Abstract
Objective:
To conduct a network meta-analysis (NMA) to assess the relative efficacy and safety of simeprevir, a second generation oral protease inhibitor (PI), compared to telaprevir and boceprevir in combination with pegylated interferon-α and ribavirin (PR) in patients with chronic hepatitis C.
Methods:
A systematic literature review and NMA of randomized controlled trials involving anti-virals added to PR were conducted. Electronic database searches and hand searches were conducted to identify relevant publications. Outcomes of interest included sustained virologic response (SVR), incidence of adverse events (AEs), and discontinuation due to AEs. Networks were based on treatment-, dose-, and duration-specific nodes. Sub-group analyses were conducted to investigate heterogeneity, based on Metavir scores, sub-genotypes 1a/1b, and prior response.
Results:
A total of 15 publications were considered for the base case of the meta-analysis. Simeprevir was associated with higher SVR rates than PR alone. Compared to telaprevir and boceprevir, SVR rates tended to be higher for simeprevir, with odds ratios ranging from 1.27 [0.81–2.00] to 2.61 [1.44–4.74] in treatment-naïve and from 1.04 [0.78–1.38] to 1.74 [0.84–3.61] in treatment-experienced patients, respectively. In terms of safety, the risks of anemia and discontinuations due to AEs were lower for simeprevir compared to PR alone, telaprevir, and boceprevir. The risk of rash was lower for simeprevir compared to telaprevir, and similar compared to PR alone and boceprevir.
Conclusion:
This NMA in genotype 1 HCV patients suggests a similar or better efficacy and tolerability profile for simeprevir compared to telaprevir and boceprevir.
Introduction
The hepatitis C virus (HCV) is an enveloped, single-stranded ribonucleic acid virus that is primarily transmitted through percutaneous or mucous membrane exposure to contaminated blood and body fluidsCitation1. Infection with HCV is a common cause of chronic liver disease, with 130–150 million people chronically infected with HCV worldwideCitation2–4. According to the European Centre for Disease Prevention and Control, ∼30% of those chronically infected with HCV will develop cirrhosis, which can then progress to decompensated liver disease and hepatocellular carcinomaCitation4. Other sources estimate the risk of developing cirrhosis within 20 years to be ∼10–20%, with some studies showing estimates up to 50%Citation5–8. The objective of therapy in those infected with HCV is to cure patients from disease. Cure of infection is defined as achieving a ‘sustained virologic response’ (SVR)—undetectable HCV RNA in the peripheral blood (i.e., HCV RNA < 25 IU/ml) typically at 12 (SVR12) or 24 (SVR24) weeks after the end of treatmentCitation2. The high concordance of SVR rates at 12 and 24 weeks has been previously reported in the literatureCitation9. Until recently, treatment of chronic HCV was based on the combination of a pegylated interferon-α (peginterferon alfa) and ribavirinCitation10–12. There are six main genotypes of HCV which affect treatment duration and dose of ribavirin. Peginterferon alfa and ribavirin combination therapy (PR) have been reported to cure ∼80% of infections in patients infected with HCV genotypes 2 (G2) or 3 (G3), but only 40–50% in patients infected with genotypes 1 (G1) or 4 (G4)Citation10–12. In addition to the low SVR rates in G1 and G4 patients, therapy is further limited by the long duration of treatment and adverse events (such as fatigue, flu-like symptoms, anemia, and rash). Direct-acting HCV antivirals were developed to improve efficacy and shorten treatment duration in specific HCV genotypes.
In 2011 the approval of the first-generation NS3/4A protease inhibitors (PI) telaprevir and boceprevir in combination with PR, allowed for shorter treatment regimens in G1 patients with higher SVR rates. However, the side-effect profiles of these triple combination therapies (among them rash, pruritus, anemia, and fatigue) were not considered favorableCitation13.
Simeprevir is a second generation oral PI and is indicated in combination with other medicinal products for the treatment of chronic hepatitis C in adult patientsCitation14. Q80K is a naturally occurring NS3 polymorphism that confers low-level resistance to simeprevir. Simeprevir + PR has been shown to have reduced efficacy in patients with HCV genotype 1a and Q80K baseline polymorphism. Therefore, Q80K polymorphism testing in patients with HCV genotype 1a is strongly recommended when considering simeprevir therapyCitation14.
Five randomized controlled trials compared simeprevir + PR with PR alone in patients with genotype 1 HCV infection who were treatment-naïve or had either failed to respond or experienced a relapse following initial PR therapyCitation15–19. These studies demonstrated significant superiority of simeprevir + PR over PR alone in terms of antiviral efficacy. One studyCitation20 compared simeprevir + PR with telaprevir + PR in patients who had previously not responded to first line PR alone, and showed non-inferiority vs telaprevir + PR with an improved tolerability profile. Different to older triple therapy which requires treatment durations for naïve and prior relapsed patients between 24–48 weeks, treatment duration with simeprevir is shortened to a fixed 24-week period, 12 weeks of simeprevir + PR followed by 12 weeks with PR alone. With its improved tolerability and shorter treatment duration for naïve and prior relapsed patients, simeprevir has the potential to advance treatment over older triple therapy with PI. In addition, simeprevir can be given in combination with sofosbuvirCitation14, offering a highly efficacious interferon-free treatment optionCitation13.
