Abstract
Objective:
To identify cost estimates related to myocardial infarction (MI) or stroke in patients with type 2 diabetes mellitus (T2DM) for use in economic models.
Methods:
A systematic literature review was conducted. Electronic databases and conference abstracts were screened against inclusion criteria, which included studies performed in patients who had T2DM before experiencing an MI or stroke. Primary cost studies and economic models were included. Costs were converted to 2012 pounds sterling.
Results:
Fifty-four studies were identified: 13 primary cost studies and 41 economic evaluations using secondary sources for complication costs. Primary studies provided costs from 10 countries. Estimates for a fatal event ranged from £2482–£5222 for MI and from £4900–£6694 for stroke. Costs for the year a non-fatal event occurred ranged from £5071–£29,249 for MI and from £5171–£38,732 for stroke. Annual follow-up costs ranged from £945–£1616 for an MI and from £4704–£12,926 for a stroke. Economic evaluations from 12 countries were identified, and costs of complications showed similar variability to the primary studies.
Discussion:
The costs identified within primary studies varied between and within countries. Many studies used costs estimated in studies not specific to patients with T2DM. Data gaps included a detailed breakdown of resource use, which affected the ability to compare data across countries.
Conclusions:
In the development of economic models for patients with T2DM, the use of accurate estimates of costs associated with MI and stroke is important. When country-specific costs are not available, clear justification for the choice of estimates should be provided.
Introduction
Type 2 diabetes (T2DM) is a metabolic disorder that increases an individual’s risk of macrovascular complicationsCitation1. Two such macrovascular complications commonly included in T2DM economic models are myocardial infarction (MI) and stroke. The costs of these severe events increase the economic burden of T2DM on healthcare systemsCitation2. Thus, in the development of economic models estimating the value of new interventions for patients with T2DM, it is important that accurate estimates of the costs associated with these complications are obtained. These cost parameters can be a key model input, particularly for models showing a benefit regarding a reduction in complications. For example, the results from these models might vary across countries if practice patterns and costs differ across countries. For new product launches that will take place in multiple countries, it is important to determine the best costs to use for an individual country for the local economic model. In that case, it is not expected that the cost data will be compared or synthesized across countries.
Current health technology assessment (HTA) guidelines provide limited guidance for how costs should be determined for economic models used in a reimbursement submission, but they do recommend a ‘systematic’ approach. For example, the National Institute for Health and Care Excellence (NICE) states that ‘For all parameters (including effectiveness, valuation of [health-related quality-of-life] and costs) economic evaluation should systematically consider possible data sources, and avoid selection bias in the choice of sources’ (p. 19)Citation3. When cost data are taken from the literature, it is therefore important that the methods used to identify the sources (e.g., literature review search strategy) are clearly defined and any discrepancies between the sources identified are explained. NICE also recommends that, when several alternative sources are available, a justification for the costs chosen should be provided, and sensitivity analysis should be performed to assess the implications for the model results of using alternative data sources.
The objective of this research was to identify and describe the methods used to generate cost estimates for patients with T2DM experiencing an MI or stroke via a systematic literature review for use in economic models in multiple countries. Both primary cost studies and economic models were included in a literature review to identify cost estimates and evaluate the approaches used for obtaining and applying costs in economic evaluations.
Methods
Studies were identified systematically using prospective search criteria and an explicit reproducible plan for literature search. The studies were located via electronic and manual searches, and available data were assessed. Data gaps in the literature identified for future research purposes the countries that did not have relevant cost data.
The inclusion criteria, defined a priori, were as follows:
Studies conducted in patients of any age with T2DM, or reporting disaggregated results specific to a T2DM sub-population; and
Studies (primary cost studies or economic evaluations) presenting cost and resource utilization related to MI and/or any type of stroke in patients with T2DM.
Exclusion criteria included studies of mixed populations (e.g., non-diabetic and T2DM, or T1DM and T2DM) that did not present separate outcomes for patients with T2DM; studies that were not primary economic studies or economic evaluations or did not present outcomes of interest; and publication types not of interest (case reports, letters, comments, editorials, and reviews). Studies using meta-analysis were reviewed to make sure all primary studies had been captured, but they were excluded to avoid duplication of data.
