Abstract
Objective:
To compare healthcare costs and utilization between commercially insured patients with type 2 diabetes mellitus (T2DM) in the United States newly initiating exenatide once weekly (QW) or liraglutide.
Methods:
This retrospective cohort study used US administrative claims data to study patients with T2DM initiating exenatide QW or liraglutide (initiated therapy = index therapy). Patients were included if they had T2DM, were glucagon-like peptide-1 receptor agonist (GLP-1RA) naïve, initiated exenatide QW or liraglutide from 1 February 2012 to 1 October 2012 (date of initiation = index), were ≥18 years at index, and had continuous enrollment for 12 months before (baseline) to 6 months after index (follow-up). Study outcomes were overall and diabetes-specific healthcare utilization and costs. Multivariable regressions compared the study outcomes between exenatide QW and liraglutide, adjusting for potential confounders. Sensitivity analyses were performed to assess liraglutide by dose (1.2 mg/1.8 mg).
Results:
The study sample included 9106 liraglutide (4188, 1.2 mg; 4918, 1.8 mg) patients and 2445 exenatide QW patients. In multivariable-adjusted analyses, compared with liraglutide patients, exenatide QW patients had statistically significantly lower odds of overall inpatient admissions (odds ratio [OR] = 0.80, p = 0.046) and diabetes-specific (OR = 0.83, p = 0.026) inpatient admissions, similar overall total costs ($7833 exenatide QW, $8296 liraglutide, p = 0.069) and diabetes-specific total costs ($3610 exenatide QW, $3736 liraglutide, p = 0.298), and statistically significantly lower overall medical costs ($3939 exenatide QW, $4652 liraglutide, p = 0.008) and diabetes-specific medical costs ($1161 exenatide QW, $1469 liraglutide, p = 0.007). Sensitivity analyses assessing liraglutide by dose were directionally consistent. Unadjusted exploratory analyses showed that exenatide QW patients obtained a greater median number of days supplied for their GLP-1RA during follow-up (141 days) than liraglutide patients (124 days).
Conclusions:
In this 6 month follow-up study, patients receiving exenatide QW had similar total healthcare costs but lower odds of inpatient admission and lower medical costs compared with patients receiving liraglutide.
Introduction
It is well known that the clinical and economic burden of type 2 diabetes mellitus (T2DM) is high and increasing. The Centers for Disease Control and Prevention report that in the United States, there are approximately 21 million individuals who have been diagnosed with T2DM and another 8.1 million individuals who have undiagnosed T2DMCitation1. The high prevalence of T2DM translates to substantial aggregate costs, with the American Diabetes Association estimating the direct healthcare costs of both type 1 diabetes mellitus (T1DM) and T2DM in the United States at $176 billion in 2012Citation2. Of these, approximately $160 billion is attributable to T2DM alone based on previously estimated relative costs of T1DM and T2DMCitation2,Citation3. Components of these direct healthcare costs include those related to the inpatient and outpatient medical management of T2DM and its micro- and macro-vascular sequelae, as well as pharmacological treatments for T2DM.
In the United States, there are numerous glucose-lowering medications available for the management of T2DMCitation4,Citation5. Evidence-based treatment guidelines typically recommend that for individuals in whom lifestyle modification (e.g., diet and exercise) alone is inadequate to control their T2DM, pharmacological treatment should be initiated with mono- or combination therapyCitation4,Citation5. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a growing class of injectable glucose-lowering medications indicated as adjunct therapy to diet and exercise to improve glycemic control in adults with T2DM. As of January 2015, in the United States, there were five GLP-1RAs approved for the treatment of T2DM: exenatide twice daily (BID), exenatide once weekly (QW), liraglutide once daily, albiglutide QW, and most recently, dulaglutide QWCitation6–Citation10.
With the expanding number of treatment options available to patients with T2DM, US payers (i.e., health insurance plans and prescription carriers/benefit managers) are increasingly relying on real-world, comparative healthcare utilization and cost data when making decisions related to their prescription coverage policiesCitation11,Citation12. Currently, there are few studies that use real-world data to compare such outcomes among exenatide QW, exenatide BID, and liraglutide. In a recently published retrospective cohort study of patients diagnosed with T2DM, significant differences were observed in adherence to GLP-1RA treatment among adult patients who newly initiated exenatide QW, exenatide BID, or liraglutide. In that study, patients initiating exenatide QW had significantly higher adjusted odds of adherence compared with patients initiating other GLP-1RAsCitation13. Another recently presented retrospective cohort study used the Quintiles Electronic Medical Record (EMR) database to evaluate the 6 month change in glycated hemoglobin (HbA1c) for adult patients initiating exenatide QW or liraglutideCitation14. The adjusted results suggest HbA1c similarly improved in patients initiating exenatide QW or liraglutide after 6 months of follow-up in an ambulatory care setting.
