Abstract
Objective:
This study aims to estimate the long-term cost-effectiveness of saxagliptin + metformin (SAXA + MET) vs glimepiride + metformin (GLI + MET) in patients with Type 2 diabetes mellitus (T2DM) inadequately controlled with MET in China.
Methods:
The Cardiff Model was used to simulate disease progression and estimate the long-term effect of treatments on patients. Systematic literature reviews and hospital surveys were conducted to obtain patients profiles, clinical data, and costs. Health insurance costs (2014¥) were estimated over a 40-year period. One-way and probabilistic sensitivity analyses were performed.
Results:
SAXA + MET had lower predicted incidences of cardiovascular and hypoglycemia events and a decreased total cost compared with GLI + MET (¥241,072,807 vs ¥285,455,177). There were increased numbers of quality-adjusted life-years (QALYs; 1.01/patient) and life-years (Lys; 0.03/patient) gained with SAXA + MET compared with GLI + MET, and the incremental cost of SAXA + MET vs GLI + MET (−¥44,382) resulted in −¥43,883/QALY and −¥1,710,926/LY gained with SAXA + MET. Sensitivity analyses confirmed that the results were robust.
Conclusion:
In patients with T2DM in China, SAXA + MET was more cost-effective and was well tolerated with fewer adverse effects (AEs) compared with GLI + MET. As a second-line therapy for T2DM, SAXA may address some of the unmet medical needs attributable to AEs in the treatment of T2DM.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic, progressive disease characterized by insulin resistance and relative insulin deficiency, either or both of which may be present at the time diabetes is diagnosed; while its etiology and pathogenesis is still not clear at presentCitation1,Citation2. T2DM may remain undetected for many years, and the diagnosis can occur at any age, which is often made when a complication occurs or a routine urine or blood glucose test is doneCitation1. It is a condition that necessitates critical management of blood glucose levels to prevent hyperglycemia, diabetes-related complications, and disability. The world’s prevalence of diabetes is high, with a total of 387 million patients in 2014, and the number is increasing in every countryCitation3. The prevalence is also rapidly rising in ChinaCitation4,Citation5, affecting ∼113.9 million (11.6%) adults aged 18 years or older in 2010Citation6; T2DM accounted for at least 90% of these casesCitation1. In addition, blood glucose control remains elusive for many patients with diabetes in China, where only 39.7% of patients have adequate blood glucose control (glycated hemoglobin, [HbA1c] < 7.0%)Citation6. T2DM imposes a considerable economic burden on patients and their families, healthcare systems, and national economiesCitation7,Citation8. In China, the direct medical costs of T2DM and its complications were estimated to be $26.0 billion in 2007, and by 2030 may be as high as $47.2 billionCitation8.
Given the central importance of long-term blood glucose control in managing risk factors and enhancing health-related quality-of-life (HRQoL) for patients with T2DMCitation9–11, a variety of anti-diabetic agents, such as metformin (MET), α-glucosidase inhibitors, sulfonylureas (SUs), dipeptidyl peptidase-4 (DPP-4) inhibitors, thiazolidinediones (TZDs), glucagon-like peptide-1 (GLP-1) mimetics, and insulin, have been introduced into clinical practice and incorporated into the treatment algorithmCitation2 in China to improve glycemic control in patients with T2DM. Among them, sulfonylureas, a second-line therapy recommended by the Chinese Diabetes Society (CDS) 2013 T2DM Clinical GuidelinesCitation2, have been commonly accepted and widely used in China for patients with T2DM who are inadequately controlled with MET aloneCitation12.
Glimepiride (GLI) is a third-generation sulfonylureas anti-diabetic drug. It stimulates insulin release through the closure of adenosine triphosphate-sensitive potassium channels on pancreatic beta cells to increase insulin levels and further lower blood glucose in patients with T2DMCitation13,Citation14. Its clinical efficacy (glucose-lowering properties) as monotherapy or in combination treatments has been well assessed and demonstratedCitation14; however, glimepiride as one of SUs is also associated with significant adverse effects, such as weight gain and an increased risk of hypoglycemic episodesCitation15–18, which may negatively affect patient HRQoL and treatment adherenceCitation19–23 and subsequently increase the economic burden imposed on patients with T2DM and their familiesCitation24–26. Therefore, there is an urgent demand for effective oral hypoglycemic agents with beneficial hypoglycemia and weight profiles.
Saxagliptin (SAXA), a potent, reversible, and competitive DPP-4 inhibitor, acts by preventing the inactivation of the incretin hormones GLP-1 and glucose-dependent insulinotropic polypeptide, and thereby increases GLP-1 levels, stimulates insulin secretion, and decreases postprandial glucagon and glucose levelsCitation27. Studies have demonstrated that SAXA as monotherapy and in combination therapy regimens can effectively lower HbA1c levels and fasting plasma and postprandial glucose concentrations in patients with T2DM, with good safety and tolerability profiles across all drug dosesCitation28,Citation29. In addition, saxagliptin has demonstrated a non-inferior treatment effect with the significant advantage of lowering HbA1c without increasing hypoglycemic episodes and weight gain as compared with SUs and other insulin secretagoguesCitation27,Citation30–32. Another second-line option for patients with T2DM recommended by the CDS 2013 T2DM Clinical Guidelines, SAXA has been gaining increasingly more attention in clinical practice in China in recent yearsCitation33.
