Abstract
Objective:
Data from the SINGLE trial demonstrated that 88% of treatment-naïve HIV-1 patients treated with dolutegravir and abacavir/lamivudine (DTG + ABC/3TC) achieved viral suppression at 48 weeks compared with 81% of patients treated with efavirenz/tenofovir disoproxil fumarate/emtricitabine (EFV/TDF/FTC). It is unclear how this difference in short-term efficacy impacts long-term cost-effectiveness of these regimens. This study sought to evaluate long-term cost-effectiveness of DTG + ABC/3TC vs EFV/TDF/FTC from a US payer perspective.
Methods:
This study is an individual discrete-event simulation which tracked the disease status and treatment pathway of HIV-1 patients. The model simulated treatment over a lifetime horizon by tracking change in patients’ CD4 count, clinical events occurrence (opportunistic infections, cancer, and cardiovascular events), treatment switch, and death. The model included up to four lines of treatment. Baseline patient characteristics, efficacy, and safety of DTG + ABC/3TC and EFV/TDF/FTC were informed by data from the SINGLE trial. The efficacy of subsequent treatment lines, clinical event risks, mortality, cost, and utility inputs were based on literature and expert opinion. Outcomes were lifetime discounted medical costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER).
Results:
Compared with EFV/TDF/FTC, DTG + ABC/3TC increased lifetime costs by $19,153 and per person survival by 0.12 QALYs, resulting in an ICER of $158,890/QALY. ICERs comparing DTG + ABC/3TC to EFV/TDF/FTC remained above the traditional, US willingness-to-pay threshold of $50,000/QALY gained in all scenarios, and above $100,000 or $150,000/QALY gained in most scenarios.
Limitations:
Due to data limitations, the treatment patterns, CD4 count during viral rebound and treatment switch, viral rebound after trial end, and long-term adverse event-related treatment discontinuation were based on assumptions, presented to and approved by clinical experts.
Conclusions:
Compared with EFV/TDF/FTC, DTG + ABC/3TC resulted in higher cost and only slightly increased QALYs over a lifetime, with an ICER that exceeded the standard cost-effectiveness threshold. This indicates that the incremental benefit in effectiveness associated with DTG + ABC/3TC may not be worth the incremental increase in costs.
Introduction
Human immunodeficiency virus (HIV) continues to be a public health concern in the US. By the end of 2011, roughly 1.2 million people in the US were living with HIV and another 50,000 become infected every year. HIV remains a significant cause of death in the US, with ∼13,712 deaths due to HIV in 2013Citation1,Citation2. However, HIV is much less acutely fatal than it was at the beginning of the HIV/AIDS (acquired immunodeficiency syndrome) epidemic; in the US, it is now managed more as a chronic condition through treatment with antiretroviral therapyCitation3. Because HIV patients live longer, the longer-term effects of the disease (such as non-AIDS-related cancers and cardiovascular disease), its treatment, and the associated life-long costs are increasingly important. Per-person, discounted, lifetime medical costs (including cost of medications and non-drug costs) were estimated to be $326,500 for a person infected with HIV at the age of 35Citation4. Given that Medicaid is estimated to cover half of all HIV patients in regular care in the USCitation5, containing these costs is a rising priority. Indeed, the US Department of Health and Human Services (DHHS) HIV guidelines include a recommendation that the cost of therapy be considered in prescribing decisions.
The main objective of long-term antiretroviral therapy is to maintain a stable, sufficiently high CD4 cell count to protect patients against opportunistic infections and malignancies. This is achieved through virologic suppression, demonstrated through an undetectable viral load. Atripla (EFV/TDF/FTC) is a first-in-class, multi-class drug that combines efavirenz (EFV, a non-nucleoside reverse transcriptase inhibitor [NNRTI]), tenofovir disoproxil fumarate (TDF, a nucleoside/nucleotide reverse transcriptase inhibitor [NRTI]), and emtricitabine (FTC; NRTI) in a single-tablet regimen, and was approved for the treatment of HIV by the US Food and Drug Administration (FDA) in July 2006Citation6. EFV/TDF/FTC is a DHHS guideline-recommended regimen for treatment-naïve patients with HIVCitation7.
