Abstract
Objective:
Improved health outcomes can result in economic savings for hospitals and payers. While effectiveness of topical hemostatic agents in cardiac surgery has been demonstrated, evaluations of their economic benefit are limited. This study quantifies the cost consequences to hospitals, based on clinical outcomes, from using a flowable hemostatic matrix vs non-flowable topical hemostatic agents in cardiac surgery.
Research design and methods:
Applying clinical outcomes from a prospective randomized clinical trial, a cost consequence framework was utilized to model the economic impact of comparator groups. From that study, clinical outcomes were obtained and analyzed for a flowable hemostatic matrix (FLOSEAL, Baxter Healthcare Corporation) vs non-flowable topical hemostats (SURGICEL Nu-Knit, Ethicon–Johnson & Johnson; GELFOAM, Pfizer). Costing analyses focused on the following outcomes: complications, blood transfusions, surgical revisions, and operating room (OR) time. Cardiac surgery costs were analyzed and expressed in 2012 US dollars based on available literature searches and US data. Comparator group variability in cost consequences (i.e., cost savings) was calculated based on annualized impact and scenario testing.
Results:
Results suggest that if a flowable hemostatic matrix (rather than a non-flowable hemostat) was utilized exclusively in 600 mixed cardiac surgeries annually, a hospital could improve patient outcomes by a reduction of 33 major complications, 76 minor complications, 54 surgical revisions, 194 transfusions, and 242 h of OR time. These outcomes correspond to a net annualized cost consequence savings of $5.38 million, with complication avoidance as the largest contributor.
Conclusions:
This cost consequence framework and supportive modeling was used to evaluate the hospital economic impact of outcomes resulting from the usage of various hemostatic agents. These analyses support that cost savings can be achieved from routine use of a flowable hemostatic matrix, rather than a non-flowable topical hemostat, in cardiac surgery.
Introduction
Inadequate hemostasis during surgery may lead to immediate and long-term complications in patients who undergo surgery. Excessive blood loss and allogeneic blood transfusions during or after surgery are associated with increased morbidity and mortalityCitation1–4. Patients experiencing bleeding-related complications including bleeding events, re-operation to control bleeding, and/or transfusion of blood products, require an increased hospital length of stay and longer ICU time resulting in an increased economic burden relative to patients without these eventsCitation5. Up to 5% of cardiac surgery patients may require surgical revision for bleeding; such procedures are associated with increased risk of operative mortalityCitation6. Moreover, enhancing a surgeon’s view of the surgical field allows for greater control of intra-operative bleeding, which can reduce operative times, prevent injuries related to sub-optimal visualization, and minimize blood transfusionsCitation3,Citation7.
Several topical adjunctive products have been developed to improve intra-operative hemostasis, as traditional methods for control of intra-operative bleeding, such as sutures and cautery, can be ineffective or impracticalCitation7. Such products include hemostatic agents, which can stop bleeding when applied directly to the site of a bleed; glues and adhesives used to attach tissues or organs; and sealants, which can create a barrier to leakage from surgical sites when applied to dry or clamped surfacesCitation8. Effectiveness of these agents is limited, particularly in patients who have received heparin and at surgical sites that are actively bleeding or difficult to reachCitation3,Citation7. As such, a gelatin matrix-thrombin composite product, the hemostatic matrix (FLOSEAL, Baxter Healthcare Corporation, Deerfield, IL), was developed to overcome the above-mentioned limitations of other agents used to control intra-operative bleedingCitation3. This agent is different from other advanced hemostatic products such as fibrin sealants patches, as this flowable hemostatic matrix (FLOSEAL) is effective in addressing intra-operative bleeding ranging from mild oozing to aggressive arterial spurtingCitation3, whereas non-flowable hemostats, fibrin sealants, and fibrin sealant patches are used to address bleeding ranging from mild oozing to moderate bleeding. These products, according to their respective package inserts, should not be used for severe or brisk arterial bleeding.
Prospective randomized clinical trials (RCT) have found that clinical efficacy of a flowable hemostatic matrix comparatively to non-flowable topical hemostat has resulted in significant reduction in time to hemostasis and greater control of bleeding by reducing patient blood lossCitation3,Citation7,Citation9.