The electronic databases PubMed, Embase, and the National Health Service Economic Evaluation Database (NHS EED) were searched. Initial database searches included only papers published in the English language from January 2001 to February 2012 and conference proceedings from January 2009 to February 2011. The database search was then updated to also include non-English-language papers. Staff at RTI Health Solutions who were fluent in the needed languages (French, German, Italian, and Polish) were informed of the research goals and required outcomes. They then reviewed the non-English-language articles for relevance. All non-English articles selected for inclusion were translated for data extraction. Bibliographies of the included studies and systematic reviews (with or without meta-analyses) were hand searched for further relevant studies.
Articles were screened by two researchers at two levels using a prospectively defined protocol. At Level 1, titles and abstracts of all articles identified were screened and included if they met the inclusion criteria or if it seemed that the full-text article might have the desired information. At Level 2, full-text papers determined to be eligible at Level 1 were reviewed to ensure that they met the inclusion criteria (e.g., separate data for patients with T2DM in mixed population studies). Any discrepancies were discussed and consensus reached.
For each eligible study that passed both levels of screening, data elements of interest were extracted from full-text documents into pre-defined data-extraction tables. Data extracted included the year of publication, cost year, country of study and currency, study type (economic evaluation or cost study), direct and/or indirect costs associated with an MI or stroke, a brief summary of the items included in reported costs, the methodology for obtaining costs, and the source of costs reported if not the original source (for the economic evaluations only). To ensure quality and accuracy of the data, a second researcher verified the data with the original sources.
A recommendation on the overall suitability of each primary cost study for use in an economic evaluation was also made using the adapted Drummond checklistCitation4. The checklist included questions regarding study design (e.g., clearly stating the research question, use of an appropriate time horizon, and thorough reporting of population characteristics), data collection (e.g., the inclusion of all relevant resources and unit costs with detailed reporting, reporting of price, and currency data), and the analysis and interpretation of results (e.g., major outcomes presented in a disaggregated and aggregated form).
To yield a common year and currency, costs were standardized to 2012 pounds sterling (GBP) using relevant purchasing-power-parity exchange rates and inflation indicesCitation5,Citation6. The tables also present the original cost data in each paper as well as the converted 2012 GBP estimates. No data synthesis was undertaken in this review.
Results
Overall results
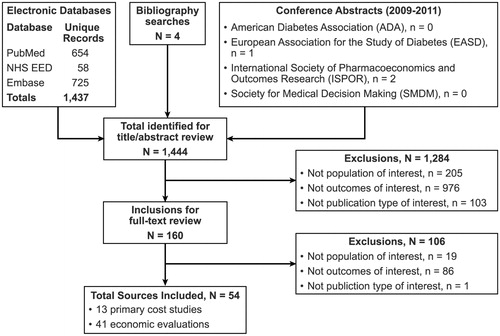
presents a flow diagram of the sources reviewed and either excluded or included for summarizing in this analysis. The literature review identified 54 studies reporting estimated costs for patients with T2DM who experienced a stroke or an MI.
Results for model review costs
Of the 54 studies included in the review, 13 were primary cost studies. summarizes key study characteristics and comments regarding the suitability of these studies for inclusion in an economic model. The remaining 41 studies were economic models including and reporting event costs for an MI or stroke.
Table 1. Summary of characteristics of key cost studies.
Myocardial infarction cost study estimates
The literature review identified 48 studies reporting costs of MI in patients with T2DM: 12 primary studies and 36 economic evaluations including MI as an adverse event. The costs are presented as mean costs per patient.
