Abstract
Objective:
To compare hospitalization rates in patients with schizophrenia treated prospectively with aripiprazole once-monthly 400 mg (AOM 400; an extended-release injectable suspension) vs the same patients’ retrospective rates with their prior oral anti-psychotic therapy.
Research design and methods:
Multi-center, open-label, mirror-image, naturalistic study in a community setting in North America. Patients who required a change in treatment and/or would benefit from long-acting injectable anti-psychotic therapy were treated prospectively for 6 months with AOM 400. Retrospective data on hospitalization rates were obtained.
Clinical trial registration:
ClinicalTrials.gov: NCT01432444.
Main outcome measures:
The proportion of patients with ≥1 psychiatric inpatient hospitalization with oral anti-psychotic therapy examined retrospectively (months –4 to –1 before oral conversion) and after switching to AOM 400 (months 4–6 after initiating AOM 400).
Results:
Psychiatric hospitalization rates were significantly lower when patients were treated with AOM 400 compared with oral anti-psychotic therapy both in the 3-month primary efficacy sample (2.7% [n = 9/336] vs 27.1% [n = 91/336], respectively; p < 0.0001) and in the total sample (6-month prospective rate: 8.8% [n = 38/433] vs 6-month retrospective rate: 38.1% [n = 165/433]; p < 0.0001). Discontinuations due to adverse events (AEs) during cross-titration were lower in patients cross-titrated on oral aripiprazole for >1 and <4 weeks (2.9% [n = 7/239]) compared with patients cross-titrated for ≤1 week (10.4% [n = 5/48]). The most common treatment-emergent AEs during the prospective treatment phase were insomnia (6.7% [n = 29/431]) and akathisia (6.5% [n = 28/431]). Patient-rated injection-site pain decreased from the first injection to the last visit.
Conclusions:
In a community setting, patients with schizophrenia demonstrated significantly lower psychiatric hospitalization rates after switching from their prior oral anti-psychotic therapy to AOM 400. Patients served as their own control, and thus an active control group was not included in this study. Confounding factors, such as insurance coverage and availability of hospital beds, were not examined here and deserve further consideration.
Introduction
Schizophrenia is estimated to affect ∼24 million individuals worldwideCitation1. Total costs related to schizophrenia were estimated at $62.7 billion in the USCitation2, and the total cost of schizophrenia and other psychotic disorders was estimated at €93.9 billion in EuropeCitation3. Direct and indirect costs may be high because schizophrenia is a chronic, lifelong condition with frequent relapsesCitation4. One factor that contributes to relapse and treatment costs is non-adherence to anti-psychotic medicationCitation5–7. Recent estimates suggest that rates of anti-psychotic non-adherence are as high as 60% early in the course of treatment in patients with schizophreniaCitation6. Furthermore, non-adherence to anti-psychotic medication is associated with an increase in the number and risk of hospitalizationsCitation5–8. Recent guidelines for the management of schizophrenia recommend improving medication adherence as a strategy to reduce hospitalization rates and costs in patients with schizophreniaCitation4,Citation9. Rates of relapse and hospitalization with long-acting injectable anti-psychotic medications are comparable or superior to oral anti-psychotic agentsCitation10–15. Results may vary because of methodologic differences in defining relapse or in study design (i.e., naturalistic vs controlled studies)Citation13–16. Nonetheless, long-acting injectable anti-psychotics provide an opportunity to improve medication adherenceCitation4,Citation9 and reduce hospitalization rates relative to treatment with oral formulations of the same compoundCitation17,Citation18. Reducing hospitalization rates and improving medication adherence are key components for improving functional outcomes in patients with schizophreniaCitation19.
Aripiprazole once-monthly 400 mg, a long-acting injectable formulation of the dopamine D2 partial agonist aripiprazoleCitation20, is approved for the treatment of schizophreniaCitation21–23. In two long-term, randomized, double-blind studies, aripiprazole once-monthly 400 mg was well tolerated, delayed time to impending relapse vs placeboCitation24, and demonstrated non-inferiority vs oral aripiprazole in reducing the rate of impending relapseCitation10. Moreover, data from a preliminary analysis of the present mirror-image study demonstrated reduced psychiatric hospitalization rates in patients with schizophrenia who switched from oral anti-psychotics to aripiprazole once-monthly 400 mgCitation25. Here, we report the full study results.
