Abstract
Objective:
Bipolar disorder imposes a high economic burden on patients and society. Lurasidone and quetiapine extended-release (XR) are atypical antipsychotic agents indicated for monotherapy treatment of bipolar depression. Lurasidone is also indicated as adjunctive therapy with lithium or valproate for depressive episodes associated with bipolar disorder. The objective of this analysis was to estimate the cost-effectiveness of lurasidone and quetiapine XR in patients with bipolar depression.
Methods:
A cost-effectiveness model was developed to compare lurasidone to quetiapine XR. The model was based on a US third-party payer perspective over a 3-month time horizon. The effectiveness measure in the model was the percentage of patients achieving remission (Montgomery-Åsberg Depression Rating Scale [MADRS] total score ≤12 by weeks 6–8). The comparison of remission rates was made through an adjusted indirect treatment comparison of lurasidone and quetiapine XR pivotal trials using placebo as the common comparator. Resource utilization for remission vs no remission was estimated from published expert panel data, and resource costs were obtained from a retrospective database study of bipolar I depression patients. Drug costs were estimated using the mean dose from clinical trials and wholesale acquisition costs.
Results:
Over the 3-month model time period, lurasidone and quetiapine XR patients, respectively, had similar mean numbers of emergency department visits (0.48 vs 0.50), inpatient days (2.1 vs 2.2), and office visits (9.3 vs 9.6). More lurasidone than quetiapine XR patients achieved remission (52.0% vs 43.2%) with slightly higher total costs ($4982 vs $4676), resulting in an incremental cost-effectiveness ratio of $3474 per remission. The probabilistic sensitivity analysis showed lurasidone had an 86% probability of being cost-effective compared to quetiapine XR at a willingness-to-pay threshold of $10,000 per remission.
Conclusions:
Lurasidone may be a cost-effective option when compared to quetiapine XR for the treatment of adults with bipolar depression.
Introduction
Bipolar disorder is a serious, chronic, and disabling mental illness that typically develops in the late teens or early adulthoodCitation1. In the US population, bipolar disorder has a lifetime prevalence of 4.4%, with a lifetime prevalence of 1.0% for bipolar I disorderCitation2. Patients with bipolar disorder have a significantly increased risk of various physical comorbidities (e.g., cardiovascular diseases, diabetes, and truncal obesity) compared to the general populationCitation3,Citation4. These patients with bipolar disorder are also at increased risk of disability, incarcerations, and suicideCitation2,Citation5,Citation6.
Bipolar I disorder is generally considered a more severe form of the disease and is characterized by manic or mixed episodes that last at least 7 days or by manic symptoms that are so severe that the person needs immediate hospital care. Patients with bipolar I disorder may also experience episodes of major depression, typically lasting at least 2 weeksCitation1.
Patients with bipolar disorder spend approximately triple the amount of time with major depressive symptoms compared to symptoms of maniaCitation7. Patients with bipolar disorder spend ∼50% of their time in a symptomatic stateCitation8,Citation9. Depressive episodes may lead to hospitalizationCitation10 and re-hospitalization with slower recoveryCitation11, and even mild depressive symptoms can lead to functional impairmentCitation12.
Bipolar disorder has a major economic impact on healthcare expenditures and is one of the most costly psychiatric disorders in the US. The total cost of bipolar disorder, including direct and indirect costs, was estimated to be ∼$151 billion in 2009Citation13. According to data from the Healthcare Cost and Utilization Project, there were ∼780,000 hospital stays in the US for patients with bipolar disorder in 2011, with an average length of stay (LOS) of 7.2 days and total costs of $5575 per stayCitation14. Bipolar disorder also imposes a considerable cost burden on patients, caregivers, and society. A large portion of these costs is related to lost employment on both the part of the caregiver and the patient. One study found that an estimated $29.8 billion could be attributed to morbidity in bipolar disorder (accounting for lost productivity of family caregivers and lost productivity due to institutionalization)Citation15. Bipolar disorder has also been associated with high costs related to short-term employee disability and work-related absencesCitation16. Accordingly, therapeutic agents that improve remission rates in bipolar disorder have the potential to confer substantial economic benefitCitation2.
Currently, two atypical antipsychotics are indicated as monotherapy for the treatment of depressive episodes associated with bipolar disorder. Seroquel XR (quetiapine extended-release [XR]), the first atypical antipsychotic agent to be approved for bipolar depression as a monotherapy, is an atypical antipsychotic in an extended-release formulation that is approved for (1) the acute treatment of manic or mixed episodes associated with bipolar I disorder, both as monotherapy and as an adjunct to lithium or divalproex, (2) acute treatment as monotherapy of depressive episodes associated with bipolar disorder, and (3) maintenance treatment of bipolar I disorder as an adjunct to lithium or divalproexCitation17. Latuda (lurasidone) is the only atypical antipsychotic approved for the treatment of major depressive episodes associated with bipolar depression as a monotherapy and as an adjunctive therapy with lithium or valproateCitation18. The objective of this analysis was to compare the cost-effectiveness of lurasidone and quetiapine XR as monotherapies for patients with bipolar I depression.