Fatal myocardial infarction
Of the 12 primary cost studies reporting costs for an MI, two estimated direct costs for a fatal MICitation2,Citation7. In Clarke et al.Citation2 cost estimates were based on data collected from 5102 patients included in the United Kingdom Prospective Diabetes Study (UKPDS), conducted from 1977–1997. The estimated annual hospital costs for a fatal MI were £1567 (cost year 1999), which equates to £2482 at 2012 UK prices. O’Reilly et al.Citation7. estimated resource use from a sample of 734,113 patients and used a two-part model to determine costs. The authors used logistic regression including variables representing patient age, gender, and indicator variables for the occurrence of diabetes-related complications to estimate the cost of a fatal event. The total costs incurred, conditional on incurring any costs, then were estimated, resulting in a converted cost of £5222 at 2012 UK prices. No cost studies were identified that estimated indirect costs due to mortality from a fatal MI.
Of the 36 economic evaluations reporting costs for an MI in patients with T2DM, 14 included costs for a fatal MI. Cost estimates reported in economic evaluations are presented in the online supplementary information (Table S1). Estimates ranged from £2713–£5590 for CanadaCitation8,Citation9, from £1959–£3635 for SwitzerlandCitation10,Citation11, and from £1606–£1736 for the UKCitation12,Citation13. One study in the US reported an estimate of £14,403Citation14.
Non-fatal myocardial infarction: year of the event
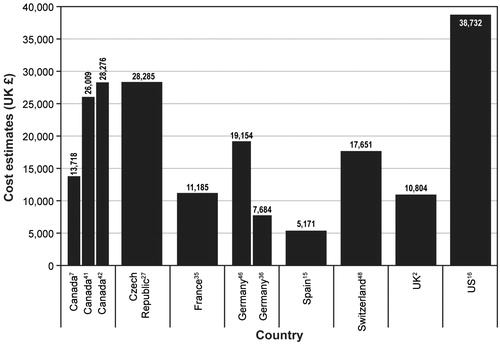
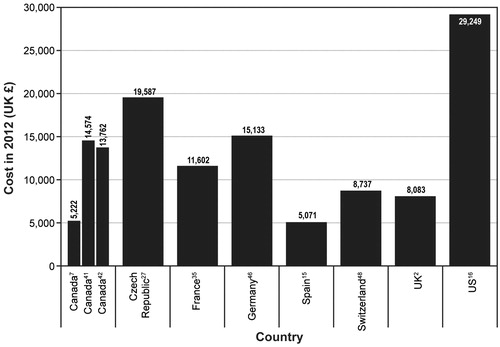
Of the 12 primary cost studies reporting direct costs for an MI in patients with T2DM, 11 reported costs for the year in which the MI occurred. Costs from eight countries (Canada, Czech Republic, France, Germany, Spain, Switzerland, UK, and the US) were identified. presents the costs reported by 10 of these studies. Estimates ranged from £5071–£29,249 for Spain and the US, respectivelyCitation15,Citation16. The eleventh study reported a national cost of MI in patients with T2DM of £25,448,745; because this study did not report the number of MI events experienced, a per-event cost could not be determined. None of the 12 primary cost studies reported indirect costs.
Figure 2. Non-fatal myocardial infarction in the year of the event. UK = United Kingdom; US = United States.
Of the 36 economic evaluations reporting costs for an MI, all included direct costs for the index year of the MI. Cost estimates reported in economic evaluations are presented in the online supplementary information (Table S2). Estimates ranged from £10,054–£15,930 for CanadaCitation17,Citation18, from £13,3459–£15,580 for GermanyCitation19,Citation20, from £3072–£4788 for SwedenCitation21,Citation22, from £8931–£15,489 for SwitzerlandCitation11,Citation23, from £5674–£7649 for the UKCitation12,Citation24, and from £5565–£33,904 for the USCitation25,Citation26.
Although four economic models took a societal perspective, only one reported indirect costs associated with an MI in patients with T2DM. Lindgren et al.Citation22. reported direct and indirect costs (costs related to lost productivity through work absence) for the first year after an MI. Indirect costs incurred by patients in the first year after an MI were estimated to be £10,256 (2012 GBP). The authors provided no detail on the methodological approach supporting this estimate.
Non-fatal myocardial infarction: subsequent years
Of the 12 primary cost studies identified as reporting direct costs for an MI in patients with T2DM, seven studies identified annual costs of follow-up for the years following the event. Cost estimates were identified for six countries (Canada, Czech Republic, Germany, Spain, Switzerland, and the US). presents these costs. Estimates ranged from £286 for the Czech Republic to £1616 for the USCitation16,Citation27.