Patients and methods
Patients
Details regarding patient inclusion and exclusion criteria were previously describedCitation25. Briefly, adults aged 18–65 years with a duration of schizophrenia of >1 year that was consistent with criteria in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), and who had ≥7 months of retrospective data were eligible for study inclusion. Patients with other DSM-IV-TR diagnoses were excluded from the study. To be considered for study inclusion, patients had to have been treated with oral anti-psychotic therapy in the 7 months before screening and, in the investigator’s opinion, required a change in treatment for any reason (e.g., poor medication adherence, tolerability issues, lack of efficacy) and potentially benefit from long-term treatment with a long-acting injectable. Eligible patients had ≥1 inpatient psychiatric hospitalization ≤4 years before study screening; patients had to be managed as outpatients for the 4 weeks before signing an informed consent form and throughout the screening periodCitation25. Patients with a history of lack of efficacy or intolerance to aripiprazole treatment were excluded from enrolling in the study.
Study design
Details regarding study design were previously describedCitation25. Briefly, this was a phase IIIb, multi-center, open-label, mirror-image, naturalistic study in a North American community setting (NCT01432444). The study sites’ ethics committees approved the protocol in accordance with the Declaration of Helsinki. The study consisted of a screening phase and three treatment phases (). During the screening phase (days 2–28), patient eligibility was determined. Retrospective psychiatric and non-psychiatric hospitalization data were obtained for the 7-month time frame before screening. This provided 6 months of hospitalization data and 4 weeks of outpatient data. Study investigators had the option of tapering off other anti-psychotic medications during the screening phase, before the first dose of oral aripiprazole, or cross-titration with oral aripiprazole during the oral conversion phase (Phase A).
Figure 1. Study design. *Patients who were already receiving oral aripiprazole treatment entered the open-label treatment phase (Phase B) without entering the oral conversion phase (Phase A).
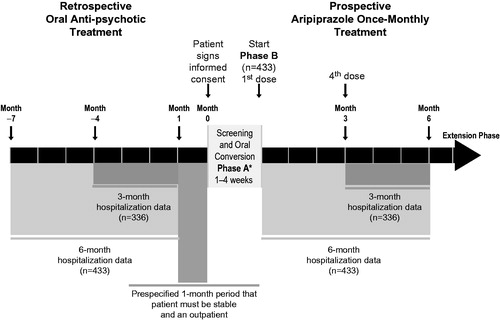
Patients receiving anti-psychotics other than oral aripiprazole and who had no prior history of tolerance to aripiprazole entered the first phase of prospective treatment, Phase A. However, those who were already being treated with oral aripiprazole at the time of study enrollment or who had a history of tolerating oral aripiprazole could directly enter the 6-month open-label treatment phase (Phase B) if the study investigator deemed the patients’ safety would be maintained. During Phase A (1–4 weeks), patients cross-titrated to oral aripiprazole (10–30 mg). The recommended starting dose for oral aripiprazole was 10 or 15 mg/day, depending on the patient’s symptoms and the investigator’s opinion. The investigators were permitted to titrate the dose up to 30 mg/day based on their clinical judgment. Patients who completed Phase A, and those who in the opinion of the investigator did not require cross-titration to oral aripiprazole, entered Phase B. During Phase B, patients were treated with aripiprazole once-monthly 400 mg by intramuscular injection in the gluteal muscle. A dose reduction to 300 mg was permitted if patients experienced tolerability issues. For the first 14 days of Phase B, patients received concomitant oral aripiprazole (10–20 mg) following their initial dose of aripiprazole once-monthly 400 mg. During these first 14 days, patients received oral aripiprazole 10 mg/day unless they were currently receiving oral aripiprazole >20–30 mg/day, in which case they received oral aripiprazole 15 mg/day for the first 14 days of treatment in Phase B. The initial doses for the first 14 days of aripiprazole once-monthly 400 mg treatment were treatment recommendations—study investigators had the option to increase the dose for efficacy to a maximum of 20 mg/day or decrease the dose for tolerability to a minimum of 10 mg/day; changes to the oral aripiprazole daily dose during the first 14 days of Phase B were made in 5-mg increments.