To date, little information has been published regarding healthcare utilization and costs among patients treated with GLP-1RAs, and no studies have reported such information for exenatide QW. Pelletier and colleagues compared exenatide BID to liraglutide on healthcare utilization and costs using an administrative claims databaseCitation15. They reported that patients initiating exenatide BID had similar total healthcare costs but significantly lower total pharmacy costs compared to patients initiating liraglutide. DeKoven and colleagues also used an administrative claims database to compare exenatide BID to liraglutide on diabetes-related pharmacy costsCitation16. They also reported that diabetes-related pharmacy costs were greater for patients initiating liraglutide compared to patients initiating exenatide BID; however, they noted that a higher proportion of patients initiating liraglutide achieved an HbA1c <7%.
Motivated by the reported adherence differences and by the reported similarities in glycemic control across liraglutide and exenatide QW in real world evidence reports, we sought to examine whether differences may be present for healthcare resource utilization and costsCitation17–Citation19. Thus, we undertook this retrospective cohort study to test the hypothesis that healthcare utilization and costs would differ among GLP-1RA-naïve patients treated with exenatide QW or liraglutide. A priori defined sensitivity analyses were performed to assess liraglutide by dose (1.2 mg/1.8 mg).
Methods
Overview of study design
This was a retrospective cohort study based on US administrative insurance claims data for a non-probability sample of individuals with employer-sponsored commercial health insurance. GLP-1RA-naïve patients with T2DM who initiated exenatide QW or liraglutide between 1 February 2012 and 1 October 2012, were followed on an intention-to-treat basis (followed regardless of switching or discontinuation of their antidiabetes medications) for 6 months to measure and compare overall and diabetes-specific healthcare utilization and costs.
Data source
The study data were administrative insurance claims data contained in the Truven Health MarketScan Commercial Claims and Encounters (Commercial) database. This database contains enrollment information, inpatient and outpatient medical data, and outpatient pharmacy claims data for approximately 40 million (annually) individuals with employer-sponsored health insurance. It has been used in multiple published evaluations related to medication adherenceCitation20.
The study database satisfies the conditions set forth in Sections 164.514 (a)-(b)1ii of the Health Insurance Portability and Accountability Act of 1996 privacy rule regarding the determination and documentation of statistically de-identified data. Because this study used only de-identified patient records and did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board approval to conduct this study was not necessary. This article does not contain any new studies with human or animal subjects performed by any of the authors.
Study variables were measured from the database using enrollment records, International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes, Current Procedural Technology, 4th edition (CPT-4) codes, Healthcare Common Procedure Coding System (HCPCS) codes, and National Drug Codes (NDCs), as appropriate.
Patient selection criteria
Patients were initially included in the study sample if they met the following inclusion criteria: filled at least one pharmacy claim for exenatide QW or liraglutide between 1 February 2012 (exenatide QW was approved in late January 2012), and 1 October 2012 (the date of the first of such claims was set as the index date); did not have pharmacy claims for more than one GLP-1RA on the index date; were 18 years or older on the index date; had at least 12 months of continuous pre-index medical and pharmacy benefits enrollment (the baseline period); had at least 6 months of continuous post-index medical and pharmacy benefits enrollment (the follow-up period); had at least one medical claim with a diagnosis code for T2DM (ICD-9-CM 250.x0 or 250.x2) in the baseline period or on the index date; and had no pharmacy claims for a GLP-1RA in the baseline period, a criterion applied in an attempt to restrict the sample to GLP-1RA-naïve patients.
Patients were excluded from the study sample if they had any medical claims with a diagnosis code for T1DM (ICD-9-CM 250.x1 or 250.x3) or gestational diabetes (ICD-9-CM 648.8x), or had any medical claims indicative of pregnancy or childbirth in the baseline or follow-up periods.