Tivicay (dolutegravir [DTG]) is an integrase inhibitor that was approved for the treatment of HIV by the FDA in August 2013Citation8. In the Phase III SINGLE trial, 88% of patients treated with DTG in combination with abacavir (ABC) and lamivudine (3TC) achieved viral suppression by 48 weeks, compared to 81% of patients on EFV/TDF/FTC (p < 0.003), as assessed by the FDA snapshot algorithmCitation9. Patients on the DTG regimen also showed higher change from baseline CD4 count compared to patients treated with EFV/TDF/FTC at 48 weeks (267 cells/mm3 vs 208 cells/mm3, p < 0.001)Citation9. Results were similar at 96 weeks of follow-up, with 80% of patients on DTG + ABC/3TC achieving viral suppression vs 72% on EFV/TDF/FTC (p < 0.006)Citation10. Overall, the statistically higher responses on DTG + ABC/3TC vs EFV/TDF/FTC were driven by a greater number of withdrawals due to adverse events in the EFV/TDF/FTC arm (11% vs 3% with DTG + ABC/3TC), regardless of baseline viral load. The major adverse events leading to withdrawal in the EFV/TDF/FTC arm were psychiatric and nervous system disorders. DTG was combined with TDF/FTC or ABC/3TC in the SPRING-1 and SPRING-2 trials in treatment-naïve populations, with similar efficacy and safety results, indicating that the backbone with which DTG is combined has little impact on such outcomesCitation9–12. This was supported by a network meta-analysis that indirectly compared 48-week virologic suppression and CD4 change outcomes of guideline-recommended third agents in treatment-naïve, HIV-1-infected individualsCitation13.
Dolutegravir regimens, whether prescribed as DTG + ABC/3TC, DTG + TDF/FTC, or the recently approved single tablet DTG/ABC/3TC, are more costly than EFV/TDF/FTC. Given the increasing importance of cost containment and the relatively low cost of EFV/TDF/FTC, this study sought to evaluate the comparative cost-effectiveness of the DTG-based regimen (DTG + ABC/3TC in the base case and DTG + TDF/FTC in a scenario) vs the EFV/TDF/FTC single tablet regimen.
Patients and methods
Model approach
A model was developed in Microsoft Excel to simulate patients on treatment for HIV. This model was designed to capture the following disease-related events and values over a patient’s lifetime, in particular:
Virologic suppression events;
Treatment discontinuation and switch events (due to virologic failure, virologic rebound, adverse events, or other administrative reasons, including but not limited to loss of access to medication and medication contraindication);
CD4 count over time;
AIDS- and non-AIDS-related events; and
Cost and utility values.
Given the dynamic nature of CD4 count and the unpredictable timing of disease- and treatment-related events, a discrete-event simulation was selected as the optimal modeling approach, using time-to-event calculations to simulate patient experiences. Each patient followed a distinct treatment and disease pathway that was used to estimate cost and quality-of-life outcomes over a lifetime time horizon (assuming a maximum age of 100 years) from a US payer perspective. Future health and cost outcomes estimated by the model were discounted by 3%, which takes inflation into accountCitation14.
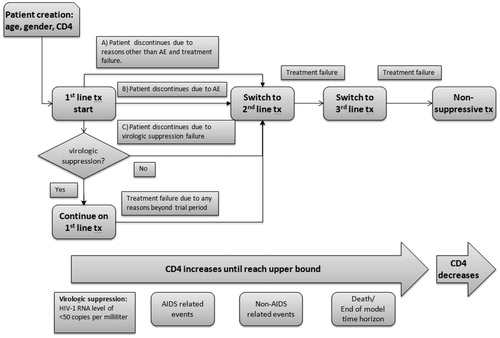
The target population considered in the model was based on the SINGLE trial population. Each patient was assigned a baseline age, gender, CD4 count, and a maximum CD4 count of 1000 cells/µL. The patient entered the model and began initial treatment with either EFV/TDF/FTC or a DTG-based regimen (DTG + ABC/3TC in the base case or DTG + TDF/FTC in a sensitivity scenario). Based on the outcomes of the SINGLE trial, a certain proportion of patients achieved virologic suppression in each arm and continued on their initial treatment. The remaining patients discontinued and switched to a subsequent line of treatment due to virologic failure, adverse events, or other reasons. For patients who achieved virologic suppression, first-line treatment was separated into a treatment initiation phase (48 weeks) and a maintenance phase (beyond 48 weeks). Patients who achieved virologic suppression within the 48-week initiation phase were subject to treatment discontinuation due to various reasons (e.g., loss of virologic suppression status [virologic rebound], adverse events, or other administrative reasons) in the following maintenance phase, all of which resulted in treatment switch. Treatment switches for patients on subsequent lines of treatment were assumed to follow a constant rate. The treatment status influenced the patients’ CD4 counts; this was tracked over the entire model time horizon using 3-month disease-monitoring schedules recommended by HIV clinical guidelinesCitation15 and was used to project the occurrence of clinical events (e.g., opportunistic infection and cancer) and death. presents the full model flow, which displays how the model tracks patients through three lines of active HIV therapy; patients who eventually fail a third line of treatment are placed on non-suppressive therapy, where they remain until death.
Comparator data
The model compared EFV/TDF/FTC with DTG + ABC/3TC in the base case and DTG + TDF/FTC in a scenario. Evidence suggests that the efficacy of DTG is similar, regardless of whether it is used in combination with TDF/FTC or ABC/3TCCitation13. Therefore, it was assumed that the efficacy of DTG + TDF/FTC was the same as DTG + ABC/3TC. For the trial period, the efficacy inputs were obtained from the SINGLE trial, which provided direct head-to-head comparison dataCitation9,Citation10.