Patients were assessed at baseline (week 0), week 1, week 2, week 4, and every 4 weeks thereafter up to 24 weeks. Patients who completed Phase B and who, in the opinion of the investigator, would benefit from continued therapy with aripiprazole once-monthly were eligible to continue treatment in an extension phase. During the extension phase, patients continued to receive aripiprazole once-monthly at 400 or 300 mg. Data from the extension phase will be reported separately as it is not part of the primary analysis.
End-points
The primary end-point was the total psychiatric hospitalization rate, defined as the proportion of patients with ≥1 psychiatric hospitalization. Rates were compared between the retrospective oral anti-psychotic treatment period (months –4 to –1 before screening) and during prospective aripiprazole once-monthly therapy (months 4–6, the last 3 months of Phase B) in patients who entered Phase B and completed 3 months of treatment (3-month primary efficacy sample).
Secondary efficacy end-points included the 6-month hospitalization rate during prospective aripiprazole once-monthly therapy (months 1–6) compared with the 6-month retrospective period (months –7 to –1) among all patients who entered Phase B (total efficacy sample); the cumulative and mean durations of inpatient psychiatric hospitalizations for months –4 to –1 and months 4 to 6; the number of psychiatric emergency room visits during months –4 to –1 and months 4 to 6; and the number, cumulative duration, and mean duration of the following end-points for months –4 to –1 and months 4 to 6: inpatient hospitalizations for psychosocial reasons, psychiatric partial hospitalizations, and psychiatric intensive outpatient programs.
Safety and tolerability end-points included adverse events (AEs), clinical laboratory tests, vital signs, electrocardiograms, physical examinations, weight, height, body mass index, extrapyramidal symptoms, investigator ratings of injection-site reactions, and patient ratings of injection-site pain using a visual analog scale. Suicidality was assessed using the Columbia Suicide Severity Rating Scale (C-SSRS).
Data analytic procedures and statistical analysis
Data analytic procedures and statistical analyses were previously describedCitation25. Briefly, total psychiatric hospitalization rates were calculated for patients who entered Phase B and completed 3 months of treatment. Comparisons between rates during the retrospective oral anti-psychotic treatment period (months –4 to –1) and the prospective aripiprazole once-monthly treatment period (months 4–6) were assessed using exact McNemar’s tests with statistical significance at an alpha level of 0.05 (two-sided). Sample size was estimated at 230 to provide ≥99% power to detect a statistically significant difference of 15% in two paired hospitalization proportions between pre-switch and post-switch periods, while the proportion of total discordant pairs was 25%; this was based on the assumption that 30% of patients would be hospitalized pre-switch and, overall, 15% would be hospitalized post-switch (10% would be hospitalized both pre-switch and post-switch and 5% with no pre-switch hospitalization). McNemar’s test was conducted on the difference in the discordant rates of total psychiatric hospitalizations, defined as the proportion of patients with ≥1 psychiatric hospitalization in the prospective treatment period and 0 hospitalizations in the retrospective period, minus the proportion of patients with 0 hospitalizations in the prospective treatment period and ≥1 hospitalization in the retrospective period. New and ongoing hospitalizations that occurred during the 3-month periods were included in the analyses. Paired t-tests were also conducted, when applicable, for the number of hospitalization stays per subject, cumulative duration of hospitalization, mean duration of hospitalization, and the number and mean duration of all other outpatient hospitalization programs during the retrospective (months –4 to –1) vs prospective (months 4–6) treatment periods for patients with ≥3 months of aripiprazole once-monthly treatment during Phase B. The rate ratio was calculated from the rates before and after switching to aripiprazole once-monthly treatment.
