. The data collected did not account for patient and center characteristics and variability on active HCP time.
Conclusions:
SC rituximab was associated with reduced active HCP time and costs vs IV rituximab, as well as reduced patient time in the treatment room. Switching from IV to SC rituximab could increase treatment room capacity and patient throughput, as well as improving the patient experience.
Introduction
Non-Hodgkin lymphoma (NHL) is a broad classification for a number of cancers originating from lymphocytes that are not categorized as Hodgkin lymphoma. Approximately 11,300 cases of NHL are diagnosed in the UK every year, with ∼4500 deathsCitation1. Rituximab (MabThera; Roche Ltd, Welwyn Garden City, UK) is a chimeric monoclonal antibody directed against the CD20 antigen used in the treatment of NHLCitation2. Rituximab was initially approved in Europe in 1998 for the treatment of relapsed or refractory low-grade or follicular, CD20-positive B-cell NHLCitation3, and for maintenance treatment for follicular lymphoma in 2010Citation4. Following these approvals, rituximab has become the standard induction or maintenance therapy for many CD20-positive lymphomas, including follicular and diffuse large B-cell lymphomaCitation5,Citation6.
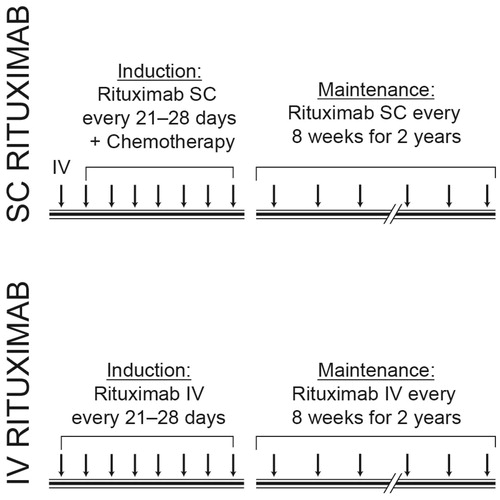
Rituximab is most commonly administered as an intravenous (IV) infusion given over 1.5–4 h at varying schedules, depending on the individual lymphoma type being treated and whether patients are receiving induction or maintenance therapyCitation2. Induction therapy consists of immunochemotherapy, with patients receiving rituximab 375 mg/m2 concurrently with a chemotherapy regimen such as cyclophosphamide, vincristine, doxorubicin, and prednisone (CHOP), usually once every 3 weeks for up to eight cyclesCitation2,Citation7. When used as maintenance therapy, rituximab is usually given as a monotherapy at 2- or 3-month intervals for a maximum of 2 yearsCitation2.
Administration of the IV infusion is resource intensive, requiring the attention of a number of healthcare professionals (HCPs) to prepare and administer the infusion, including monitoring of patients during and after administration. Recently, a subcutaneous (SC) formulation of rituximab has been developed, which will be available from June 2014. This formulation includes a recombinant hyaluronidase enzyme which temporarily modifies the extracellular matrix in the skin and allows SC injection of larger volumesCitation8. The recent SABRINA study compared the original IV formulation with the newer SC formulation, and demonstrated equivalence with respect to efficacy, while the time taken to deliver the SC formulation was significantly reduced to less than 10 minCitation9,Citation10.
Although there is a marked difference in the absolute time taken to administer rituximab as an IV infusion vs a SC injection, it is difficult to anticipate the impact a switch from an IV infusion to SC injection would have on real-world workflow. Furthermore, since the IV infusion is administered in a hospital setting on an outpatient basis, such a change in treatment protocols could also be expected to impact on hospital resource use and the time patients spend in hospital, particularly when assessed over the duration of a full course of therapy.
A commonly used method of assessing the effects of such a change on workflow and resource use is a time and motion study. Time and motion studies are non-interventional studies which aim to quantify efficiency-related outcomes, and are performed by breaking a process down into measurable tasks, using repeated on-site observations of the time taken to perform each individual task to assess the impact of the change on the time taken to perform the overall processCitation11.
This study reports the outcomes of a time and motion study in NHS day care units comparing HCP time used in the preparation and administration of IV and SC rituximab for the treatment of patients with NHL. In addition, observations of HCP time were used to estimate the staff costs associated with preparation and administration of rituximab, while patient time spent in the treatment room, including time spent in the infusion chair, was recorded so as to gain a clear picture of the impact of SC administration on healthcare resource use.
