Abstract
Introduction:
Triple therapy using a protease inhibitor (PI) with peginterferon and ribavirin (PR) is increasingly used in patients with chronic hepatitis C virus (HCV) infection. The most recently introduced PI, simeprevir (SMV), offers high levels of viral eradication combined with a reduced overall duration of therapy. The objective of this study was to compare the cost-effectiveness of SMV + PR vs PR alone or in combination with telaprevir (TVR) or boceprevir (BOC) in patients infected with genotype 1 HCV
Method:
A cost-utility model was constructed, incorporating two phases, capturing the efficacy of therapy in an initial treatment phase, followed by a long-term post-treatment Markov phase, capturing lifetime outcomes according to whether a sustained viral response (SVR) had been achieved on treatment. Dosage regimens were based on the EMA approved label for each treatment. SVR estimates and adverse event rates were derived from a mixed treatment comparison. Baseline characteristics were drawn from an analysis of a UK HCV data-set and clinician opinion. Health state transition probabilities, utilities, and health state costs were drawn from previously published economic analyses. The model considered direct health costs only, and the perspective was that of the UK National Health Service.
Results:
The model yielded an ICER for SMV + PR vs PR alone of £9725/QALY for treatment-naïve and £7819/QALY for treatment-experienced. Benefit was driven by increased likelihood of achieving SVR, with consequent long-term utility gains. SMV + PR dominated TVR + PR and BOC + PR in both patient groups. This principally reflected the QALY benefit of an increased likelihood of SVR with SMV, combined with lower overall drug costs, due to reduced mean treatment duration.
Conclusion:
Compared to other currently licensed treatment options, SMV + PR represents a cost effective treatment option for patients with chronic genotype 1 HCV infection.
Introduction
Hepatitis C virus (HCV) is one of the most common causes of chronic liver disease globally, with an estimated 185 million infected individuals, of whom ∼350,000 will die each yearCitation1,Citation2. Prevalence varies widely in the developed world. In Western Europe ∼0.5–1.5% of the general population have detectable HCV antibody, although in Italy and Romania rates well in excess of this have been reportedCitation3. In the US it is estimated that 3.2 million people are infected, representing a general population prevalence of 1.0%Citation4.
Although acute infection with HCV is generally mild and may pass unnoticed, in the majority of patients (55–85%) viraemia persists in the form of chronic infectionCitation5,Citation6. If untreated, ∼15–30% of these patients will progress to cirrhosis over a 20 year periodCitation7–9. Once diagnosed with cirrhosis, 2–4% per year will go on to develop hepatocellular carcinomaCitation10. In consequence, mortality due to HCV has now overtaken that of HIV in the USCitation11.
Once diagnosed, eradication of HCV using antiviral therapy prevents further hepatic injury and patients usually remain infection-free unless re-exposed. If HCV is undetectable in the blood 12–24 weeks after completion of treatment, the likelihood of recurrence of infection is very low. This state is termed sustained virological response (SVR) and is the standard measure of treatment success used in clinical trials of antiviral therapy. First line use of a combination of peginterferon + ribavirin (PR) has been shown to yield SVR in 56% of patients in well managed populationsCitation12, although the rates achieved in clinical practice vary with the drug regimen used, the HCV genotype, prior exposure to antiviral agents, and patient adherence to treatmentCitation13.
The recent introduction of protease inhibitors (PI) to HCV treatment regimens based on PR has significantly increased SVR in primary treatment, compared with PR alone. It also offers an additional option for those who had an inadequate response to initial treatment with PR or suffered a subsequent relapseCitation14. In Europe, boceprevir (BOC) and telaprevir (TVR) currently account for the majority of PI prescribing for HCV, but the availability of simeprevir (SMV) offers further clinical benefits, with high efficacy rates and the potential for a reduced duration of treatment.
SMV has been investigated across a range of patient types in its research programme—most extensively in those with genotype 1 HCV infection. Three studies (PILLAR, QUEST-1, and QUEST-2)Citation15–17 compared SMV + PR in treatment naïve patients (n = 830), and two further studies (ASPIRE and PROMISE)Citation18,Citation19 compared SMV with PR alone in patients who had either failed to respond or experienced a relapse following initial PR therapy (n = 656). ATTAINCitation20 compared SMV + PR with TVR + PR in patients who had previously not responded to first line PR alone (n = 763).