A sensitivity analysis was also conducted to assess the potential impact of discontinuation on total psychiatric hospitalization rates. Missing data were imputed for total psychiatric hospitalization rates between the 6-month retrospective and prospective Phase B periods for all patients who received aripiprazole once-monthly treatment. For the sensitivity analysis, patients who discontinued aripiprazole once-monthly during Phase B for psychiatric AEs (excluding insomnia) or lack of efficacy were treated as having one imputed psychiatric hospitalization during the 6-month prospective period. Moreover, to examine the effect of duration of cross-titration, the rate of discontinuations due to AEs in Phase A was analyzed in a post-hoc analysis, stratified by duration of oral aripiprazole cross-titration period (≤1 week vs >1 and ≤4 weeks).
Results
Patients
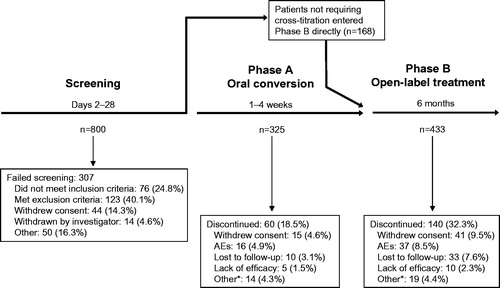
Of 800 patients screened, 493 were eligible for study inclusion and enrolled in the study. A total of 325 patients entered the study in Phase A (), the oral conversion phase, and 168 patients did not require cross-titration to oral aripiprazole based on their history with oral aripiprazole therapy and the investigator’s opinion, and thus entered the study in Phase B, the 6-month open-label treatment period with aripiprazole once-monthly. Sixty patients discontinued during Phase A (); the primary reasons were AEs (4.9%) and withdrawn consent to participate (4.6%). There were 433 patients in Phase B (intent-to-treat population), 431 of whom received at least one aripiprazole once-monthly injection (Phase B safety population). 336 patients entered the fourth month of Phase B and were part of the primary efficacy analysis, and 293 completed treatment. There were 140 patients who discontinued during Phase B; primary reasons for discontinuation were withdrawn consent to participate (9.5%) and AEs (8.5%). Of those patients who completed Phase B, there were 192 patients who, in the opinion of the investigator, would benefit from continued aripiprazole once-monthly therapy and, thus, continued treatment in the extension phase (results not shown).
Figure 2. Patient disposition during the prospective period. *Patients met withdrawal criteria, were withdrawn by the study investigator, or had a protocol deviation. AE = adverse event.
Ninety-nine per cent (290/293) of patients who completed Phase B initiated treatment on aripiprazole once-monthly at 400 mg; three patients initiated aripiprazole once-monthly therapy at 300 mg. Most patients (240/293 [82%]) who completed Phase B started and remained on the 400-mg dose of aripiprazole once-monthly throughout Phase B. At the last (6th) injection, 86% of patients received aripiprazole once-monthly 400 mg, and the remainder received 300 mg. Thirteen per cent of patients (n = 39/293) who completed Phase B had their dose decreased from 400 to 300 mg, and 4% (n = 11/293) had their dose decreased from 400 to 300 mg and then increased thereafter to 400 mg. Three patients started on aripiprazole once-monthly 300 mg, which were protocol deviations; two of these patients remained on aripiprazole once-monthly 300 mg, and one patient had a dose increase to 400 mg.
Baseline demographic and disease characteristics by study phase for patients who entered each respective phase are reported in . The majority of the patients were male (n = 301/433, 70%), half (n = 216/433) were white, and 46% (n = 198/433) were black. Disease severity at Phase B baseline was moderate (Clinical Global Impression-Severity of Illness scale, 3.7 ± 0.8); common concomitant central nervous system medications taken during Phase B included psycholeptics (including aripiprazole) in 206 patients (47.6%), psycho-analeptics in 169 patients (39.0%), and anti-parkinsonian medications in 103 patients (23.8%).
Table 1. Demographics and baseline characteristics by study phase.