Methods
Study design
This paper reports the results obtained from UK centers participating in a prospective, observational, time and motion study. The time and motion study was conducted alongside a phase IIl randomized, open label, multinational study assessing SC rituximab as induction and maintenance therapy in patients with relapsed or refractory indolent NHL (ClinicalTrials.gov identifier: NCT01461928). The parent study was conducted in the outpatient consultation/day hospital visit setting, and enrolled 700 patients across 180 centers in 24 countries.
The UK time and motion study was conducted in three centers (Plymouth, London, and Oxford). Ideally, only monotherapy administration of rituximab as maintenance therapy was to be observed during the time and motion study, so as to avoid introduction of bias caused by administration of other chemotherapy agents, infusion reactions following the first dose of rituximab and delays caused by complications of therapy. However, for rituximab SC, collection of data during administration of induction therapy with rituximab (followed by same day chemotherapy) as well as maintenance rituximab monotherapy were allowed. The design of the time and motion study is shown in . Patient inclusion/exclusion criteria for patients receiving SC rituximab were inherited from the parent study, with no time and motion study-specific inclusion or exclusion criteria. Observations for administration of IV rituximab were collected during patients’ routine clinical care.
Definition of study variables and development of case report forms
No publications describing the processes of preparing and administering IV and SC rituximab in detail could be identified. Thus, the steps in the IV and SC processes were based on an interview with a clinical expert identified by a clinical investigator participating in the parent study. The interview was used to elaborate the principal tasks making up each process and details of sub-tasks for each activity, as well as to provide insight into potential differences between the IV and SC processes. The identified differences between the two processes were included in the time and motion sub-study as target variables.
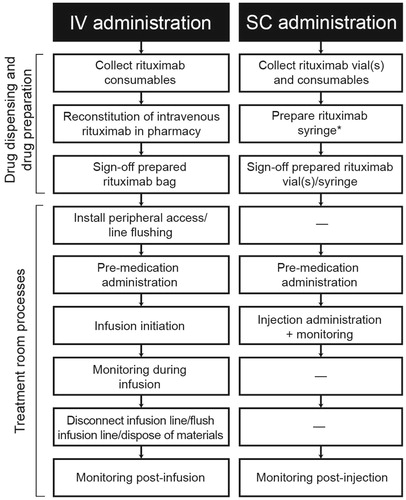
Three generic case report forms were developed, listing the identified tasks making up each process in chronological order, with ‘start’ to ‘stop’ descriptions for each task. The three case report forms were rituximab IV infusion (treatment room), rituximab SC injection (treatment room), and IV or SC rituximab preparation (pharmacy/drug preparation area). The tasks associated with IV and SC administration of rituximab are shown in . In addition to collecting active HCP time, the case report forms also collected the type and quantity of consumables used. In addition to the data collected using case report forms, a qualitative interview was also conducted with key staff at each of the three centers.
End-points
Five end-points were assessed based on differences in IV and SC processes. These end-points were: active HCP time per pre-specified task; quantity of consumable per pre-specified task; patient time in the treatment room; patient time in the chair; and patient time in the hospital.
Data were analyzed using Statistical Analysis Software (SAS) version 9.2. For the IV and SC processes, costs associated with each task were calculated by multiplying HCP time by the corresponding unit cost per hour for each HCP, obtained from Personal Social Services Research Unit (PSSRU) Costs of Health and Social Care 2012Citation1Citation2. PSSRU data reflect directly attributable costs, such as salary and benefit costs, as well as fixed costs associated with performance of HCP duties, such as qualifications, on-going training, and overheads (e.g., staff, non-staff, and capital costs). PSSRU data were available for registered nurses, junior doctors, hospital pharmacists, and clinical support workers. PSSRU unit costs were adjusted to 2012/2013 levels using the appropriate GDP deflators at market prices. Unit costs for other HCPs were obtained from the NHS Agenda for Change pay rates for 2013/2014 when PSSRU costs were unavailableCitation13. Unit costs used in the base case scenario are shown in .
Table 1. Base unit costs.
Two types of time measurement were used during the study. Stopwatch time (minutes and seconds) was used to measure active HCP time, and time of day (hours and minutes) was used to measure patient chair and treatment room time.
Scenarios
Active HCP time was measured and recorded for all tasks, as specified in , and costs for each task calculated accordingly. However, practice of the task ‘monitoring during infusion’ varies between hospitals; as such, the extent to which an HCP might remain fully engaged with the patient during a rituximab infusion is likely to differ. To address this, three analyses were conducted with differing definitions of active HCP time for the task ‘monitoring during infusion’.