Methods
Model design
A decision analytic model was developed in Microsoft Excel to estimate the cost-effectiveness of lurasidone monotherapy vs quetiapine XR monotherapy for the acute treatment of major depressive episodes in adult patients diagnosed with bipolar depression. The model compared the direct mental healthcare costs of lurasidone with quetiapine XR over a 3-month time horizon from a US payer perspective.
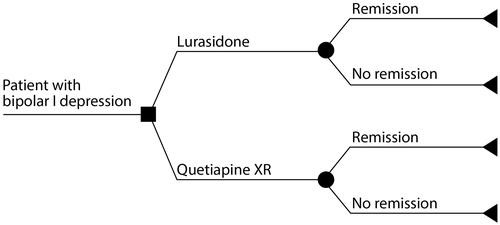
The model begins with a cohort of patients diagnosed with bipolar I depression in a state of acute depression. Patients receive treatment for 6 weeks, at which time treatment effectiveness is determined. After 6 weeks of treatment, patients either achieve remission or do not achieve remission and still remain in a state of acute depression (). Applying health states previously defined by an expert panelCitation19, levels of resource utilization vary based on patient clinical response (remission status) such that patients who achieve remission consume fewer healthcare resources compared to patients who remain in the depressive state. Resource utilization is then multiplied by the corresponding unit costs to obtain the total costs per patient. Modeled costs include pharmacy and medical (inpatient, outpatient, physician’s office, and emergency department [ED]) costs associated with the management of patients with bipolar I depression. No discounting of costs or outcomes was done due to the short time horizon of the model. A one-way deterministic sensitivity analysis (DSA) and probabilistic sensitivity analysis (PSA) were performed to assess the sensitivity of model cost outcomes to model parameters.
Data inputs
Effectiveness parameters
Treatment effectiveness was determined by the percentage of patients achieving remission vs no remission. Remission rates for lurasidone and quetiapine XR were based on rates from published studies shown in , where remission was defined as a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of ≤12 by week 6 (lurasidone) or 8 (quetiapine XR).
Table 1. Mean rates of remission for lurasidone, quetiapine XR, and placebo in the short-term treatment of bipolar disorder.
Our evaluation of the literature did not identify any study directly comparing lurasidone with quetiapine XR. In light of this review, an adjusted indirect treatment comparison was conducted, using placebo as the common comparator, to provide treatment effectiveness estimates as model inputs. In the indirect treatment comparison analysis, remission rates for lurasidone were pooled across two different lurasidone daily dose ranges (20–60 mg and 80–120 mg) from a 6-week, double-blind, placebo-controlled, fixed-flexible dose, parallel-group study in patients with bipolar I depressionCitation20. Remission rates for quetiapine XR were obtained from a similar 8-week, double-blind, placebo-controlled study in acutely depressed adults with bipolar I or II disorderCitation21. The populations between these two studies were slightly different in that the quetiapine XR trial included bipolar II disorder patients. However, the studies were similar in the inclusion/exclusion criteria, requiring that patients be adults with their most recent episode depressed as defined by the DSM-IV criteria. In addition, patients must have qualified for each of the studies by having a total score of ≤12 on the Young Mania Rating Scale (YMRS). Baseline demographics showed similar intervention and placebo group baseline MADRS total scores between the two study populations. Assessments and interpretation of remission as assessed by MADRS score were also the sameCitation20,Citation21.
Resource utilization parameters
Resource utilization parameters () were obtained from a study reporting results of an expert panel that estimated the resource use of patients with bipolar disorder in different health states in the USCitation19. Patients who achieved remission utilized fewer resources (ED visits, hospitalizations, and physician office visits) compared with patients who did not achieve remission. Resource use for patients not achieving remission was estimated based on patients in the acute depressive state, and resource use for patients achieving remission was based on patients in the recovery state. During the initial 6 weeks on medication, all patients were assumed to be in the acute depressive state. presents the resource utilization for patients for the remission or recovery states per 6-week time period, with the standard error assumed to be ±10% of the parameter value.
Table 2. Resource utilization parameters (per 6 week time period).
As adherence is known to affect treatment outcomes and can differ in a real-world setting, an assumption was made that patients achieving remission were fully adherent to their medication and that patients not achieving remission were only 80% adherent. Adherence in patients with bipolar disorder has been found to be ∼64%Citation22; therefore, the assumption of full adherence in patients achieving remission and 80% adherence in those not achieving remission is a conservative assumption, as it increased costs associated with remission relative to patients who remained in the acute depressive state. The effect of this assumption was also tested in the sensitivity analysis.
Cost parameters
Cost estimates for drugs were based on the mean daily doses reported in the clinical trials for lurasidone (57 mg) and quetiapine XR (300 mg) and the reported wholesale acquisition cost of eachCitation20,Citation21,Citation23. Cost estimates for resources were based on a retrospective database study of patients diagnosed with bipolar I depression using a Truven Health MarketScan Commercial and Medicare Supplemental database (). The study evaluated healthcare expenditures for patients (n = 43,049) with visits associated with bipolar I depression between July 1, 2011 and June 30, 2012Citation24.
Table 3. Cost parameters.