A patient-centric treatment strategy for patients with T2DM should be determined by the patient’s characteristics and related complications, and optimized for efficacy, tolerability, safety, and treatment costsCitation15,Citation34. Because hypoglycemia and weight gain resulting from T2DM treatment may increase the risk of cardiovascular diseaseCitation2,Citation4, as well as treatment costs, a therapeutic regimen with beneficial hypoglycemia and weight profiles, which provides long-term blood glucose control, like SAXA, may represent an advancement in treatment and cost-effectiveness compared with SUsCitation35–37. However, to our knowledge, there are no direct head-to-head studies comparing both the long-term benefits and costs of SAXA with GLI as second-line treatment added to MET in a Chinese population. Therefore, the aim of this study was to estimate the long-term outcomes and cost-effectiveness of treatment with the DPP-4 inhibitor SAXA as second-line therapy in combination with MET compared with a sulfonylureas GLI plus MET, after failure of MET as monotherapy in China, from the perspective of payers (health insurance).
Methods
Cost-effectiveness model
This study used a previously published long-term simulation model, the Cardiff Diabetes ModelCitation38–41, which is designed specifically to assess therapeutic regimens in diabetes and has been validated against other available T2DM modelsCitation42. It is capable of running in two key modes: deterministic (mean values) and probabilistic sensitivity analysis (PSA). Underlying each mode is a Monte Carlo simulation that simulates the progress of each patient within a cohort over a maximum of 40 years. It also allows users to perform univariate sensitivity analyses in a succinct fashion by using its inside Tornado model, which provides the ability to set alternative input values for certain parameters and perform multiple simulation scenarios in order to provide results relating to each choice of sensitivity analysis. The Cardiff model utilizes published United Kingdom Prospective Diabetes Study (UKPDS) 68 or UKPDS 82 risk equations to estimate the risk of clinical end-points associated with T2DMCitation43,Citation44. We adopted UKPDS 68 equations to simulate disease progression and update model parameters in this study, because they have been widely used in diabetes modeling and extensively validated compared with UKPDS 82 equationsCitation35–37,Citation45. The simulation cohort consisted of 1000 patients over a period of 40 years (a lifetime horizon because the baseline age is 48.51 years)Citation46; patients discontinued the simulation only in the event of death or when the time horizon was reached. The outputs for the model include the incidence of microvascular (e.g., amputation, blindness, end-stage renal disease) and macrovascular (e.g., congestive heart failure, myocardial infarction, stroke, and ischemic heart disease) events, hypoglycemia, diabetes-specific mortality, and all-cause mortality. The costs and quality-adjusted life-years (QALYs) associated with each treatment strategy were calculated from a payer’s perspective, using only direct medical costs. The annual discount rates for costs and benefits were set to be 3.0%, based on the World Health Organization (WHO) guidelineCitation47. The final results from the model are expressed as incremental cost-effectiveness ratios (ICERs), which is calculated as the incremental cost (cost in treatment group minus costs in control group) divided by the incremental QALYs (QALYs in treatment group minus QALYs in control group).
Based on the UKPDS series studies, the Cardiff Diabetes Model used time-dependent risk factor profiles to determine the risk of diabetes-related complications. The model differentiates between symptomatic and severe hypoglycemic events (severe hypoglycemia is defined as a major symptomatic event requiring medical assistance owing to severe impairment in consciousness and is associated with healthcare costs)Citation48–50. Because hypoglycemia is a transient event that is relatively infrequent in patients with T2DM treated with oral anti-hyperglycemic agents, fear of hypoglycemia can be a crucial driver of disutility, with a strong relevance to severe episodesCitation22,Citation51. Another characteristic of this model is the use of weight gain associated with diabetes treatment, which may have a negative effect on patients HRQoL and their long-term risk of cardiovascular complicationsCitation24, and may, furthermore, lead to increased healthcare costsCitation22,Citation52,Citation53.
Data required in the model
Patient profiles, the clinical efficacy of each treatment, costs related to treatment strategies and diabetes-related events, and utilities related to events are included in the model. We conducted a literature review and hospital survey to obtain the required data.
Literature review and hospital survey
A systematic literature search was carried out in both English (PubMed and Web of Science) and Chinese (China National Knowledge Infrastructure, Wanfang Data, and Chongqing VIP) language databases to identify relevant randomized controlled trials or observational studies providing direct head-to-head comparisons of the treatment efficacy of SAXA + MET and GLI + MET in Chinese patients with T2DM (18 years or older). The search terms included saxagliptin, Onglyza, glimepiride, Amaryl, type 2 diabetes mellitus, non-insulin-dependent diabetes mellitus, Chinese, and China. The keywords were combined and adapted to search the above-mentioned databases for publications dating from 1995–2014 to ensure a comprehensive search.
The included studies had HbA1c as the primary outcome indicator, with a follow-up duration (up to 12 weeks) appropriate for each comparison. Other than the target treatments, only diet and/or physical exercise were allowed for the study patients. After removal of duplicated publications, we retrieved 1218 and 56 studies from the Chinese and English language databases, respectively, on the topic of SAXA or GLI in T2DM treatment; however, after title and abstract screening and further examination of the full-text articles, only one Chinese-language clinical study was identifiedCitation32 that provided a direct head-to-head comparison between SAXA and GLI as add-on therapy to MET in a randomized controlled trial of patients with T2DM. This study selected 75 Chinese patients from a hospital between September 2012 and May 2013. The patients were divided into two groups: treatment group (37 cases) given SAXA and MET, and control group (38 cases) given GLI and MET for 24 weeks. The changes of Fasting Plasma Glucose (FPG), HbA1c, body mass index (BMI), Insulin, and pro-insulin-to-insulin ratio levels were observed before and after treatment. The adverse events were investigated during the treatment.