Nasso et al.Citation10 performed a prospective RCT of patients undergoing mixed cardiac surgery and also examined the clinical efficacy of a flowable hemostatic matrix compared to non-flowable topical hemostats. However, unlike prior FLOSEAL studies in cardiac surgery, the authors closely studied additional clinical outcomes (i.e., complications, revisions for surgery, resource consumption). Patients in the control group were treated with non-flowable topical hemostatic patches or sponges of oxidized regenerated cellulose or purified porcine gelatin (SURGICEL Nu-Knit, Ethicon–Johnson & Johnson, Somerville, NJ, and GELFOAM, Pfizer, New York, NY), whereas the comparator group was treated with a flowable hemostatic matrix (FLOSEAL). The study found that patients with intra-operative bleeding in the flowable hemostatic matrix (FLOSEAL)-treated group experienced significant reductions in a range of various clinical outcomes—hemostasis time, surgical revisions for bleeding, transfusions, and minor complications—compared to the control groupCitation10.
This study demonstrated that the clinical value of a flowable hemostatic matrix cannot only be tied to rapid hemostasis and reduction in blood loss, but can also improve outcomes such as reductions in complications, transfusions, re-operation rates, and resource consumption. These clinical outcomes tied to impaired hemostasis suggest that improved control of intra-operative bleeding produces enhanced outcomes, which in return could produce cost savings to hospitals and payers. However, an economic evaluation on such effectiveness has not yet been conducted.
Our objective is to understand the cost consequence (i.e., direct cost savings) associated with the usage of a flowable hemostatic matrix comparatively to a non-flowable topical hemostat when treating intra-operative bleeding during surgery. Given the results of the Nasso et al.Citation10 study, we anticipate there to be qualitative benefits from the flowable hemostatic matrix group. However, to what extent these qualitative benefits will transpose to quantitative costs between the two groups are unknown and, therefore, the rationale for this study.
As such, a cost consequence framework and supportive model was developed to comparatively evaluate direct costs and savings from product usage based on a prospective RCT reported by Nasso et al.Citation10. The framework methodology to perform this evaluation will be further detailed in our methods section, but the high-level steps are as follows: (1) determine clinical outcomes and inputs to be applied to the evaluation from the prospective RCT, Nasso et al. study, (2) obtain and apply key costs inputs related to clinical outcomes from US databases and literature, (3) perform cost consequence evaluation modeling, and (4) perform quantitative sensitivity analyses and representative ‘real-world’ hospital simulations.
Methods
Overview
A cost consequence model was developed using Microsoft Excel (Redmond, WA) to estimate clinical and economic outcomes associated with exclusive use of a flowable hemostatic matrix (FLOSEAL) or another non-flowable hemostatic agent (SURGICEL Nu-Knit or GELFOAM) during cardiac surgery. Estimates were then calculated to represent an annualized impact based on surgery population size, rates of clinical outcomes in this population, and costs associated with each outcome.
Surgical population size is defined by the number of mixed cardiac surgeries (e.g., isolated coronary surgery, isolated valvular surgery, combined coronary/valvular surgery, and aortic surgery, alone or in combination) performed in a hospital system. In the model base-case evaluation, the surgical population size was set at 600 patients, representing an average size cardiac surgery program in a single hospital. Clinical outcomes for the surgical population utilized results from Nasso et al.Citation10, based on the usage of flowable (FLOSEAL) and non-flowable hemostatic agents (SURGICEL Nu-Knit or GELFOAM) for intra-operative bleeding.
Treatment costs included associated complications, surgical revisions for bleeding, blood transfusions, and operating room (OR) times; these were applied to the model’s surgical population. Modeling analyzed the estimated annualized outcomes and cost savings. Model base-case costs were obtained from US database analyses and published literature, and are expressed in 2012 US dollars (USD). Surgery- and care-related costs were economically adjusted based on regional market influences.
Modeling framework
Two key assumptions were applied:
Cost consequence components included in the model were assumed to account for all significant differential costs between the two groups.
Difference in hemostasis time per case between comparative groups was assumed to represent an equivalent absolute difference in total OR time.
Clinical inputs
Key clinical inputs were applied from the prospective RCT or through personal communication with study investigatorsCitation10. Clinical inputs are detailed below. The input values used in the model are summarized in .
