Abstract
Background and aims:
Randomized controlled trials have shown that a once-daily prolonged-release (PR) tacrolimus formulation (PR tacrolimus; Advagraf), is non-inferior to a twice-daily immediate-release (IR) tacrolimus formulation (IR tacrolimus; Prograf) in terms of biopsy-proven acute rejection, graft failure and mortality in renal transplant recipients. However, relative to IR tacrolimus, PR tacrolimus exhibits reduced tacrolimus trough concentration variability, which has been associated with reduced graft failure. Based on these data, the present study evaluated the cost of switching UK renal transplant patients from IR tacrolimus to PR tacrolimus.
Methods:
UK-specific data on acute rejection, graft failure, and mortality were used to construct a budget impact model to assess the costs of switching from IR tacrolimus to PR tacrolimus on a 1:1 mg:mg basis. The model assumed that 3.1% of patients on PR tacrolimus had high tacrolimus trough concentration variability compared with 17.4% on IR tacrolimus, based on a study comparing PR tacrolimus and IR tacrolimus pharmacokinetics. A relative graft failure risk of 2.38 was applied to high variability patients based on data from a tacrolimus variability study in which 10/148 patients with low variability experienced graft failure, compared with 24/149 in the high variability group. Cost data were taken from the British National Formulary and 2012–2013 NHS tariff information.
Results:
The mean per-patient cost (including tacrolimus, concomitant immunosuppressive medications, dialysis after graft failure, and treatment for acute rejection) was GBP 26,941 (standard deviation [SD] = GBP 2765) with PR tacrolimus vs GBP 30,356 (SD = GBP 3085) for IR tacrolimus over a 5-year period, corresponding to a saving of GBP 3415 (SD = GBP 516) per patient or GBP 341,500 in a hypothetical 100-patient transplant center. Cost savings were driven primarily by lower dialysis costs resulting from the lower proportion of PR tacrolimus patients with high tacrolimus trough concentration variability (leading to lower graft failure risk).
Limitations:
The main limitation of the study was the use of heterogeneous data sources to capture the effect of within-patient variability on graft failure. The most important difference between the studies was the definition of the threshold between low and high within-patient variability. This was explored in sensitivity analyses in which the inter-arm difference in the inter-arm proportions of patients with high and low variability was abolished.
Conclusions:
Converting UK renal transplant recipients from IR tacrolimus to PR tacrolimus was associated with lower pharmacy and dialysis costs.
Introduction
Since its approval for the prophylaxis of liver allograft rejection by the Food and Drug Administration in 1994, tacrolimus has also been used as the primary maintenance immunosuppressive medication in heart, small bowel, pancreas, bone marrow, kidney and lung allograft recipientsCitation1,Citation2. In the EU, the highly-potent macrolide immunosuppressant is indicated for the prophylaxis of transplant rejection in kidney or liver allograft recipients and, in adult patients, it has typically been prescribed as a twice-daily capsule with a starting dose of 0.2–0.3 mg/kg/day in kidney transplant recipientsCitation2,Citation3.
While studies have demonstrated that tacrolimus has a well-characterized efficacy and safety profile in patients at risk of post-transplantation graft rejection, doses that result in tacrolimus exposure outside of the therapeutic range can result in an increased risk of rejection (with low tacrolimus concentrations) or toxicity (with high tacrolimus concentrations)Citation4–6. These potential undesirable effects, combined with the high within-patient variability in tacrolimus pharmacokinetics and the relatively narrow therapeutic index, necessitate the use of regular therapeutic drug monitoring of whole-blood tacrolimus trough concentrations (i.e., the concentration immediately before taking another dose) to establish the appropriate dose for a given patientCitation7. In kidney transplant recipients, the therapeutic range (expressed as the tacrolimus trough concentration) typically varies by time after transplantation: 15–20 ng/mL in the first month, dropping to 10–15 ng/mL in months 2 and 3 and 5–12 ng/mL in subsequent months, with a typical maintenance trough level of 5–8 ng/mLCitation2,Citation8.