These studies provide clear evidence that SMV + PR is significantly superior to PR alone in terms of antiviral efficacy, and the ATTAIN studyCitation20 demonstrated non-inferiority vs TVR + PR in patients who were non-responders to prior treatment with PR, with an improved tolerability profile. An additional regulatory requirement in many healthcare systems, prior to acceptance and/or reimbursement, is to demonstrate the cost-effectiveness of a new treatment vs treatments currently in use. The objective of this study was, therefore, to carry out a cost-utility analysis to compare the cost-effectiveness of SMV + PR vs PR alone and also vs PI-containing regimens (TVR + PR and BOC + PR) in both treatment-naïve and treatment-experienced patients chronically infected with genotype 1 HCV in the UK.
Methods
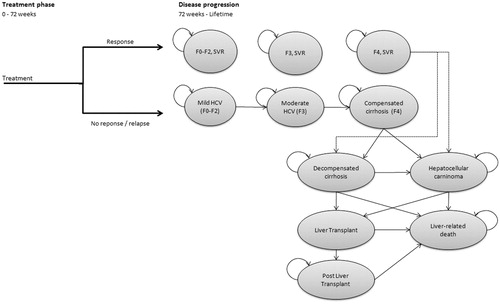
A cost-utility model was developed to assess the cost-effectiveness ratio of SMV + PR vs PR used either alone or in combination with other protease inhibitors (TVR or BOC) in patients with genotype 1 HCV infection. The model shares an overall approach with previously published economic analyses in the field of hepatitis C treatmentCitation21–23. It is composed of two phases. The first—‘treatment’ phase—relates to the initial anti-viral treatment period (). This phase includes up to 48 weeks of treatment followed by a 12–24 week post-treatment period, at which point viral response is assessed. Based on the outcome, patients move into a second ‘post-treatment’ Markov phase of the model after 72 weeks, which captures long-term outcomes over the patients’ remaining lifespan.
Patients not reaching SVR in the first phase are at risk of progression to more advanced liver disease stages, depending on their baseline disease severity, as classified by their Metavir gradeCitation24: no or mild fibrosis (F0–2), moderate fibrosis (F3), and compensated cirrhosis (F4). The model assumes that patients with grades F4 can progress directly to more advanced liver disease stages, while those in grade F0–3 can only progress indirectly via Metavir grade F4. SVR prevents progression from milder grades to F4, and reduces the risk of progressing from F4 to decompensated cirrhosis (DCC) and hepatocellular carcinoma (HCC).
Cycle length in the Markov phase is 1 year, with a lifetime horizon. Half cycle correction is applied. Both costs and benefits are discounted at 3.5% per year and are assessed from the perspective of the National Health Service in England.
Patient population
The patient population was defined as adults chronically infected with genotype 1 HCV, excluding genotype 1a patients who exhibit the Q80K polymorphism, following SMV + PR label recommendationsCitation25. Two parallel populations were analysed based on whether they were treatment naïve or treatment experienced to PR, including prior relapsers, prior non-responders, and prior partial responders. Information on gender distribution and mean age and weight were sourced from the HCV Research UK database. In the absence of epidemiological data, baseline Metavir grade distribution was based on the opinion of a UK clinician advisory board. summarizes the values used for all baseline patient characteristics.
Table 1. Baseline characteristics of patient population.
Comparative treatments
All treatments were modeled in accordance with their EMA licensed dosage and recommended duration. Treatment duration is an important differentiator between regimens and has potential consequences for both patient quality-of-life and cost of treatment. Treatment duration for SMV + PR is 24 weeks (12 weeks SMV + PR followed by 12 weeks PR alone) for treatment naïve patients and those with a prior relapse. Prior non-responders receive an additional 24 weeks of PR treatment. In the PR, TVR + PR, and BOC + PR regimens, all treatments are given for 48 weeks to all patient types. TVR and BOC can be reduced to 24 or 28 weeks (response guided therapy, RGT) only when patients show a rapid early response to therapy. Full details are shown in Supplementary Table 1.