Approach towards modeling efficacy
Treatment efficacy data were assigned separately for four specific treatment phases: 0–48 weeks of first-line treatment; 48–96 weeks of first-line treatment; first-line treatment beyond 96 weeks; and subsequent lines of treatment. The first two treatment phases were informed by the SINGLE trial results, and the following two treatment phases were estimated through assumptions based on long-term projections and previously published studies. The primary efficacy measures captured for each treatment phase in the economic model were the achievement of virologic suppression and CD4 count. presents the data inputs and sources for modeling treatment efficacy in all phases of treatment.
Table 1. Clinical inputs.
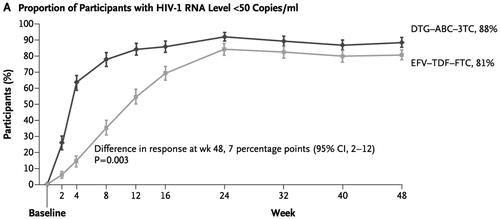
Time to virologic suppression on first-line treatment was modeled based on empirical Kaplan-Meier data from the SINGLE trial (). Patients on any line of treatment who failed to achieve virologic suppression or discontinued due to other reasons were assumed to discontinue their current treatment and immediately switch to a subsequent line of treatment. As a result, it was assumed that the CD4 count remained unchanged from treatment discontinuation to subsequent treatment initiation. For patients who had confirmed virologic failure, a proportion (71%Citation24) were assumed to achieve the same CD4 count increase as patients who did achieve suppression, while the remainder stayed at their baseline CD4 count. For patients who achieved virologic suppression, CD4 count was sampled at the viral suppression time, as well as at the 48- and 96-week trial end-points based on the reported mean (267 per mm3) and standard error (9.18 per mm3) of the adjusted change from baseline CD4 count during the SINGLE trial. During the simulation run, the CD4 count at each 3-month disease monitoring schedule was extrapolated with respect to the sampled points within the first 96 weeks. After that, a constant rate of change was assumed until the CD4 count upper bound was reached. For patients on subsequent lines of treatment, CD4 count was also assumed to follow a constant rate of change.
Figure 2. Time to virologic suppression on first-line treatmentCitation7.
Time to treatment discontinuation varied by reason for discontinuation. Adverse events that resulted in treatment discontinuation were assumed to occur within the first month after treatment initiation based on data from clinical trials and clinical expert comments. Patients who discontinued due to other reasons (e.g., who were censored in the trial) were assumed to switch treatment at time zero and would, therefore, incur no first-line treatment costs. For patients in either discontinuation group, the model assumed that no benefit in CD4 count was incurred for first-line treatment, due to the short treatment duration period. The rate at which virologic suppression was lost during the 48–96-week SINGLE trial period was used as a proxy for estimating treatment discontinuation during the treatment maintenance phase of the first-line treatment. A constant rate was extrapolated beyond the 96-week trial period.
The subsequent lines of treatment for both arms were assumed to be the same. According to suggestions from treatment guidelines and clinical experts, and since the HIV virus remains susceptible to protease inhibitors (PI) after treatment with both EFV/TDF/FTC and DTG based regimens, a protease inhibitor-based regimen (atazanavir/ritonavir + two NRTIs or darunavir/ritonavir + two NRTIs) was assigned as second-line therapy, and raltegravir + etravirine + darunavir/ritonavir + two NRTIs was assigned as third-line therapy, as DTG is reported to have limited cross-resistance to raltegravir in this patient populationCitation7. The annual probabilities of treatment switch for subsequent lines of treatment were estimated from the Kaplan-Meier data of patients whose treatment remained successful over time, as observed in a UK cohort study and other published studiesCitation17–20. The efficacy of subsequent-line treatment was obtained from pivotal trials (i.e., HIV STAR study, the TRIO trial) and the estimated annual CD4 slope of individuals who failed three antiretroviral-drug classesCitation17,Citation19. The non-suppressive treatment regimen was assumed to be the same as the third-line treatment, but, instead of leading to CD4 improvement, it resulted in a gradual decline of CD4 countCitation23. Patients were assumed to remain on non-suppressive treatment until death.
Patients were at risk for AIDS- and non-AIDS-related clinical events (including non-AIDS-related infection, non-AIDS-related cancer, and cardiovascular events) throughout the model. Patients were limited to experience up to two opportunistic infections, but could experience multiple cardiovascular (CV) events in their lifetime. The probability of CV events was impacted by treatment. As shown in , the probability of AIDS- and non-AIDS-related events was stratified by CD4 count and updated every 3 months.
Table 2. Risks of clinical, adverse, and mortality events.