Measurement of healthcare utilization and costs
The study outcomes were overall and diabetes-specific healthcare utilization – with particular focus on inpatient admissions – and costs, which were measured during the 6 month intent-to-treat follow-up period. Overall healthcare utilization and costs were measured using all medical and outpatient pharmacy claims incurred during the 6 month follow-up period. Diabetes-specific healthcare utilization and costs were measured using all medical claims with a diagnosis of T2DM (ICD-9-CM 250.x0, 250.x2) in any position and outpatient pharmacy claims for GLP-1RAs and other glucose-lowering medications incurred during the 6 month follow-up period.
Healthcare costs were expressed in 2012 constant dollars, adjusted using the Medical Care component of the Consumer Price Index. Healthcare costs were measured using the financial fields on administrative claims in the MarketScan databases. Healthcare costs included the gross covered payments for all healthcare services or products, which includes both payer-paid costs and patient-paid out-of-pocket costs, i.e., the amount eligible for payment after applying pricing guidelines such as fee schedules and discounts, but including deductibles, copayments, and coordination of benefits.
Costs for services provided under capitated payment arrangements were imputed using a Truven Health Analytics algorithm that computes a payment proxy for healthcare services used, based on the average payments for noncapitated claims at the region, year, and procedure level within the MarketScan databases. This algorithm has been widely used in the published health-economic literature for multiple disease areasCitation20.
GLP-1RA classification and covariates
Patients were classified according to the GLP-1RA that they initiated on the index date. For liraglutide-treated patients, a priori sensitivity analyses related to dose were also conducted, in which patients were classified as being treated with either liraglutide 1.2 mg (defined as having only prescriptions indicative of 1.2 mg dose on the basis of days supplied and metric quantity during the 6 month follow-up period) or liraglutide 1.8 mg (defined as having any prescriptions indicative of 1.8 mg dose on the basis of days supplied and metric quantity during the 6 month follow-up period).
The study covariates included baseline variables such as patient demographics, clinical characteristics, and baseline healthcare utilization and costs, which may potentially confound the relationship between choice of GLP-1RA and the healthcare utilization and cost outcomes. Patient demographics were measured at the index date, and patient clinical characteristics and baseline healthcare utilization and costs were measured throughout the baseline period. Patient demographics are listed in , and patient clinical characteristics are listed in .
Table 1. Patient demographics.
Table 2. Patient baseline clinical characteristics.
Statistical analyses
Bivariate analyses were used to display summaries of variable distributions, stratified by liraglutide vs. exenatide QW and by dose for patients treated with liraglutide. Multivariable logistic regression models were used to compare the odds of overall and diabetes-specific inpatient admission, separately. Multivariable generalized linear models with a log link and gamma error distribution were used to compare overall (total, medical), diabetes-specific (total, medical), and GLP-1RA (i.e., for the medication itself) costs. All models adjusted for all patient demographics and clinical characteristics listed in and . The variance inflation factor was used to assess multi-collinearity of the models’ independent variables. For the healthcare cost models, adjusted predicted costs were generated through the recycled prediction methodCitation21. One set of models was fitted comparing exenatide QW to liraglutide (overall), and another set was fitted comparing exenatide QW to liraglutide, separating liraglutide by dose (1.2 mg/1.8 mg). Data extraction was performed using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA). Statistical analyses were performed using StataMP 12 (StataCorp, College Station, Texas, USA). P values < 0.05 were considered, a priori, to be statistically significant.
Results
Patient selection and characteristics
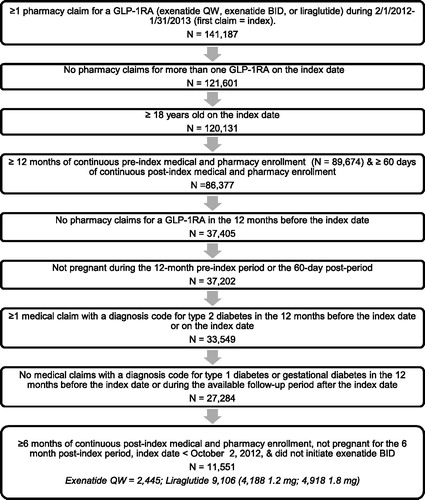
displays the changes in sample size associated with the application of each inclusion and exclusion criterion. A total of 2445 patients treated with exenatide QW and 9106 patients treated with liraglutide (4188, 1.2 mg; 4918, 1.8 mg) ultimately met all patient selection criteria.