Table 2. Annual cost estimates for follow-up subsequent to the first year after a non-fatal myocardial infarction reported in cost studies.
Of the 36 economic evaluations reporting costs for MI, 21 included annual direct costs for MI follow-up for subsequent years. Cost estimates reported in economic evaluations are presented in the online supplementary information (Table S3). Estimates ranged from £769–£5489 for CanadaCitation8,Citation18, from £1047–£2062 for GermanyCitation19,Citation20, from £939–£1399 for SwitzerlandCitation10,Citation23, from £699–£962 for the UKCitation13,Citation28, and from £1185–£1558 for the USCitation29,Citation30.
Stroke cost study estimates
The literature review identified 48 studies reporting costs for patients with T2DM who had experienced a stroke: 13 primary studies and 35 economic evaluations in patients with T2DM that included stroke as an adverse event. The costs are presented as mean cost per patient.
Fatal stroke
Of the 13 primary cost studies identified as reporting costs for any type of stroke, only two reported the costs for a fatal event. Clarke et al.Citation2. reported a cost of £6694, when converted to 2012 GBP, and O’Reilly et al.Citation7 reported a converted estimate of £4900 at 2012 UK prices.
Of the 35 economic evaluations reporting costs for stroke in patients with T2DM, 18 reported costs for a fatal event. Cost estimates reported in economic evaluations are presented in the online supplementary information (Table S4). Estimates ranged from £4985–£14,830 for CanadaCitation17,Citation18, from £8075–£8421 for GermanyCitation19,Citation31, from £1936–£4244 for SwitzerlandCitation11,Citation23, and from £4509–£4717 for the UKCitation12,Citation28; one estimate of £3689 was identified for the USCitation14. No cost studies were identified that estimated indirect costs due to mortality for a fatal stroke.
Non-fatal stroke
Of the 13 primary cost studies identified as reporting direct costs for a stroke in patients with T2DM, 11 reported a per-patient cost for the year in which the event occurred. Cost estimates were identified for eight countries (Canada, Czech Republic, France, Germany, Spain, Switzerland, the UK, and the US). presents these costs. Estimates from the 11 studies ranged from £5171 for Spain to £38,732 for the USCitation15,Citation16. All of the 35 economic evaluations reporting costs for a stroke in patients with T2DM reported costs for the initial stroke event or index year.
None of the 11 primary studies reporting costs for a non-fatal stroke estimated indirect costs.
Cost estimates reported in economic evaluations are presented in the online supplementary information (Table S5). Estimates ranged from £13,760–£33,586 for CanadaCitation17,Citation18, from £16,238–£18,104 for GermanyCitation20,Citation30, from £3300–£10,059 for the UKCitation12,Citation24, and from £2109–£50,047 for the USCitation25,Citation32.
Non-fatal stroke follow-up
Of the 13 primary studies, nine studies estimated direct costs for annual follow-up following a stroke. Costs were reported for seven countries (Canada, Czech Republic, Germany, Spain, Switzerland, UK, and the US). presents these costs. Estimates ranged from £286 for the Czech Republic to £12,962 for the USCitation16,Citation27.
Table 3. Annual cost estimates for follow-up subsequent to the first year after a non-fatal stroke reported in cost studies.
Of the 35 economic evaluations reporting costs for a stroke in patients with T2DM, 28 studies reported costs for subsequent years from the second year onward following a stroke in patients with T2DM. Cost estimates reported in economic evaluations are presented in the online supplementary information (Table S6). Estimates ranged from £1909–£5653 for CanadaCitation8,Citation17, from £3919–£5666 for GermanyCitation20,Citation31, from £4116–£7190 for SwitzerlandCitation11,Citation23, from £348–£676 for the UKCitation12,Citation33, and from £1927–£19,732 for the USCitation14,Citation25.