Efficacy and safety
In addition to placebo-controlled trials for each of the licensed PIs, only one head-to-head clinical trial comparing SMV + PR with TVR + PR has been publishedCitation20. In order to derive relative SVR data for all treatments, a mixed treatment comparison (MTC) was carried out, incorporating both direct and indirect evidenceCitation26,Citation27. Following a systematic review, 15 studies were identified comparing triple therapy using SMV, TVR, or BOC + PR vs PR alone, in addition to the SMV + PR vs TVR + PR comparison mentioned aboveCitation20. Eight studies were carried out in treatment naïve patients and seven in treatment experienced. Using a Bayesian approach, the odds ratio for achieving SVR for each triple therapy regimen and population was estimated relative to PR. This odds ratio was then applied to the SVR for PR alone, in order to arrive at adjusted SVR values for each triple therapy.
The SVR estimates for SMV + PR were based on the Q80K negative population, reflecting its label recommendation. The efficacy of the BOC + PR RGT regimen was conservatively based on data from the fixed duration arm, showing a greater SVR rateCitation26,Citation27.
SVR data by treatment population and regimen are presented in .
Table 2. SVR12/24 results derived from the mixed treatment comparison.
Adverse events are a prominent feature of any PR-containing regimen, with the PI component contributing a lesser effect. The four adverse events most typically observed in clinical trials of triple therapy are anemia, neutropenia, pruritus, and rash. Data on the incidence of each of these events were collected and pooled within the MTC, in a similar fashion as was applied to the SVR rates, with the exception that, for SMV, data for the full study population are presented, regardless of Q80k status. Results of this analysis are presented in Supplementary Table 2.
Health state transitions
Transition probabilities reflecting advanced liver disease risks were sourced from the literature. The majority of results were drawn from a recent comprehensive systematic review of the economic literature in the field, reflecting values used in 34 economic models published between 2000–2011Citation29. Where data were lacking, they were sourced from individual published papersCitation23,Citation30. The model assumed time-independent disease transition probabilities, in line with current understanding that the length of time in a particular health state is not associated with the probability of disease progressionCitation31. Values used are listed in .
Table 3. Annual transition probabilities.
Utilities
On-treatment disutilities
The impact of drug-associated adverse events, exerting a significant effect on quality-of-life during antiviral treatment, was well studied in the PI trials using the EQ-5D valuation index. Treatment-specific utility decrements were calculated by comparing baseline (day 1) EQ-5D scores with the average scores captured during the period of treatment and applying UK preference tariffs to arrive at a utility estimate. Values from each study were pooled to arrive at a mean estimate—data for TVR and BOC were obtained from European HTA assessmentsCitation32,Citation33. Values for utility decrements are shown in .
Table 4. On-treatment and health state utilities.
Health-state utilities
Similar to recent UK health-economic analysis, utilities were derived from EQ-5D scores for patients in the UK Mild HCV trialCitation21. For health states pre- and post- liver transplant, values elicited in a UK transplantation study were usedCitation34. Utility improvements following SVR achievement at any Metavir stage (+0.05) was based on Hartwell et al.Citation23. A summary of the health state utility values used is presented in .
Costs
Antiviral treatment-related costs
Current UK list prices were used for all drug costs, based on the licensed doses, applied for the duration of therapy. Weekly costs for SMV and TVR are £1866.50 and for BOC £700. Two PR regimens are in common use: Peginterferon alfa-2a + Copegus® and Peginterferon alfa-2b + Rebetol®. Based on clinician advice, a mean cost of £218.57 per week for PR was estimated based on 82.5% of the former combination and 17.5% of the latter. Treatment monitoring costs were based on the 2003 resources utilization estimates and monitoring schedule used in a previously published economic analysisCitation22 Uprated to current values. Screening cost for the Q80K polymorphism in SMV patients was estimated at £150. The costs associated with the management of AEs (anemia, neutropenia, rash, and pruritus) were derived from a recent published budget impact analysis of BOC and TVR treatmentCitation35. Estimates were obtained by contacting pharmacies and by interviewing practising hepatologists and their clinical staff.