Figure 1. Patient selection diagram. BID = twice daily; GLP-1RA = glucagon-like peptide-1 receptor agonist; QW = once weekly.
and display patients’ demographics and baseline clinical characteristics, respectively. Compared with patients treated with exenatide QW, patients treated with liraglutide had significantly (P < 0.05 for all comparisons) higher proportions of women, lower rates of baseline microvascular complications, lower rates of baseline dyslipidemia, lower mean out-of-pocket monthly cost, and lower likelihood of visiting an endocrinologist at baseline. When the liraglutide patients were separately examined by dose groups it was evident that these differences arose from those in the liraglutide 1.2 mg group. Liraglutide 1.8 mg patients were generally similar to patients treated with exenatide QW.
Overall healthcare utilization and costs
and display unadjusted data on overall healthcare utilization and costs, respectively, during the 6 month follow-up period. Overall healthcare utilization and costs generally did not differ significantly across the GLP-1RAs. In all cohorts, payers paid for approximately 88% of overall total costs, and patients paid for approximately 12% of these costs.
Table 3. Unadjusted overall healthcare utilization during 6 month follow-up period.
Table 4. Unadjusted overall healthcare costs during 6 month follow-up period.
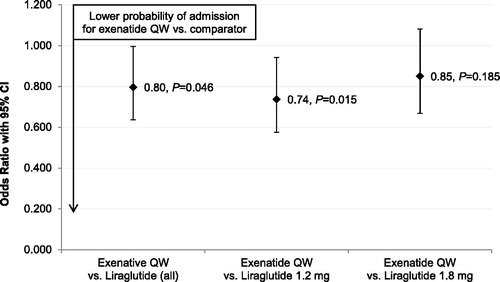
displays the multivariable logistic regression-adjusted odds of overall inpatient admission during the 6 month follow-up period. Compared with patients treated with liraglutide, patients treated with exenatide QW had statistically significantly lower odds of experiencing an overall inpatient admission. This difference was driven primarily by patients treated with liraglutide 1.2 mg.
Figure 2. Multivariable logistic regression-adjusted odds of overall inpatient admission during 6 month follow-up period, exenatide QW as reference category (N = 11,551). CI = confidence interval; QW = once weekly.
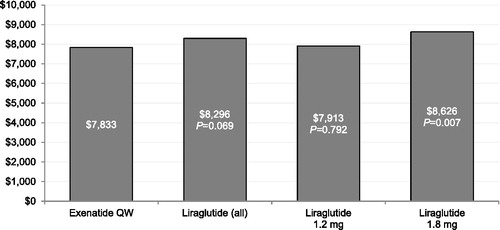
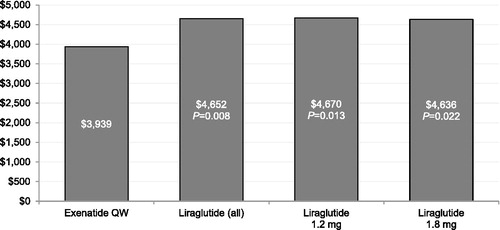
and display the multivariable regression-adjusted overall total and medical costs, respectively, during the 6 month follow-up period. Compared with patients treated with liraglutide 1.8 mg, patients treated with exenatide QW had statistically significantly lower overall total healthcare costs during the 6 month follow-up period. Patients treated with exenatide QW had statistically significantly lower overall medical costs during the 6 month follow-up period compared with patients treated with liraglutide, collectively and in individual dose groups.
Diabetes-specific utilization and costs
and display unadjusted data on diabetes-specific healthcare utilization and costs, respectively, during the 6 month follow-up period. Diabetes-specific healthcare utilization and costs generally did not differ significantly across the GLP-1RAs. As was the case with overall total costs, in all cohorts, payers paid for approximately 88% of diabetes-specific total costs, and patients paid for approximately 12% of these costs.
Table 5. Unadjusted diabetes-specific healthcare utilization during 6 month follow-up period.
Table 6. Unadjusted diabetes-specific healthcare costs during 6 month follow-up period.