Methods
Study question and search strategy
In many healthcare systems a requirement to obtain funding for a new treatment is to demonstrate the clinical (and cost) effectiveness vs already established therapies. Due to the limited availability of head-to-head studies, an indirect comparison in the form of a network meta-analysis (NMA) was conducted to assess the relative efficacy and safety of simeprevir + PR vs other treatments for HCV genotype 1.
A systematic literature review was conducted to retrieve all relevant clinical trials available from the literature until November 2013. The following databases were searched: MEDLINE, MEDLINE-IN-PROCESS, EMBASE, and the Cochrane Library. The search strategy was divided into three components: the study type (restricted to clinical trials), the disease, and the interventions of interest. The search was restricted to English publications. Major conference proceedings in the field of HCV were searched (i.e., American Association for the Study of Liver Diseases, British Association for the Study of the Liver, Conference on Retroviruses and Opportunistic Infections, Digestive Disease Week, European AIDS Clinical Society, and European Association for the Study of the Liver). In addition, the clinical study reports related to simeprevir triple therapy were reviewed. These included: PILLAR, PROMISE, QUEST1, QUEST2, ASPIRE, and ATTAINCitation15–20.
Study selection
The pre-specified inclusion/exclusion criteria for the NMA included randomized clinical trials which reported results for adults with chronic HCV G1. Treatments of interest included triple therapy: simeprevir, telaprevir, or boceprevir (with PR); or a dual therapy including PR. At least one of the following outcomes had to be reported: SVR at 12 or 24 weeks, adverse events (AEs) of specific interest (anemia, neutropenia, pruritus, and rash), or discontinuation. Studies were also required to report results by prior treatment experience (treatment naïve or treatment experienced). Randomized controlled trials included mono-infected HCV patients with G1 as no RCTs assessing simeprevir in a HIV co-infected cohort were identified. Clinical trials or trial arms assessing PR treatment durations or doses not licensed in Europe were also excluded. This resulted in the exclusion of some study arms from PILLAR and studies in Japanese patients. A separate NMA of Japanese studies with simeprevir and boceprevir has previously been publishedCitation21. The addition of newer agents such as sofosbuvir to the NMA was endeavored through a systematic literature review update (August 2014); however, no randomized controlled studies were identified that met the study inclusion criteria.
According to systematic review guidelinesCitation22, screening was conducted in two phases. The first phase involved the screening of titles and abstracts, and the second required articles to be reviewed in full for articles relevant to the study question. A quality check of the screening was conducted by a second reviewer and any discrepancy resolved by discussion.
Data extraction and quality assessment
Data were extracted based on the study question. For each eligible clinical trial, the extracted data included fields such as publication details, study design, study population, and baseline characteristics (e.g., age, gender, Metavir score, and viral load). Key efficacy outcomes were SVR at 12 or 24 weeks (depending on the primary end-point or the trial). Safety outcomes included AEs of specific interest (anemia, neutropenia, pruritus, and rash), overall discontinuation, and discontinuation due to AEs. Clinical trials were critically appraised according to the quality assessment tool recommended by NICECitation23. Extracted data were checked by a second reviewerCitation22,Citation23.
Statistical analyses
A Bayesian NMA was conducted to combine direct and indirect evidence while preserving the randomization of each trial, in line with guidelines from ISPOR and NICECitation23–25. The NMA was based on a logit model for binomial data. Non-informative prior distributions were used to produce results driven by the data (normal distributions with a mean of 0 and a variance of 10,000 for treatments effects and intercepts and a uniform distribution for the between-trial standard deviation, with a range of 0–2). The selection of using a fixed or random effects model was based on the Deviance Information Criterion (DIC), which measures the relative goodness of fit between models. The model with the smallest DIC was considered as the model with the best compromise between adequacy and complexityCitation26.
Separate NMAs were conducted in treatment-naïve and treatment-experienced patients. Networks were based on treatment-, dose-, and duration-specific nodes for all treatments, except peginterferon alfa 2a/2b, for which a class effect was assumed in the base case analysis based on clinical expert opinion (this assumption was subsequently assessed in sensitivity analyses). Q80K-positive patients were excluded from simeprevir arms for the analysis of SVR rates in line with EMA label considerations for simeprevir. Q80K-positive patients were included in other treatment arms as Q80K-status is not known as a treatment-effect modifier for other HCV treatments. For the analyses of safety outcomes, the full ITT population was considered in all treatment arms, as there is no clinical rationale to justify why Q80K status would have an impact on the safety of simeprevir.