Average hemostasis time per surgery (i.e., operative time from decannulation to closure of the sternum).
Rate of major complications (i.e., stroke, sepsis, shock, and myocardial infarction [MI] and any combination of two or more major complications).
Rate of minor complications (i.e., renal failure, respiratory insufficiency, inotropic support lasting more than 24 h, and any combination of two or more minor complications).
Rate of perioperative events of surgical revision after initial transfer to the ICU.
Rate of blood transfusions.
Comparative between-group differences in the rates of all primary end-points noted were statistically significant (p < 0.05) except for the composite end-point of any major complication (p = 0.34).
Surgical population case types were assumed to have similar distribution of scheduled elective primary cardiac surgery (coronary [36%], valvular [29%], aortic [17%], or combined [18%]) as witnessed by the Nasso et al.Citation10 study with outcomes from the intra-operative bleeding (n = 214) cohort.
Patients (n = 415) were randomized to either the flowable hemostatic matrix group or the non-flowable hemostat group, which was further divided into SURGICEL Nu-Knit absorbable hemostat (60.2%) and GELFOAM (39.8%). (Detailed study information supplied by personal communication from Dr Nasso.)
Table 1. Study end-points and results among patients with intra-operative bleedingCitation10.
Cost inputs
Key costs related to clinical outcomes were applied based on published literature and analyses of 2010 US hospital discharge data from six statesCitation11–16. The selected state databases capture variation in healthcare costs and together provide a representative national sample. These databases contain merged discharge-level demographic, clinical, and economic data for all hospital discharges within a given year for patients of all ages and covered by all payers (e.g., Medicare or private). Mean hospital charges obtained from the database analysis were adjusted by applying a cost-to-charge ratio and an inflation adjustment of 1.065 per the Medical Care Consumer Price Index to report 2012 USD costs. Cost inputs are detailed below. The input values used in the model are summarized in .
Initial surgery
Costs associated for cardiac surgery type.
Criteria for inclusion in the database analysis were based on pre-specified procedure codes in discharge records.
Complications
Incremental costs of surgery including management of peri- and post-operative clinical events.
Major and minor complications, surgical revision for bleeding, and complications present or with revision for bleeding were isolated and categorized, respectively.
‘Complicated’ cases were those associated with post-hospital admission secondary discharge diagnosis codes (International classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) for MI (410.x0, 410.x1), stroke (434–434.99, 436, 433.x1), sepsis (995.91, 995.92), shock (785.50–785.59), renal failure (586, 584–584.9), respiratory insufficiency (786.09, 518.81, 518.82, 518.5), or low output syndrome (428.9) representing treatment of inotropic support (>24 h).
Incremental cost of complication was calculated as the average cost of the initial surgery subtracted from the average cost of the initial surgery with complication.
Resource utilization
OR time
Base-case cost of $360.00 per 15 min of OR time was calculated from an average per-minute cost for OR time across five different high-volume surgical proceduresCitation17.
Blood transfusions
Total cost of a blood transfusion was calculated using an estimated incremental hospital cost of $2223 (expressed in 2012 USD) per single-unit allogeneic blood transfusion; reflecting costs of adverse effects and increased resource use associated with allogeneic bloodCitation18.
Base-case average blood units transfused per surgery were 2.8 unitsCitation10.
Other hospital costs such as hospital and ICU length of stay (LOS) and supplies were not implicitly captured in other components of the model as these incremental costs are accounted for within the complication costs. For example, hospital and ICU LOS difference was implicitly captured in the incremental costs associated with complications.
Economic adjustment
All costs were adjusted based on regional economic indices obtained from the Centers for Medicare & Medicaid Services (CMS) (2012)Citation19. The economic index for the base-case model is 1.0, representing the US national average.
Table 2. Model cost inputs.