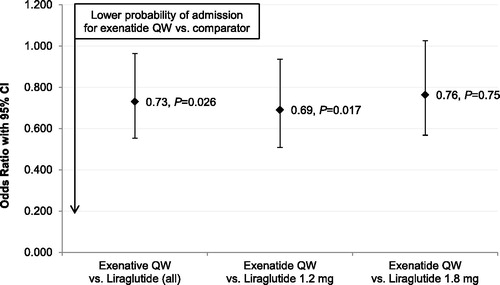
displays the multivariable logistic regression-adjusted odds of diabetes-specific inpatient admission during the 6 month follow-up period. Compared with patients treated with liraglutide, patients treated with exenatide QW had statistically significantly lower odds of experiencing a diabetes-specific inpatient admission. This difference was again driven primarily by patients treated with liraglutide 1.2 mg.
Figure 5. Multivariable logistic regression-adjusted odds of diabetes-specific inpatient admission during 6 month follow-up period, exenatide QW as reference category (N = 11,551). CI = confidence interval; QW = once weekly.
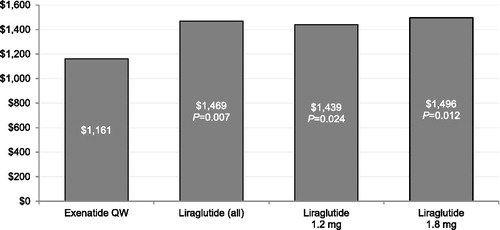
and display the multivariable regression-adjusted diabetes-specific total and medical costs, respectively, during the 6 month follow-up period. Compared with patients treated with liraglutide 1.8 mg, patients treated with exenatide QW had statistically significantly lower diabetes-specific total healthcare costs during the 6 month follow-up period. Compared with patients treated with liraglutide, liraglutide 1.2 mg, or liraglutide 1.8 mg, patients treated with exenatide QW had statistically significantly lower diabetes-specific medical costs during the 6 month follow-up period.
Figure 6. Multivariable regression-adjusted diabetes-specific total costs during 6 month follow-up period (N = 11,551). QW = once weekly.
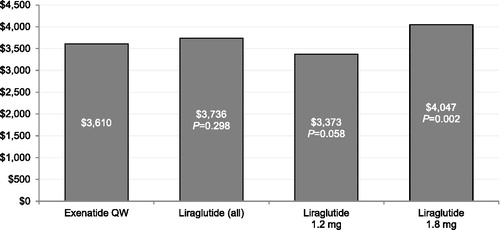
Figure 7. Multivariable regression-adjusted diabetes-specific medical costs during 6 month follow-up period (N = 11,551). QW = once weekly.
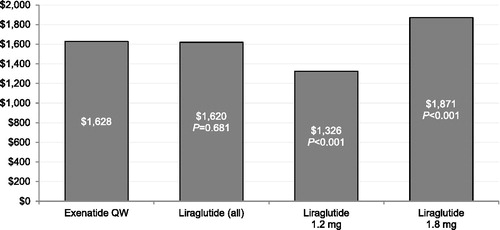
displays the multivariable regression-adjusted GLP-1RA costs during the 6 month follow-up period. Compared with patients treated with liraglutide 1.8 mg, patients treated with exenatide QW had statistically significantly lower GLP-1RA medication costs during the 6 month follow-up period. Compared with patients treated with liraglutide 1.2 mg, patients treated with exenatide QW had statistically significantly higher GLP-1RA medication costs during the 6 month follow-up period.
Discussion
To our knowledge, this retrospective cohort study is the first comparison of healthcare utilization and costs between GLP-1RA-naïve patients with T2DM newly initiating either liraglutide or exenatide QW. This study found that the two groups had similar overall healthcare costs; however, patients in the liraglutide cohort had 18% higher overall medical costs than exenatide QW patients. Patients initiating liraglutide incurred 26% higher diabetes-specific medical costs compared to exenatide QW patients. This study also found that patients initiating exenatide QW consistently had numerically lower odds of overall and diabetes-specific hospitalization when compared with patients initiating liraglutide, both overall and when separating liraglutide by dose. These differences were statistically significant for the overall liraglutide group and the patients treated with liraglutide 1.2 mg, in whom the difference was largest.
