Abstract
Objective:
The objective of this economic model was to estimate the difference in medical costs among patients treated with paliperidone palmitate once-monthly injectable antipsychotic (PP1M) vs placebo, based on clinical event rates reported in the 15-month randomized, double-blind, placebo-controlled, parallel-group study of paliperidone palmitate evaluating time to relapse in subjects with schizoaffective disorder.
Research design and methods:
Rates of psychotic, depressive, and/or manic relapses and serious and non-serious treatment-emergent adverse events (TEAEs) were obtained from the long-term paliperidone palmitate vs placebo relapse prevention study. The total annual medical cost for a relapse from a US payer perspective was obtained from published literature and the costs for serious and non-serious TEAEs were based on Common Procedure Terminology codes. Total annual medical cost differences for patients treated with PP1M vs placebo were then estimated. Additionally, one-way and Monte Carlo sensitivity analyses were conducted.
Results:
Lower rates of relapse (−18.3%) and serious TEAEs (−3.9%) were associated with use of PP1M vs placebo as reported in the long-term paliperidone palmitate vs placebo relapse prevention study. As a result of the reduction in these clinical event rates, the total annual medical cost was reduced by $7140 per patient treated with PP1M vs placebo. One-way sensitivity analysis showed that variations in relapse rates had the greatest impact on the estimated medical cost differences (range: −$9786, −$4670). Of the 10,000 random cycles of Monte Carlo simulations, 100% showed a medical cost difference <$0 (reduction) for patients using PPIM vs placebo. The average total annual medical differences per patient were −$8321 for PP1M monotherapy and −$6031 for PPIM adjunctive therapy.
Conclusions:
Use of PP1M for treatment of patients with schizoaffective disorder was associated with a significantly lower rate of relapse and a reduction in medical costs compared to placebo. Further evaluation in the real-world setting is warranted.
Introduction
Schizoaffective disorder is a chronic mental disorder with symptoms of both schizophrenia and a major mood disorder, including psychosis, mania, or depression. The lifetime prevalence of schizoaffective disorder using a general population survey was estimated at 0.32%, which was approximately one-third of that estimated for schizophreniaCitation1. Although the overlap of symptoms of schizoaffective disorder with that of other mood disorders presents a diagnostic challenge and a likely under-estimation of its prevalence, its diagnosis as a distinct entity from other serious mental illnesses has strengthened over time with specific characteristics and clinical features identifiedCitation2,Citation3. A retrospective study by Olfson et al.Citation4 of Medicaid claims data from two US states has reported that among 55,000 adults treated for schizoaffective disorder and schizophrenia, approximately one-third were treated for schizoaffective disorder.
Atypical antipsychotics, mood stabilizers, and antidepressants are typically used for treatment of patients with schizoaffective disorder based on clinical trial experiencesCitation5. However, the complexity of the disease makes sub-standard management (e.g. polypharmacy) of patients with schizoaffective disorder a high likelihoodCitation6,Citation7. The Olfson et al.Citation4 study did observe that patients with schizoaffective disorder were significantly more likely to be prescribed mood stabilizers, anxiolytics, and antidepressants than patients with schizophrenia. Additionally, studies have demonstrated that patients with schizoaffective disorder have greater risk for hospitalization, substance abuse, and suicide in comparison to patients with schizophrenia, suggesting a more severe clinical course and/or sub-optimal treatmentCitation2,Citation4.
Adherence to treatment is critical for patients with schizoaffective disorder to avoid relapse; however, a recent study found that 44% of patients with schizoaffective disorder have poor medication adherenceCitation6,Citation8. A study of 1193 Medicaid enrolled patients with schizoaffective disorder reported that adherence levels (mean rate = 46%) to disease-related medications, as measured by the proportion of days covered, are lowest in the 60-day period prior to a hospitalization for a relapseCitation6. Additionally, this study observed that, in the 60-day period after hospitalization for relapse, re-hospitalization was common and healthcare costs were highest, suggesting post-discharge interventions may be an effective strategy to improve patient outcomes and reduce costsCitation6. Irregular use of medications among patients with severe mental illness is reported as one of the strongest predictors of hospitalizationCitation9. A review of several studies of chronic illnesses, including mental disorders, identified treatment regimen complexity as a determinant of adherenceCitation10. As patients with schizoaffective disorder commonly have complex treatment regimens, strategies to simplify treatment may provide many advantages for these patients and improve patient outcomes and reduce costs.