The process of ascertaining the appropriate dose for a given patient is complicated by the intra-patient pharmacokinetic variability exhibited by tacrolimus. Variously referred to as within-patient, intra-subject, intra-individual or within-patient variability, the term refers to day-to-day differences in either tacrolimus exposure or tacrolimus clearance in a given patient taking the same daily dose. Intra-patient variability is typically measured using the coefficient of variability (CoV), which is calculated by dividing the standard deviation of the trough concentration by the mean and expressing the result as a percentageCitation9. Any reduction in CoV would be likely to reduce the complexity associated with establishing a suitable dose, while potentially also improving patient outcomes by maintaining the whole-blood tacrolimus concentration within the therapeutic range. In 2007, the European Commission granted EU-wide marketing authorization for a once-daily, prolonged-release (PR) formulation of tacrolimus (Advagraf, PR tacrolimus)Citation3. In the scientific discussion that accompanied the marketing authorization, it was noted that phase II trial data had already demonstrated that conversion from an existing twice-daily, immediate-release (IR) tacrolimus formulation; (Prograf, IR tacrolimus) to PR tacrolimus had resulted in reduced intra-subject variabilityCitation10. More recently, a 2011 prospective study showed that, when stable renal transplant recipients switched from IR tacrolimus to PR tacrolimus, the proportion of patients with a tacrolimus CoV greater than 22.5% decreased from 17.4% to 3.1% (p < 0.01)Citation11. The threshold of 22.5% was selected based on a receiver-operator characteristic (ROC) curve presenting the performance of baseline tacrolimus trough concentration variability as a predictor of reduced trough concentration 7 days after conversion to PR tacrolimus. Youden’s J-statistic (the inflection point of the curve, closest to the point at which sensitivity and specificity equal 1) was then used to select the optimal cut-off value. Similar findings have been reported in liver transplant recipients by Florman et al.Citation12, who reported that variability in tacrolimus exposure was 40.6% lower with PR tacrolimus than for IR tacrolimus (p = 0.044).
Published evidence also indicates that there is a relationship between high within-patient variability and poor clinical outcomes, including graft failure and late rejection. Notably, in 2010, Borra et al.Citation13 published a retrospective analysis into the relationship between tacrolimus clearance variability and a composite end-point of graft loss, biopsy-proven chronic allograft nephropathy and doubling of plasma creatinine concentration. The study found that significantly more renal transplant recipients with high within-patient variability (defined as a tacrolimus clearance variability higher than the whole-population median of 14.9%) reached the composite end-point than those with low within-patient variability (16.1% vs 6.8%). Similarly, Pollock-Barziv et al.Citation14 reported that increased standard deviation in intra-patient tacrolimus blood levels was an independent risk factor for late rejection (odds ratio of 1.6, p = 0.02), and that graft survival (conditional on 1-year patient survival) in patients with a tacrolimus concentration standard deviation <2 ng/mL was 98%, compared with 70% in patients with standard deviation >2 ng/mL (p = 0.003).
Based on the reported differences in within-patient variability between IR tacrolimus and PR tacrolimus and the reported increase in graft failure risk in patients with high within-patient variability, the present study aimed to utilize these data, along with UK-specific clinical and cost data, to evaluate the budgetary implications of switching renal transplant recipients from IR tacrolimus to PR tacrolimus from the perspective of a healthcare payer in the UK setting. The comparison with IR tacrolimus was conducted on the grounds that IR tacrolimus represents the current standard of care in the UK setting as per the most recent National Institute of Health and Care Excellence (NICE) technology appraisal of immunosuppressive therapy for renal transplantation in adultsCitation15. The head-to-head comparison with PR tacrolimus was performed on the basis of the relative ease of conversion to a different formulation of tacrolimus (as opposed to a different class of immunosuppressant). Importantly, the same therapeutic drug monitoring techniques can be used for IR and PR tacrolimus, with both formulations showing a similar correlation between tacrolimus trough concentration and tacrolimus exposure (as measured by AUC0–24)Citation16,Citation17.