Health state costs
On-going annual costs associated with HCV infected patients in each health state were obtained from previously published economic analyses based on an observational costing study conducted in the UKCitation21. For consistency with previous analysesCitation23, SVR health-state costs after F0/F2 and F3 were applied only in the year following treatment. The SVR cost for patients with compensated cirrhosis (F4) is applied for 5 years (cost was assumed half of cirrhosis health state cost). Liver transplant associated costs were derived from a UK assessment of cost-effectivenessCitation36. presents an overview of all cost inputs. All costs were inflated to 2013 prices using the HCHS Pay and Prices indexCitation37.
Table 5. Treatment monitoring and adverse event costs.
Sensitivity analysis
Deterministic and probabilistic sensitivity analyses were carried out to determine the influence of uncertainty surrounding input parameters. All variables listed in and Supplementary Tables 2 and 3 were tested within univariate sensitivity analyses. Values for parameter estimates were varied within the uncertainty distributions that best reflected the nature and uncertainty of each specific parameter. The standard error was assumed to vary by ±20% in cases where uncertainty information on variance was lacking. Results were expressed as tornado diagrams, showing the 15 variables in each case that exerted the greatest effect on the resulting incremental cost-effectiveness ratio (ICER).
Table 6. Results of base case analyses (discounted).
In the multivariate probabilistic sensitivity analysis (PSA), 3000 simulations were processed to represent the uncertainty of model results by varying the parameters by random draws from their assumed distributions. For the relative SVR and AE estimates, 3000 simulations were directly extracted from WinBugs. Based on the simulations, a scatterplot and an acceptability curve were drawn to estimate the probability of SMV + PR being considered cost-effective against its comparator treatments across different willingness-to-pay (WTP) thresholds per QALY gained.
Two scenario analyses were also carried out to explore the effect of varying multiple variables simultaneously. To explore the impact on the results and to allow back-compatibility with previously published models (1) age, gender and Metavir profiles and (2) health state transition probabilities were matched to Hartwell et al.Citation23. Our decision not to use the Hartwell assumptions reflect the uncertain equivalence of the assumptions used in the baseline patient characteristics to currently treated UK populations, combined with the availability of more comprehensive and up-to-date data on health state transitions that are now availableCitation29.
Results
Base case analysis
Base case results are summarized in . SMV + PR was associated with an ICER of £9725/QALY vs PR alone in treatment-naïve patients and £7819/QALY in treatment experienced. SMV + PR was the dominant treatment option vs TVR + PR and BOC + PR in both naïve and experienced patients.
For the comparison vs PR alone, the QALY gains and long-term cost savings are driven by improvements in SVR, preventing the development of disabling and expensive advanced liver disease stages. Obviously, SMV + PR introduces additional PI costs compared to PR alone. Versus BOC + PR and TVR + PR, QALY gains of SMV + PR are also largely driven by improved SVR, albeit somewhat lower in magnitude than the increment seen vs PR alone. This is supplemented with a QALY gain attributable to a reduced on-treatment decrement, reflecting the shorter mean duration of treatment. The latter also underlies the cost advantage that SMV + PR has over the other two triple regimens. Differences in adverse event profiles between the regimens exerted a relatively small effect on the incremental costs, ranging from £279 for SMV + PR to £517 for TVR + PR, the difference being largely driven by anemia treatment costs.
The acquisition cost of SMV is identical to that of TVR (£266.64 per day). However, thanks to the shorter mean duration of PR therapy required with SMV compared with TVR—24.0 vs 36.8 weeks in treatment naïve and 38.4 vs 43.6 weeks in treatment experienced—the overall cost of treatment is lower with SMV: £2795 in treatment naïve and £1137 in treatment experienced.
Deterministic sensitivity analyses
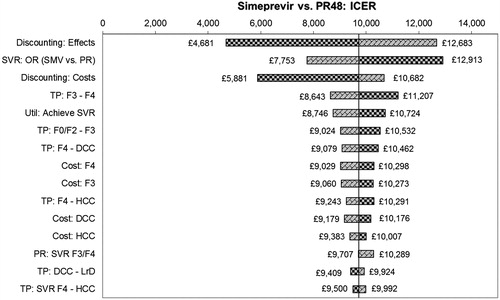
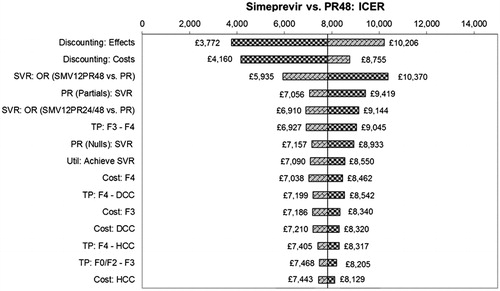
The deterministic univariate sensitivity analysis showed that incremental QALYs and costs are most sensitive to the percentage of patients achieving SVR for all treatments, discounting, the post-treatment transition probabilities and their associated healthcare costs, together with the utility associated with achieving SVR in both treatment naïve and experienced populations. For the comparison vs PR alone in treatment naive, the ICER remains below £12,913/QALY in all cases, while in treatment experienced all ICERs are less than £10,370. Tornado diagrams for the 15 most sensitive parameters are presented in and .