The base case analysis was the most optimistic scenario, with HCPs assumed to be actively treating a patient for the total duration of rituximab infusion and the individual patient undergoing treatment assumed to be the sole focus of the HCP’s attention, accounting for 100% of their active time. The parameters of the base case scenario in the present study were chosen so as to be consistent with those used in a previously published time and motion study, which investigated active HCP time and costs associated with switching from IV to SC trastuzumab in patients with HER2-positive early breast cancerCitation14.
As internal protocols are likely to result in considerable variation in active HCP time between hospitals, two additional scenario analyses were performed. For the first scenario analysis, HCPs were assumed to be monitoring administration of IV rituximab to an average of 3.5 patients at any given time and, therefore, a single patient would account for ∼29% of the HCP’s active time within the task ‘monitoring during infusion’. This value was chosen based on one of the qualitative interview questions where centers were asked ‘how many patients is a nurse typically responsible for at any given time?’.
For the second scenario analysis, the definition of active HCP time within the task ‘monitoring during infusion’ was defined as only the time that the HCP was physically with the patient, as per the study protocol.
Statistical analyses
The time and motion sub-study was designed for descriptive analysis of the variables of interest. As the sample size was constrained by the limited number of centers included, this study did not have sufficient power for formal statistical analysis of differences between IV and SC administration of rituximab.
Regulatory approvals and informed consent
The protocol for the parent study was submitted to the Medicines and Healthcare Products Regulatory Agency and the National Research Ethics Service East of England–Cambridge South committee. Written informed consent was required for inclusion of patients receiving investigational rituximab SC in the study; informed consent for data collection was also obtained from patients receiving IV rituximab as routine treatment.
Results
Time and motion data were collected from the three participating centers between December 2012 and June 2013. The characteristics of the three participating centers are shown in . From these centers, 31 treatment observations and 20 drug preparation area observations were recorded for SC rituximab and 38 treatment room observations and 13 drug preparation area observations were recorded for IV rituximab ().
Table 2. Characteristics of centers in the UK time and motion sub-study.
Table 3. Number of observations of rituximab administration.
Active HCP time
SC rituximab was associated with a reduction in active HCP time compared with IV rituximab. shows that, in the base case, mean total active HCP time was 48.5 min (95% CI = 45.5–51.6) for SC rituximab vs 223.3 min (95% confidence interval [CI] = 218.0–228.7) for IV rituximab, resulting in a time saving of 174.8 min (95% CI = 172.5–177.1). The mean total time spent on treatment room tasks per patient was 15.2 min (95% CI = 13.1–19.1) for SC injection vs 184.4 min (95% CI = 163.8–209.0) for IV infusion, a time saving of 169.2 min. Drug preparation area tasks took a mean total of 33.3 min (95% CI = 28.9–38.6) for SC injections vs 38.9 min (95% CI = 26.1–61.7) for IV infusions, a time saving of 5.6 min.
Table 4. Comparison of active HCP time and costs per patient for IV rituximab infusion and SC rituximab injection for the base case analysis.
Patient time
SC rituximab was also associated with a reduction in time spent in the treatment room by each patient compared with IV rituximab. Mean total patient time in the treatment room per session for patients receiving SC rituximab was 70.0 min (95% CI = 57.1–87.2), compared with 263.8 min (95% CI = 236.6–294.3) for IV rituximab (). The majority of the time saved was time spent in the treatment chair. Mean total patient time in hospital per session was reduced from 303.8 min for patients receiving IV rituximab to 110.0 min for patients receiving SC rituximab.
Table 5. Comparison of patient time in the treatment room associated with IV and SC rituximab treatment.
Costs
In the base case scenario, presented in , the estimated total mean staff costs for one session of SC and IV rituximab were £31.26 (95% CI = 27.07–37.28) and £146.43 (95% CI = 126.02–174.21), respectively, resulting in an estimated cost saving of £115.17 (95% CI = 98.95–136.93) for SC rituximab. Of this, staff costs for treatment room-related tasks were £10.60 (95% CI = 9.12–13.32) for SC rituximab and £128.10 (95% CI = 113.74–145.13) for IV rituximab, resulting in an estimated cost saving of £117.51 (95% CI = 104.62–131.81) for SC rituximab, mostly attributable to the reductions in injection time and requirement for patient monitoring. Drug preparation and dispensing area costs were £20.67 (95% CI = 17.95–23.96) for SC rituximab and £18.33 (95% CI = 12.28–29.08) for IV rituximab, an increase in costs per session of £2.34 (95% CI = −5.67–5.12) for SC rituximab. The higher costs for preparation of the SC formulation were related to the time and motion study being undertaken alongside a clinical trial using an investigational drug. A greater proportion of the preparation of SC rituximab was performed by pharmacists and senior pharmacy technicians, whose time is more expensive than the pharmacy technicians and assistant pharmacy technicians who undertook most of the preparation tasks for the IV formulation (). Extrapolated over a full course of induction therapy (eight sessions, with the first session always administered as an IV infusion), estimated staff costs per patient were £365.25 for SC rituximab and £1171.44 for IV rituximab, a cost saving of £806.19 per patient per year. Over a full course of maintenance therapy (on average five sessions per year), estimated staff costs per patient were £156.30 for SC rituximab and £732.15 for IV rituximab, a cost saving of £575.85 per patient per year.