Findings from primary cost studies
The costs identified varied both across and within countries (see ). One of the main reasons for the differences could be the types of resources included in the cost estimates. However, many studies did not provide a detailed breakdown of the resources included in their analysis. Studies tended to report a final cost, but rarely provided a list of the resources included with the corresponding unit costs applied. It was noted that the costs seemed consistently higher in the US, which could be due to better ambulance services leading to lower immediate mortality rates, greater interventional services, and/or higher per-unit costs for medical interventionsCitation34.
The review of primary studies also identified key data gaps. No studies estimated costs according to the severity of the event, and all studies were performed in adult populations. Moreover, no studies estimated the indirect costs (e.g., work time lost, productivity losses) associated with an MI or stroke, which should be an agenda for future research.
Findings from economic models
In several of the economic evaluations identified, the event costs were estimated from a cohort of patients with T2DM; however, a significant number of studies used costs estimated in studies not specific to this population. For example, Table S1 of the supplementary evidence presents the 14 estimates identified for a fatal MI within the economic evaluations. Twelve of these estimates lie within the range of £1606–£5588. The remaining two studies (Hayashino et al.Citation34 [Japan]; Ly et al.Citation14 [US]) reported estimates outside this range, equivalent to £204,366 and £14,403. Both of these studies used estimates taken from studies with general population samples, as opposed to a sample of patients with T2DM. Although these two costs are markedly different from the other 12 values, without detailed studies comparing the resource use and costs of an MI in patients with T2DM vs patients without T2DM, the impact of using these non-specific estimates is difficult to ascertain.
Although several of the identified economic evaluations reported a societal perspective, they did not estimate the proportion of indirect costs (e.g., productivity losses through work absences) that was directly attributable to the adverse event (MI or stroke). Only one study was identified that estimated or reported the indirect costs associated with an MI or stroke in patients with T2DM, and this study provided limited details on the methodology used to determine the costsCitation22.
As with the primary cost studies, no economic evaluations were identified that estimated costs for MI or stroke according to the severity of the event in a cohort of patients with T2DM.
Discussion
How to determine the best data for a core economic model
Selection of cost estimates for an economic model will primarily depend on the country of analysis. The 13 identified primary cost studies provided estimates for Canada, Croatia, the Czech Republic, France, Germany, Mexico, Spain, Switzerland, the UK, and the US. Economic models were also identified that provided estimates for five of these countries (Canada, Germany, Switzerland, the UK, and the US). Updating the review to include also non-English-language papers increased the comprehensiveness of the review and identified three additional studies: two cost studies, one performed in FranceCitation35 and one performed in GermanyCitation36, and one economic evaluationCitation37, which was also performed in Germany.
The choice of cost estimates may be based on several criteria. As this review has shown, for some countries, there are suitable estimates available that have been used within published economic models (e.g., the use of Clarke et al.Citation2 for UK studies). However, for some countries, no primary studies were identified. The review identified three economic models that were developed for SwedenCitation21,Citation22,Citation38, which used costs from studies that were performed in Sweden but were not specific to patients with T2DM. A further study undertaken in PolandCitation39 used an estimate cited as sourced from personal communication. The ideal solution to this would be to perform a cost study in the country of interest using the specific patient sample. However, this approach is not always an option given financial and time constraints. Therefore, some justification should be provided for the choice of estimates when no primary studies have been performed in the country of the analysis. The choice of which estimate to use could be based on multiple alternative criteria; for example:
Use of the most comprehensive cost estimate;
Use of the most conservative estimate; and
Use of data from a country with available data and a similar healthcare system.
Once the choice of cost parameter has been made and the reasons for this choice documented, an important modeling component (particularly when no country-specific estimates are available) is a sensitivity analysis. Sensitivity analysis considers the uncertainty around certain parameters and the impact the value has on cost-effectiveness outcomesCitation40; it also can be used to determine the impact of alternative choices of cost estimates on the model outcomes.
Recommendations for future research
This review has indicated that, although several primary cost studies are evaluating the cost of complications in patients with T2DM, there are significant data gaps. These gaps include a detailed breakdown of the resource use with accompanying unit costs, costs for a pediatric population, costs according to the severity of the event, and the inclusion of indirect costs.