Outputs included the odds ratio and corresponding 95% Credible Interval (95% CrI), the probability for simeprevir to perform better than each comparator (P), and the ranking of treatments based on the Surface Under the Cumulative Ranking (SUCRA)Citation27. SUCRA values range between 0–100%, where 100% indicates that an intervention is certainly the best, while an intervention that is certainly the worst would have a SUCRA value of 0%. Results were interpreted as follows: if the probability of performing better for treatment A compared to treatment B was higher than 85%, then A was assessed as better than B. If this probability was between 15–85%, A and B were reported as similar, and if the probability was lower than 15% then B was described as better than ACitation28. It is important to note that the reported probability values (e.g., the probability that one treatment has a higher proportion of patients achieving SVR than the other) have a different interpretation than classical p values, and credible intervals should not be interpreted as a test statistic (confidence interval)Citation29.
The statistical analyses were conducted in WinBUGS version 1.4 using MCMC (Markov chain Monte Carlo) simulations and SAS version 9.3. Three chains were simulated and 20,000 iterations were used as burn-in followed by 20,000 iterations to monitor the parameters for fixed effects model, and 100,000 iterations as burn-in and 100,000 to monitor the parameters for random effects models. Studies causing heterogeneity (identified through the I2 statistic) were identified statistically, as well as studies causing inconsistencies (identified through the comparison of direct and indirect evidence within each closed loop of the network)Citation30. Sub-group analyses were conducted to investigate heterogeneity, based on Metavir scores, sub-genotypes 1a/1b, and prior response status (i.e., relapsers, null, and partial responders). Sensitivity analyses excluding trials which were sources of heterogeneity and trials of lower quality were conducted. A sensitivity analysis assuming no class effect for peginterferon alfa 2a/2b was also conducted as well as a sensitivity analysis focusing on SVR at 24 weeks for all studies.
Results
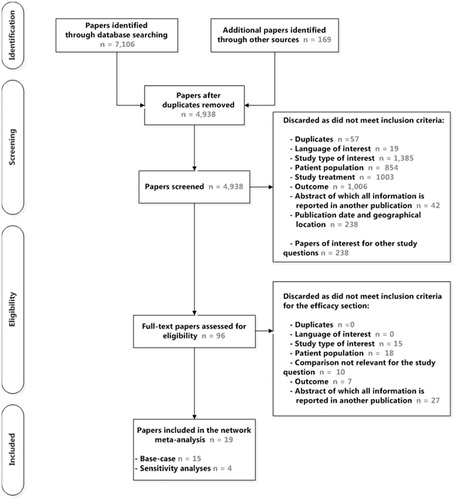
As shown in , a total of 15 publications were considered for the base case meta-analysisCitation15–20,Citation31–39. The studies were of good quality, with the possible exception of one study which was open-label. This study was excluded in a sensitivity analysis. Four additional studies were included in sensitivity analyses (three studies comparing peginterferon alfa 2a vs 2b and one study assessing telaprevir in combination with ribavirin and with peginterferon alfa 2a or with peginterferon alfa 2bCitation40–43).
Based on the reduced number of trials included in the analysis and on fit criterion (differences in DIC associated with the fixed and random effects model lower than five points for all end-points), a fixed effects model was appropriate for the analysis of all end-points. Results based on the random effects model are available in Supplementary material.
Treatment-naïve patients
Eight trials focused on treatment-naïve individuals. Separate treatment nodes were defined for different treatment regimens (). The network of evidence was linked through PR alone for 48 weeks (PR48) as it was the comparator arm used in all trials. Three telaprevir nodes, three boceprevir nodes, and one simeprevir node were considered in the analysis.
Figure 2. Global network of evidence, treatment-naïve patients. Note: TxPRy: telaprevir for x weeks in combination with PR for y weeks; BxPRy: boceprevir for x weeks in combination with PR for y weeks; SMVxPRy: simeprevir for x weeks in combination with PR for y weeks; PR response guided therapy noted as 24/48 (simeprevir, telaprevir) or 28/48 (boceprevir).
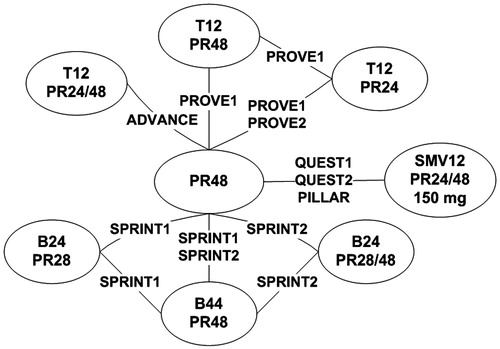
Studies included in the network were homogeneous in terms of sex, age, and viral load at baseline. Some differences were observed in terms of distribution of genotype, with a lower proportion of genotype 1a patients in trials PILLARCitation15, PROVE2Citation32 and QUEST2Citation17 (∼40% compared to more than 55%). The differences in genotype 1a/1b distribution were likely to be driven by the countries and regions for the recruitment of patients in clinical trials. For example, in North America, Australia, and New-Zealand, genotype 1a patients are predominant, whereas in most European countries patients with genotype 1b are predominant. In all trials, the proportion of patients with F0–F2 Metavir scores was high (i.e., more than 65%). There were no F4 patients in trials PILLAR, PROVE1, and PROVE2, and, in the other studies, the proportion of F4 patients was low, ranging from 4–13%.