Total psychiatric hospitalization rates
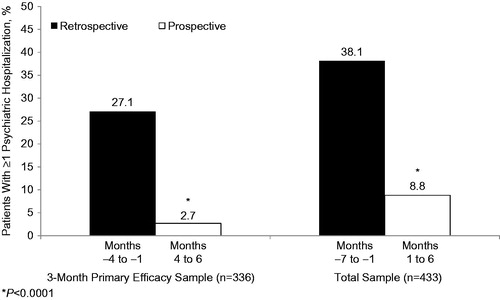
Inpatient psychiatric hospitalization rates in patients who were treated with aripiprazole once-monthly for ≥3 months were significantly lower during prospective months 4–6, when patients were treated with aripiprazole once-monthly at 400 mg, compared with their retrospective rates during months –4 to –1, when treated with oral anti-psychotic therapy (2.7% [n = 9/336] vs 27.1% [n = 91/336], respectively; p < 0.0001; ). Rates were also significantly lower for all Phase B patients during the 6-month prospective period vs their rates during the 6-month retrospective period (8.8% [n = 38/433] vs 38.1% [n = 165/433], p < 0.0001; ). The rate ratios for hospitalization during the prospective vs retrospective periods were 0.10 for patients who completed ≥3 months of treatment on aripiprazole once-monthly and 0.23 for all patients who entered Phase B.
Figure 3. Total psychiatric hospitalization rates following the switch to aripiprazole once-monthly 400 mg (prospective) compared with the same patients treated with oral antipsychotics (retrospective). P value derived from exact McNemar test. AOM 400 = aripiprazole once-monthly 400 mg.
The sensitivity analysis included data for 55 patients with observed or imputed (based on study discontinuation) psychiatric inpatient hospitalizations during the prospective period. This analysis also demonstrated significant reductions in total psychiatric hospitalizations during months 1–6 of prospective treatment with aripiprazole once-monthly compared with retrospective treatment during months –7 to –1 with oral anti-psychotic therapy (12.7% [n = 55/433] vs 38.1% [n = 165/433], respectively, p < 0.0001).
Other secondary efficacy end-points
A breakdown of hospital resource use is provided in . The data are summarized for patients having the event during the retrospective (months –4 to –1) and prospective (months 4–6) periods. Mean cumulative duration of inpatient psychiatric hospitalizations per patient was reduced by ∼4 days in the prospective aripiprazole once-monthly 400 mg treatment period (7.0 days) vs the retrospective period (11.1 days; ). No patient was hospitalized for psychosocial reasons or participated in psychiatric intensive outpatient or partial inpatient programs during the prospective period. In contrast, seven patients were hospitalized once for psychosocial reasons, with a mean cumulative duration of 15.6 days, four patients participated in psychiatric intensive outpatient programs, and two patients participated in psychiatric partial inpatient programs in the retrospective period. Likewise, the number of psychiatric emergency room visits and the mean number of visits per patient were both lower in the prospective period compared with the retrospective period.
Table 2. Hospitalization data.
Safety
Of the 433 patients who entered Phase B, 431 received ≥1 dose of aripiprazole once-monthly and were thus included in the safety analysis. The incidence of treatment-emergent AEs (TEAEs) during Phase B that occurred in ≥2% of patients is reported in . Sixty-one per cent of patients (n = 265/431) experienced a TEAE. Many TEAEs occurred <1 month after treatment initiation (n = 162/431 [37.6%]), and the incidence decreased each month thereafter (months 1–2: n = 79/420 [18.8%], months 2–3: n = 66/397 [16.6%], months 3–4: n = 57/364 [15.7%], months 4–5: n = 35/342 [10.2%], months 5–6: n = 35/317 [11.0%], >6 months: 2/298 [0.7%]). Most TEAEs were mild or moderate in severity during Phase B. There were 42 (9.7%) patients who had a severe TEAE during Phase B; the most common severe TEAEs were psychotic disorder (7/431 [1.6%]) and schizophrenia (5/431 [1.2%]).
Table 3. Incidence of treatment-emergent adverse events occurring in ≥2% of all patients treated in phase B (n = 431).