Particularly in countries where no primary cost studies have been performed, economic evaluations tend to use estimates from studies not specific to the T2DM population. The impact of their use is difficult to ascertain without further studies comparing the cost estimates for patients with T2DM to those for the general population.
Conclusion
Determining the costs associated with complications, particularly in chronic diseases, will continue to be an important component of developing economic models. Based on the findings of this review, several standards should be established when estimating or identifying these costs:
Completion of a systematic literature review to identify potential sources of cost parameters;
Detailed review of the primary cost studies identified to review for alignment with the model structure and health states, patient population, and healthcare system of interest;
Detailed reporting of the reasons and justification for the choice of source data; and
Sensitivity analysis of cost estimates to determine the impact of the estimate on model outcomes.
Globally, T2DM continues to be a highly prevalent disease, and its potential complications can have a significant impact on the economic burden of a healthcare system. It is, therefore, important that, in the development of economic models for patients with T2DM, accurate estimates of the costs associated with these complications are obtained.
Transparency
Declaration of funding
This study was funded by Boehringer Ingelheim GmbH. V. Brennan and C. Copley-Merriman drafted this manuscript, and all authors subsequently reviewed and modified the draft to develop this submitted manuscript.
Declaration of financial/other relationships
R. Palencia and B. Hass are employees of Boehringer Ingelheim GmbH. V. Brennan, A. Colosia, C. Copley-Merriman, and J. Mauskopf are employees of RTI Health Solutions. This study was conducted by RTI Health Solutions under the direction of Boehringer Ingelheim GmbH and was funded by Boehringer Ingelheim GmbH. JME Peer Reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplementary Material
Download PDF (151.8 KB)Acknowledgements
The authors thank Maria Fernandez, Cheryl Coon, Jake Libiecki, and Doreen McBride for translation of non-English articles, and Matthew Cawson for assistance with standardizing costs.
References
- Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279-89
- Clarke P, Gray A, Legood R, et al. The impact of diabetes-related complications on healthcare costs: results from the United Kingdom Prospective Diabetes Study (UKPDS Study No. 65). Diabet Med 2003;20:442-50
- Guide to the methods of technology appraisal 2013. London: National Institute for Health and Care Excellence (NICE), 2013. http://www.nice.org.uk/media/D45/1E/GuideToMethodsTechnologyAppraisal2013.pdf. Accessed January 10, 2014
- Drummond M, Sculpher MJ, Torrance GW, et al. Methods for the economic evaluation of health care programmes. 2nd edn. Oxford: Oxford University Press, 1997
- Purchasing power parities (PPPs) data. Paris: Organisation for Economic Co-operation and Development (OECD), 2012. http://www.oecd.org/document/47/0,3746,en_2649_34347_36202863_1_1_1_1,00.html. Accessed January 10, 2014
- Curtis L. Unit costs of health and social care. Canterbury: Personal Social Services Research Unit, 2012. http://www.pssru.ac.uk/project-pages/unit-costs/2012/. Accessed January 10, 2014
- O’Reilly D, Hopkins R, Blackhouse G, et al. Development of an Ontario Diabetes Economic Model (ODEM) and application to a multidisciplinary primary care diabetes management program. Hamilton, ON: Program for Assessment of Technology in Health (PATH), 2006. http://www.path-hta.ca/Libraries/Reports/Development_of_an_Ontario_Diabetes_Economic_Model_ODEM_and_Application_to_a_Multidisciplinary_Primary_Care_Diabetes_Management_Program.sflb.ashx. Accessed January 10, 2014