For the comparisons vs TVR, the ICER remains dominant in both patient groups for all variables except the SVR rate for SMV + PR; at the extreme lower end of the tested range (where the SVR rate for SMV + PR drops below that of TVR + PR), the ICER moves into the south-west quadrant. For the comparisons vs BOC, the ICER remains dominant in treatment naïve patients for all variables. For treatment experienced, at the extreme lower end of the tested range for SVR for SMV, the ICER moves into the south-west quadrant.
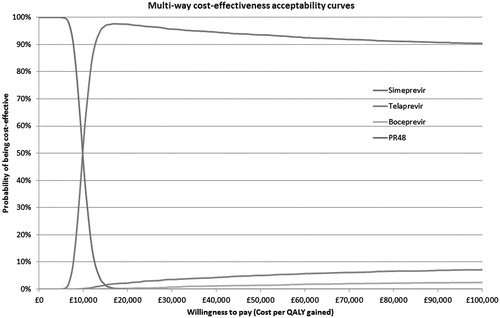
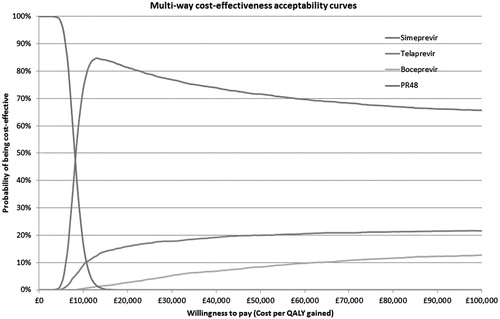
Probabilistic sensitivity analysis
Cost-effectiveness acceptability plots for both patient groups are presented in and . For treatment naïve patients, at a WTP threshold of £20,000/QALY, the probabilities that each treatment will be cost-effective are: SMV + PR = 97.4%; TVR + PR = 2.1%; BOC + PR = 0.4%; PR alone = 0.03%. At a WTP threshold of £30,000/QALY, the corresponding figures are 96.1%, 3.3%, 0.6%, and 0%, respectively.
For treatment experienced patients, at a WTP threshold of £20,000/QALY, the probabilities that each treatment will be cost-effective are: SMV + PR = 82.1%; TVR + PR = 15.0%; BOC + PR = 2.8%; PR alone = 0%. At a WTP threshold of £30,000/QALY, the corresponding figures are 77.0%, 17.6%, 5.4%, and 0%, respectively.
Scenario analyses
Applying age, gender, and Metavir profiles from Hartwell et al.Citation23 reduced the ICER vs PR alone from £9725/QALY to £8910/QALY for treatment naïve and increased from £7819 to £8099 for treatment experienced patients. Applying health state transition probabilities from Hartwell et al. increased the ICER vs PR alone to £12,695/QALY for treatment naïve and to £9694 for treatment experienced patients.
Discussion
This is the first published economic analysis of SMV + PR vs PR alone and the first to compare the cost-effectiveness of one PI-containing triple therapy regimen with other PIs. We have demonstrated that SMV + PR is a cost-effective treatment option when compared with PR alone, with ICERs of £9725/QALY and £7819/QALY in treatment naïve and treatment experienced populations, respectively. These conclusions are robust to a wide range of sensitivity analyses, with no scenario investigated yielding an ICER that exceeds the conventionally accepted WTP threshold of £20,000/QALY.