Model analyses
Modeling outputs were expressed in terms of annualized comparative clinical and economic outcomes avoided from exclusive use of a flowable hemostatic matrix (FLOSEAL) vs non-flowable topical hemostats (SURGICEL Nu-Knit or GELFOAM) for cardiac surgery. Comparative clinical and economic outcomes analyzed cumulative differences in major and minor complications, surgical revisions for bleeding, transfusions, and OR time (hours). Net cost impact of ±20% adjustments to model inputs values was evaluated for a deterministic sensitivity analysis (SA). To ensure practicality, several input parameters were coupled in the sensitivity analyses—that is, exclusive values were adjusted and evaluated simultaneously. A series of scenarios reflecting possible real-world inputs were evaluated.
Results
Cost consequence evaluation modeling estimates for a cardiac surgery population of 600 patients resulted in fewer clinical and economic consequences—major complications (33 fewer resulting in $1.07 million saved), minor complications (76 fewer resulting in $1.43M saved), surgical revisions for bleeding (54 fewer resulting in $1.32 M saved), blood transfusions (194 fewer resulting in $1.21M saved), and hours of OR time (242 fewer resulting in $0.35M saved)—while noting that major complications were not statistically significant in the Nasso et al.Citation10 study. Cumulative outcomes resulted in $5.38M of cost consequences using the non-flowable topical hemostat group. These results are presented in .
Table 3. Annualized clinical and economic consequences for a single hospital with 600 cardiac surgeries per year.
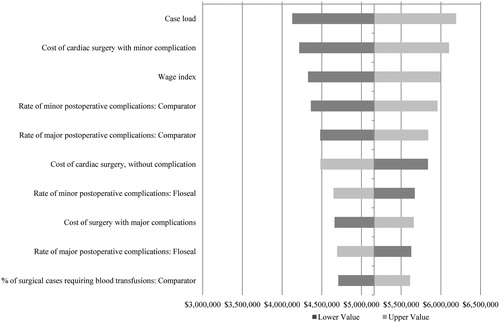
A deterministic SA was conducted to assess the impact of ±20% adjustment in the value of clinical and cost inputs. The 10 model inputs to which the cost impact was most sensitive are shown in . Additionally, several relevant scenarios were tested to understand the clinical and economic impact of practical cross-sectional adjustments to case load, US geographic region, and clinical and cost model parameters on a US hospital. These representative scenarios are shown in .
Table 4. Model variant scenario analyses, presenting varying case loads, regional adjustments, and clinical outcomes, on the resultant cost consequence.
Based on US national averages, an avoidable cost consequence would result until a surgical case hemostat acquisition price differential (i.e., difference between flowable hemostatic matrix and the non-flowable topical hemostat) reached $8960.36 per average case. At this acquisition price differential, while a clinical outcomes consequence would still exist for the non-flowable topical hemostat group, there would no longer be an economic cost consequence between the comparative groups.
Discussion
Topical hemostats are often used by surgeons to treat bleeding during surgery when bleeding cannot be controlled by conventional methods (e.g., suturing, cautery, or manual compression) or when conventional methods are impracticalCitation10. Topical hemostats can be categorized as passive or active, referring to the mechanism of action the agent provides during surgery. Passive agents act via bleeding site contact activation and promotion of platelet aggregation, and include collagens, cellulose, gelatins, and polysaccharide spheres. Active agents act biologically on the clotting cascade and include thrombin. Categories comprised of active agents are flowable topical hemostats and sealants, including fibrin sealants, polyethylene glycol (PEG) polymers, albumin and glutaraldehyde, and cyanoacrylateCitation3,Citation7. Studies have shown that higher efficacy is observed from active agents vs passive mechanical agents in human trials. One such prospective RCT was the Nasso et al.Citation10 trial which compared an active flowable hemostatic matrix containing thrombin (FLOSEAL) to a passive non-flowable hemostat agent (SURGICEL Nu-Knit and GELFOAM). This study proved to be valuable as it represented the first FLOSEAL study in cardiac surgery that evaluated clinical outcomes outside of hemostasis (e.g., major and minor complications, revisions for bleeding, and transfusions).
We developed a cost consequence model to estimate the potential cost savings of using an active flowable hemostatic matrix (FLOSEAL) to control intra-operative bleeding during cardiac surgery. The base-case analysis suggests that, in a cardiac surgery population of 600 patients, the net cost impact of the flowable hemostatic matrix comparative to non-flowable topical hemostat is substantial to a hospital and payer, given reductions in avoidable costs related to complications, surgical revisions, blood transfusions, and OR time.