Triple therapies with boceprevir, telaprevir, or simeprevir in combination with PR had a higher proportion of patients achieving SVR than PR alone, with odds ratios ranging from 1.85 (95% CrI = 1.13–3.05) for B24PR28 to 4.83 (95% CrI = 3.50–6.70) for simeprevir (see Supplementary material). Simeprevir had probabilities higher than 85% of better efficacy compared to telaprevir, with odds ratios ranging from 1.27 (95% CrI = 0.81–2.00) vs T12PR24/48 to 2.00 (95% CrI = 1.14–3.49) vs T12PR24. Compared to boceprevir, simeprevir had probabilities higher than 90% of better efficacy. Odds ratios for SVR ranged between 1.36 (95% CrI = 0.89–2.09) for simeprevir vs B44PR48 and 2.61 (95% CrI = 1.44–4.74) vs B24PR28. Simeprevir ranked first on the basis of SUCRA, which further supports the higher probability of better efficacy ( and Supplementary material).
Figure 3. Results for SVR: OR [95% CrI] and probabilities (Prob) for SMV to perform better than comparators.
![Figure 3. Results for SVR: OR [95% CrI] and probabilities (Prob) for SMV to perform better than comparators.](/cms/asset/ed489930-a35f-4b70-b787-30ab0be8bdd7/ijme_a_1046880_f0003_b.jpg)
In terms of safety, the risk of anemia and discontinuations due to AEs was lower for simeprevir than for PR alone, with probabilities of at least 90% for having a better outcome, odds ratios were 0.80 (95% CrI = 0.57–1.12) for anemia and 0.39 (95% CrI = 0.21–0.70) for discontinuations due to AE, and similar or higher risk for rash, pruritus, and neutropenia, odds ratios ranged from 1.16 (95% CrI = 0.82–1.63) to 1.24 (95% CrI = 0.88–1.78). When comparing simeprevir vs telaprevir, simeprevir had lower risks of anemia, rash pruritus, and discontinuations due to AE, with odds ratios vs T12PR24/48 between 0.27 (95% CrI = 0.12–0.60) for discontinuation due to AE and 0.67 (95% CRI = 0.44–1.04) for pruritus. The risk of neutropenia was higher for simeprevir (odds ratio = 1.77, 95% CrI = 1.05–3.02). Compared to boceprevir, simeprevir had lower or similar risk of anemia, rash, and neutropenia, with odds ratios vs B4PR48 between 0.34 (95% CrI = 0.22–0.52) for anemia and 1.08 (95% CrI = 0.67–1.76) for rash, while the risk of pruritus was higher for simeprevir (highest odds ratio vs B24PR24/28 = 1.41, 95% CrI = 0.89–2.22). The risk of discontinuations due to AEs was lower for simeprevir compared to boceprevir, with probabilities of having a better outcome between 96–100% ( and Supplementary material).
Figure 4. Safety results—OR [95% CrI] and probabilities (Prob) for SMV to perform better than comparators.
![Figure 4. Safety results—OR [95% CrI] and probabilities (Prob) for SMV to perform better than comparators.](/cms/asset/fc6a6d44-7d0c-480d-b295-d3d230d3ccaf/ijme_a_1046880_f0004_b.jpg)
Sensitivity and sub-group analyses had minor to no impact on the results and did not change conclusions regarding simeprevir efficacy compared to PR48, telaprevir, and boceprevir in treatment-naïve patients (Supplementary material).
Treatment-experienced patients
Treatment-experienced patients were evaluated in seven studies (). All studies were of good quality. The network of evidence included six PR48 controlled studies and one study comparing simeprevir and telaprevir as a head-to-head comparison (ATTAIN). Two telaprevir regimens, two boceprevir regimens, and two simeprevir regimens were considered in the analysis of treatment-experienced patients ().
Figure 5. Global network of evidence, treatment-experienced patients. Note: TxPRy: telaprevir for x weeks in combination with PR for y weeks; BxPRy: boceprevir for x weeks in combination with PR for y weeks; SMVxPRy: simeprevir for x weeks in combination with PR for y weeks; PR response guided therapy noted as 24/48 (simeprevir, telaprevir) or 36/48 (boceprevir).