The 15-month, randomized, double-blind, placebo-controlled, parallel-group study of paliperidone palmitate evaluating time to relapse in subjects with schizoaffective disorder investigated the efficacy, safety, and tolerability of the once-monthly, atypical, long-acting, antipsychotic, paliperidone palmitate compared with placebo in the delay of relapse of the symptoms of schizoaffective disorderCitation11,Citation12. This long-term study demonstrated that paliperidone palmitate treatment at doses of 78–234 mg significantly delayed relapse and reduced risk of relapse in patients with schizoaffective disorderCitation11,Citation12. It was efficacious as both a monotherapy and as an adjunctive therapy to antidepressants or mood stabilizersCitation11,Citation12. Additionally, paliperidone palmitate treatment demonstrated efficacy in improving patient functioning, as assessed by the Personal and Social Performance ScaleCitation11,Citation12. Paliperidone (orally administered) was approved by the FDA in 2009 for the treatment of schizoaffective disorder. Recently, the atypical long-acting injectable paliperidone palmitate has received FDA approval for the treatment of schizoaffective disorder as a monotherapy and as an adjunct to mood stabilizers or antidepressantsCitation13.
As the long-term paliperidone palmitate vs placebo relapse prevention study showed that treatment of patients with schizoaffective disorder markedly reduces the risk of relapse, it is important to determine the amount of medical costs that are avoided among this patient populationCitation11,Citation12. The objective of this economic model was to evaluate the difference in medical costs when paliperidone palmitate is used for the treatment of schizoaffective disorder based on the clinical event rates reported in the long-term paliperidone palmitate vs placebo relapse prevention study.
Patients and methods
The long-term paliperidone palmitate vs placebo relapse prevention study (NCT01193153) was an international, randomized, clinical trial that evaluated the efficacy, safety, and tolerability of paliperidone palmitate as a monotherapy or adjunctive therapy to mood stabilizers or antidepressants vs placebo for the treatment of schizoaffective disorderCitation11,Citation12. It consisted of four periods, a screening period, lead-in period (13 weeks), stabilization period (12 weeks), and relapse prevention period (15 months)Citation11,Citation12. Symptomatic patients with a confirmed diagnosis of schizoaffective disorder were enrolled in this long-term studyCitation11,Citation12. After the lead-in and stabilization periods, in which all patients received paliperidone palmitate, stable patients were randomized in a 1:1 ratio, with 164 patients allocated to receive paliperidone palmitate and 170 patients to receive placeboCitation11,Citation12. Paliperidone palmitate (78–234 mg; 50 mg eq to 150 mg eq) or placebo were administered by intramuscular injection once every 4 weeks for 15 monthsCitation11,Citation12. The detailed study design and key results are described elsewhereCitation12.
The primary efficacy end-point was relapse. The study additionally measured the impact of paliperidone palmitate treatment vs placebo on healthcare resource utilization as assessed by a resource utilization questionnaire (RUQ) as pre-specified in the study protocol. The RUQ assessed the number of hospitalizations and duration of hospitalization (refers to ≥1 overnight stay), emergency room (ER) visits, and day or night clinic stays, and outpatient visits, which were objectively verified through medical records and emergency/crisis center documents obtained by investigative site staff when availableCitation11,Citation12. The study protocol and amendment(s) were reviewed by an Independent Ethics Committee and Institutional Review Board and was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Clinical Practices and applicable regulatory requirementsCitation11,Citation12.
Estimation of clinical event rates
The clinical events include relapse, serious treatment-emergent adverse events (TEAE), and other non-serious TEAEs. The event rates were calculated as the percentage of patients with each of the clinical events during the study periodCitation11,Citation12. Event rates were further calculated for patients treated with placebo, all patients treated with paliperidone palmitate, and for the sub-groups of patients treated with paliperidone palmitate monotherapy and paliperidone adjunctive therapy to mood stabilizers or antidepressants. Since the serious TEAE and non-serious TEAE were only reported for the overall populations, such rates were assumed to be the same among patients treated with paliperidone palmitate monotherapy and adjunctive therapy.