Scenario analyses
In the first scenario analysis, presented in , the assumption that a HCP would treat 3.5 patients at any given time reduced the time taken to monitor an infusion of IV rituximab to 42.2 min (95% CI = 38.0–46.9) per patient per session, resulting in a mean total active HCP time for administration of IV rituximab of 117.9 min (95% CI = 112.5–123.2) per patient per session. Active HCP time for SC rituximab was unchanged from the base case scenario (48.5 min [95% CI = 45.5–51.6]), leading to a difference in total mean active HCP time of 69.4 min (95% CI = 67.1–71.7) per patient per session, favouring SC rituximab. The total mean cost for administration of IV rituximab was £73.21 (95% CI = 59.99–92.71) per patient, while the total mean cost of SC rituximab was unchanged (£31.26 [95% CI = 27.07–37.28]), resulting in an estimated cost saving for SC vs IV rituximab of £41.94 (95% CI = 32.92–55.43) per patient per session. Over a full course of induction therapy, estimated staff costs per patient were £292.03 for a full course of SC rituximab and £585.68 for a course of IV rituximab, a cost saving of £293.65 per patient per year. For a full course of maintenance therapy, estimated staff costs per patient were £156.30 for SC rituximab and £366.05 for IV rituximab, a saving of £209.75 per patient per year.
Table 6. Comparison of active HCP time and costs per patient for IV rituximab infusion and SC rituximab injection for scenario 1.
In the second scenario analysis, shown in , only the time during which HCPs were physically present with patients was classified as active HCP time for the task ‘monitoring during infusion’ as per the study protocol, and, as such, this was substituted for the total infusion duration used in the base case analysis. Compared with the base case analysis, the time taken to monitor an infusion of IV rituximab was reduced to 3.7 min (95% CI = 2.7–5.2), resulting in a total mean process duration for administration of IV rituximab of 79.4 min (95% CI = 74.0–84.8) per session. As active HCP time associated with SC rituximab administration was again unchanged from the base case scenario (48.5 min [95% CI = 45.5–51.6]), SC rituximab was associated with a reduction in total mean active HCP time of 30.9 min (95% CI = 28.5–33.2) per patient. The total mean cost for administration of IV rituximab was £46.49 (95% CI = 35.5–63.7) per patient per session, while the cost of administering SC rituximab was unchanged (£31.26 [95% CI = 27.07–37.28]), with an estimated cost saving for SC rituximab vs IV rituximab of £15.22 (95% CI = 8.39–26.46) per patient. Extrapolated over a full course of induction therapy, the total staff costs per patient were £265.31 for SC rituximab and £371.92 for IV rituximab, a cost saving of £106.61 per patient per year. For a full course of maintenance therapy, total staff costs per patient were £156.30 for SC rituximab and £232.45 for IV rituximab, with a cost saving per patient per year of £76.15 over a full course of treatment of SC rituximab.
Table 7. Comparison of active HCP time and costs per patient for IV rituximab infusion and SC rituximab injection for scenario 2.
Discussion
SC rituximab was associated with time and cost savings compared with IV rituximab in patients with NHL. In the base case scenario, the most optimistic of the three scenarios, SC rituximab was associated with a total saving of 174.8 min of active HCP time per patient compared with IV rituximab, resulting in an estimated cost saving of £115.17 per patient per session.
Two scenario analyses were performed as part of the current study. The first scenario analysis assessed how total HCP time and costs were affected by changes in the number of patients treated by a HCP at any one time. The second scenario analysis assessed the effects of changing the definition of active HCP time spent monitoring patients during rituximab infusion to the time a HCP was actually present with the patient. As might be expected, the difference in total mean active HCP time between SC and IV rituximab was reduced compared with the base case analysis in both scenarios, although SC rituximab was consistently associated with time and cost savings compared with IV rituximab.