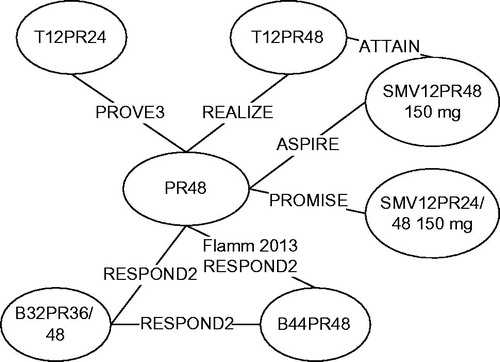
Studies included were similar in terms of proportion of males, mean age at baseline, distribution of Metavir score at baseline, and viral load at baseline. As in the treatment-naïve analysis, trials differed in terms of genotype 1a/1b distribution due to different countries and regions for the recruitment of patients.
All triple therapies were associated with probabilities of better efficacy vs PR48 of 100% (see Supplementary material). When comparing to telaprevir, simeprevir was associated with odds ratios ranging from 1.04 (95% CrI = 0.78–1.38) for SMV12PR48 vs T12PR48 to 1.37 (95% CrI = 0.60–3.09) for SMR12PR24/48 vs T12PR24 for the proportion of patients achieving SVR. Simeprevir had probabilities higher than 70% of better efficacy compared to boceprevir. Odds ratios for SVR ranged between 1.21 (95% CrI = 0.62–2.37) for SMV12PR48 vs B44PR48 and 1.74 (95% CrI = 0.84–3.61) for SMV12PR24/48 vs B24PR28 ( and Supplementary material).
Simeprevir SMV12PR24/48 and SMV12PR48 ranked first and second, respectively, on the basis of the SUCRA, supporting the probability of better efficacy with simeprevir compared to the other treatment regimens.
In terms of safety, the risk of anemia and discontinuations due to AEs was similar or lower in those treated with simeprevir compared to PR alone, with median odds ratios ranging from 0. 50 (95% CrI = 0.15–1.68) to 1. 02 (95% CrI = 0.37–2.91) (see Supplementary material). Simeprevir had lower risks of anemia, rash, pruritus, and discontinuations due to AEs compared to telaprevir. Versus T12PR48, for adverse events, odds ratios ranged from 0.34 (95% CrI = 0.18–0.63) in pruritus to 0.39 (95% CrI = 0.19–0.80) in anemia for SMV12PR24/48. For SMV12PR48, the range was from 0.47 (95% CrI = 0.35–0.63) in anemia to 0.69 (95% CrI = 0.52–0.91) in pruritus. Odds ratios for discontinuation due to AE vs T12PR48 were 0.22 (95% CrI = 0.05–1.00) for SMV12PR24/48 and 0.45 (95% CrI = 0.24–0.80) for SMV12PR48.
When comparing simeprevir to boceprevir, simeprevir had lower or similar risk of anemia and rash, with odds ratios between 0.20 (95% CrI = 0.07–0.58) and 0.41 (95% CrI = 0.16–1.00). The risk of discontinuations due to AEs was lower for simeprevir compared to boceprevir. For pruritus, the response-guided therapy regimen of simeprevir had similar risks as boceprevir, while the fixed-duration regimen was associated with a higher risk than boceprevir. Results for neutropenia mainly reflected the different durations of interferon ( and Supplementary material).
To account for the potential impact of heterogeneity due to different types of prior response (i.e., relapse, partial response, or null response to prior PR treatment) in the different studies, sub-group analyses by prior response were conducted. All estimates for null responders were lower in the sub-group analysis than in the base case analysis for simeprevir vs comparators. The odds ratio for simeprevir vs PR alone and telaprevir were higher in partial responders compared to the base case analysis, but was lower vs boceprevir. The model convergence was not optimal for this sub-group analysis and lead to very broad credible intervals. When focusing on relapsers, odds ratios for simeprevir vs telaprevir were lower in the sub-group analysis compared to the base case analysis and similar when compared with PR48 and boceprevir. Overall credible intervals were broader in the sub-group analyses compared to the base case analysis, as fewer trials were included in the sub-group analyses (due to lack of data by prior response) and also because of a lower sample size in each treatment arm.
Sensitivity analyses and other sub-group analyses had little to no impact on the results and did not change conclusions from the base case analysis regarding simeprevir efficacy compared to PR48, telaprevir, and boceprevir.
Discussion
The most robust way to assess relative efficacy between treatments would be through direct comparison. With an increasing number of treatment options, it becomes, however, very unlikely that one, and even less likely more than one, study will include all treatments of interest. Indeed, no study compared all four of the treatments under consideration. While one study compared simeprevir directly with telaprevir, all other PI studies used PR as comparator. Still, in the absence of head-to-head trials involving a direct comparison of all treatments of interest, an indirect comparison based on Bayesian NMA can provide useful insights to clinicians and reimbursement decision-makers on the relative efficacy among competing interventionsCitation25.