Estimation of differences in medical costs associated with clinical outcomes
An Excel-based model was developed to estimate the medical cost differences among patients with schizoaffective disorder treated with paliperidone palmitate vs placebo. Annual medical costs, defined as the incremental inpatient and outpatient costs to a US health payer of a patient experiencing a relapse during 1 year following the initial event were obtained from a retrospective Medicaid claims analysis of patients with schizophrenia between 1997–2010 by Lafeuille et al.Citation14. The medical cost, for a serious TEAE was assumed to be the cost of one emergency room visit followed by four office visits with Common Procedure Terminology (CPT) coding 99215, level 5 established office patient visit. The costs of such medical services were obtained from the Medical Expenditure Panel Survey and the Medicare Fee Schedule, Payment, and Reimbursement Benefit GuidelineCitation15,Citation16. The medical cost for a non-serious TEAE, from a US payer perspective, was based on CPT code 99214, level 4 established office patient visit, and obtained from the Medicare Fee Schedule, Payment and Reimbursement Benefit GuidelineCitation16. All medical costs associated with clinical events were inflation adjusted to 2014 cost levels via the Consumer Price Index medical care componentCitation17. Based on the event rates for each of the clinical end-points, the total annual medical cost differences associated with use of paliperidone palmitate (overall, monotherapy, and adjunctive therapy) vs corresponding placebo groups were determined. Our study focused on the medical cost reduction driven by clinical outcomes, with drug costs not included in this analysis.
Sensitivity analyses
Univariate (one-way) sensitivity analyses were conducted to determine the effects of varying a single clinical event rate or the corresponding medical cost on the total annual medical cost differences associated with paliperidone palmitate treatment (overall, monotherapy, and adjunctive therapy) vs placebo. In the univariate sensitivity analysis, clinical event rates and cost estimates were varied between the ranges of their respective 95% confidence intervals when such confidence intervals were known. For the incremental cost estimates where the confidence intervals (e.g. serious and non-serious TEAE) were not known the estimates were varied by ±30%Citation18.
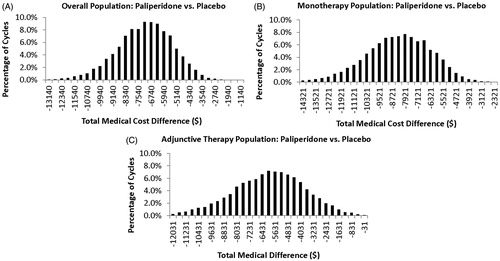
Since these variables are often interdependent, Monte Carlo analyses were also conducted as multivariable sensitivity analyses. Gaussian distributions were assumed for the estimates of clinical event rates and the corresponding cost estimates. For each cycle of a Monte Carlo analysis, the values of the clinical event rates were taken randomly from a Gaussian distribution of the mean and standard deviation of the corresponding variables. When standard deviations of the cost estimates (e.g. serious and non-serious TEAE) were not known, they were estimated based on the assumption that the confidence intervals of such parameters were ±30%, as in the univariate sensitivity analysis. Ten thousand Monte Carlo iterations were conducted for each comparison. Descriptive statistics of the total annual medical cost differences were measured from the results of the 10,000 random Monte Carlo cycles. The 95% confidence intervals of the mean total annual medical cost differences were evaluated as the range between the 2.5–97.5 percentile of total annual medical costs from the 10,000 random cycles of Monte Carlo simulation.
Exploratory analysis
As an exploratory analysis, differences in the number of psychiatric hospitalizations and ER visits among patients treated with paliperidone palmitate and placebo were determined from RUQ evaluation during the study periodCitation11,Citation12. The number of recorded psychiatric hospitalizations and ER visits of paliperidone palmitate and placebo treated patients during the trial period were measured as per patient-year. The relative changes in psychiatric hospitalizations and ER visits were then determined based on the absolute differences in psychiatric hospitalizations and ER visits between paliperidone-treated patients and placebo-treated patients.