One of the most substantial differences between liraglutide and exenatide QW is the lower frequency with which exenatide QW is administered (once weekly as opposed to once daily). Prior analyses have shown that glucose-lowering medication regimens with less frequent dosing are associated with increased adherenceCitation22–Citation27. Furthermore, a prior discrete choice experiment demonstrated that among injection-naïve patients with T2DM, the most important feature of injectable treatments when choosing among hypothetical treatments was injection frequency; patients preferred weekly injections over daily injectionsCitation28. Medication adherence is a significant consideration in the management of diabetes because of mounting evidence that nonadherence is prevalent and associated with adverse outcomes and higher costs of careCitation17,Citation29. In a previous study using the same health insurance plan database, Johnston et al.Citation13 reported that patients treated with exenatide QW had a statistically significantly greater probability of achieving adherence of ≥80% and ≥90% – two clinically significant adherence thresholds in diabetes that are predictive of reduced hospitalization and mortality – when compared with patients treated with either exenatide BID or liraglutide, a finding that was consistent across liraglutide doses of 1.2 mg and 1.8 mgCitation17,Citation30–32. Although adherence was not directly assessed in this study, in an exploratory examination of treatment duration, we found that patients treated with exenatide QW had a greater median number of days supplied for their GLP-1RA during follow-up (141 days) than patients treated with liraglutide (124 days). The longer duration of therapy experienced by exenatide QW patients in this study was consistent with the previous adherence study and drove higher GLP-1RA costs for exenatide QW patients compared with patients in the overall liraglutide cohort. These higher GLP-1RA costs for exenatide QW patients also help to explain the finding of lower medical costs for exenatide QW but similar overall and diabetes-specific total healthcare costs when compared to the overall liraglutide cohort. It is notable, however, that patients treated with the more expensive option of liraglutide 1.8 mg had higher GLP-1RA costs as well as higher overall and diabetes-specific total healthcare costs compared with exenatide QW patients.
High adherence levels have also been demonstrated to be associated with decreased hospitalizationsCitation17. In this study, patients in the exenatide QW cohort experienced significantly lower odds of hospitalizations (overall and diabetes-specific) than liraglutide patients. These differences were statistically significant for the overall liraglutide group and the patients treated with liraglutide 1.2 mg, in whom the difference was largest. These findings are important because not only do hospitalizations generally represent undesirable and often severe acute contact with the healthcare system, they have been documented as a major cost driver among patients with T2DM – representing approximately 43% of the total nationwide medical cost of diabetesCitation2. Indeed, in the present study’s sample, costs for hospitalization represented approximately 51–55% of the unadjusted overall total healthcare costs and 33–41% of the unadjusted diabetes-specific healthcare costs. It is important to note that because the present study did not measure hospital length of stay, further research would be useful to understand whether the lower odds of hospitalizations seen for patients in the exenatide QW cohort would also translate to lower hospital length of stay.
Based on prior evidence, it is plausible that differences in glycemic control between the study cohorts could also partially explain some of the differential hospitalization rates across study cohorts. In a network meta-analysis that included 11,049 patients with T2DM from 22 studies, Scott and colleagues reported significantly greater reductions in HbA1c for patients initiating exenatide QW compared with liraglutide 1.2 mgCitation33. Such differential effects on HbA1c would be consistent with the present study’s finding that patients treated with liraglutide 1.2 mg had the greatest odds of both overall and diabetes-related inpatient admissions. Furthermore, in a previous study by Menzin et al., higher average HbA1c levels were associated with significantly higher rates of diabetes-related hospital utilization and, among those with at least one hospitalization, significantly higher estimated hospitalization costsCitation34.
The differences in the odds of hospitalization across the study cohorts likely underlie our findings that exenatide QW had statistically significant lower overall and diabetes-specific medical costs than liraglutide. These costs may correspond to the efforts taken to medically manage these patients, including services such as physician visits, laboratory tests, and outpatient procedures, among others. The findings from the present study may plausibly be interpreted as a medical cost offset in exenatide QW relative to liraglutide, which resulted in significantly lower diabetes-specific healthcare costs for exenatide QW patients.
With respect to clinical application in practice, the choice of a medication for a given patient will be based on a provider’s clinical judgment regarding the benefits and risks of treatment, the patient’s preference for route of administration, frequency of administration, and/or potential side effects, as well as healthcare coverage and costs for the medication, among other factors. Exenatide QW and liraglutide differ on a number of these factors: for example, exenatide QW may cause injection site nodules, while liraglutide may cause comparatively more gastrointestinal tolerability problemsCitation6,Citation35. Although the economic focus of this study does not necessarily inform these particular aspects of clinical practice, our results are nevertheless helpful for understanding a broad range of potential healthcare utilization and cost implications when prescribing these medications.