At the time of this study, this NMA was the first to compare all currently approved PI’s in both naïve and experienced HCV patients in genotype 1, whilst focusing on label or near-label treatment regimens. Combination therapies with sofosbuvir or other new direct antiviral agents could not be included in this analysis due to the lack of randomized controlled studies that evaluated these agents in genotype 1 HCV patients according to their licensed treatment regimen.
There have been five published meta-analyses focusing on triple therapies with PR in treatment-naïve and treatment-experienced genotype 1 HCV patientsCitation44–48.
Meta-analyses by Cooper et al.Citation44, Kieran et al.Citation45, and Cure et al.Citation46 did not include simeprevir in the networks of evidence. The impact of the inclusion of simeprevir analyses on the results for telaprevir and boceprevir vs PR was minor. Results from our analysis for telaprevir and boceprevir vs PR48 were close to the results obtained in analyses by Cooper et al.Citation44, Kieran et al.Citation45, and Cure et al.Citation46.
Publications by Bryden et al.Citation47 and Druyts et al.Citation48 included simeprevir in the network of evidence focusing on treatment-naïve patients. When comparing simeprevir to PR, odds ratios for SVR were 3.67 [2.66; 5.10] in Druyts et al.Citation48, 3.76 [2.80; 5.09] in Bryden et al.Citation47, and 4.83 [3.50; 6.71] in our analysis. The difference can be explained by the inclusion of Q80K-positive patients in the simeprevir arms in these analyses.
Further, a report by Tice et al.Citation49 included sofosbuvir in a NMA for treatment-naïve patients, using uncontrolled data and data from a randomized controlled phase 2 study of sofosbuvir + PR outside its labeled dosingCitation50. Results (excluding Q80K-positive patients) were comparable to results from our analysis for simeprevir vs telaprevir, boceprevir, and PR, and similar efficacy was found for simeprevir + PR and sofosbuvir + PR.
The number of studies included in the analysis was small, particularly in the treatment-experienced patient population. In consequence, the estimate of the between-study variance had poor precision, making it difficult to differentiate heterogeneity from differences caused by the different treatments. Therefore, a fixed-effect model was chosen. Model fit measures also supported the choice for the fixed-effect model. Nevertheless, random-effect models were explored and findings were consistent with the results from the fixed effect model. As expected, the credible intervals around the random effects estimates were wider (Supplementary material).
The main limitation of the study was that SVR rates analyses were based on the Q80K-negative patient sub-group for simeprevir arms (recommendations of the simeprevir CHMP label). The consequence of using results from a sub-group analysis is that randomization is no longer guaranteed.
The restriction of the search to English-language publications can be seen as a further limitation. However, an exploratory search on the clinicaltrials.com website did not yield additional studies that would have met the inclusion criteria.
It was not feasible to conduct a meta-regression to account for potential imbalances in baseline characteristics or study designs, given the limited number of trials included in the networksCitation51. While in both networks, patient characteristics were reasonably similar across studies, differences between studies regarding prior treatment response exist in the experienced network. A limitation of this study was, therefore, that overall odds ratios were considered for all treatment-experienced patients in the base case. The impact of heterogeneity due to different types of prior response was investigated with sub-group analyses based on prior treatment response. These analyses were, however, not robust due to low sample sizes and low number of patients achieving SVR in the PR arms.
Further, at the start of the NMA, data for simeprevir were not yet available from peer-reviewed publications which can be considered a significant limitation. Since then, all simeprevir studies included in this analysis were published in peer-reviewed journals and concordance of NMA inputs with published data was ensured for all base case analyses except for the PILLAR phase 2 study where data for SVR rates excluding Q80-K positive patients for the SMV12PR24/48 regimen were not available from peer reviewed sources, and most sensitivity analysis with the exception of sub-groups by Metavir score, where no SVR data for patients excluding Q80K are published. An additional sensitivity analysis excluding PILLAR was conducted which yielded slightly higher odds ratios for simeprevir for SVR than in the base case, suggesting that the inclusion of non-peer reviewed data did not lead to bias in favor of simeprevir (Supplementary material).
Finally, both SVR12 and SVR24 results were used as the end-point, depending on what was defined as the primary end-point in the individual studies. To explore possible bias, a sensitivity analysis using SVR24 results throughout was conducted. However, since no results were available for SVR at 24 weeks for the ATTAIN trial; results at 12 weeks were used in this sensitivity analysis. For both networks, results were similar to the base case, suggesting that the use of SVR 12 where it was the primary end-point did not introduce bias. The high concordance of SVR rates at 12 and 24 weeks which has been previously reported in the literatureCitation9 supports the findings of the sensitivity analysis (Supplementary material).
While other new treatments for HCV have recently been licensed, in a number of countries, these currently receive no or restricted funding; therefore, PIs are still the only broadly available class of direct-acting antiviral treatments for chronic HCV in many locations. Hence, this analysis offers relevant information for decision-makers. However, the emergence of highly effective interferon-free treatment options—including combinations of simeprevir with sofosbuvir, daclatasvir with sofosbuvir, sofosbuvir/ledipasvir, and ombitasvir/paritaprevir/ritonavir plus dasabuvir—is expected to change the HCV treatment landscape in the coming years, and has already done so in some countries. A comparison of these new interferon free combination treatments would be of interest.