The investigational nature of SC rituximab resulted in higher costs associated with drug preparation than IV rituximab as a result of preparation by more senior pharmacy staff. However, as only the investigational formulation is subject to higher preparation costs, this is not expected to affect the validity of the study results. In addition, it is reasonable to expect that the costs associated with preparation of SC rituximab would be lower if the drug was licensed and in wider use.
Impact on the NHS
In 2013, an estimated 8475 NHL patients were treated with rituximab in the UK, with 6044 patients receiving induction therapy and 2431 receiving maintenance therapyCitation15. In the base case, if all patients were treated with SC rather than IV rituximab, this would result in savings to the NHS valued at in excess of £6,200,000 per year. In scenarios 1 and 2, savings to the NHS are valued at £2,300,000 and £830,000, respectively, per year. Furthermore, these yearly cost savings were estimated with the assumption that patients would not receive induction and maintenance therapy in the same year. Thus, yearly savings in each scenario would potentially be greater if one accounted for induction therapy patients beginning maintenance therapy 2 months after their last dose of induction therapy (as per the protocol described in the MabThera Summary of Product Characteristics)Citation2. As these savings are valued in terms of active HCP time gained, it should be recognized that savings would only become tangible if staff numbers were reduced; thus, these values represent gains to the NHS occurring as a result of being able to transfer resources elsewhere. Furthermore, although this study focuses on resource costs and did not include drug costs, it should be noted that SC rituximab is administered as a fixed dose and would not result in drug wastage.
In addition to the reduction in active HCP time, SC rituximab also had a considerable impact on the time patients spent in the infusion chair, with a timesaving of 193.1 for each individual session. This equates to being able to treat three patients with SC rituximab for every one patient treated with IV rituximab over a full course of induction therapy. Over a full course of maintenance therapy, this equates to being able to treat five patients with SC for every one patient treated with IV. Although this study examines switching from IV to SC rituximab from the perspective of the NHS, such a switch could potentially have societal benefits as well, as reductions in patient time spent in the treatment room, and thus in hospital, would be of considerable benefit to patients and their caregivers.
These results are consistent with a similar time and motion study which assessed the impact of a switch from IV to SC trastuzumab in patients with HER2-positive early breast cancer, with the study showing savings in active HCP time and costs, as well as savings in patient time favouring SC administrationCitation14.
Study limitations
As this was a non-interventional sub-study of a randomized trial, the number of patients available for observation, drug preparation protocols and treatment protocols were conditioned by the parent trial. Despite this, the statistics collected are expected to be a good proxy for real-world data; because SC rituximab is currently an investigational drug, the observations recorded in the clinical trial setting are as close as possible to real-world use. Patients were treated within the same environment and by the same staff as they would be if they were not taking part in a clinical study. This applied to both arms of the study. Because staff are currently unfamiliar with the SC formulation, it is likely that the time taken to handle and deliver it was longer than might be the case when the drug is available for clinical use; this was not the case with the IV formulation. However, it should be noted that this sub-study does not have sufficient statistical power for formal analysis of differences between the SC and IV groups. Pharmacy research staff costs were used because these were the staff participating in the trial, but it would be reasonable to expect these costs to be lower in the ‘real world’.
The study excluded induction therapy with IV rituximab because comparing maintenance regimes provided a clean head-to-head comparison of the two formulations. Had induction therapy been included, reactions due to first dose rituximab could have biased measured resource use against the IV formulation. A valid comparison could have been made if all patients had been receiving the same chemotherapy backbone, but patients were receiving a variety of regimens, creating large variations in the time required to administer rituximab. Excluding administration of rituximab in patients receiving chemotherapy also avoids inclusion of delays caused by complications of therapy.
Mapping of the processes for administration of SC and IV rituximab reveals one potential limitation of the current study. As no previously published processes for administration of IV and SC rituximab were available, it was not possible to prospectively define relevant predictors of process time, such as center or patient-related characteristics, or to identify potential confounders of time between the IV and SC processes. Furthermore, administration of SC rituximab is a new process, for which real-world experience is not yet available. As no patient information was collected during this study, it was not possible to assess the impact of patient characteristics on either active HCP time during rituximab administration or patient time spent in hospital.