Our study has several important limitations related to the use of administrative claims data. First, administrative claims data are not collected for research purposes, and the coding on administrative claims is recorded by physicians to support reimbursement. Diagnoses on claims may be coded incorrectly or missing, which may introduce measurement error with respect to ICD-9-CM-based variables. In particular, as with any administrative claims analysis in which disease-specific costs are of interest, such errors may affect the classification of costs as being diabetes-specific, where some costs may have been missed while others may have been erroneously included. Second, although this study used the largest non-probability sample available in proprietary databases, findings are generalizable primarily to the commercially insured US population, but not necessarily to individuals with other forms of insurance or uninsured individuals. Third, we classified patients treated with liraglutide into 1.2 mg or 1.8 mg on the basis of days supplied and metric quantity recorded on the pharmacy claims. Because liraglutide is delivered in a self-adjustable prefilled dosing pen, it is possible that patients may have self-administered more or less liraglutide than would be indicated for a given prescription’s days supplied and metric quantity. Fourth, because of the limited duration of history for which data are available on exenatide QW, our study examined a relatively short follow-up period of 6 months. Also, the patterns of exenatide QW use reflected only the first year of market availability, and these patterns may change over time. Thus, future analyses are needed to better understand the comparative long-term adherence to each GLP-1RA. Fifth, this study did not include all available GLP-1RAs, as albiglutide and dulaglutide were approved after the study period ended. Sixth, observational analyses such as the present study may be subject to residual confounding despite multivariable adjustment. However, the patient groups did not differ substantially on observed characteristics and the differences that were presented tended to be indicative of poorer overall health for patients treated with exenatide QW (e.g., higher values of comorbidity indices, higher baseline prevalence of comorbidities). This suggests that confounding would tend to bias the results in a direction favoring liraglutide.
Conclusion
This retrospective cohort study compared healthcare utilization and costs between GLP-1RA-naïve patients with T2DM newly initiating either liraglutide or exenatide QW. Compared with liraglutide patients, exenatide QW patients had similar overall and diabetes-specific total healthcare costs but lower overall and diabetes-specific odds of inpatient admission and lower overall and diabetes-specific medical costs. Further research is needed to evaluate the associations between GLP-1RA adherence, the costs of diabetes care, and diabetes-specific utilization over a longer period.
Transparency
Declaration of funding
This study was sponsored by AstraZeneca, Fort Washington, PA, USA and Bristol-Myers Squibb, Plainsboro, NJ, USA.
Declaration of financial/other relationships
S.S.J., K.C., J.K.N., and B.-C.C. have disclosed that they are employees of Truven Health Analytics, which was paid by the study sponsors to conduct this study. H.N. and I.K. have disclosed that they are employees of AstraZeneca and were employees of Bristol-Myers Squibb at the time this study was conducted. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Boris Ivanov, employee of Truven Health Analytics, provided statistical programming support for this study. Eugene Felber, an employee of Bristol-Myers Squibb, contributed to the study design.