The model captured mortality due to events (both acute and chronic) and background mortality. Time to death due to each clinical event was estimated upon occurrence of each event. To calculate the background mortality for the HIV population, death due to specific events was subtracted from the general population mortality, which was then adjusted for CD4 count categories. Deaths due to various reasons were handled as competing events in the model; however, all time-to-event and clinical measurements were updated in the model based on a quarterly time unit, given the 3-month disease monitoring schedule suggested by HIV clinical guidelines. All clinical assumptions were verified by clinical experts.
Approach towards modeling quality-of-life
Utility in the model was captured through a CD4 count-dependent baseline utility that was decremented according to clinical and adverse events (utility values presented in ). Utility values were updated yearly with age and CD4 count, and stratified by genderCitation29. A utility decrement of 0.033, reflecting the impact of grade 2–4 dizziness, the only adverse events reported with greater than 5% frequency in either arm, was applied to the 5% of patients who were receiving EFV/TDF/FTC and the 0.5% of patients who were receiving a DTG-based regimenCitation29. Treatment-specific utility decrements were not included in the base case, but were included in a sensitivity analysis to test the impact of a disutility associated with taking multiple tablets instead of a single tablet.
Table 3. Utility inputs.
Costs and resource use
The model included treatment-related costs, monitoring costs, and event costs, including adverse events, AIDS-related events, and non-AIDS-related events. Treatment adherence was not explicitly included in the model, as the SINGLE trial reported similar adherence between the two treatment arms. Patients on HIV treatment require frequent visits to healthcare providers and tests to monitor CD4 count and viral load. The cost of these visits and monitoring tests were modeled as recurrent, routine costs every 3 months. Cost inputs are presented in .
Table 4. Cost inputs.
Results
Base case results
Results of the base-case analysis are presented in , evaluating total lifetime discounted costs and health outcomes for each treatment arm, as well as incremental cost and health outcomes, which were calculated as the differences between the two treatment arms. Results suggest that, over a lifetime of treatment, health outcomes associated with EFV/TDF/FTC vs DTG-based regimens are very similar, with DTG-based regimens achieving only a 0.12 incremental quality-adjusted life-year (QALY) gain and a 0.16 life-year (LY) gain over treatment with EFV/TDF/FTC. Lifetime costs were $19,153 and $32,687 higher for patients initially treated with DTG + ABC/3TC and DTG + TDF/FTC, respectively, compared to patients initially treated with EFV/TDF/FTC. These findings resulted in base case incremental cost-effectiveness ratios (ICERs) of $158,890 and $272,389 comparing DTG + ABC/3TC and DTG + TDF/FTC, respectively, with EFV/TDF/FTC. While there is no formal willingness-to-pay threshold in the US, a recent study recommends considering a range of thresholds, using $100,000 or $150,000 as an ICER thresholdCitation31. Base case ICERs fall above even the higher threshold suggested. Drug costs comprised the majority of both regimens’ costs; costs associated with events and disease monitoring were relatively small and roughly equivalent across regimens.
Table 5. Base-case results.
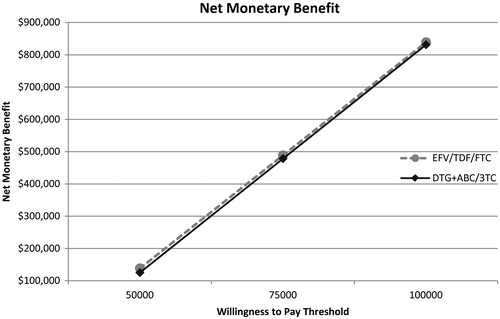
presents the base case results in the form of a net monetary benefit assessment. The two lines representing EFV/TDF/FTC and DTG + ABC/3TC are nearly parallel, indicating that the efficacy benefit of the two treatments is nearly identical. Furthermore, the EFV/TDF/FTC line remains slightly higher than DTG + ABC/3TC at different willingness-to-pay thresholds, indicating that it is marginally more cost-effective. This trend of results remains consistent across numerous sensitivity and scenario analyses designed to favor DTG/ABC/3TC, indicating the results are robust.
Figure 3. Base case results presented as net monetary benefit analysis. The two lines representing EFV/TDF/FTC and DTG + ABC/3TC are nearly parallel (indicating that the efficacy benefit of the two treatments is nearly identical), with the EFV/TDF/FTC line remaining very slightly higher, indicating it is marginally more cost-effective.