Various sensitivity and scenario analyses suggested the net cost impact of the flowable hemostatic matrix was positive across a broad range of cardiac surgical case loads, US geographic regions, rates of clinical outcomes, and costs drivers. In particular, exclusion of costs associated with major complications, which did not differ significantly between groups in the RCTCitation10, still resulted in an avoidable net cost savings of $4.3M from usage of the flowable hemostatic matrix group. Additional costs may be realized beyond these figures, as modeling did not reflect opportunity costs associated with improved OR throughput (i.e., OR capacity improvement) and reductions to inpatient bed capacity constraints (i.e., freeing inpatient or critical care beds once occupied by surgical complications).
While the clinical inputs of the model were obtained from one randomized trialCitation10, the flowable hemostatic matrix has been clinically examined in numerous surgical settings. In prospective RCTs, a flowable hemostatic matrix has been shown to be more effective than other methods of controlling bleeding in patients undergoing cardiovascular, vascular, and orthopedic surgeryCitation3; spinal surgeryCitation7; vascular surgeryCitation9; sinus surgeryCitation20; abdominal myomectomyCitation21; and total thyroidectomyCitation22.
Additionally, Krishnan et al.Citation23 performed a database analysis examining hospital length of stay (LOS) in patients who underwent cardiac surgery, suggesting that use of the flowable hemostatic matrix (FLOSEAL) was associated with economic benefits. In this study, cardiac surgery patients who were treated with the flowable hemostatic matrix (FLOSEAL) were significantly less likely to exceed their expected LOS (odds ratio (OR) = 0.791, p < 0.01), and the incidence of a longer-than-expected LOS was lower (incidence rate ratio (IRR) = 0.891, p < 0.01) comparative to those who received other treatments for hemostasisCitation23. Like our cost consequence analysis, these data suggest that the use of the flowable hemostatic matrix to control bleeding during cardiac surgery may lead to better economic outcomes. However, evaluations are recommended to further build on this evidence by performing prospective or retrospective studies to better understand utilization and cost consequences from various hemostatic agents when used in surgery; analyzing complications, healthcare resource consumptions, and associated costs from individual hospitals across the US.
Several limitations to this cost consequence analysis exist, including: (1) the use of incremental costs for complications assumes the difference represents attributable costs of having the complication; (2) the cost of a unit of blood can vary depending on the definition; however, transfusion cost represents only 20% of the savings in the base case; and (3) generalizability of the results to other settings may be limited given that clinical outcomes data was from a prospective RCT conducted in two hospitals in Italy.
Conclusion
Base-case modeling suggested use of an active flowable hemostatic matrix (FLOSEAL) during cardiac surgery was associated with improved clinical and economic outcomes as compared to two other passive non-flowable topical hemostats. This resulted in net savings compared with the comparator group due to reductions in complications, revisions, transfusions, and OR time. Results were consistent, even when outcomes rates and costs were changed. It is recommended that further studies are performed to better understand utilization and cost consequences from various hemostatic agents when used in surgery.
Transparency
Declaration of funding
This study was funded by Baxter Healthcare Corporation.
Declaration of financial/other relationships
Scott Tackett and Huub Kreuwel are paid employees of Baxter Healthcare Corporation. Rebecca Sugarman and Piedad Alvarez are paid employees of Evidera, which received funding from Baxter Healthcare Corporation for this work. Dr. Giuseppe Nasso was a former paid consultant for Baxter Healthcare Corporation, but received no payments related to this study.
Acknowledgments
The authors would like to acknowledge Michael Byrnes for his contributions to the preparation of this manuscript.