Methods
Model
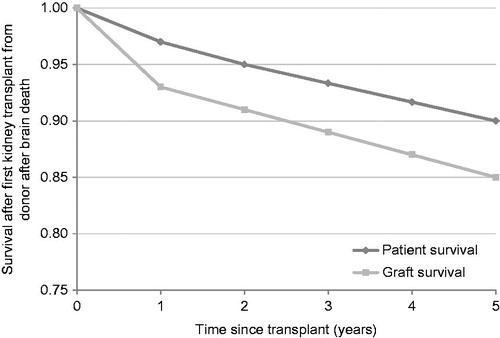
A budget impact model was constructed to evaluate the direct medical costs associated with using PR tacrolimus relative to IR tacrolimus in patients undergoing de novo kidney transplant in the UK setting. The model was constructed in Microsoft Excel (Microsoft Corporation, Redmond, WA) and was designed to report the clinical end-points of graft failure, acute rejection and patient mortality. The underlying incidence of graft failure and patient mortality was based on data from the 2011–2012 National Health Service Blood and Transplant (NHSBT) Organ Donation and Transplantation Activity ReportCitation18. Specifically, data from 2469 UK patients who underwent first kidney transplantation (from donors after brain death) in 2004–2006 were used to establish the ‘baseline’ proportion of grafts and patients surviving at years 1, 2 and 5 after transplant. Graft and patient survival in years 3 and 4 were interpolated linearly between years 2 and 5 (). As the incidence of acute rejection was not reported in the NHSBT annual report, data on acute rejection were taken from a 2006 UK database analysis by McEwan et al.Citation19
Figure 1. Graft and patient survival from the National Health Service Blood and Transplant 2011–2012 Organ Donation and Transplantation Activity ReportCitation18. Data at years 3 and 4 were linearly interpolated using values from years 2 and 5.
In the base case analysis, the cohort and dosing assumptions were based on a multi-center randomized trial, published in 2010 by Krämer et al.Citation20, that compared PR tacrolimus and IR tacrolimus in 667 de novo renal transplant recipients. Based on the study, the cohort was assumed to have a mean body weight of 70.3 kg. The model used mean bodyweight to calculate the mean daily dose of tacrolimus, mycophenolate mofetil (MMF) and corticosteroids (betamethasone) using dosing data from the same study. In the case of tacrolimus, doses were based on the dose at study day 365. Since patients in the Krämer et al.Citation20 study were initiated on the same dose (of 0.2 mg/kg/day) and subsequent differences arising from titration to a pre-specified serum trough concentration were not reported as significant, the PR tacrolimus and IR tacrolimus doses were taken to be the same in both arms (0.075 mg/kg/day). Mean MMF doses were taken to be 1450 mg/day in year 1 (based on the mid-point of the doses at baseline and day 365) and 960 mg/day in subsequent years (based on the dose at day 365). All patients were assumed to continue taking MMF for the duration of the modeling analysis. Corticosteroid doses of 16.6 mg/day in year 1 and 4.9 mg/day in subsequent years were calculated on the same basis as MMF. However, in contrast to MMF, the proportion of patients taking corticosteroids was assumed to be 94.7% in the first year, dropping to 89.3% in subsequent years, also based on the Krämer et al.Citation20 study.
The proportion of patients with high within-patient variability and the effects of high within-patient variability on the incidence of graft failure were modeled using data from two studies. The first, published in 2011 by Wu et al.Citation11, reported that, after conversion of stable renal transplant recipients (n = 129) from IR tacrolimus to PR tacrolimus, the proportion of patients with a trough concentration CoV greater than 22.5% decreased from 17.4% to 3.1% (p < 0.01 at 3 months after conversion). For the purposes of the analysis, these proportions of patients were considered to have ‘high’ within-patient variability. The second study, by Borra et al.Citation13, was a retrospective analysis of 297 patients who had undergone kidney transplant between 2001–2004 and had a functioning graft at 12 months after transplantation. The patients were divided into two groups down the median tacrolimus clearance CoV (calculated as the variability in plasma concentration divided by the dose) to give ‘low’ and ‘high’ within-patient variability groups and the incidence of the primary composite end-point (of graft loss, biopsy-proven chronic allograft nephropathy and doubling of plasma creatinine concentration) was calculated for each group. The mean follow-up period was 1849 days (range = 1029–2811 days), over which time 34 patients reached the primary end-point. Of these, 10 occurred in the low variability group (n = 148) and 24 occurred in the high variability group (n = 149), giving a relative risk of reaching the composite end-point of 2.38 in patients with high within-patient variability relative to those with low within-patient variability. In the proportion of patients with high within-patient variability, this relative risk was applied to the baseline annual incidence of graft failure, as derived from the underlying NHSBT data.