For the hospital survey, cost data for diabetes-related events occurring between 2010–2014, were collected from two representative hospitals in eastern China (one secondary hospital and one tertiary hospital). These data were a summation of direct medical costs, such as physician visits, drug and laboratory tests, hospital beds, examinations, drugs, operations, and nursing. Costs collected from both hospitals were synthesized to form a combined cost profile; however, because these costs were not comprehensive owing to the unavailability of outpatient data, we used them only as an alternative cost profile in sensitivity analyses to test and corroborate the data collected from the published literature.
Patient profile and treatment strategies
The patients in this analysis were characterized by the mean cohort values for demographics (e.g., age, percentage female) and risk factors (e.g., HbA1c level, total cholesterol) reported by Zhu and SongCitation32 in a head-to-head study conducted in China (). Because only BMI rather than body weight or height was reported in this head-to-head study, the baseline weight was calculated from the baseline BMI by assuming that the average height in the investigated patient populations was 1.63 mCitation54. For other data that were not available in the head-to-head study, such as the duration of diabetes, proportion of smokers, and high-density lipoprotein cholesterol levels, we used data from cross-sectional, multi-center, observational survey studies with nationally representative populations of patients with T2DM in ChinaCitation6,Citation55.
Table 1. Demographics and risk factors.
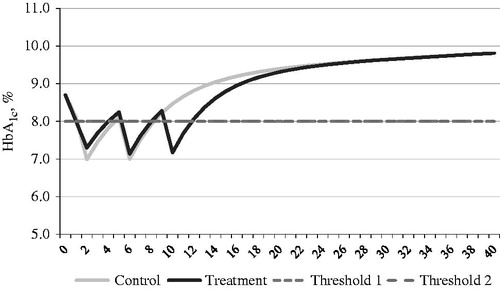
Type 2 diabetes mellitus is a progressive disease. Consequently, even in patients receiving anti-diabetic treatment, HbA1c levels increase over time, resulting in a positive slope of the blood glucose curve. The shape of blood glucose profile determines when the therapy escalation occurs because the treatment effect of T2DM is primarily driven by blood glucose control. The treatment strategy considered in this analysis was that patients inadequately controlled on MET alone started with the combination therapy of SAXA + MET (treatment group) or GLI + MET (control group), and that therapy escalations occurred when the pre-specified HbA1c threshold of 8.0% was reached.
Clinical data
Clinical results and costs were estimated based on the following treatment strategies in the head-to-head study reported by Zhu and SongCitation32: the SAXA dose was 5 mg/day, GLI was titrated from 1–4 mg/day as needed (the mean dosage was 2.8 mg/day), and MET was used in open-label individual doses.
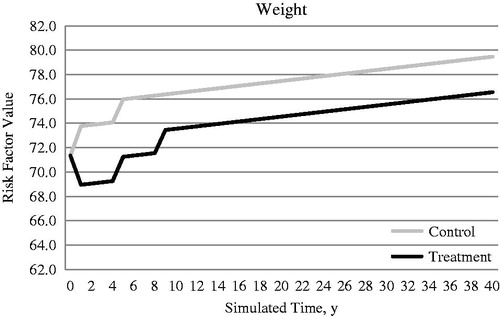
The primary treatment effect parameters considered in this analysis were changes in HbA1c level and body weight and the occurrence of hypoglycemia (). No treatment-associated effect on blood pressure or lipid levels was assumed because it had not been reported in the head-to-head studyCitation32. SAXA + MET was associated with weight loss, whereas GLI + MET was associated with weight gain in the head-to-head study. Change in body weight was calculated from change of BMI, by assuming that average height in investigated patient populations was 1.63 mCitation54.
Table 2. Clinical input variables.
Hypoglycemia reported by Zhu and SongCitation32 was not clearly differentiated between symptomatic and severe episodes. Because patients are more closely monitored and may be better educated about hypoglycemia in clinical trials compared with the usual care setting, they may report few or no occurrences of severe hypoglycemic events. In the usual care setting, however, patients may be more likely to experience severe hypoglycemia. Therefore, we modified the data from the head-to-head study by referring to an observational survey studyCitation50 in which the percentage of severe cases was 2.18% of all hypoglycemic events.
The HbA1c and body weight profiles changed over time, and the effects of the treatments applied at the relevant thresholds are presented in and . Weight increases were estimated to be 0.1 kg/year for the control and treatment cohorts; this estimate was based on UKPDS data after accounting for the initial treatment-related weight changeCitation56.
Costs
In this analysis, only country-specific direct medical costs were estimated. All costs were converted into 2014 Chinese Yuan (¥) using the Chinese Consumer Price Index (CPI)Citation57. The exchange rate of Chinese Yuan/US dollar was 6.143 in 2014Citation58. Costs related to macrovascular and microvascular events were classified as fatal or non-fatal and applied to the year in which the event occurred. Maintenance costs for individuals who survived were applied in all subsequent years until the end of the simulation horizon or until the patient died. The costs were primarily derived from published literature in ChinaCitation59. Because the costs for ulcers were not available in the literature, we estimated these costs by synthesizing data from the hospital survey and published studies ()Citation60,Citation61.
Table 3. Annual direct medical costs for diabetes-related complications and severe hypoglycemia (2014¥).