Notes
*MarketScan is a registered trade name of Truven Health, Ann Arbor, MI, USA
References
- Centers for Disease Control and Prevention. National Diabetes Statistics Report, 2014. Available at: http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf [Last accessed 26 June 2014]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care 2013;36:1033-46
- Dall TM, Mann SE, Zhang Y, et al. Distinguishing the economic costs associated with type 1 and type 2 diabetes. Popul Health Manag 2009;12:103-10
- American Diabetes Association. Standards of medical care in diabetes – 2014. Diabetes Care 2014;37(Suppl 1):S14-80
- Garber AJ, Abrahamson MJ, Barzilay JI, et al. American Association of Clinical Edocrinologists Comprehensive Diabetes Management Algorithm 2013 Consensus Statement. Endocr Pract 2013;19(Suppl 2):1-48
- Bydureon [package insert]. San Diego, CA: Amylin Pharmaceuticals Inc., 2012
- Byetta [package insert]. San Diego, CA: Amylin Pharmaceuticals Inc., 2011
- Victoza [package insert]. Plainsboro, NJ: Novo Nordisk, 2013
- Tanzeum [package insert]. Wilmington, DE: GlaxoSmithKline LLC, 2014
- Trulicity [package insert]. Indianapolis, IA: Eli Lilly & Co, 2014
- What payers want: viewing payers as customers. Deloitte Life Sciences, 2009. Available at: http://www.deloitte.com/assets/Dcom-UnitedStates/Local%20Assets/Documents/us_lshc_WhatPayersWant_091109.pdf [Last accessed 22 August 2013]
- Managed Care. Payers Step in With ‘Real-World’ Comparative Effectiveness Research. 2011. Available at: http://www.managedcaremag.com/archives/1106/1106.cer.html [Last accessed 22 August 2013]
- Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther 2014;31:1119-33
- Saunders B, Nguyen H, Kalsekar I. Real-world comparative effectiveness of exenatide once weekly and liraglutide in patients with type 2 diabetes mellitus. Presented at the American Diabetes Association (ADA) 74th Scientific Sessions, 13–17 June 2014, San Francisco, CA, USA
- Pelletier EM, Pawaskar M, Smith PJ, et al. Economic outcomes of exenatide vs liraglutide in type 2 diabetes patients in the United States: results from a retrospective claims database analysis. J Med Econ 2012;15:1039-50
- DeKoven M, Lee WC, Bouchard J, et al. Real-world cost-effectiveness: lower cost of treating patients to glycemic goal with liraglutide versus exenatide. Adv Ther 2014;31:202-16
- Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther 2011;33:74-109
- Wild H. The economic rationale for adherence in the treatment of type 2 diabetes mellitus. Am J Manag Care 2012;18(3 Suppl):S43-8
- Salas M, Hughes D, Zuluaga A, et al. Costs of medication nonadherence in patients with diabetes mellitus: a systematic review and critical analysis of the literature. Value Health 2009;12:915-22
- Truven Health Analytics. MarketScan Bibliography. Available at: http://marketscan.truvenhealth.com/marketscanuniversity/publications/2012%20Truven%20Health%20MarketScan%20Bibliography.pdf [Last accessed 20 June 2014]
- Kleinman LC, Norton EC. What’s the risk? A simple approach for estimating adjusted risk measures from nonlinear models including logistic regression. Health Serv Res 2009;44:288-302
- Rubin RR. Adherence to pharmacologic therapy in patients with type 2 diabetes mellitus. Am J Med 2005;118(Suppl 5A):27-34S
- Zhang L, Zakharyan A, Stockl KM, et al. Mail-order pharmacy use and medication adherence among Medicare Part D beneficiaries with diabetes. J Med Econ 2011;14:562-7
- Dezii CM, Kawabata H, Tran M. Effects of once-daily and twice-daily dosing on adherence with prescribed glipizide oral therapy for type 2 diabetes. South Med J 2002;95:68-71
- Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med 2008;31:213-24
- Melikian C, White TJ, Vanderplas A, et al. Adherence to oral antidiabetic therapy in a managed care organization: a comparison of monotherapy, combination therapy, and fixed-dose combination therapy. Clin Ther 2002;24:460-7
- Pollack M, Chastek B, Williams SA, et al. Impact of treatment complexity on adherence and glycemic control: an analysis of oral antidiabetic agents. J Clin Outcomes Manage 2010;17:257-65
- Hauber B, Nguyen H, Posner J, et al. Patient preferences for frequency of glucagon-like peptide-1 receptor agonist (GLP-1RA) injections in the treatment of type 2 diabetes. Presented at the ISPOR 19th Annual International Meeting, 31 May–4 June 2014, Montreal, QC, Canada
- Osterberg L, Blaschke T. Adherence to medication. N Engl J Med 2005;353:487-97
- Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836-41
- Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care 2004;27:2149-53
- Karve S, Cleves MA, Helm M, et al. Good and poor adherence: optimal cut-point for adherence measures using administrative claims data. Curr Med Res Opin 2009;25:2303-10
- Scott DA, Boye KS, Timlin L, et al. A network meta-analysis to compare glycaemic control in patients with type 2 diabetes treated with exenatide once weekly or liraglutide once daily in comparison with insulin glargine, exenatide twice daily or placebo. Diabetes Obes Metab 2013;15:213-23
- Menzin J, Korn JR, Cohen J, et al. Relationship between glycemic control and diabetes-related hospital costs in patients with type 1 or type 2 diabetes mellitus. J Manag Care Pharm 2010;16:264-75
- Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet 2013;381:117-24