Discontinuation rates due to AEs varied with duration of cross-titration in Phase A before initiating aripiprazole once-monthly at 400 mg. Among those who cross-titrated to oral aripiprazole for ≤1 week, 10.4% (n = 5/48) discontinued during Phase A because of AEs, whereas 2.9% (n = 7/239) of patients who cross-titrated to oral aripiprazole for >1 and ≤4 weeks discontinued because of AEs.
The incidence of potentially clinically relevant changes in lipid parameters, prolactin level, and weight in Phase B are listed in . Although some patients experienced weight gain ≥7% (n = 22 [6.1%]) or weight loss ≥7% (n = 25 [7.0%]), and 10 patients (2.3%) reported weight increased as an AE during phase B, there were no clinically relevant mean changes from baseline in weight and other vital signs (including blood pressure and heart rate). Mean ± SD weight change from Phase B baseline to last visit was minimal (–0.2 ± 3.9 kg) and similar between black (−0.3 ± 4.0 kg, n = 165) and non-black patients (−0.1 ± 3.9 kg, n = 194). There were no clinically relevant mean changes from baseline in laboratory values, electrocardiogram parameters, extrapyramidal symptoms, or suicidality during phase B. Mean ± SD C-SSRS suicidal ideation total scores remained stable from Phase B baseline (0.1 ± 1.1) to last visit (0.4 ± 2.1). Patient-rated injection-site pain decreased from Phase B baseline (7.2 ± 13.4) to Phase B last visit (5.6 ± 11.4). Physicians rated the majority of their patients’ injection-site pain as absent at all assessments during Phase B.
Table 4. Incidence of potentially clinically relevant treatment-emergent changes in lipids, prolactin levels, and weight in phase B (n = 431).
Discussion
Results of the present final analysis confirm the findings from the preliminary analysis demonstrating a reduction in the number of psychiatric hospitalizations after switching to aripiprazole once-monthly at 400 mg vs prior treatment with oral anti-psychotics before study enrollmentCitation25. The significant improvement in other secondary outcomes (e.g., per-patient psychiatric emergency room visits, mean length of hospitalization) in the prospective vs the retrospective period further supports the effectiveness and potential for cost savings when using aripiprazole once-monthly at 400 mg in a community setting in patients switching from oral anti-psychotic therapy. The AE profile was comparable to prospective, randomized controlled trials of aripiprazole once-monthly at 400 mg as maintenance treatment in patients with schizophreniaCitation10,Citation24 and as treatment of acute exacerbations of psychotic symptoms in patients with schizophreniaCitation26. Furthermore, the lower rate of discontinuation due to AEs in patients who cross-titrated to oral aripiprazole for >1 and ≤4 weeks vs those whose length of cross-titration was ≤1 week in Phase A suggests that a >1- to 4-week length of cross-titration is preferable.
The findings on the effectiveness of aripiprazole once-monthly at 400 mg are also consistent with other clinical studies and analyses of claims databases demonstrating reduced psychiatric hospitalizations with long-acting injectable anti-psychotics relative to oral anti-psychotic therapyCitation18,Citation27–30. In a meta-analysis, 15 of 16 mirror-image studies showed that long-acting injectable anti-psychotics reduced the risk of hospitalization relative to oral anti-psychoticsCitation15. Another meta-analysis of 13 studies showed that research design affects the assessment of comparative effectiveness (e.g., hospitalization rates), with observational studies being more sensitive to differences than randomized controlled trials where adherence is fostered through regular study visitsCitation16.
Fewer psychiatric hospitalizations may also contribute to substantial cost savings with long-acting injectable anti-psychotic therapyCitation27,Citation29,Citation31,Citation32. For example, in another mirror-image study using a US commercial claims database, patients with schizophrenia who initiated depot anti-psychotic medication had lower all-cause and schizophrenia-related inpatient costs relative to their pre-initiation index period, although outpatient costs remained comparableCitation31. In addition to reduced total hospitalizations, the present results showed that the average duration of psychiatric inpatient hospitalization was 4 days shorter after patients switched to aripiprazole once-monthly. Other potential indirect cost benefits with the use of long-acting injectable anti-psychotics may derive from fewer disruptions in work and family/social functioning due to fewer relapses and hospitalizations. Some health insurance providers may limit access to or reimbursement for long-acting injectable anti-psychotic therapy to minimize prescription costsCitation33. However, the potential direct and indirect cost savings through reduced psychiatric hospitalizations, per-patient psychiatric emergency room visits, mean length of hospitalization, and hospitalizations for psychosocial reasons may offset prescription costs.