Sensitivity analysis
A series of one-way sensitivity analyses were conducted to test the effect of varying individual parameters on model results. The results () indicated that the ICER comparing DTG + ABC/3TC vs EFV/TDF/FTC remains high, regardless of variations in model parameters. The lowest ICER from a one-way sensitivity analysis occurred when the single tablet price of DTG/ABC/3TC was tested. The ICER resulting from this sensitivity analysis was $70,945. This scenario was included because DTG/ABC/3TC has a different price as a single tablet, but this was not used in the base case since the SINGLE trial data was based on a two-tablet regimen. The ICER comparing DTG + ABC/3TC vs EFV/TDF/FTC increased significantly in certain scenarios, including under shorter time horizons ($2,282,759/QALY over 5 years, and $2,071,933/QALY over 20 years of treatment), and when alternative assumptions were made regarding discontinuation due to adverse events.
Table 6. Results of one-way sensitivity scenarios (EFV/TDF/FTC vs DTG/ABC/3TC).
While adherence was not considered in the base case scenario, a sensitivity analysis was performed in which drug adherence was assumed to be 89%, based on EFV/TDF/FTC’s 144 week follow-up dataCitation32. A recent study showed no significant difference in likelihood of viral suppression for EFV/TDF/FTC patients with 85–89% adherence vs those with >95%Citation33. Therefore, adherence was modeled to impact drug costs, but efficacy inputs were not varied in this sensitivity test. The resulting ICER comparing DTG + ABC/3TC and EFV/TDF/FTC was $285,274, significantly higher than the base case result.
In the base case scenario, it was also assumed that patients who discontinue treatment immediately start their next line of treatment, which may not reflect real-world practice. A sensitivity analysis that assumed a 2-month waiting period between the discontinuation of the previous treatment and the initiation of the next line of treatment resulted in little impact on the ICER.
These base case and sensitivity analysis results were derived using the current price of EFV/TDF/FTC, which may or may not be reduced in the near future after the US patent for EFV expires. Such a price reduction would lead to incrementally higher ICERs comparing DTG + ABC/3TC to EFV/TDF/FTC across the board. A scenario in which EFV/TDF/FTC’s price was reduced by 20% was tested in order to evaluate the impact of this potential price change, resulting in an ICER of $394,331 (a 148% increase) comparing DTG + ABC/3TC to EFV/TDF/FTC.
Results of the probabilistic sensitivity analysis
A probabilistic sensitivity analysis (PSA) was conducted to evaluate the impact of varying cost and utility parameters simultaneously on the results. The PSA was run for 100 replications.
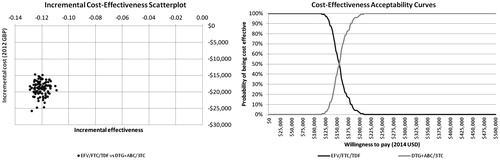
The resulting incremental-cost effectiveness scatter plot () shows a tight grouping of points, indicating that the results of the model are robust to variation in parameter values. The cloud of points sits decidedly in the third quadrant of the cost-effectiveness plane; this is consistent with the deterministic results, which indicate that EFV/TDF/FTC is consistently cost-saving with a slightly lower incremental health benefit. These findings are mirrored in the cost-effectiveness acceptability curve, which shows that EFV/TDF/FTC has a 100% probability of being more cost-effective than DTG/ABC/3TC at willingness-to-pay thresholds up to $120,000 per QALY gained and has superior cost-effectiveness up to a willingness-to-pay threshold of $155,000 per QALY gained.
Figure 4. Incremental cost-effectiveness scatterplot and cost-effectiveness acceptability curves. In the scatterplot, the tight grouping of points indicates model results are robust to variations in parameter values and that EFV/TDF/FTC is consistently cost-saving with a slightly lower incremental health benefit. This is mirrored in the cost-effectiveness acceptability curve, which shows EFV/TDF/FTC to have a 100% probability of being cost-effective compared to DTG + ABC/3TC at accepted willingness-to-pay thresholds.
Discussion
EFV/TDF/FTC was the first approved single-tablet regimen for HIV and has been extensively studied in treatment-naïve patients. DTG is a newly approved agent that is reported to have superior efficacy to EFV/TDF/FTC when taken in combination with ABC/3TCCitation9,Citation10. DTG was also added to the guidelines as a part of a recommended regimen for treatment-naïve patients, either with ABC/3TC or TDF/FTC. Given the life-long nature of HIV and its treatment, and the high associated economic burden, decision-makers must increasingly consider questions of cost and value for money. Base case results indicate that the incremental benefit in efficacy demonstrated by DTG + ABC/3TC in the SINGLE trial translates into minimal long-term health gains, amounting to 1.92 months of survival and 1.44 quality-adjusted months of life over a lifetime. The high ICER when comparing DTG + ABC/3TC to EFV/TDF/FTC indicates that DTG + ABC/3TC is not a cost-effective treatment option, or, in other words, that the small incremental benefit it provides is not justified by the higher cost of treatment.