Conclusions
In both the treatment-naïve and treatment-experienced patients, simeprevir had a better efficacy than PR alone and a similar or higher efficacy compared to telaprevir and boceprevir. In terms of safety, the risks of AEs and discontinuations due to AEs were lower for simeprevir compared to telaprevir and lower or similar compared to boceprevir. Sensitivity analyses had little to no impact on the results and did not change conclusions regarding simeprevir efficacy compared to telaprevir and boceprevir.
Transparency
Declaration of funding
This work was supported by Janssen Pharmaceutica NV, Beerse, Belgium.
Declaration of financial/other relationships
VT, MPa, SH, and SP are employees of Amaris, consultancy company, which conducted the analysis for Janssen Pharmaceutica. SVS, MPi, and AM are employees of Janssen Pharmaceutica. AU has received speaker and advisory fees from Janssen, Gilead, Abbvie, MSD and BMS. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material.pdf
Download PDF (98.5 KB)Acknowledgments
The authors would like to thank Professor Keith Abrams for his technical advice, Urbano Sbarigia, MSc, for his contribution to the objectives and design of this study, and Dr Jonathan Belsey and Katie Pascoe, PhD, for critical review of earlier drafts of the NMA report.
References
- Simmonds P. Genetic diversity and evolution of hepatitis C virus–15 years on. J Gen Virol 2004;85:3173-88
- European Association for Study of Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2014;60:392-420
- World Health Organization. Hepatitis C - Fact sheet N°164 2014. WHO. http://www.who.int/mediacentre/factsheets/fs164/en/. Accessed April 27, 2015
- European Centre for Disease Prevention and Control (ECDC). Hepatitis C. 2014. ECDC. http://ecdc.europa.eu/en/healthtopics/hepatitis_c/pages/index.aspx. Accessed April 27, 2015
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. Lancet 1997;349:825-32
- Wiese M, Berr F, Lafrenz M, et al. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in Germany: a 20-year multicenter study. Hepatology 2000;32:91-6
- Sangiovanni A, Prati GM, et al. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology 2006;43:1303-10
- de Ledinghen V, Trimoulet P, Mannant PR, et al. Outbreak of hepatitis C virus infection during sclerotherapy of varicose veins: long-term follow-up of 196 patients (4535 patient-years). J Hepatol 2007;46:19-25
- Chen J, Florian J, Carter W, et al. Earlier sustained virologic response end points for regulatory approval and dose selection of hepatitis C therapies. Gastroenterology 2013;144:1450-55
- Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-82
- Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-65
- Hadziyannis SJ, Sette H, Jr Morgan TR, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004;140:346-55
- European Association for the Study of the Liver. EASL recommendations on treatment of Hepatitis C. 2014. EASL. http://files.easl.eu/easl-recommendations-on-treatment-of-hepatitis-C.pdf. Accessed April 27, 2015
- European Medicines Agency. Olysio - Summary of Product Characteristics. 2014. EMA. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002777/WC500167867.pdf. Accessed April 27, 2015
- Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naive genotype 1 hepatitis C: the randomized PILLAR study. Hepatology 2013;58:1918-29
- Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014;384:403-13
- Manns M, Marcellin P, Poordad F, et al. Simeprevir with pegylated interferon alfa 2a or 2b plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-2): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2014;384:414-26
- Forns X, Lawitz E, Zeuzem S, et al. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology 2014;146:1669-79
- Zeuzem S, Berg T, Gane E, et al. Simeprevir increases rate of sustained virologic response among treatment-experienced patients with HCV genotype-1 infection: a phase IIb trial. Gastroenterology 2014;146:430-41
- Reddy KR, Zeuzem S, Zoulim F, et al. Simeprevir versus telaprevir with peginterferon and ribavirin in previous null or partial responders with chronic hepatitis C virus genotype 1 infection (ATTAIN): a randomised, double-blind, non-inferiority phase 3 trial. Lancet Infect Dis 2015;15:27-35
- Quigley JM, Bryden PA, Scott DA et al. Relative efficacy and safety of simeprevir and telaprevir in treatment-naïve hepatitis C-infected patients in a Japanese population: a Bayesian network meta-analysis. Hepatol Res 2015; DOI: 10.1111/hepr.12467
- Centre for Reviews and Dissemination. University of York. Systematic reviews: CRD's guidance for undertaking systematic reviews in health care, 2009. Centre for Reviews and Dissemination. http://www.york.ac.uk/crd/guidance/. Accessed April 27, 2015