The study was also limited with regards to the measurement of active HCP time, both in terms of accounting for all tasks associated with administration of IV and SC rituximab, and the accuracy of measuring the time taken to perform each task. To minimize the potential for variability, observers were provided with a clear definition of active HCP time and underwent standardized training before the study began. In addition, the same observers were used to monitor active HCP time for both IV and SC rituximab administration at each center. However, there is still the potential for variation in the time individual HCPs take to perform tasks, which was not accounted for in this study.
A number of other factors also have the potential to limit the current study. As the three study sites were activated at different time points during the parent study, there was an imbalance in the number of observations at each center, both in terms of the total number of treatment sessions observed and in the number of patients receiving IV and SC rituximab at each center. Furthermore, there were differences in task descriptions between treatment centers, with some drug dispensing tasks taking place in the treatment room, and trial-driven procedures resulting in increased time taken to dispense SC rituximab at one center. Use of pooled data from all three centers helped to mitigate the impact of these variations.
Conclusions
In patients with NHL, administration of SC rituximab is associated with reductions in active HCP time and costs compared with IV rituximab. Furthermore, SC rituximab is associated with a considerable reduction in the time patients spend in the infusion chair and, thus, in the treatment room. This suggests that switching to SC administration of rituximab could reduce the burden of treatment on patients and their families, while also freeing up capacity in the treatment room, potentially allowing a greater throughput of patients.
Transparency
Declaration of funding
This study was funded by Roche Products Ltd.
Declaration of financial/other relationships
K. Samanta is a Roche employee; G. Collins has been in receipt of honoraria and travel grants from Roche; S. Rule had been in receipt of honoraria and research funding from Roche.
Acknowledgments
The authors would like to thank the participating centres in Plymouth, London, and Oxford, particularly Dr. David Cunningham, lead investigator of the London center. Assistance with data analysis was provided by UBC, and was funded by Roche Products Ltd. Editorial assistance with the preparation of this manuscript was provided by Succinct Medical Communications, also funded by Roche Products Ltd. Full editorial control was retained by the authors.
Notes
*MabThera is a registered trade name of Roche Ltd, Welwyn Garden City, UK.
References
- International Agency for Research on Cancer. GLOBOCAN. http://globocan.iarc.fr. Accessed January 15, 2014
- Roche. MabThera Summary of Product Characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000165/WC500025821.pdf. Accessed January 15, 2014
- Roche. MabThera® receives marketing authorization in the European Union. http://www.roche.com/static/app/news/media-news-1998-06-03-e.pdf. Accessed January 15, 2014
- Roche. MabThera approved in Europe for first line maintenance treatment of follicular lymphoma, a common type of blood cancer. http://www.roche.com/media/media_releases/med-cor-2010-10-29.htm. Accessed January 15, 2014
- Tilly H, Vitolo U, Walewski J, et al. Diffuse large B-cell lymphoma (DLBCL): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii78-vii82
- Dreyling M, Ghielmini M, Marcus R, et al. Newly diagnosed and relapsed follicular lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22:vi59-vi63
- Hiddemann W, Kneba M, Dreyling M, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 2005;106:3725-32
- Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv 2007;4:427-40
- Davies A, Merli F, Mihaljevik B, et al. Pharmacokinetics (PK), safety and overall response rate (ORR) achieved with subcutaneous (SC) administration of rituximab in combination with chemotherapy were comparable to those achieved with intravenous (IV) administration in patients (pts) with follicular lymphoma (FL) in the first-line setting: stage 1 results of the phase III SABRINA study (BO22334). ASH Annual Meeting Abstracts 2012;120:1629
- Salar A, Bouabdallah R, McIntyre C, et al. A two-stage phase Ib study to investigate the pharmacokinetics, safety and tolerability of subcutaneous rituximab in patients with follicular lymphoma as part of maintenance treatment. ASH Annual Meeting Abstracts 2010;116:2858
- Barnes RM. Motion and time study: design and measurement of work. 7th edn. New York: John Wiley & Sons, 1980
- Curtis L. Unit costs of health and social care 2012. http://www.pssru.ac.uk. Accessed January 15, 2014
- NHS Agenda for Change - pay rates. http://www.nhscareers.nhs.uk/working-in-the-nhs/pay-and-benefits/agenda-for-change-pay-rates. Accessed January 15, 2014
- Burcombe R, Chan S, Simcock R, et al. Subcutaneous trastuzumab (Herceptin®): a UK time and motion study in comparison with intravenous formulation for the treatment of patients with HER2-positive early breast cancer. Adv Breast Cancer Res 2013;2:133-40
- Roche. Data on file; RXUKDATA00137. 2013