The SINGLE trial’s primary efficacy end-point was viral suppression as measured by the FDA snapshot algorithm, which treats patients who discontinue treatment, switch treatment, or who have missing data as virologic failures. By this measure, the difference in efficacy between EFV/TDF/FTC and DTG + ABC/3TC in the SINGLE trial was primarily due to the difference in discontinuations due to adverse events between the two arms. Ten percent of patients treated with EFV/TDF/FTC compared to only 2% of patients treated with DTG + ABC/3TC discontinued due to adverse events, whereas discontinuation due to lack of efficacy was the same (2%) in both trial armsCitation9,Citation10. Such high rates of withdrawals due to adverse events have not been documented in other EFV/TDF/FTC trialsCitation16,Citation34. Furthermore, the top four adverse events that led to discontinuation in the SINGLE trial are included in EFV/TDF/FTC’s label, so prescribing physicians would be aware of such events and may not subscribe EFV/TDF/FTC to patients at risk. In light of these factors, a scenario was run that assumed equal (2.4%) discontinuation due to all adverse events from both DTG + ABC/3TC and EFV/TDF/FTC. This resulted in an incremental QALY gain of 0.03 for DTG + ABC/3TC and an ICER of $757,706/QALY.
The biggest drivers of model results were shorter time horizon and alternative assumptions regarding discontinuation due to adverse events. A 5-year time horizon increased the ICER for DTG/ABC/3TC vs EFV/TDF/FTC 14-fold, which could be relevant to US commercial insurance payers who often consider shorter time horizons. The assumption that discontinuation due to AEs was zero in both treatment arms increased the ICER 6-fold. Using the single tablet pricing of DTG/ABC/3TC led to the greatest reduction in the ICER for DTG/ABC/3TC vs EFV/TDF/FTC, with a reduction of 53%.
Interpretation of model results relies on willingness-to-pay thresholds, which do not formally exist in the US. A threshold of $50,000/QALY gained has been used historically; a recent study suggested considering a range of different thresholds, ultimately recommending either $100,000 or $150,000/QALY gainedCitation31. However, this new recommendation includes the caveat that resource constraints and opportunity costs should be considered when determining an ICER threshold. Given the current trend towards cost containment generally in the US and the fact that roughly half of HIV patients in the US are covered by Medicaid, an upper limit of $100,000/QALY gained is perhaps a more appropriate metric to determine value-for-money in the context of HIV treatment.
Results of the model were validated in several ways. It was confirmed that the CD4 count distribution and percentage of patients with viral suppression matched those reported in the SINGLE trial for EFV/TDF/FTC and DTG + ABC/3TC at 48 and 96 weeks, serving as an internal validation that clinical trial results can be duplicated by the simulation. Furthermore, the model estimated a life expectancy of 80 years for patients who maintained a high CD4 count and responded to treatment, which aligns with a studyCitation35 that reported that HIV patients whose viral load is well controlled can have life expectancy similar to that of the general population. No published studies were identified that directly evaluated the cost-effectiveness of EFV/TDF/FTC vs DTG regimens, or that compared the cost-effectiveness of DTG to any other HIV regimen. However, the LY and QALY gains projected by the current model were very similar to those of other evaluations of EFV/TDF/FTC. A 2013 cost-effectiveness evaluation of EFV/TDF/FTC by Juday et al.Citation36 reported a 16.83 gain in LYs and a 14.96 gain in QALYs across a lifetime time horizon. Similarly, Brogan et al.Citation37 reported a 17.22 LY gain and 15.75 QALY gain for patients treated with EFV/TDF/FTC over a lifetime time horizon. These findings are similar to the results of the current model.
This economic analysis has a number of strengths. The discrete event simulation approach provides flexibility and transparency over the alternative Markov approach and is a more intuitive framework for clinical stakeholders, as it more closely simulates the course of disease and treatment patterns. Discrete event simulation is able to simulate continuous changes in disease and treatment status, and allows for tracking of disease history and treatment history for each individual patient. The treatment switching of patients with differing viral suppression status could be modeled separately using this tracking feature. The model structure also provides flexibility to allow for various long-term projection assumptions. Multiple clinical events related to the disease (e.g., AIDS-related events, cancer, and non-AIDS-related infection) and related to the treatment (e.g., cardiovascular disease) were included in the model to capture all aspects of consequences of treatment effect.