- National Institute for Health and Care Excellence. Guide to the methods of technology appraisal. London: National Institute for Clinical Excellence (NICE), 2013.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomised controlled trials. 2011. National Institute for Health and Care Excellence Decision Support Unit. http://www.nicedsu.org.uk/Evidence-Synthesis-TSD-series%282391675%29.htm. Accessed April 2015
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health 2011;14:417-28
- Spiegelhalter DJ, Best NG, Carlin BP, et al. Bayesian measures of model complexity and fit. J Roy Stat Soc B 2002;64:583-639
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163-71
- Cope S, Donohue JF, Jansen JP, et al. Comparative efficacy of long-acting bronchodilators for COPD: a network meta-analysis. Respir Res 2013;14:100
- Jansen JP, Trikalinos T, Cappelleri JC, et al. Indirect treatment comparison/network meta-analysis study questionnaire to assess relevance and credibility to inform health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health 2014;17:157-73
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials. 2011. National Institute for Health and Care Excellence Decision Support Unit. http://www.nicedsu.org.uk/Evidence-Synthesis-TSD-series%282391675%29.htm. Accessed April 2015
- Hezode C, Forestier N, Dusheiko G, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med 2009;360:1839-50
- Jacobson IM, McHutchison JG, Dusheiko G, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011;364:2405-16
- Kwo PY, Lawitz EJ, McCone J, et al. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet 2010;376:705-16
- McHutchison JG, Everson GT, Gordon SC, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. N Engl J Med 2009;360:1827-38
- Poordad F, McCone J, Jr Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195-206
- Bacon BR, Gordon SC, Lawitz E, et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1207-17
- Flamm SL, Lawitz E, Jacobson I, et al. Boceprevir with peginterferon alfa-2a-ribavirin is effective for previously treated chronic hepatitis C genotype 1 infection. Clin Gastroenterol Hepatol 2013;11:81-7
- McHutchison JG, Manns MP, Muir AJ, et al. Telaprevir for previously treated chronic HCV infection. N Engl J Med 2010;362:1292-303
- Zeuzem S, Andreone P, Pol S, et al. Telaprevir for retreatment of HCV infection. N Engl J Med 2011;364:2417-28
- McHutchison JG, Lawitz EJ, Shiffman ML, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med 2009;361:580-93
- Rumi MG, Aghemo A, Prati GM, et al. Randomized study of peginterferon-alpha2a plus ribavirin vs peginterferon-alpha2b plus ribavirin in chronic hepatitis C. Gastroenterology 2010;138:108-15
- Yenice N, Mehtap O, Gumrah M, et al. The efficacy of pegylated interferon alpha 2a or 2b plus ribavirin in chronic hepatitis C patients. Turk J Gastroenterol 2006;17:94-8
- Marcellin P, Forns X, Goeser T, et al. Telaprevir is effective given every 8 or 12 hours with ribavirin and peginterferon alfa-2a or -2b to patients with chronic hepatitis C. Gastroenterology 2011;140:459-68
- Cooper C, Lester R, Thorlund K, et al. Direct-acting antiviral therapies for hepatitis C genotype 1 infection: a multiple treatment comparison meta-analysis. QJ Med 2013;106:153-63
- Kieran J, Schmitz S, O'Leary A, et al. The relative efficacy of boceprevir and telaprevir in the treatment of Hepatitis C virus genotype 1. Clin Infect Dis 2013;56:228-35
- Cure S, Diels J, Gavart S, et al. Efficacy of telaprevir and boceprevir in treatment-naive and treatment-experienced genotype 1 chronic hepatitis C patients: an indirect comparison using Bayesian network meta-analysis. Cur Med Res Opin 2012;28:1841-56
- Bryden PA, Quigley JM, Padhiar A, et al. The relative efficacy and safety of simeprevir-based triple therapy compared to boceprevir and telaprevir in treatment-naïve patients chronically infected with hepatitis C virus genotype 1: Bayesian network meta-analyses. Hepatology 2013;58(1 Suppl):756A
- Druyts E, Lorenzi M, Toor K, et al. Network meta-analysis of direct-acting antivirals in combination with peginterferon-ribavirin for previously untreated patients with hepatitis C genotype 1 infection. QJM-Int J Med 2014; DOI: http://dx.doi.org/10.1093/qjmed/hcu202.
- Tice J, Ollendorf D, Pearson S. The comparative clinical effectiveness and value of simeprevir and sofosbuvir in the treatment of chronic Hepatitis C Infection - A technology assessment: Institute for Clinical and Economic Review (ICER). 2014. Institute for Clinical and Economic Review. http://ctaf.org/reports/treatments-hepatitis-c. Accessed April 27, 2015
- Lawitz E, Lalezari JP, Hassanein T, et al. Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial. Lancet Infect Dis 2013;13:401-8
- Gagnier JJ, Morgenstern H, Altman DG, et al. Consensus-based recommendations for investigating clinical heterogeneity in systematic reviews. BMC Med Res Methodol 2013;13:106