Results
Differences in clinical event rates
Differences in all clinical event rates evaluated based on the long-term paliperidone palmitate vs placebo relapse prevention study are presented in . Among the overall patient populations treated with paliperidone palmitate and those treated with placebo, the absolute risks of relapse were 15.2% and 33.5%, respectively. Among the sub-groups of patients treated with paliperidone palmitate as monotherapy and those treated with corresponding placebo treatment, the absolute risks of relapse were 11.5% and 32.9%, respectively. Among the sub-groups of patients treated with paliperidone palmitate as adjunctive therapy and those treated with corresponding placebo treatment, the absolute risks of relapse were 18.6% and 34.0%, respectively. There was a reduction of relapse rates of 18.3%, 21.3%, and 15.4%, for patients treated with paliperidone palmitate, and sub-groups of patients treated with paliperidone palmitate as monotherapy, and paliperidone palmitate as adjunctive therapy vs placebo, respectively. The rates for serious TEAEs for paliperidone palmitate-treated patients and placebo-treated patients were 5.5% and 9.4%, respectively, and for non-serious TEAEs were 59.2% and 46.6%, respectively, as reported in the original trial dataCitation11.
Table 1. Differences in clinical event rates among patients with schizoaffective disorder treated with paliperidone palmitate (PP1M) vs placebo.
Differences in total annual medical costs
The total annual medical costs of clinical events in 2014 dollars to a US payer were estimated as the following: relapse = $38,672Citation14, serious TEAE = $2087Citation15,Citation16, and non-serious TEAE = $102Citation16. The total annual medical cost was reduced by $7140 per patient treated with paliperidone palmitate vs placebo (). The total annual medical cost differences per patient were −$8321 for paliperidone palmitate monotherapy and −$6031 for paliperidone palmitate adjunctive therapy ().
Table 2. Annual medical cost differences among patients with schizoaffective disorder: paliperidone palmitate (PP1M) vs placebo.
Univariate and multivariable sensitivity analyses
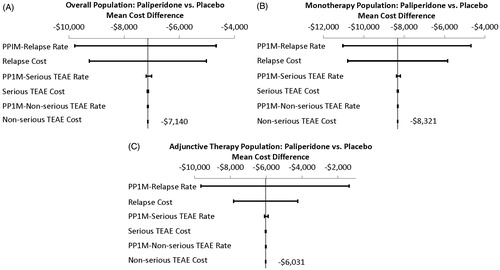
Univariate sensitivity analysis was carried out to assess the impact of each single model parameter on the total annual medical cost differences estimated from the model. indicates how such variations in clinical event rates and event costs influenced the estimated cost differences of paliperidone palmitate (overall, monotherapy, and adjunctive therapy) vs corresponding placebo groups. Variations in relapse rate had the greatest impact on the estimated total annual medical cost differences, which ranged from −$9786 to −$4670 for the overall patients treated with paliperidone palmitate, from −$11,037 to −$4685 for patients treated with paliperidone palmitate monotherapy, and from −$9650 to −$1373 for patients treated with paliperidone palmitate adjunctive therapy. Variations in the cost of relapse also had a large impact on the estimated total annual medical cost differences, which ranged from −$9262 to −$5019 for the overall patients treated with paliperidone palmitate, from −$10,797 to −$5845 for patients treated with paliperidone palmitate monotherapy, and from −$7819 to −$4242 for patients treated with paliperidone palmitate adjunctive therapy. Variations in other event rates and costs had only minor impacts on the estimates of the total annual medical cost differences.
Figure 1. Univariate sensitivity analyses of total annual medical cost differences: paliperidone palmitate (PP1M) vs placebo.