This analysis, however, is not without limitations. Because of the lack of patient-level data and any long-term data on clinical trial outcomes, the model relied on several assumptions, which can be limiting or introduce uncertainty. Areas that are not well understood are the behavior of the CD4 count during viral rebound and treatment switch, the likelihood of rebound during long-term maintenance therapy, and long-term treatment discontinuation due to adverse events. To ensure the model was based on clinically appropriate assumptions for these parameters, modeling assumptions were presented to and approved by clinical experts. Treatment adherence was not explicitly included in the model, despite being a factor that can impact treatment efficacy and viral resistance. Adherence was excluded primarily because the available data is based on randomized-controlled trials (which generally do not accurately capture adherence behavior) and because there is a general lack of real-world data to inform long-term adherence patterns. A sensitivity analysis in which an 89% adherence rate was applied to drug costs resulted in a much higher ICER compared to baseline (). Furthermore, the analysis was limited by the patient population of the SINGLE trial from which model inputs were derived. The SINGLE trial population may not reflect the general population of people living with HIV/AIDS in the US, especially those covered by Medicaid to whom cost containment issues may be most relevant. However, the incremental differences in efficacy and safety between the regimens tested, which drive results of the economic analyses, are likely to be generalizable to other populations. In an effort to make the analysis more relevant to the Medicaid perspective, a scenario was tested in which drug costs were discounted by 20%, resulting in similar conclusions to the base case (). In reality, Medicaid rebate pricing is dependent on the number of years a drug has been on the market. Therefore, a 20% discount is conservative for EFV/TDF/FTC relative to DTG which has only recently entered the US market. Finally, the model simulated treatment patterns following first-line treatment with either EFV/TDF/FTC or DTG in combination with two likely backbones. However, in real-world clinical practice, other regimens may be used in naïve patients and differing agents may be used for second and third line as compared to what was assumed in the model.
Conclusion
Given persistently high ICERs associated with DTG-based regimens in the base case and across all sensitivity scenarios, it could not be demonstrated that DTG’s efficacy benefit shown in the SINGLE trial is worth the higher cost of treatment. From a net benefit perspective, EFV/TDF/FTC offers marginally greater benefit over a range of willingness-to-pay thresholds. These cost considerations are significant, given the life-long nature of HIV and its treatment, and the increasing attention to cost of care in the US.
Transparency
Declaration of funding
Funding for this study was provided by Bristol-Myers Squibb Co.
Declaration of financial/other relationships
Taffazoli, Peng, Dorman, and Sorensen were employees of Evidera, which provides consulting and other research services to pharmaceutical, device, government, and non-government organizations. In their salaried positions, they work with a variety of companies and organizations, and are precluded from receiving any payment or honoraria directly from these organizations for services rendered. Evidera received funding from Bristol-Myers Squibb Co. for this study. Rosenblatt and Villasis-Keever were employees of Bristol-Myers Squibb Co.
Acknowledgments
The authors are grateful for the expert opinion provided by Daniel Seekins and the methodologic review provided by Josephine Mauskopf.
Notes
†Atripla is a registered trademark of Bristol-Myers Squibb & Gilead Sciences, LLC (New York, NY, USA).
‡Tivicay is a registered trademark of ViiV Healthcare (Brentford, Greater London, UK).
References
- Centers for Disease Control and Prevention (CDC). Monitoring selected National HIV prevention and care objectives by using HIV Surveillance Data—United States and 6 Dependent Areas—2011. HIV Surveillance Supplemental Report 2013;18(No. 5). Atlanta, GA: CDC, 2013. http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed April 15, 2015
- Centers for Disease Control and Prevention (CDC). Diagnoses of HIV Infection in the United States and dependent areas, 2013. HIV Surveillance Report, 2013; vol. 25. Atlanta, GA: CDC, 2015. http://www.cdc.gov/hiv/library/reports/surveillance/. Accessed April 15, 2015
- Centers for Disease Control and Prevention (CDC). CDC fact sheet: new HIV infections in the US 2007-2010. Atlanta, GA: CDC, 2013. http://www.cdc.gov/hiv/basics/statistics.html. Accessed August 9, 2013
- Schackman BR, Fleishman JA, Su AE, et al. The lifetime medical cost savings from preventing HIV in the United States. Med Care 2015;53:293-301
- The Henry J. Kaiser Family Foundation. Quick Take: an update on the ACA & HIV: Medicaid Health Homes. Menlo Park, CA, 2012. http://kff.org/health-reform/fact-sheet/quick-take-an-update-on-the-aca/. Accessed June 2, 2014
- FDA. FDA approval of Atripla, 3-drug fixed dose combination antiretroviral. Silver Spring, MD: US Food and Drug Administration, 2006. http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm124444.htm. Accessed August 20, 2013