Only the costs of severe hypoglycemic events were estimated in this analysis, because mild-to-moderate events, like symptomatic hypoglycemia, do not require medical assistanceCitation48,Citation49. This cost was based on direct medical costs for episodes of hypoglycemia in ChinaCitation50, and the annual cost of severe hypoglycemia was assumed to be ¥3829.96 for each patient ().
For drug cost acquisition, the highest retail price according to the latest official drug price list of the Price Bureau in eastern ChinaCitation62,Citation63 was considered. Because MET was used in open-label single doses that could not be averaged in the head-to-head study, we cited the average annual drug cost of MET according to a published study ()Citation64.
Table 4. Annual treatment costs for different drugs.
The BMI-related costs which are relate to increased prescribing costs per BMI unit were calculated and estimated based on a follow-up observation study in ChinaCitation65, and they were assumed to be identical between men and women ().
Table 5. BMI-related costs.*†.
Utilities
Utilities related to complications, hypoglycemia, and weight change are presented in . Given the absence of utility data in China, we used the utilities from the UKPDS 62Citation66, with the exception of end-stage renal disease (ESRD) and blindnessCitation67, hypoglycemiaCitation19, and per unit change in BMICitation53.
Table 6. Utility decrements.
Sensitivity analyses
In sensitivity analysis, we first ran the Tornado model for univariate sensitivity analysis in order to investigate key model drivers and parameters among input parameters, including baseline demographics (e.g., HbA1c, age, total cholesterol), costs and utilities related to diabetes-related events and BMI changes, etc. Then, a series of 1-way sensitivity analyses were conducted to further examine the effects of these key model drivers on ICER. The combined effect of variability around model inputs was assessed using a probabilistic sensitivity analysis (PSA) performed as a second-order Monte-Carlo simulation in the Cardiff diabetes model. Treatment-related HbA1c effects, weight changes, and symptomatic hypoglycemic events are sampled from a normal distribution, while the probability of severe hypoglycemic events and utility decrements are sampled from a beta distribution, and costs from a gamma distribution. The range of values is expressed by the 95% confidence intervals for each parameter. All of the sensitivity analyses were performed for 1000 patients. The PSA was used to generate a scatter plot of the ICER and a cost-effectiveness acceptability curve (CEAC).
Results
Predicted health events
In the base-case analysis, predicted incidences of macrovascular and microvascular events (except nephropathy) were lower in the SAXA + MET vs GLI + MET group. Similarly, the model also predicted fewer deaths caused by macrovascular events in SAXA + MET. Over a patient’s lifetime, the main differences between the SAXA + MET and GLI + MET groups were the adverse effect profiles. Patients in the SAXA + MET group experienced fewer hypoglycemic symptomatic (11,557 vs 13,981) and severe (411 vs 498) events than those in the GLI + MET group. Furthermore, patients in the SAXA + MET group demonstrated weight loss, whereas those in the GLI + MET group experienced an increase in body weight ().
Table 7. Results for SAXA + MET vs GLI + MET.
Predicted costs
Compared with GLI + MET, SAXA + MET was associated with lower total costs. Although the cost for nephropathy was higher with SAXA + MET than GLI + MET, this difference might not significantly affect the total costs of long-term diabetes treatments because the costs for most diabetes-related events (except nephropathy) and hypoglycemia episodes were all lower with SAXA + MET. The major differences between SAXA + MET and GLI + MET were the target drug treatment costs and BMI-related costs, which had a notable effect on the total cost. The drug treatment costs were higher in SAXA + MET (¥68,357,021) than GLI + MET (¥63,754,426); however, this difference was immediately offset by a much bigger difference in BMI-related costs between SAXA + MET (¥122,596,983) and GLI + MET (¥171,089,513) ().
Incremental cost-effectiveness ratio
The QALY gained with SAXA + MET compared with GLI + MET was 1.01 per patient, and the life-years (LYs) gained was 0.03 per patient. Thus, the main benefits of SAXA + MET was derived from better QALY as well as added LYs. Moreover, there was greater cost savings with SAXA + MET in T2DM treatment than with GLI + MET. The mean incremental cost for SAXA + MET compared with GLI + MET was −¥44,382, and the mean cost savings per QALY gained was ¥43,883 and per LY gained was ¥1,710,926, which indicated that SAXA + MET would lead to better utility and decreased costs for patients. In general, the results showed that SAXA + MET exceeded GLI + MET in cost savings per QALY and per LY ().
Parameters influencing the incremental cost-effectiveness ratio
In the sensitivity analyses, results from tornado model indicated that the key model drivers were associated to utility, HbA1c, BMI, and age ().
Figure 3. Tornado diagram of the univariate sensitivity analysis. BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; ICER, incremental cost effectiveness ratio; SBP, systolic blood pressure.
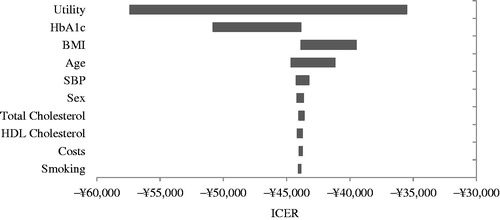
Most of the results of the 1-way sensitivity analyses (except one scenario considering BMI cost to be 0) showed that SAXA + MET was superior to GLI + MET in both cost savings and benefit; the cost savings per QALY gained with SAXA + MET ranged from −¥127,225 to −¥7,923 ().
Table 8. Sensitivity analyses for SAXA + MET vs GLI + MET (results per patient).