References
- Levi M, Cromheecke ME, de Jonge E, et al. Pharmacological strategies to decrease excessive blood loss in cardiac surgery: a meta-analysis of clinically relevant endpoints. Lancet 1999;354:1940-7
- Murphy GJ, Reeves BC, Rogers CA, et al. Increased mortality, postoperative morbidity, and cost after red blood cell transfusion in patients having cardiac surgery. Circulation 2007;116:2544-52
- Oz MC, Cosgrove DM 3rd, Badduke BR, et al. Controlled clinical trial of a novel hemostatic agent in cardiac surgery. The Fusion Matrix Study Group. Ann Thorac Surg 2000;69:1376-82
- Rady MY, Ryan T, Starr NJ. Perioperative determinants of morbidity and mortality in elderly patients undergoing cardiac surgery. Crit Care Med 1998;26:225-35
- Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res 2011;11:135
- Moulton MJ, Creswell LL, Mackey ME, et al. Reexploration for bleeding is a risk factor for adverse outcomes after cardiac operations. J Thorac Cardiovasc Surg 1996;111:1037-46
- Renkens KL Jr., Payner TD, Leipzig TJ, et al. A multicenter, prospective, randomized trial evaluating a new hemostatic agent for spinal surgery. Spine (Phila Pa 1976) 2001;26:1645-50
- Meijer DW. Hemostats, adhesives, and sealants in endoscopic surgery. J Laparoendosc Adv Surg Tech A 2002;12:393-4
- Weaver FA, Hood DB, Zatina M, et al. Gelatin-thrombin-based hemostatic sealant for intraoperative bleeding in vascular surgery. Ann Vasc Surg 2002;16:286-93
- Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg 2009;88:1520-6
- California OSHPD. California Public Patient Discharge Data. Sacramento, CA: Office of Statewide Health Planning and Development, State of California, 2010. Available at: http://www.oshpd.ca.gov/HID/Products/PatDischargeData/PublicDataSet/index.html. Last accessed December 2012
- Florida AHCA. Florida 2010 Hospital Patient Discharge Data. Tallahassee, FL: Agency for Health Care Administration, State of Florida, 2010. Available at: http://www.floridahealthfinder.gov/Researchers/OrderData/orderdata.aspx. Last accessed December 2012
- Massachusetts CHIA. Massachusetts Hospital Inpatient Discharge Database. Boston, MA: Center for Health Information and Analysis (CHIA), Commonwealth of Massachusetts, 2010. Available at: http://www.mass.gov/chia/researcher/hcf-data-resources/case-mix/information-about-casemix-data.html. Last accessed December 2012
- Maryland HSCRC. Maryland Inpatient Discharge Research Data File. Baltimore, MD: Health Services Cost Review Commission (HSCRC), State of Maryland, 2010. Available at: http://www.hscrc.state.md.us/caseMixData_requests.cfm. Last accessed December 2012
- HCUP. New Jersey State Inpatient Database. Rockville, MD: Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality (AHRQ), 2010. Available at: http://www.hcup-us.ahrq.gov/sidoverview.jsp. Last accessed December 2012
- Washington CHARS. Washington Public Hospital Inpatient Discharge Data File. Olympia, WA: Comprehensive Hospital Abstract Reporting System (CHARS), Washington State Department of Health, 2010. Available at: http://www.doh.wa.gov/DataandStatisticalReports/HealthcareinWashington/HospitalandPatientData/HospitalDischargeDataCHARS.aspx. Last accessed December 2012
- Chatterjee A, Payette MJ, Demas CP, et al. Opportunity cost: a systematic application to surgery. Surgery 2009;146:18-22
- Boucher BA, Hannon TJ. Blood management: a primer for clinicians. Pharmacotherapy 2007;27:1394-411
- CMS. Fiscal Year 2012 Wage Index Home Page: Wage index factors. Baltimore, MD: Centers for Medicare and Medicaid Services, 2012. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files-Items/CMS1239640.html. Last accessed December 2012
- Jameson M, Gross CW, Kountakis SE. FloSeal use in endoscopic sinus surgery: effect on postoperative bleeding and synechiae formation. Am J Otolaryngol 2006;27:86-90
- Raga F, Sanz-Cortes M, Bonilla F, et al. Reducing blood loss at myomectomy with use of a gelatin-thrombin matrix hemostatic sealant. Fertil Steril 2009;92:356-60
- Testini M, Marzaioli R, Lissidini G, et al. The effectiveness of FloSeal matrix hemostatic agent in thyroid surgery: a prospective, randomized, control study. Langenbecks Arch Surg 2009;394:837-42
- Krishnan S, Conner TM, Leslie R, et al. Choice of hemostatic agent and hospital length of stay in cardiovascular surgery. Semin Cardiothorac Vasc Anesth 2009;13:225-30