In the base case analysis, the model assumed that patients experiencing graft failure all started dialysis (rather than undergoing kidney re-transplantation). Based on the mid-point of estimates from the NICE clinical guideline on peritoneal dialysisCitation21, 15% of patients were assumed to be on peritoneal dialysis, with the remaining 85% undergoing hemodialysis. Patients on hemodialysis were assumed to have an average of three dialysis sessions per week, based on the Renal Association hemodialysis guidelinesCitation22.
While the model was constructed to allow different risks of acute rejection and mortality to be applied to patients on PR tacrolimus and IR tacrolimus, the relative risks were set to one in both arms in the base case analysis. The incidence of acute rejection and mortality was, therefore, equivalent in both arms.
Costs
All drug costs were taken from the March 2013 British National Formulary (BNF), while costs associated with dialysis were taken from 2012–2013 National Health Service tariff information (). Costs of acute rejection episodes were based on the assumption that patients would have 3 days of intravenous methylprednisolone therapy at 250 mg/day. Rejection episodes refractory to such treatment were assumed to be treated with a 10-day intravenous infusion of anti-thymocyte immunoglobulin at 1.5 mg/kg/day.
Table 1. Costs used in the base case analysis.
Perspective, time horizon and discounting
The base case analysis was conducted over a 5-year time horizon and, in line with budget impact modeling guidance from the International Society for Pharmacoeconomic and Outcomes Research (ISPOR), outcomes were not discounted (i.e. the discount rate was set to 0% per annum)Citation23. However, sensitivity analyses were performed in which the discount rate was set to 3.5% as per the recommendations for discounting the cost component of cost-effectiveness analyses laid out in the National Institute for Care Excellence (NICE) Guide to the Methods of Technology AppraisalCitation24. All analyses were performed from the perspective of a UK healthcare payer.
Sensitivity analyses
A series of sensitivity analyses were performed around the base case to establish the magnitude of effect of various drivers on the absolute and incremental outcomes. Analyses were performed in which the discount rate was set to 3.5% and the time horizon reduced to 3 years.
The effect of a proportion of graft failure patients undergoing re-transplantation rather than dialysis was also explored in an analysis utilizing re-transplantation incidence data from the McEwan et al.Citation19 database analysis. In the re-transplantation sensitivity analysis, patients not undergoing re-transplantation underwent dialysis in line with the base case proportions of 15% peritoneal dialysis, 85% hemodialysis. The cost of kidney re-transplantation, which does not have an associated Healthcare Resource Group (HRG) code, was taken to be GBP 22,080, based on a 2011 UK cost-effectiveness analysis that reported kidney transplantation costsCitation25.
One sensitivity analysis was performed in which the proportion of patients with high within-patient variability in the PR tacrolimus arm was set to the same as in the IR tacrolimus arm (17.4%), while a separate analysis assumed no difference in the relative risk of graft failure in patients with high vs low within-patient variability. In addition to the one-way sensitivity analyses, a series of five analyses were performed to establish the relationship between relative risk of graft failure in patients with high within-patient variability and the incremental cost of PR tacrolimus relative to IR tacrolimus, assuming that 3.1% of PR tacrolimus patients and 17.4% of IR tacrolimus patients had ‘high’ within-patient variability. In this analysis, the relative risk of graft failure with high within-patient variability was varied linearly between 1 (no change in risk) and 2.38 (the base case value). Finally, a sensitivity analysis was performed in which the per-milligram cost of PR tacrolimus was set to the same as the per-milligram cost of IR tacrolimus.
Probabilistic sensitivity analysis (PSA) was conducted in the base case and around all one-way sensitivity analyses. In each analysis, the model performed 10,000 iterations, with each iteration sampling from distributions around key model parameters. Normal distributions around bodyweight and tacrolimus, MMF and corticosteroid dosing were sampled based on data from the Krämer et al.Citation20 clinical trial and a uniform distribution of the proportion of graft failure patients undergoing peritoneal dialysis (0–30%) and hemodialysis (70–100%) was sampled based on the range provided in NICE Clinical Guideline 125Citation21. All analysis results were reported as mean cost and standard deviation (SD) in each arm and the mean and SD incremental cost.