The highest absolute value of the cost per QALY (−¥127,225) for SAXA + MET vs GLI + MET was attained under the extreme assumption that the utility decrement (first and subsequent years) per unit BMI gain was reduced by 75%, which provided a 190% increase in cost savings per QALY gained by SAXA + MET compared with the base case (−¥43,883). In the scenario in which the utility decrement per unit BMI gain was halved, the resulting cost savings per QALY by SAXA + MET remained high at −¥77,906 (an absolute increase of 78%). When adopting an alternative weight utility associated with body weight changesCitation68, in which the absolute value of the weight utility related to the gain or loss per unit BMI was set to be identical (both were 0.014), SAXA + MET again yielded a dominant cost savings per QALY, increasing QALYs and reducing costs ().
Although a decrease in BMI-related costs would have a negative effect on cost savings per QALY gained by SAXA + MET, the results still favored SAXA + MET vs GLI + MET. When BMI-related costs decreased by half, the magnitude of the cost savings per QALY gained by SAXA + MET decreased by ∼55% (−¥19,910 vs −¥43,883); when reduced by 75%, the magnitude decreased by 82% (−¥7923 vs −¥43,883). Only when BMI-related costs were not taken into consideration, there presented a reverse picture where the cost per QALY gained with SAXA + MET was ¥4064 compared with GLI + MET; however, it was still within the acceptable range of gross domestic product (GDP) per capita of China (). The GDP per capita in China was ¥43,320 in 2013Citation69.
Although hypoglycemia is associated with utility decrement and, for severe hypoglycemia, a related costCitation50, the adjustment of hypoglycemia events and the utility associated with them in alternative scenarios resulted in only a negligible effect on the magnitude of cost savings by SAXA + MET, indicating that episodes of hypoglycemia might not be an important factor in our analysis. Both HbA1c and age were identified as important factors by the tornado model; however, after reducing the base-case HbA1c level by 1%, assuming that the reduction of HbA1c in GLI + MET was equal to that in SAXA + MET, or increasing the base-case age by 25% or 50%, the conclusion remained unchanged ().
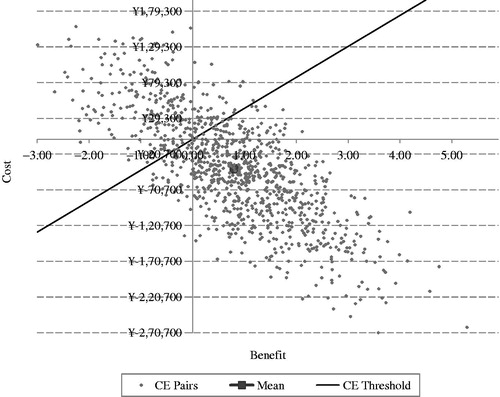
In the PSA, SAXA + MET generated a mean QALY gained of 0.80 and an incremental cost of −¥41,265 over a patient’s lifetime compared with the QALY gained and incremental cost associated with GLI + MET, which was a little lower than that determined in the base case (1.01 and −¥44,382, respectively). However, the overall cost savings per QALY for SAXA + MET vs GLI + MET was slightly higher in the PSA (−¥51,588 vs −¥43,883; ).
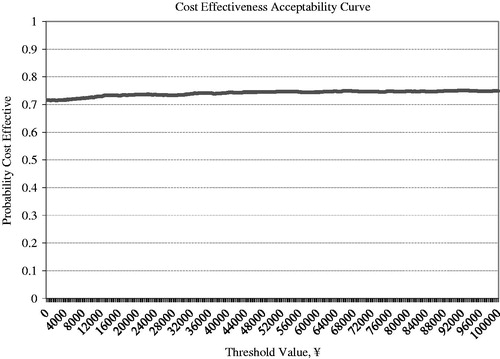
SAXA + MET provides an additional benefit as well as lower costs compared with GLI + MET. Most simulations were located in the southeast quadrant of the ICER scatter plot figure, which means SAXA + MET gained more benefits and took less costs than GLI + MET. SAXA + MET was cost-effective in 74.3% of the simulations by a threshold value of GDP per capita ( and ).
Discussion
This study is the first pharmacoeconomic analysis conducted using the Cardiff Diabetes Model to evaluate long-term outcomes and cost-effectiveness of SAXA vs GLI as add-on therapy to MET in a cohort of patients with T2DM in China. We found that the combination therapy of SAXA + MET resulted in better QALY and LY gained, as well as a lower total cost compared with GLI + MET. In addition, the cost-effectiveness results were robust, remaining stable to changes in various assumptions of input parameters.
Although no similar studies have been conducted in China, our findings partially support the results of studies performed in SwedenCitation35, GermanyCitation36, and ArgentinaCitation37, which used a similar Cardiff simulation model and demonstrated that the SAXA + MET treatment strategy can improve both QALYs and LY compared with an SU + MET. Compared with SU + MET, our study has shown that SAXA + MET can reduce total costs; however, in the above-mentioned studies, SAXA + MET was found to potentially increase costs. These discrepancies may be related to the differences in BMI-related costs. Because BMI-related costs were not included in their studies, the large difference in BMI-related costs between SAXA + MET and SU + MET in our study might have contributed to this difference.
A patient-centric treatment strategy for patients with T2DM should be determined based on the patient characteristics, and optimized for efficacy, tolerability, safety, and treatment costsCitation15,Citation34. The CDS 2013 T2DM Clinical GuidelinesCitation2 suggest that, when choosing among different therapies, their advantages and disadvantages should be considered. Our analysis highlights that it is essential to consider not only the effect of a drug on long-term blood glucose, but also its effect on hypoglycemia and body weight in the treatment of T2DM.