When compared with the TVR + PR and BOC + PR, SMV + PR is the dominant treatment option in both patient populations. At a willingness-to-pay threshold of £20,000, the probability of SMV + PR being cost-effective for treatment-naïve genotype 1 HCV patients is estimated at 97.4%; in treatment experienced patients the probability is 82.1%. The only circumstance explored in which SMV + PR does not dominate the other treatments is at the extreme lower end of the estimates of SVR derived from the indirect treatment comparison that underpins the model.
The results for SMV + PR vs PR alone are consistent with the published evidence base relating to BOC and TVR. A recently published systematic review identified 11 economic analyses evaluating triple therapy regimens containing one of these two PIs vs PR aloneCitation38. In the majority of patient sub-groups assessed within these analyses, treatment with one or other triple therapy regimen was found to be cost-effective vs PR alone at a WTP threshold of £20,000/QALY.
Strengths
A major strength of this economic evaluation is that the approach adopted is very similar to that used for previously published cost-effectiveness analyses of treatments for HCV, including models previously developed for comparators assessed in the current evaluation (TVR + PR, BOC + PR, and PR alone)Citation23,Citation32,Citation39. The results described in this analysis are derived using virtually identical methodology to these past evaluations and, therefore, the results can easily be compared to previous models.
The model fully complies with the 36 criteria listed in the critical appraisal tool recommended by NICE to assess the quality of economic analysesCitation40. The underlying structure reflects the natural history of the disease and many of the values used for the estimation of QALYs are identical to those used in previous appraisals. This approach has been developed over a number of years and is considered both robust and representative of the disease. Extensive sensitivity analyses and scenario analyses within each model demonstrate robustness of results about a wide range of values.
The robust Phase III clinical trial program underpins the evidence used in the economic evaluations and contributes evidence (both indirect and directly) to the MTC used for the calculation of point estimates of relative efficacy and safety for all treatment options. The head-to-head study for SMV/PR vs TPV/PR enhanced the reliability of the MTC results and confirmed the utility benefits that were calculated from the phase III studies vs PR alone.
Limitations
This model was explicitly designed to compare SMV + PR with other PR-containing regimens, on the basis that these are the treatment strategies most commonly in use and does not include any interferon-free regimens. In the past 12 months, regimens using sofosbuvir with either SMV or ribavirin alone have been licensed. By avoiding the use of peginterferon, which is a frequent cause of adverse events, it is expected that both patient acceptability and adherence will be improved. Sofosbuvir + R is currently not recommended by NICE for use in patients with genotype 1 HCV infectionCitation41, although they have yet to issue guidance on Sofosbuvir + SMV. This combination is the subject of a separate cost-utility model, that will be submitted for publication in due course.
Baseline patient characteristics are generally based on UK data, but at the time of developing the model information on the relative proportions of genotype 1a:1b and the percentage of 1a patients with the Q80K polymorphism were not available. The latter figure is important, as simeprevir is less effective in patients with the Q80K variant. Consequently, clinical trial-derived figures were used for these parameters. A recently published studyCitation42 looking at a European population of 3349 patients with HCV, has provided some confirmatory evidence for these estimates. The authors found a prevalence of 35.8% genotype 1a across Europe, somewhat greater than our estimate of 27.3%. However, substituting 35.8% into our model resulted in a 0.1% increase in the ICER, suggesting this is not a major driver of health economic outcomes.
The same paperCitation42 reports that 19.8% of European genotype 1a patients express the Q80K polymorphism, compared with the figure of 29.2% derived from our clinical trials. Based on the two prevalence figures, our model suggested an overall prevalence of Q80K in genotype 1 of 8.0% (29.2% × 17.3%), while the data from the new paper suggests 7.1% (19.8% × 35.8%). These results seem mutually consistent and, therefore, we are confident that the results of the analysis are likely to be applicable to a European population.
Despite the availability of one head-to-head study, the majority of clinical inputs to the model depended on the use of an indirect comparison. This is potentially a source of bias for the estimates of treatment effect, as, although the Bayesian approach adopted mitigated the effect of between-studies heterogeneity, a residual confounding effect cannot be excluded. Given that the SVR12 value for SMV + PR was the sole factor that qualitatively altered the results of the analysis—albeit only at the extremes of the 95% credibility intervals—this is an area of the model that should be refined as subsequent data becomes available.