Monte Carlo multivariate analyses, in which each variable of the univariate analyses were allowed to vary simultaneously for 10,000 random cycles, were used to further test the consistency of the estimates of the total annual medical cost differences for paliperidone palmitate treatment relative to placebo (). The mean (95% confidence interval (CI)) total annual medical cost reductions associated with paliperidone palmitate use vs placebo were as follows: overall: −$7154 (95% CI = −$10,862 to −$4092], monotherapy: −$8346 [95% CI = −$12,692 to −$4661], adjunctive therapy: −$6039 [95% CI = −$10,940 to −$1838]. Of the 10,000 random Monte-Carlo simulation cycles, 100%, 100%, and 99.8% had a net cost reduction (cost difference <$0) for the overall patient population treated with paliperidone palmitate, patients treated with paliperidone palmitate monotherapy, and patients treated with paliperidone palmitate adjunctive therapy, respectively. The means of such 10,000 random cycles of Monte Carlo simulations were very close to the estimated total annual medical cost differences in the default model analysis. This showed that the results of the original estimated total annual medical cost differences are relatively robust given the uncertainties explored.
Exploratory analysis
The exploratory analysis was conducted to assess, with different clinical inputs, if the data are consistent in terms of direction. Based on the results of the long-term paliperidone palmitate vs placebo relapse prevention study, the relative reductions in the number of psychiatric hospitalizations and ER visits for patients treated with paliperidone palmitate vs placebo were 46% and 43%, respectively, and were generally consistent with the reduction in the number of relapses among the trial populationCitation11,Citation12.
Discussion
This economic model using clinical trial event rates showed that total annual medical costs were estimated to be lower (−$7140) for patients with schizoaffective disorder who are treated with paliperidone palmitate vs those who are treated with placebo. The placebo treated group of the long-term paliperidone palmitate vs placebo relapse prevention study may mimic the real-world situation when patients are not adherent to their medication. Reductions in total annual medical costs were observed for both patients treated with paliperidone palmitate as a monotherapy (−$8321) and patients treated with paliperidone palmitate as an adjunctive therapy (−$6031). The estimates of the medical cost reductions associated with paliperidone palmitate treatment relative to placebo were consistent in sensitivity analyses. From the 10,000 probability distribution inputs in each of the Monte Carlo multivariable analyses the mean total annual medical cost difference associated with use of paliperidone palmitate treatment vs placebo was similar to that estimated in the default analysis, with 100.0% showing a net cost reduction (cost difference <$0) vs placebo. Furthermore, the results of the exploratory analysis suggest that data collected by a resource utilization questionnaire are consistent with trial primary end-point findings on the association of paliperidone palmitate treatment with reductions in schizoaffective relapses.
Only a few studies have examined hospitalization rates specifically among patients with schizoaffective disorder in the real-world settingCitation6,Citation9. Svarstad et al.Citation9 reported a hospitalization rate of 23% for patients with schizoaffective disorder in a 12-month period from 1989–1990. A more recent study by Karve et al.Citation6 reported that, in the first 60 days post-discharge of a relapse, ∼11% of patients with schizoaffective disorder had at least one disease-related inpatient stay. In the long-term paliperidone palmitate vs placebo relapse prevention study the rates of relapse during the 15-month double-blind period of the trial were 15% for patients taking paliperidone palmitate and 34% for patients taking placeboCitation11,Citation12. Relapses, in the context of schizophrenia, are personally debilitating and have large societal and economic burdens, with first episode average costs reported at $38,672 (2014 cost level)Citation14. It is important to study the clinical course of this disease, independently of other psychotic disorders, to improve patient management and reduce the risk of relapse specifically among this patient population.