Hypoglycemia and fear of hypoglycemia impede the attainment of stringent blood glucose control, either through sub-optimal dosing and/or poor treatment adherence, which increases the risk of macrovascular complicationsCitation21,Citation51,Citation70. Although episodes of hypoglycemia did not affect the outcomes significantly in our analysis, this might be explained by the minor difference in the occurrence of symptomatic and severe episodes between SAXA + MET and GLI + MET treatments, which could result in a similar risk of developing macrovascular complications for both treatments.
Being overweight, or obese, was common among patients with T2DM and was usually associated with hypertension and dyslipidemia, which is significantly associated with an increased risk of developing cardiovascular diseaseCitation2,Citation55. SAXA + MET was associated with body weight loss and a low risk of hypoglycemia, whereas GLI + MET was associated with weight gain and a relatively higher risk of hypoglycemiaCitation32, which could lead to a major difference in diabetes-related complications as well as the corresponding utility and costs associated with the change in BMI between these two treatments. Therefore, SAXA + MET had a cost-effectiveness advantage compared with GLI + MET. Hence, from the perspective of payers, adding SAXA to MET might be preferable to adding an SU in patients with inadequate blood glucose control on MET alone, especially patients who are overweight or at higher risk of hypoglycemia.
In the context of improving the management of diabetes in China, as well as decreasing the disease burden, the use of treatments like SAXA could provide a benefit by addressing some of the unmet medical needs in T2DM treatment that are attributable to undesirable adverse effects (e.g., hypoglycemia and weight gain) caused by SUs, reduce the long-term disease burden on patients and their families, and lessen health expenditures for diabetes treatments for the healthcare system and government in China.
The present study is limited by the paucity of head-to-head studies; only one short-term head-to-head trial comparing the treatment effect of SAXA + MET vs GLI + MET in China was identifiedCitation32, causing uncertainty in the input parameters. In addition, as with other Cardiff modeling studiesCitation35,Citation36,Citation71, our study projects long-term outcomes based on clinical input parameters from short-term trials owing to a shortage of lifetime follow-up data from a well-designed epidemiologic or clinical study. However, the use of UKPDS 68 equations to simulate a range of long-term outcomes provided a disease progression that closely matched that observed in the UKPDS studyCitation43, potentially resulting in a more realistic simulation and reducing the bias to a certain extent.
Because this study is from the perspective of payers, only direct medical costs are estimated, although diabetes-related events, weight gain, and hypoglycemia may have a considerable effect on productivity (indirect costs) at the base age of 48.51 years. The costs of diabetes-related complications are mainly drawn from a studyCitation59 conducted in China that abstracted cost data from previously published literature providing an economic evaluation of treatments in the Chinese diabetes population. The costs were established in 2010, which might bias and limit the generalizability of the results. Therefore, another set of costs derived from a hospital survey in eastern China in 2014, along with literature review data as a supplement, was investigated in an alternative scenario.
The sensitivity analysis corroborated the robustness of the results obtained in the base case. Although it is more reasonable to apply a country-specific utility for diabetes-related complications, hypoglycemia, and weight change, there are no studies related to utilities covering all events, especially in China. We used utilities from the UKPDS 62 and other published studies of foreign populations in this study, which may have resulted in some bias.
All the concerns above suggest that the results of our analysis should be applied with caution to avoid misleading conclusions rather than to deny their real value for decision-makers and administrators.
Conclusion
The combination of SAXA + MET is a preferred option from the perspective of payers, demonstrating greater effectiveness and lower costs compared with GLI + MET. SAXA is likely to represent a durable, well-tolerated, and cost-effective treatment option for physicians and decision-makers as second-line therapy in combination with MET for T2DM inadequately controlled with MET alone in China, and it may address some of the unmet medical needs in the treatment of T2DM attributable to adverse effects (e.g., hypoglycemia and weight gain) and reduce the economic burden of diabetes.
Transparency
Declaration of funding
This study was sponsored by AstraZeneca.
Declaration of financial/other relationships
SG, JD, LS, YM, and HD have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
Acknowledgments
The authors thank Xuemei Zhen and Yuhang Zeng (Zhejiang University School of Public Health, Hangzhou, China) for their help in collecting data.
References
- International Diabetes Federation. About diabetes: types of diabetes. Brussels, Belgium. http://www.idf.org/about-diabetes. Accessed March 10, 2014.