The health state transition probabilities in the post-treatment phase are subject to some uncertainty. Although the majority of the values used were derived from a wide range of published models, many of these are based on populations being treated many years ago and may not reflect current disease patterns. Although scenario analysis suggested that using a substantially different range of transition probabilities did not materially alter the conclusions of the analysis, this is an area that future modelers would benefit from re-assessing.
The model did not incorporate the impact of futility rules. The dosage regimens for triple therapy allow for clinicians to stop treatment if there is no sign of benefit at an early assessment point—usually 4 weeks. This reduces overall drug expenditure and is, thus, an attractive element to include in an economic model. However, because of the way in which the clinical trials have been designed and reported, it was not possible to disaggregate this group of patients from the overall efficacy results, in order to assess their impact on SVR.
Finally, the impact of a reduction in onward HCV transmission within the population consequent on improved viral eradication has not been incorporated in this economic evaluation. This is a conservative omission, and likely under-estimates the QALY gain and wider societal benefit that may be observed following the introduction of SMV. Currently, the bulk of clinical trial data for the treatment of HCV focus on the eradication of HCV RNA over the first 1–2 years of treatment. There is an assumption that this eradication will yield sustained health benefits over the residual lifespan of these patients. The quality and quantity of data to support this assumption is currently limited. If future research can clarify this issue, incorporation of these data into the model would further enhance the analysis.
Conclusions
There is a large and growing burden of HCV infection in the UK. Given the relatively high costs of disease management, treatment decisions need to consider the cost-effectiveness of new therapy options. This study has demonstrated that the combination of SMV + PR is a superior treatment option to PR alone for all patient groups with G1 HCV infection, at an acceptable level of cost-effectiveness. At a WTP threshold of £20,000/QALY the probability that SMV + PR is cost-effective is 97.4% for treatment-naïve and 82.1% for treatment-experienced populations. This robust model demonstrates that the most cost-effective treatment option available is SMV + PR, regardless of prior treatment experience.
Transparency
Declaration of funding
The study was funded by Janssen EMEA.
Declaration of financial/other relationships
KW and MT received payment from Janssen EMEA to develop the economic model. AM is an employee of Janssen EMEA. KP was an employee of Janssen-Cilag UK at the time the work was carried out. IL is an employee of Janssen-Cilag UK. JB received payment from Janssen EMEA to write up the paper. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material.pdf
Download PDF (85.4 KB)Acknowledgments
The authors would like to acknowledge Karin Cerri , Ph.D., Janssen Pharmaceutica NV, Beerse, Belgium, for her input into the development of the core model.
References
- Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333-42
- Lavanchy D. The global burden of hepatitis C. Liver Int 2009;29(1 Suppl):74-81
- European Centres for Disease Prevention and Control. Hepatitis B and C in the EU neighbourhood: prevalence, burden of disease and screening policies. Stokholm 2010
- Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm#. Accessed March 27, 2015
- Thomson EC, Fleming VM, Main J, et al. Predicting spontaneous clearance of acute hepatitis C virus in a large cohort of HIV-1-infected men. Gut 2011;60:837-45
- Gerlach JT, Diepolder HM, Zachoval R, et al. Acute hepatitis C: high rate of both spontaneous and treatment induced viral clearance. Gastroenterology 2003;125:80-8
- Tong MJ, el-Farra NS, Reikes AR, et al. Clinical outcomes after transfusionassociated hepatitis C. New Engl J Med 1995;332:1463-6
- Tremolada F, Casarin C, Alberti A, et al. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol 1992;16:273-81
- Thein HH, Yi Q, Dore GJ, et al. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology 2008;48:418-31
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 2007;132:2557-76
- Ly KN, Xing J, Klevens RM, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med 2012;156:271-8
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-82
- World Health Organisation. Guidelines for the screening, care and treatment of persons with hepatitis C infection. Geneva: World Health Organisation, 2014