Safety and tolerability after initiation of aripiprazole once-monthly at 400 mg were consistent with previous experience with aripiprazole tabletsCitation34. Patients may benefit from active management of more common AEs such as insomnia and akathisia. Minimal mean weight change was observed in the current study (−0.2 ± 3.9 kg), and notably, black patients showed no mean weight gain. The lack of weight gain among black patients may relate to the fact that all patients were relatively stable upon study entry, and few were hospitalized during the prospective period. A previous study of aripiprazole once-monthly at 400 mg for the treatment of acute exacerbations of psychotic symptoms found significant weight gain correlated with time in hospitalCitation26. Minimal weight change during outpatient maintenance treatment is consistent with other maintenance studies with aripiprazole once-monthly at 400 mgCitation10,Citation24. The favorable metabolic profile of aripiprazole relative to other atypical anti-psychotics may partially explain its continued use despite availability of generic atypical anti-psychoticss, risperidone in particularCitation35. Regardless, all patients treated with any atypical anti-psychotic therapy should be regularly monitored for changes in metabolic parametersCitation36.
Limitations of the current study include lack of an active control group and non-blinded treatment, both of which limit the ability to assess the impact of the study design and study participation on outcomesCitation25. Confounding factors such as insurance coverage, hospital bed availability, and physician willingness to hospitalize patients were not examined in the current analysesCitation25.
Conclusions
In a community setting, patients with schizophrenia demonstrated significantly lower rates of psychiatric hospitalization after switching to aripiprazole once-monthly 400 mg compared with their rates from retrospective data during prior oral anti-psychotic therapy. Results are consistent with findings from a preliminary analysis and demonstrate the potential for cost savings when switching patients from oral anti-psychotic therapy to aripiprazole once-monthly at 400 mg.
Transparency
Declaration of funding
This research was supported by Otsuka Pharmaceutical Development & Commercialization, Inc., and H. Lundbeck A/S.
Declaration of financial/other relationships
JK has received honoraria for lectures and/or consulting from Alkermes, Amgen, Bristol-Myers Squibb, Cephalon, Eisai, Boehringer Ingelheim, Eli Lilly, Intracellular Therapeutics, Janssen, Johnson & Johnson, Lundbeck, Merck, Novartis, Otsuka, Pfizer, Pierre Fabre, Proteus, Roche, Sunovion, and Targacept and is a shareholder of MedAvante. RS, CZ, BJ, RB, RM, TP-S & AD are employees of Otsuka Pharmaceutical Development and Commercialization, Inc. AE is an employee of Lundbeck LLC. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Editorial support for the preparation of this manuscript was provided by Amy Roth Shaberman, PhD, of C4 MedSolutions, LLC, a CHC Group company (Yardley, PA, USA), with funding from Otsuka Pharmaceutical Development & Commercialization, Inc. and H. Lundbeck A/S.
Previous presentations
• The 4th Schizophrenia International Research Society Conference, April 5–9, 2014; Florence, Italy.
• 2014 American Society of Clinical Psychopharmacology Annual Meeting, June 16–19, 2014; Hollywood, FL, USA.
• US Psychiatric and Mental Health Congress Conference & Exhibition, September 20–23, 2014; Orlando, FL, USA.
• The 27th European College of Neuropsychopharmacology Congress, October 18–21, 2014; Berlin, Germany.
• Neuroscience Education Institute Psychopharmacology Congress, November 13–16, 2014; Colorado Springs, CO, USA.
• Preliminary analysis published in Journal of Medical Economics (Kane JM, et al. J Med Econ. 2013;16(7):917–25).