- Chinese Diabetes Society. Chinese guideline for Type 2 diabetes prevention (2013). Chinese J Diabetes 2014;22:2-42
- International Diabetes Federation. IDF Diabetes Atlas, 6th edn. Brussels, Belgium. 2014. http://www.idf.org/diabetesatlas/update-2014. Accessed 16 April 2015
- Yang WY, Lu JM, Weng JP, et al. Prevalence of diabetes among men and women in China. New Engl J Med 2010;362:1090-101
- Dong Y, Gao W, Nan H, et al. Prevalence of Type 2 diabetes in urban and rural Chinese populations in Qingdao, China. Diabet Med 2005;22:1427-33
- Xu Y, Wang LM, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948-58
- Tang L, Chen XB, Chen HY, et al. Economic burden of Type 2 Diabetes mellitus and its complications in Urban China. Chinese Health Econ 2003;22:21-3
- Wang W, McGreevey WP, Fu C, et al. Type 2 diabetes mellitus in China: a preventable economic burden. Am J Manag Care 2009;15:593-601
- Turner R, Holman R, Stratton I, et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703-13
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. New Engl J Med 2008;359:1577-89
- Stratton IM, Adler AI, Neil H, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Brit Med J 2000;321:405-12
- Ji L, Lu J, Weng J, et al. China type 2 diabetes treatment status survey of treatment pattern of oral drugs users. J Diabetes 2015;7:166-73
- Langtry HD, Balfour JA. Glimepiride. A review of its use in the management of type 2 diabetes mellitus. Drugs 1998;55:563-84
- Massi-Benedetti M. Glimepiride in type 2 diabetes mellitus: a review of the worldwide therapeutic experience. Clin Ther 2003;25:799-816
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577-96
- Phung OJ, Scholle JM, Talwar M, et al. Effect of noninsulin antidiabetic drugs added to metformin therapy on glycemic control, weight gain, and hypoglycemia in type 2 Diabetes. JAMA 2010;303:1410-18
- Peters AL. Patient and treatment perspectives: revisiting the link between type 2 diabetes, weight gain, and cardiovascular risk. Clev Clin J Med 2009;76(5 Suppl):S20-S27
- Ferrannini E, Fonseca V, Zinman B, et al. Fifty-two-week efficacy and safety of vildagliptin vs. glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin monotherapy. Diabetes Obes Metab 2009;11:157-66
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34
- Anderson JW, Kendall CW, Jenkins DJ. Importance of weight management in type 2 diabetes: review with meta-analysis of clinical studies. J Am Coll Nutr 2003;22:331-9
- Alvarez GF, Tofe PS, Krishnarajah G, et al. Hypoglycaemic symptoms, treatment satisfaction, adherence and their associations with glycaemic goal in patients with type 2 diabetes mellitus: findings from the Real-Life Effectiveness and Care Patterns of Diabetes Management (RECAP-DM) Study. Diabetes Obes Metab 2008;10(1 Suppl):25-32
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251-65
- Pi-Sunyer FX. The impact of weight gain on motivation, compliance, and metabolic control in patients with type 2 diabetes mellitus. Postgrad Med 2009;121:94-107
- Bodegard J, Sundstrom J, Svennblad B, et al. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabetes Metab 2013;39:306-13
- Bolin K, Gip C, Mork AC, et al. Diabetes, healthcare cost and loss of productivity in Sweden 1987 and 2005–a register-based approach. Diabet Med 2009;26:928-34
- Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetic agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin 2007;23:2157-69
- Dhillon S, Weber J. Saxagliptin. Drugs 2009;69:2103-14
- Rosenstock J, Sankoh S, List JF. Glucose-lowering activity of the dipeptidyl peptidase-4 inhibitor saxagliptin in drug-naive patients with type 2 diabetes. Diabetes Obes Metab 2008;10:376-86
- DeFronzo RA, Hissa MN, Garber AJ, et al. The efficacy and safety of saxagliptin when added to metformin therapy in patients with inadequately controlled type 2 Diabetes with metformin alone. Diabetes Care 2009;32:1649-55
- Kulasa K, Edelman S. Saxagliptin: the evidence for its place in the treatment of type 2 diabetes mellitus. Core Evid 2010;5:23-37
- Goke B, Gallwitz B, Eriksson J, et al. Saxagliptin is non-inferior to glipizide in patients with type 2 diabetes mellitus inadequately controlled on metformin alone: a 52-week randomised controlled trial. Int J Clin Pract 2010;64:1619-31
- Zhu F, Song XY. Therapeutic effects of dipeptidyl peptidase-4 inhibitor saxagliptin on patients with type 2 diabetes and its influences on β-cell function. Chinese J Diabetes 2014;22:494-6
- Xu Z, Lu M, Zhou Y, et al. Analysis of the use of oral antidiabetic drugs in our hospital from 2009 to 2012. Drug Eval 2013;10:14-18, 44
- Freeman JS. Managing hyperglycemia in patients with type 2 diabetes mellitus: rationale for the use of dipeptidyl peptidase-4 inhibitors in combination with other oral antidiabetic drugs. J Am Osteopath Assoc 2010;110:528-37
- Granstrom O, Bergenheim K, McEwan P, et al. Cost-effectiveness of saxagliptin (Onglyza(R)) in type 2 diabetes in Sweden. Prim Care Diabetes 2012;6:127-36
- Erhardt W, Bergenheim K, Duprat-Lomon I, et al. Cost effectiveness of saxagliptin and metformin versus sulfonylurea and metformin in the treatment of type 2 diabetes mellitus in Germany: a Cardiff diabetes model analysis. Clin Drug Investig 2012;32:189-202
- Elgart JF, Caporale JE, Gonzalez L, et al. Treatment of type 2 diabetes with saxagliptin: a pharmacoeconomic evaluation in Argentina. Health Econ Rev 2013;3:11-19
- McEwan P, Peters JR, Bergenheim K, et al. Evaluation of the costs and outcomes from changes in risk factors in type 2 diabetes using the Cardiff stochastic simulation cost-utility model (DiabForecaster). Curr Med Res Opin 2006;22:121-9