- Chou R, Hartung D, Rahman B, et al. Comparative effectiveness of antiviral treatment for hepatitis C virus infection in adults: a systematic review. Ann Intern Med 2013;158:114-23
- Fried M, Buti M, Dore G et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: the randomized PILLAR study. Hepatology 2013;58:1918-29
- Jacobson I, Dore G, Foster G, et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype 1 infection in treatment-naive patients: The phase 3, randomised, double-blind, placebo-controlled QUEST-1 trial. Lancet 2014;384:403-13
- Manns M, Marcellin P, Poordad F, et al. Simeprevir (TMC435) with peginterferon α-2a or α-2b/ribavirin for the treatment of chronic HCV genotype 1 infection in treatment-naive patients: QUEST-2, a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2014;384:414-26
- Zeuzem S, Berg T, Gane E, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology 2014;146:430-41
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with Peginterferon and Ribavirin leads to high rates of SVR in patients with HCV Genotype 1 who relapsed after previous therapy: a Phase 3 Trial. Gastroenterology 2014;146:1669-79
- Reddy K, Zeuzem S, Zoulim F, et al. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis 2015;15:27-35
- Wright M, Grieve R, Roberts J, et al. Health benefits of antiviral therapy for mild chronic hepatitis C: randomised controlled trial and economic evaluation. Health Technol Assess 2006;10:1-113
- Shepherd J, Jones J, Hartwell D, et al. Interferon alpha (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2007;11:1-205
- Hartwell D, Jones J, Baxter L, et al. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess 2011;15:1-210
- Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 1996;24:289-93
- European Medicines Agency. CHMP assessment report OLYSIO International non-proprietary name: SIMEPREVIR Procedure No. EMEA/H/C/002777/0000. London 2014
- Taieb V, Pacou M, Van Sanden S, et al. Mixed treatment comparison (MTC) to assess the relative efficacy and safety of simeprevir in combination with PEG-interferon alpha and ribavirin in treatment-naïve patients infected with hepatitis C virus (HCV) genotype 1. Poster presentation at AASLD/EASL Special Conference on Hepatitis C. New York, 12-13 September 2014
- Taieb V, Pacou M, Van Sanden S, et al. Mixed treatment comparison (MTC) to assess the relative efficacy and safety of simeprevir in combination with PEG-interferon alpha and ribavirin in treatment-experienced patients infected with hepatitis C virus (HCV) genotype 1. Poster presentation at AASLD/EASL Special Conference on Hepatitis C. New York, 12-13 September 2014
- Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology 2013;144:1450-5
- Townsend R, McEwan P, Kim R, et al. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health 2011;14:1068-77.
- Fernandez-Rodriguez CM, Alonso S, Martinez SM, et al. Peginterferon Plus Ribavirin and Sustained Virological Response in HCV-Related Cirrhosis: outcomes and factors predicting response. Am J Gastroenterol 2010;105:2164-72
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825-32
- National Institute for Health and Clinical Excellence (NICE). Final appraisal determination - Telaprevir for the treatment of genotype 1 chronic hepatitis C. London 2012
- Gemeinsamer Bundesausschuss (G-BA). MSD SHARP & DOHME GmbH. Dossier zur Nutzenbewertung. Modul 4. Boceprevir (VICTRELIS). Berlin 2011
- Ratcliffe J, Longworth L, Young T, et al. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver transplantation. 2002;8:263-70
- Thorlund K, Druyts E, El Khoury AC, et al. Budget impact analysis of boceprevir and telaprevir for the treatment of hepatitis C genotype 1 infection. Clinicoecon Outcomes Res 2012;4:349-5
- Longworth L, Young T, Buxton M. Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transplantation 2003;9:1295-307
- PSSRU. Unit costs of health and social care 2013. University of Kent, Canterbury, 2014
- San Miguel R, Gimeno-Ballester V, Mar J. Cost-effectiveness of protease inhibitor based regimens for chronic hepatitis C: a systematic review of published literature. Expert Rev Pharmacoecon Outcomes Res 2014;14:387-402
- National Institute for Health and Clinical Excellence (NICE). Final appraisal determination - Boceprevir for the treatment of genotype 1 chronic hepatitis C. London 2012
- Drummond M, Jefferson T. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83
- National Institute for Health and Clinical Excellence (NICE). TA330 Sofosbuvir for treating chronic hepatitis C. London 2015
- Sarrazin C, Lathouwers E, Peeters M, et al. Prevalence of the hepatitis C virus NS3 polymorphism Q80K in genotype 1 patients in the European region. Antiviral Res 2015;116:10-16