Results
In the base case analysis (which applied a relative risk of graft failure of 2.38 for patients with high within-patient variability relative to low within-patient variability), 14.8% of patients in the PR tacrolimus arm had experienced graft failure after 5 years, relative to 17.6% in the IR tacrolimus arm. Driven primarily by this reduced incidence of graft failure, patients treated with PR tacrolimus incurred costs of GBP 26,941 per patient (SD = GBP 2765) over 5 years, compared with GBP 30,356 (SD = GBP 3085) in patients treated with IR tacrolimus, representing a saving of GBP 3415 (SD = GBP 516) per patient or GBP 341,500 (SD = 51,600) in a hypothetical 100-patient transplant center (). Cost savings of GBP 1856 were driven by the reduced incidence of graft failure in the lower proportion of patients in the PR tacrolimus arm with high variability. There were no differences in mortality, incidence of acute rejection, or concomitant medication costs in the base case analysis.
Table 2. Base case results expressed as per-patient costs over a 5-year time horizon.
Sensitivity analysis showed that shortening the model time horizon lowered the cost savings with PR tacrolimus to GBP 1893 (SD = GBP 314) over a 3-year time horizon (). Increasing the discount rate to 3.5% reduced the cost savings with PR tacrolimus to GBP 3190 (SD = GBP 482) per patient. In the re-transplantation analysis (in which a proportion of graft failure patients undergo re-transplantation instead of dialysis), the cost savings with PR tacrolimus relative to IR tacrolimus decreased to GBP 2928 (SD = GBP 521). Analyses in which the proportions of patients with high within-patient variability were the same in both arms and in which the relative risk of graft failure in high within-patient variability patients was set to 1 resulted in the same incremental cost of GBP 1563 (SD = GBP 513), albeit with lower absolute costs in the latter scenario, owing to the reduction in the proportion of patients on dialysis. Finally, the analysis in which the relative risk of graft failure with high within-patient variability was varied between 1 and 2.38 showed a linear relationship between the relative risk of graft failure and the incremental cost of PR tacrolimus relative to IR tacrolimus ().
Figure 2. Incremental cost of PR tacrolimus vs IR tacrolimus over a range of relative risks of graft failure with high tacrolimus trough concentration variability.
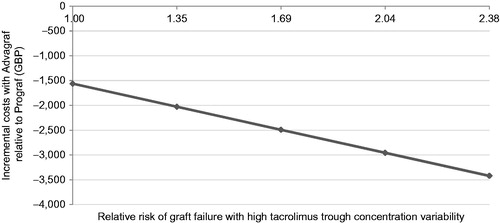
Table 3. One-way sensitivity analysis results.
Discussion
The present study showed that, over a 5-year time horizon, using PR tacrolimus, a prolonged-release tacrolimus formulation in renal transplant recipients could result in substantial cost savings in the UK setting relative to immediate-release tacrolimus. Cost savings were driven primarily by a reduction in the incidence of graft failure in patients with lower within-patient variability, of which there were more in the prolonged-release arm than the immediate-release arm (96.9% of patients in the prolonged-release arm vs 82.6% in the immediate-release arm). Over the 5-year time horizon, 14.8% of patients in the PR tacrolimus arm had experienced graft failure after 5 years, relative to 17.6% in the IR tacrolimus arm. The analysis used a straightforward and transparent modeling approach; recent, robust, and UK-specific data to model graft rejection and mortality; and up-to-date UK cost data and resource-use assumptions. However, as in all modeling studies, there are limitations that should be considered when interpreting the findings. First, despite using data primarily from the UK setting, there was some unavoidable heterogeneity in the data sources used. For instance, two distinct data sources were used to capture differences in the incidence of graft failure based on within-patient variability. The study used to define the proportion of patients with ‘high’ and ‘low’ within-patient variability was a prospective study conducted in 129 Taiwanese patients in 2010. As the authors noted, the study was based on a single-arm design in which all patients were converted from IR tacrolimus to PR tacrolimus. The proportion of patients