Patients who have schizoaffective disorder have an inter-play of psychosis and mood symptoms and are frequently treated with complex treatment regimens, which can include more than one antipsychotic medicationCitation4,Citation5. A large study of antipsychotic drug utilization across multiple healthcare systems revealed that approximately one-fifth of patients with schizophrenia and other antipsychotic disorders received antipsychotic polypharmacyCitation19. There is limited evidence that antipsychotic polypharmacy is efficacious for the treatment of psychotic disordersCitation20,Citation21. A review of the literature conducted by Lochmann van Bennekorn et al.Citation20 reported that antipsychotic polypharmacy is associated with increased mortality, metabolic syndrome, decreased cognitive function, non-adherence, and increased healthcare costs. A second review found that antipsychotic polypharmacy is also associated with increased global side-effect burden, including Parkinsonian side-effects, anti-cholinergic use, sexual dysfunction, and diabetesCitation21. Although patients with schizophrenia and other psychotic disorders are also frequently treated with multiple disease-related medications, the Olfson et al.Citation4 study observed that use of complex pharmacologic regimens was more prominent among patients with schizoaffective disorder than patients with schizophrenia. It was demonstrated in the long-term paliperidone palmitate vs placebo relapse prevention study that paliperidone palmitate is efficacious for the treatment of schizoaffective disorder as a monotherapy and its use may greatly simplify treatment regimens of patients with schizoaffective disorderCitation11,Citation12. The rates of relapse among patients treated with paliperidone palmitate monotherapy were less than those treated with adjunctive therapy, which may reflect partial efficacy of adjunctive treatment and/or more refractory illness in patients requiring adjunctive therapies. Moreover, serious TEAEs were observed at relatively low rates in the trial, with patients treated with placebo having more serious TEAEs than patients treated with paliperidone palmitate, mainly driven by more psychiatric hospitalizations due to relapses. During this study, subjects were required to be adherent to the study medication, and they were withdrawn from the study if more than 6 weeks had elapsed since the time of their last injection. Information on adherence to once-monthly treatments among patients with schizoaffective disorder in the real-world setting is not available, and a future research study is warranted and should provide valuable information.
Patients with schizoaffective disorder may represent a population in which strategies to simplify and improve treatment may have major impacts on patient medication adherence, clinical outcomes, and healthcare costs; however, more studies are warranted, especially in the real-world setting.
Limitations
Our economic model was based on clinical event rates reported in the long-term paliperidone palmitate vs placebo relapse prevention study and, therefore, paliperidone palmitate was only compared with placebo and not an active comparator. However, patients in this study were allowed to continue with other psychotropic medications such as antidepressants and mood stabilizers, which are commonly used in schizoaffective disorder. This increases the generalizability of these findings, even in the absence of a true active comparator design. Also, our economic model has limitations in that drug costs, as well as the long-term burden of clinical events, indirect costs, and quality-of-life, all of which may be impacted by more efficacious pharmacotherapy for schizoaffective disorder, were not taken into account. Further analyses incorporating all of these costs may provide valuable information for healthcare providers, patients, and health policy-makers. Such analyses will likely be best accomplished using a real-world observational design. The annual medical cost for relapse was obtained from the retrospective Medicaid claims analysis by Lafeuille et al.Citation14, in which patients had schizophrenia and not schizoaffective disorder. Thus, the annual medical cost for relapse may not be completely generalizable to patients with schizoaffective disorder or those with other types of health insurance, such as commercial insurance. Also, the costs of other events, such as TEAEs, were based on that derived from clinical input and obtained from the Medical Expenditure Panel Survey and the Medicare Fee Schedule, Payment and Reimbursement Benefit Guideline, which may not be consistent with other insurance types. Although we did conduct one-way and multivariable sensitivity analyses to demonstrate the estimates of the medical cost, differences are relatively robust when costs of clinical events are varied. The rate of relapse among patients with schizoaffective disorder may be different in real-world settings and, therefore, the direct application of the results to routine clinical practice, where many factors including relapse risk may vary, will require further assessment. On the other hand, the results of our sensitivity analysis indicate that, even when such estimates are varied, the estimated total annual medical cost differences are relatively robust to such variations of model input parameters.
Conclusion
This economic model using clinical trial event rates estimated that there is a potential total annual medical cost reduction (−$7140) for patients with schizoaffective disorder who are treated with paliperidone palmitate vs placebo. Further evaluation in the real-world setting is warranted.
Transparency
Declaration of funding
This research and preparation of this manuscript was supported by Janssen Scientific Affairs.