- McEwan P, Bergenheim K, Yuan Y, et al. Assessing the relationship between computational speed and precision: a case study comparing an interpreted versus compiled programming language using a stochastic simulation model in diabetes care. Pharmacoeconomics 2010;28:665-74
- McEwan P, Evans M, Bergenheim K. A population model evaluating the costs and benefits associated with different oral treatment strategies in people with type 2 diabetes. Diabetes Obes Metab 2010;12:623-30
- McEwan P, Evans M, Kan H, et al. Understanding the inter-relationship between improved glycaemic control, hypoglycaemia and weight change within a long-term economic model. Diabetes Obes Metab 2010;2:431-6
- Mount Hood Modeling Group. Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638-46
- Clarke PM, Gray AM, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59
- Hayes AJ, Leal J, Gray AM, et al. UKPDS outcomes model 2: a new version of a model to simulate lifetime health outcomes of patients with type 2 diabetes mellitus using data from the 30 year United Kingdom Prospective Diabetes Study: UKPDS 82. Diabetologia 2013;56:1925-33
- Sabale U, Ekman M, Granstrom O, et al. Cost-effectiveness of dapagliflozin (Forxiga(R)) added to metformin compared with sulfonylurea added to metformin in type 2 diabetes in the Nordic countries. Prim Care Diabetes 2015;9:39-47
- National Health and Family Planning Commission of the People's Republic of China. China health statistics yearbook 2013. Beijing, China. 2013. http://www.nhfpc.gov.cn/htmlfiles/zwgkzt/ptjnj/year2013/index2013.html. Accessed 20 April 2015
- World Health Organization. The world health report 2002- Chapter 5 (Some strategies to reduce risk– Technical considerations for cost-effectiveness analysis). Geneva, Switzerland. 2002. http://www.who.int/whr/2002/en/Chapter5.pdf?ua=1. Accessed 9 April 2015
- Marrett E, Radican L, Davies MJ, et al. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: a survey study. BMC Res Notes 2011;4:251
- Amiel SA, Dixon T, Mann R, et al. Hypoglycaemia in Type 2 diabetes. Diabetic Med 2008;25:245-54
- Zheng YM, Wu J, Xie K. Incidence and cost of hypoglycemia episode in patients with type 2 diabetes mellitus (T2DM). Chinese Rural Health Service Admin 2012;32:1195-8
- Lundkvist J, Berne C, Bolinder B, et al. The economic and quality of life impact of hypoglycemia. Eur J Health Econ 2005;6:197-202
- Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health 2008;11:478-86
- Lane S, Levy AR, Mukherjee J, et al. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin 2014;30:1267-73
- General Administration of Sport of China. National Physique Monitoring Bulletin (2010). Beijing, China. 2011. http://www.sport.gov.cn/n16/n1077/n297454/2052709.html. Accessed 29 December 2014
- Ji LN, Hu DY, Pan CY, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 Diabetes patients. Am J Med 2013;126:911-25
- UK PDSG. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet (N Am Ed) 1998;352:837-53
- National Bureau of Statistics of the People's Republic of China. Consumer Price Index of Residents. Beijing, China. 2014. http://data.stats.gov.cn/workspace/index?m=hgnd. Accessed 28 November 2014
- The Organisation for Economic Co-operation and Development OECD. Conversion rates- Exchange rates. Paris, France. 2014. https://data.oecd.org/conversion/exchange-rates.htm. Accessed 10 April 2015
- Gao L, Zhao FL, Li SC. Cost-utility analysis of liraglutide versus glimepiride as add-on to metformin in type 2 diabetes patients in China. Int J Technol Assess Health Care 2012;28:436-44
- Li HC, Xu F, Wang F. Cost-effectiveness of biphasic insulin aspart 30 combined with metformin in patients with type 2 diabetes mellitus. Chinese J New Drugs 2011;20:2163-70
- Palmer JL, Gibbs M, Scheijbeler H, et al. Cost-effectiveness of switching to biphasic insulin aspart in poorly-controlled type 2 diabetes patients in China. Adv Ther 2008;25:752-74
- Price Bureau. Official drug price list for saxagliptin. Zhejiang, China. 2014. http://www.zjpi.gov.cn/main/html/2014/CT10046/eec3f13a8b2d46258a93966273078df7.html. Accessed 27 December 2014
- Price Bureau. Official drug price list for glimepiride. Zhejiang, China. 2014. http://www.zjpi.gov.cn/main/html/2014/CT10046/dd72e4240a7e4c1da820a849642f7b37.html. Accessed 27 December 2014
- Hou XY, Zen Z, Tao X, et al. Cost-effectiveness analysis of 2 dosage forms of metformin hydrochloride in the treatment of type 2 Diabetes. China Pharmacy 2014;25:1844-7
- Guo H, Li J, Jiang ZL. Follow-up effects of the increased physical activity on the glucolipid metabolic factors and medical costs in type 2 diabetic patients. Chinese J Rehab Med 2007;22:395-8
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9
- Currie CJ, McEwan P, Peters JR, et al. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90
- Caro JJ, Stillman IO, Danel A, et al. Cost effectiveness of rimonabant use in patients at increased cardiometabolic risk: estimates from a Markov model. J Med Econ 2007;10:239-54
- National Bureau of Statistics of China. Gross Domestic Product (GDP) per capita. Beijing, China. 2013. http://data.stats.gov.cn/search/keywordlist2;jsessionid=387F523D9AF3519917849B593F8A3093?keyword=gdp. Accessed 20 April 2015
- Jonsson L, Bolinder B, Lundkvist J. Cost of hypoglycemia in patients with Type 2 diabetes in Sweden. Value Health 2006;9:193-8
- Schwarz B, Gouveia M, Chen J, et al. Cost-effectiveness of sitagliptin-based treatment regimens in European patients with type 2 diabetes and haemoglobin A1c above target on metformin monotherapy. Diabetes Obes Metab 2008;10(1 Suppl):43-55