with high within-patient variability on IR tacrolimus and PR tacrolimus is, therefore, derived from the same patient group before and after switching. More importantly, the study from which the relative risk of graft failure with high within-patient variability was derived used the median tacrolimus CoV to segregate patients into high and low within-patient variability group. In the whole patient group, the median CoV in tacrolimus clearance was 14.9% (mean 17%), substantially lower than the 22.5% tacrolimus trough concentration CoV cut-off used in the Wu et al.Citation11 study. As the model treated these values (tacrolimus clearance variability and tacrolimus trough concentration variability) as equivalent, a key assumption of the study is that, in a given patient on a given tacrolimus regimen, the dose, dose interval, time to maximum plasma concentration, volume of distribution and elimination rate constant would be constant and that variability in tacrolimus trough concentration would, therefore, be proportionate to the variability in tacrolimus clearance. While we acknowledge that these are notable limitations of the study, the sensitivity analysis in which the proportion of patients with high within-patient variability is set to the same in both arms, in concert with the analysis presented in , illustrate that PR tacrolimus was cost saving regardless of the proportion of patients with high within-patient variability. Furthermore, the relative risks used in the modeling analysis were conservative and the projected cost savings may, therefore, represent an under-estimate.
Finally, another potential limitation of the present study is the conservative range of factors that drove cost differences in the base case analysis, namely the pharmacy costs and increased risk of graft failure in patients with high within-patient variability. For instance, a recent study by Ro et al.Citation26 reported a hazard ratio of 2.655 (95% confidence interval = 1.394–5.056) for the incidence of biopsy-proven acute rejection in patients with high within-patient variability relative to those with low within-patient variability, having controlled for mean tacrolimus concentration, human leukocyte antigen mismatch, induction therapy, donor type and CYP3A5 polymorphism (the primary focus of the study).
Conclusion
The present analysis demonstrated that, relative to IR tacrolimus, PR tacrolimus is cost-saving in renal transplant recipients in the UK. Savings were driven primarily by the lower costs associated with dialysis after graft failure, in turn driven by a reduced incidence of graft failure in the greater proportion of patients taking PR tacrolimus with low within-patient variability. PR tacrolimus is an established immunosuppressant with a well-characterized safety and efficacy profile in the prophylaxis of graft rejection. The present study provides evidence that it may also result in substantial cost savings in the UK setting. Given that recent research indicates that 99.4% of patients prefer once-daily immunosuppressive medication to twice dailyCitation27, there should be no clinical or economic barriers to its use in renal transplant recipients in the UK.
Transparency
Declaration of funding
Funding for the development of the model and preparation of the manuscript was provided by Astellas Pharma Europe Limited, Chertsey, UK.
Declaration of financial/other relationships
Gorden Muduma and Isaac Odeyemi are full-time employees of Astellas Pharma Europe Limited. Richard Pollock is a full-time employee of Ossian Health Economics and Communications GmbH, a company that received funding from Astellas for their role in developing this study. The JME Peer Reviewer on this manuscript is part of the JME Editorial Board, but has no relevant financial relationships to disclose.
Acknowledgments
No assistance in the preparation of this article is to be declared.
Notes
*Advagraf is a registered trademark of Astellas Pharma Inc, Tokyo, Japan.
†Prograf is a registered trademark of Astellas Pharma Inc, Tokyo, Japan.
*Advagraf is a registered trademark of Astellas Pharma Inc, Tokyo, Japan.
†Prograf is a registered trademark of Astellas Pharma Inc, Tokyo, Japan.