- Grima DT, Thompson MF, Sauriol L. Modelling cost effectiveness of insulin glargine for the treatment of type 1 and 2 diabetes in Canada. Pharmacoeconomics 2007;25:253-66
- Cameron CG, Bennett HA. Cost-effectiveness of insulin analogues for diabetes mellitus. Can Med Assoc J 2009;180:400-7
- Palmer AJ, Sendi PP, Spinas GA. Applying some UK Prospective Diabetes Study results to Switzerland: the cost-effectiveness of intensive glycaemic control with metformin versus conventional control in overweight patients with type-2 diabetes. Schweiz Med Wochenschr 2000;130:1034-40
- Brändle M, Azoulay M, Greiner RA. Cost-effectiveness of insulin glargine versus NPH insulin for the treatment of Type 2 diabetes mellitus, modeling the interaction between hypoglycemia and glycemic control in Switzerland. Int J Clin Pharmacol Ther 2011;49:217-30
- Woehl A, Evans M, Tetlow AP, et al. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled type 2 diabetes in the United Kingdom (structured abstract). Cardiovasc Diabetol 2008;7:24
- Clarke PM, Gray AM, Briggs A, et al. Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72) (structured abstract). Diabetologia 2005;48:868-77
- Ly D, Fu AZ, Hebert C. Cost effectiveness analysis of a hypertension management program in patients with type 2 diabetes (structured abstract). J Clin Hypertens 2009;11:116-24
- Neeser K, Weber C. Cost impact of self-measurement of blood glucose on complications of type 2 diabetes: the Spanish perspective. Diabetes Technol Ther 2009;11:509-16
- O’Brien JA, Patrick AR, Caro J. Estimates of direct medical costs for microvascular and macrovascular complications resulting from type 2 diabetes mellitus in the United States in 2000. Clin Ther 2003;25:1017-38
- Cameron C, Coyle D, Ur E, et al. Cost-effectiveness of self-monitoring of blood glucose in patients with type 2 diabetes mellitus managed without insulin (structured abstract). CMAJ 2010;182:28-34
- Coyle D, Palmer AJ, Tam R. Economic evaluation of pioglitazone hydrochloride in the management of type 2 diabetes mellitus in Canada. Pharmacoeconomics 2002;20(1 Suppl):31-42
- Roze S, Valentine WJ, Evers T, et al. Acarbose in addition to existing treatments in patients with type 2 diabetes: health economic analysis in a German setting (structured abstract). Curr Med Res Opin 2006;22:1415-24
- Schaufler TM, Wolff M. Cost effectiveness of preventive screening programmes for type 2 diabetes mellitus in Germany. Appl Health Econ Health Policy 2010;8:191-202
- Goodall G, Jendle JH, Valentine WJ, et al. Biphasic insulin aspart 70/30 vs. insulin glargine in insulin naive type 2 diabetes patients: modelling the long-term health economic implications in a Swedish setting. Int J Clin Pract 2008;62:869-76
- Lindgren P, Lindstrom J, Tuomilehto J, et al. Lifestyle intervention to prevent diabetes in men and women with impaired glucose tolerance is cost-effective. Int J Technol Assess Health Care 2007;23:177-83
- Brändle M, Erny-Albrecht KM, Goodall G, et al. Exenatide versus insulin glargine: a cost-effectiveness evaluation in patients with type 2 diabetes in Switzerland. Int J Clin Pharmacol Ther 2009;47:501-15
- Valentine WJ, Bottomley JM, Palmer AJ, et al. PROactive 06: cost-effectiveness of pioglitazone in Type 2 diabetes in the UK (structured abstract). Diabetic Med 2007;24:982-1002
- Li R, Zhang P, Barker LE, et al. Cost-effectiveness of aspirin use among persons with newly diagnosed type 2 diabetes. Diabetes Care 2010;33:1193-9
- Ramsey SD, Clarke LD, Roberts CS, et al. An economic evaluation of atorvastatin for primary prevention of cardiovascular events in type 2 diabetes. Pharmacoeconomics 2008;26:329-39
- Weber C, Kocher S, Neeser K, et al. Impact of self-measurement of blood glucose on complications of type 2 diabetes: Economic analysis from a Czech perspective. Curr Med Res Opin 2010;26:289-96
- Ray JA, Boye KS, Yurgin N, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes (structured abstract). Curr Med Res Opin 2007;23:609-22