- Department of Health and Human Services. Guidelines for the use of Antiretroviral Agents in HIV-1-Infected adults and adolescents: Panel on Antiretroviral Guidelines for Adults and Adolescents. Rockville, MD: Department of Health and Human Services, 2014. http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Accessed June 2, 2014
- FDA. FDA approves new drug (Tivicay [dolutegravir]) to treat HIV infection. Silver Spring, MD: US Food and Drug Administration, 2013. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm364744.htm. Accessed February 20, 2014
- Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med 2013;369:1807-18
- Walmsey S, Berenguer J, Khuong-Josses M, et al. Dolutegravir regimen statistically superior to efavirenz/tenofovir/emtricitabine: 96-week results from the SINGLE Study (ING114467) Conference on Retroviruses and Opportunistic Infections (CROI) 2014. Boston, MA; 2014
- Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naïve adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet 2013;381:735-43
- Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naïve adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis 2013;13:927-35
- Patel DA, Snedecor SJ, Tang WY, et al. 48-week efficacy of dolutegravir relative to commonly used 3rd agents in treatment-naïve HIV-1-Infected patients: a systematic review and network meta-analysis. 14th European AIDS Conference Brussels, Belgium 2013
- Gold M, Siegel J, Russell L, et al. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press, 1996
- Gunthard HF, Aberg JA, Eron JJ, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA Panel. JAMA 2014;312:410-25
- Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naïve HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr 2013;63:77-85
- Jansen K, Sonnerborg A, Brockmeyer N, et al. Long-term efficacy and safety of atazanavir/ritonavir treatment in a real-life cohort of treatment-experienced patients with HIV type 1 infection. AIDS Res Hum Retroviruses 2013;29:564-73
- Svedhem-Johansson V, Pugliese P, Brockmeyer NH, et al. Long-term gender-based outcomes for atazanavir/ritonavir (ATV/r)- containing regimens in treatment-experienced patients with HIV. Curr HIV Res 2013;11:333-41
- Arasteh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther 2009;14:859-64
- Banhegyi D, Katlama C, da Cunha CA, et al. Week 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment-experienced patients in TITAN. Curr HIV Res 2012;10:171-81
- Beck EJ, Mandalia S, Youle M, et al. Treatment outcome and cost-effectiveness of different highly active antiretroviral therapy regimens in the UK (1996–2002). Int J STD AIDS 2008;19:297-304
- Fagard C, Colin C, Charpentier C, et al. Long-term efficacy and safety of raltegravir, etravirine, and darunavir/ritonavir in treatment-experienced patients: week 96 results from the ANRS 139 TRIO trial. J Acquir Immune Defic Syndr 2012;59:489-93
- Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet 2004;364:51-62
- Anude CJ, Eze E, Onyegbutulem HC, et al. Immuno-virologic outcomes and immuno-virologic discordance among adults alive and on anti-retroviral therapy at 12 months in Nigeria. BMC Infect Dis 2013;13:113
- Simpson KN, Pei PP, Moller J, et al. Lopinavir/ritonavir versus darunavir plus ritonavir for HIV infection: a cost-effectiveness analysis for the United States. Pharmacoeconomics 2013;31:427-44
- Choi AI, Vittinghoff E, Deeks SG, et al. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS 2011;25:1289-98
- Rodger AJ, Lodwick R, Schechter M, et al. Mortality in well controlled HIV in the continuous antiretroviral therapy arms of the SMART and ESPRIT trials compared with the general population. AIDS 2013;27:973-9
- Marin B, Thiebaut R, Bucher HC, et al. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 2009;23:1743-53
- Kauf TL, Roskell N, Shearer A, et al. A predictive model of health state utilities for HIV patients in the modern era of highly active antiretroviral therapy. Value Health 2008;11:1144-53
- Truven Health Analytics. RED BOOK Online® via MicroMedex Gateway. New York, NY: Truven Health Analytics, 2013. http://www.micromedexsolutions.com/micromedex2/librarian/ND_T/evidencexpert/ND_PR/evidencexpert/CS/D1626C/ND_AppProduct/evidencexpert/DUPLICATIONSHIELDSYNC/86DBB2/ND_PG/evidencexpert/ND_B/evidencexpert/ND_P/evidencexpert/PFActionId/pf.Logout. Accessed February 20, 2014
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness–the curious resilience of the $50,000-per-QALY threshold. N Engl J Med 2014;371:796-7
- Arribas JR, Pozniak AL, Gallant JE, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz compared with zidovudine/lamivudine and efavirenz in treatment-naive patients: 144-week analysis. J Acquir Immune Defic Syndr 2008;47:74-8
- Viswanathan S, Justice AC, Alexander GC, et al. Adherence and HIV RNA suppression in the current Era of Highly Active Antiretroviral Therapy (HAART). J Acquir Immune Defic Syndr 2015;69:493-8
- Zolopa A, Sax PE, DeJesus E, et al. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J Acquir Immune Defic Syndr 2013;63:96-100
- Samji H, Cescon A, Hogg mail RS, et al. Closing the Gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355
- Juday T, Correll T, Anene A, et al. Cost-effectiveness of the once-daily efavirenz/emtricitabine/tenofovir tablet compared with the once-daily elvitegravir/cobicistat/emtricitabine/tenofovir tablet as first-line antiretroviral therapy in HIV-infected adults in the US. Clinicoecon Outcomes Res 2013;5:437-45
- Brogan AJ, Talbird SE, Cohen C. Cost-effectiveness of nucleoside reverse transcriptase inhibitor pairs in efavirenz-based regimens for treatment-naive adults with HIV infection in the United States. Value Health 2011;14:657-64