References
- US Food and Drug Administration. Prograf Drug Approval Package. Silver Spring, MA, USA. 1994. http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/050708_prograf_toc.cfm. Accessed June 4, 2013
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004;43:623–53
- European Medicines Agency. Advagraf European Public Assessment Report Annex I: Summary of Product Characteristics. London, UK. 2007. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000712/WC500022234.pdf. Accessed June 4, 2013
- Kershner RP, Fitzsimmons WE. Relationship of FK506 whole blood concentrations and efficacy and toxicity after liver and kidney transplantation. Transplantation 1996;62:920–6
- Borobia AM, Romero I, Jimenez C, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit 2009;31:436–42
- taatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol Dial Transplant 2001;16:1905–9
- Scott LJ, McKeage K, Keam SJ, et al. Tacrolimus: a further update of its use in the management of organ transplantation. Drugs 2003;63:1247–97
- Ekberg H, van Gelder T, Kaplan B, et al. Relationship of tacrolimus exposure and mycophenolate mofetil dose with renal function after renal transplantation. Transplantation 2011;92:82–7
- Kahan BD, Welsh M, Urbauer DL, et al. Low intraindividual variability of cyclosporine A exposure reduces chronic rejection incidence and health care cost. J Am Soc Nephrol 2000;11:1122–31
- European Medicines Agency. Advagraf European Public Assessment Report: Scientific Discussion. London, UK. 2007. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000712/WC500022237.pdf. Accessed June 5, 2013
- Wu MJ, Cheng CY, Chen CH, et al. Lower variability of tacrolimus trough concentration after conversion from prograf to advagraf in stable kidney transplant recipients. Transplantation 2011;92:648–52
- Florman S, Alloway R, Kalayoglu M, et al. Conversion of stable liver transplant recipients from a twice-daily Prograf-based regimen to a once-daily modified release tacrolimus-based regimen. Transplant Proc 2005;37:1211–3
- Borra LC, Roodnat JI, Kal JA, et al. High within-patient variability in the clearance of tacrolimus is a risk factor for poor long-term outcome after kidney transplantation. Nephrol Dial Transplant 2010;25:2757–63
- Pollock-Barziv SM, Finkelstein Y, Manlhiot C, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant 2010;14:968–75
- National Institute for Clinical Excellence. Immunosuppressive therapy for renal transplantation in adults. Technical Appraisal 85. London, UK. 2004. http://www.nice.org.uk/TA85. Accessed 3 March, 2014
- Wlodarczyk Z, Squifflet JP, Ostrowski M, et al. Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 2009;9:2505–13
- Alloway R, Steinberg S, Khalil K, et al. Conversion of stable kidney transplant recipients from a twice daily Prograf-based regimen to a once daily modified release tacrolimus-based regimen. Transplant Proc 2005;37:867–70
- NHS Blood and Transplant. Transplant Activity in the UK. Activity report 2011–12. Watford, UK. 2012. http://www.organdonation.nhs.uk/statistics/transplant_activity_report/current_activity_reports/ukt/activity_report_2011_12.pdf. Accessed June 4, 2013
- McEwan P, Dixon S, Baboolal K, et al. Evaluation of the cost effectiveness of sirolimus versus tacrolimus for immunosuppression following renal transplantation in the UK. Pharmacoeconomics 2006;24:67–79
- Krämer BK, and the Tacrolimus Prolonged Release Renal Study Group. Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplan 2010;10:2632–43
- National Institute for Health and Clinical Excellence. Peritoneal dialysis in the treatment of stage 5 chronic kidney disease. London, UK. 2012. http://guidance.nice.org.uk/CG125/NICEGuidance/pdf/English. Accessed June 4, 2013
- The Renal Association. RA Guidelines – Haemodialysis. Petersfield, UK. 2009. http://www.renal.org/clinical/guidelinessection/haemodialysis.aspx. Accessed June 4, 2013
- Mauskopf JA, Sullivan SD, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices–budget impact analysis. Value Health 2007;10:336–47
- National Institute for Health and Clinical Excellence. Guide to the methods of technology appraisal. London, UK. 2008. http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. Accessed June 4, 2013
- Beaudet A, Palmer JL, Timlin L, et al. Cost-utility of exenatide once weekly compared with insulin glargine in patients with type 2 diabetes in the UK. J Med Econ 2011;14:357–66
- Ro H, Min SI, Yang J, et al. Impact of tacrolimusintraindividual variability and CYP3A5 genetic polymorphism on acute rejection in kidney transplantation. Ther Drug Monit 2012;34:680–5
- Guirado L, and the GREAT Study Group. Efficacy and safety of conversion from twice-daily to once-daily tacrolimus in a large cohort of stable kidney transplant recipients. Am J Transplant 2011;11:1965–71