- Maetzel A, Ruof J, Covington M, et al. Economic evaluation of orlistat in overweight and obese patients with type 2 diabetes mellitus (structured abstract). Pharmacoeconomics 2003;21:501-12
- Ray JA, Valentine WJ, Roze S, et al. Insulin therapy in type 2 diabetes patients failing oral agents: cost-effectiveness of biphasic insulin aspart 70/30 vs insulin glargine in the US (structured abstract). Diabetes Obes Metab 2007;9:103-13
- Valentine WJ, Goodall G, Aagren M, et al. Evaluating the cost-effectiveness of therapy conversion to insulin detemir in patients with type 2 diabetes in Germany: a modelling study of long-term clinical and cost outcomes. Adv Ther 2008;25:567-84
- Herman WH, Thomas MP, Hoerger TJ, et al. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance (structured abstract). Ann Intern Med 2005;142:323-32
- Valentine WJ, Palmer AJ, Lammert M, et al. Long-term clinical and cost outcomes of treatment with biphasic insulin aspart 30/70 versus insulin glargine in insulin naive type 2 diabetes patients: cost-effectiveness analysis in the UK setting (structured abstract). Curr Med Res Opin 2005;21:2063-71
- Hayashino Y, Nagata-Kobayashi S, Morimoto T, et al. Cost-effectiveness of screening for coronary artery disease in asymptomatic patients with type 2 diabetes and additional atherogenic risk factors. J Gen Intern Med 2004;19:1181-91
- Marissal JP, Gueron B, Dervaux B. The cost of complications: implications for the measurement of the cost of type II diabetes mellitus. Rev Epidemiol Sante Publ 2006;54:137-47
- Liebl A, Spannheimer A, Reitberger U, et al. Costs of long-term complications in type 2 diabetes patients in Germany. Results of the CODE-2(registered trademark) study. Med Klin 2002;97:713-19
- Lucioni C, Mazzi S, Neeser K. Cost-effectiveness analysis of pioglitazone therapy in DMT2. Pharmaco Econ Ital Res Artic 2004;6:81-93
- Ringborg A, Yin DD, Martinell M, et al. The impact of acute myocardial infarction and stroke on health care costs in patients with type 2 diabetes in Sweden. Eur J Cardiovasc Prev Rehabil 2009;16:576-82
- Grzeszczak W, Czupryniak L, Kolasa K, et al. The cost-effectiveness of saxagliptin versus NPH insulin when used in combination with other oral antidiabetes agents in the treatment of type 2 diabetes mellitus in Poland. Diabetes Technol Ther 2012;14:65-73
- Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd edn. Oxford: Oxford University Press, 2005
- O’Brien JA, Patrick AR, Caro JJ. Cost of managing complications resulting from type 2 diabetes mellitus in Canada. BMC Health Serv Res 2003;3:7
- O’Brien JA, Caro I, Getsios D, et al. Diabetes in Canada: direct medical costs of major macrovascular complications. Value Health 2001;4:258-65
- Saric T, Benkovic V, Poljicanin T, et al. Cost of diabetes in Croatia impact of complications on the costs of type II diabetes. Madrid: International Society of Pharmacoeconomics and Outcomes Research 14th Annual European Congress, 2011. Abstract and poster 27649
- Marissal JP, Sailly JC, Crainich D, et al. Évaluation de l’impact budgétaire de l’application des recommendations de bonne pratique dans le diabète de type II en France. Rev Epidemiol Sante Publique 2005;53:1567-78
- Ramsey SD, Newton K, Blough D, et al. Patient-level estimates of the cost of compliucationsin diabetes in a managed-care population. Pharmacoeconomics 1999;16:285-95
- Weber C, Neeser K, Wenzel H, et al. Cost of type 2 diabetes in Germany over 8 years: the Rosso study no. 2. J Med Econ 2006;9:45-53
- Meléndez G, Cabra HA, Zanela OO, et al. Cost of type 2 diabetes complications in Mexico. Rio de Janeiro: International Society of Pharmacoeconomics and Outcomes Research 2nd Latin America Conference, 2009. Abstract and poster 21971
- Weber C, Schneider B, Lodwig V, et al. Cost impact of blood glucose self-monitoring on complications of type 2 diabetes: a Swiss perspective (ROSSO study No. 11). Swiss Med Wkly 2007;137:545-50