Declaration of financial/other relationships
KJ and DJF are employees of Janssen Scientific Affairs and own stock in the company. JL and MLS are employees of Novosys Health, which has received research funds from Janssen Scientific Affairs in connection with conducting this study and development of this manuscript. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar 1 disorders in a general population. Arch Gen Psychiatry 2007;64:19-28
- Cheniaux E, Landeira-Fernandez J, Telles LL, et al. Does schizoaffective disorder really exist? A systematic review of the studies that compared schizoaffective disorder with schizophrenia or mood disorders. J Affective Disord 2008;106:209-17
- Peralta V, Cuesta MJ. Exploring the borders of the schizoaffective spectrum: a categorical and dimensional approach. J Affective Disord 2008;108:71-86
- Olfson M, Marcus SC, Wan GJ. Treatment patterns for schizoaffective disorder and schizophrenia among Medicaid patients. Psychiatr Serv 2009;60:210-16
- Vieta E. Developing an individualized treatment plan for patients with schizoaffective disorder: from pharmacotherapy to psychoeducation. J Clin Psychiatry 2010;71(2 Suppl):14-19
- Karve S, Markowitz M, Fu DJ, et al. Assessing medication adherence and healthcare utilization and cost patterns among hospital-discharged patients with schizoaffective disorder. Appl Health Econ Health Policy 2014;12:335-46
- Lake CR, Hurwitz N. Schizoaffective disorder merges schizophrenia and bipolar disorders as one disease—there is no schizoaffective disorder. Curr Opin Psychiatry 2007;20:365-79
- Murru A, Pacchiarotti I, Amann BL, et al. Treatment adherence in bipolar I and schizoaffective disorder, bipolar type. J Affect Disord 2013;151:1003-8
- Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv 2001;52:805-11
- Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of the literature. J Behav Med 2008;31:213-24
- Janssen Scientific Affairs. Clinical Study Report. A randomized, double-blind, placebo-controlled, parallel-group study of paliperidone palmitate evaluating time to relapse in subjects with schizoaffective disorder (PRIME). Data on File. Janssen Scientific Affairs, LLC, Raritan, NJ 2014
- Fu DJ, Turkoz I, Simonson BR, et al. Paliperidone palmitate once-monthly reduces risk of relapse of psychotic, depressive, and manic symptoms and maintains functioning in a double-blind, randomized study of schizoaffective disorder. J Clin Psychiatry 2015;76:253-62
- Janssen Scientific Affairs. Invega Sustenna Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc., 2014
- Lafeuille MH, Gravel J, Lefebvre P, et al. Patterns of relapse and associated cost burden in schizophrenia patients receiving atypical antipsychotics. J Med Econ 2013;16:1290-9
- U.S. Department of Health & Human Services. Medical Expenditure Panel Survey. Emergency Room Services. Rockville, MD: US Department of Health & Human Services, 2014 http://meps.ahrq.gov/mepsweb/data_stats/download_data_files_detail.jsp?cboPufNumber=HC-135E. Accessed September 30, 2014
- Centers for Medicare and Medicaid Services. Medicare Fee Schedule, Payment and Reimbursement Benefit Guideline, CPT Code Billing. Baltimore, MD: Centers for Medicare and Medicaid Services, http://www.medicarepaymentandreimbursement.com/p/medicar-fee-schedule-update.html. Accessed September 30, 2014
- U.S. Department of Labor. Consumer Price Index December 2014. USDL-14-1711. Washington DC: Bureau of Labor Statistics U.S. Department of Labor, http://www.bls.gov/news.release/pdf/cpi.pdf. Accessed September 30, 2014
- Deitelzweig S, Amin A, Jing Y, et al. Medical cost reductions associated with the usage of novel oral anticoagulants vs warfarin among atrial fibrillation patients, based on the RE-LY, ROCKET-AF, and ARISTOTLE trials. J Med Econ 2012;15:776-85
- Sun F, Stock EM, Copeland LA, et al. Polypharmacy with antipsychotic drugs in patients with schizophrenia: trends in multiple health care systems. Am J Health Syst Pharm 2014;71:728-38
- Lochmann van Bennekorn MW, Gijsman HJ, Zitman FG. Antipsychotic polypharmacy in psychotic disorders: a critical review of neurobiology, efficacy, tolerability and cost effectiveness. J Psychopharmacol 2013;27:327-36
- Gallego JA, Nielsen J, De Hert M, et al. Safety and tolerability of antipsychotic polypharmacy. Expert Opin Drug Saf 2012;11:527-42