References
- World Health Organization. Schizophrenia. Geneva, Switzerland: World Health Organization, 2014. http://www.who.int/mental_health/management/schizophrenia/en/. Accessed February 10, 2014
- Wu EQ, Birnbaum HG, Shi L, et al. The economic burden of schizophrenia in the United States in 2002. J Clin Psychiatry 2005;66:1122-9
- Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol 2011;21:718-79
- Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry 2013;14:2-44
- Novick D, Haro JM, Suarez D, et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res 2010;176:109-13
- Offord S, Lin J, Mirski D, et al. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther 2013;30:286-97
- Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry 2004;161:692-9
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886-91
- Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 2009;70:1-46; quiz 47-8
- Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: a double-blind, randomised, non-inferiority study. Br J Psychiatry 2014;205:135-44
- Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005;15:111-7
- Detke HC, Zhao F, Garhyan P, et al. Dose correspondence between olanzapine long-acting injection and oral olanzapine: recommendations for switching. Int Clin Psychopharmacol 2011;26:35-42
- Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia–a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res 2011;127:83-92
- Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull 2014;40:192-213
- Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry 2013;74:957-65
- Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry 2013;74:568-75
- Asseburg C, Willis M, Lothgren M, et al. Hospitalisation utilisation and costs in schizophrenia patients in Finland before and after initiation of risperidone long-acting injection. Schizophr Res Treatment 2012;2012:791468
- Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry 2011;168:603-9
- Keith S. Advances in psychotropic formulations. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:996-1008
- Burris KD, Molski TF, Xu C, et al. Aripiprazole, a novel antipsychotic, is a high-affinity partial agonist at human dopamine D2 receptors. J Pharmacol Exp Ther 2002;302:381-9.
- Abilify Maintena® US (aripiprazole). Full Prescribing Information. Tokyo, Japan: Otsuka Pharmaceutical Co., Ltd., 2013
- Abilify Maintena® EU (aripiprazole). Full Prescribing Information. Wexham, UK: Otsuka Pharmaceutical Europe Ltd., 2013
- Abilify Maintena™ CAN (aripiprazole). Full Prescribing Information. Saint-Laurent, QC, Canada: Otsuka Canada Pharmaceutical Inc., 2014
- Kane J, Sanchez R, Perry P, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012;73:617-24
- Kane JM, Sanchez R, Zhao J, et al. Hospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophrenia. J Med Econ 2013;16:917-25
- Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2014;August 19 [Epub ahead of print]
- Bera R, Offord S, Zubek D, et al. Impact on healthcare resource usage and costs among Medicaid-insured schizophrenia patients after initiation of treatment with long-acting injectable antipsychotics. J Med Econ 2013;16:522-8
- Fuller M, Shermock K, Russo P, et al. Hospitalisation and resource utilisation in patients with schizophrenia following initiation of risperidone long-acting therapy in the Veterans Affairs Healthcare System. J Med Econ 2009;12:317-24
- Peng X, Ascher-Svanum H, Faries D, et al. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res 2011;3:9-14
- Taylor D, Olofinjana O. Long-acting paliperidone palmitate - interim results of an observational study of its effect on hospitalization. Int Clin Psychopharmacol 2014;29:229-34
- Lin J, Wong B, Offord S, et al. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res 2013;40:355-66
- Lafeuille MH, Laliberte-Auger F, Lefebvre P, et al. Impact of atypical long-acting injectable versus oral antipsychotics on rehospitalization rates and emergency room visits among relapsed schizophrenia patients: a retrospective database analysis. BMC Psychiatry 2013;13:221
- Godman B, De Bruyn K, Miranda J, et al. Generic atypical antipsychotic drugs in Belgium: their influence and implications. J Comp Eff Res 2013;2:551-61
- Marder SR, McQuade RD, Stock E, et al. Aripiprazole in the treatment of schizophrenia: safety and tolerability in short-term, placebo-controlled trials. Schizophr Res 2003;61:123-36
- Godman B, Petzold M, Bennett K, et al. Can authorities appreciably enhance the prescribing of oral generic risperidone to conserve resources? Findings from across Europe and their implications. BMC Med 2014;12:98
- Lehman AF, Lieberman JA, Dixon LB, et al. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry 2004;161:1-56