Sensitivity analysis
Parameter sensitivity was evaluated using a one-way DSA and PSA. The one-way DSA was conducted to quantify the impact of parameter uncertainty on the model outcomes by changing the value of a single model parameter at a time. For all model parameters, the low and high values were based on the 95% confidence interval (CI), estimated using the mean and standard error (SE) or based on a specified percentage range where the SE was not available.
A PSA was conducted to simultaneously quantify the impact of uncertainty of all model parameters by sampling all parameters in 1000 Monte Carlo simulations. Samples for all model parameters (e.g., response/remission rates, resource utilization rates, and costs) were obtained by sampling from a distribution using the mean and SE. Beta distributions were used for remission rates and normal distributions were used for resource utilization and cost parameters.
Results
Over the 3-month model time horizon, the adjusted indirect treatment comparison results showed that 52.0% of lurasidone patients and 43.2% of quetiapine XR patients achieved remission (). The mean numbers of ED visits, inpatient days, and office visits were similar between lurasidone patients (0.48, 2.1, 9.3) and quetiapine XR patients (0.50, 2.2, 9.6), respectively. The mean pharmacy costs for lurasidone patients were $1899 (95% CI = $1573, $2241) and for quetiapine XR patients were $1455 (95% CI = $1260, $3469), and the medical costs were $3083 (95% CI = $2101, $4195) for lurasidone patients and $3222 (95% CI = $2207, $4359) for quetiapine XR patients. Mean total costs were slightly higher for lurasidone patients ($4982; 95% CI = $3965, $6135) than quetiapine XR patients ($4676; 95% CI = $3632, $5835). In the incremental cost-effectiveness analysis, lurasidone results in an incremental cost-effectiveness ratio (ICER) of $3474 per remission gained compared to quetiapine XR ().
Table 4. Cost-effectiveness results.
Sensitivity analysis
The impact of inputs on model outcomes was evaluated via one-way sensitivity analyses by varying the model parameters using the 95% CIs. The one-way sensitivity analysis results are displayed in for the parameters that had the largest impact on ICERs. The results were most sensitive to remission rates and costs of lurasidone and quetiapine XR. Parameters not shown in had little impact on the model results.
Table 5. One-way sensitivity analysis results.
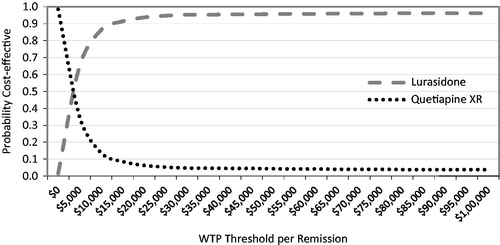
The PSA estimated the robustness at various willingness-to-pay thresholds. Results suggest that the model was fairly robust and that lurasidone had a 65% probability of being cost-effective compared with quetiapine XR at a willingness-to-pay (WTP) threshold of $5000 per remission gained, increasing to 86% at a WTP of $10,000 per remission gained and over 90% at WTP thresholds of $15,000 and higher ().
Discussion
Our model demonstrates that lurasidone may be a cost-effective treatment option for patients with bipolar depression compared with quetiapine XR. Lurasidone results in an ICER of $3474 per remission gained compared with quetiapine XR. Whether this is considered cost-effective by a particular healthcare payer would depend upon their WTP threshold for a remission, the primary clinical treatment goal in bipolar depression.
Remission is associated with reduced healthcare utilization and, ultimately, lower healthcare costs. While no real-world analyses are available in the literature that evaluate the comparative costs and consequences of bipolar depression treatments in terms of remissions gained, previous studies have shown significant increases in the cost of medical services in outpatients with depressive symptomsCitation26–28. Our study is not only the first health economic study to evaluate lurasidone for treatment of bipolar depression, but it is also the first health economic model to focus on remission in bipolar depression.
Few economic models have been published on the use of atypical antipsychotics in bipolar disorder. A systematic review published in 2007 identified only 14 health economic studies in bipolar disorder, including seven claims data analyses, four trial-based economic evaluations, and three cost-effectiveness analysesCitation28. A more recently published pharmacoeconomic review of quetiapine in bipolar disorderCitation29 reviewed three cost-effectiveness analyses that evaluated quetiapine as adjunctive maintenance therapy for bipolar I disorder from the healthcare payer perspective in the US and the UK. Of these, two were studies of quetiapine immediate-release (IR) and one was of quetiapine XR. Each of the studies employed a Markov methodology, utilizing the health states of euthymia, acute mania, and acute depression, with 3-month cycles over a 2-year time horizon. These studies found that adjunctive quetiapine was cost-effective and was either dominant or had a generally acceptable ICERCitation30–32. The parameters with the greatest effect on the results were the proportion of patients being hospitalized, the length of hospitalization, and the cost of quetiapineCitation29. In contrast to the present analysis that focuses on depressive symptoms, prior research included general bipolar I populations.
The comparative cost-effectiveness of quetiapine XR and olanzapine for patients with bipolar depression has also been studied over a 5-year time horizonCitation33. A discrete event simulation from a UK payer perspective found that quetiapine XR had an ICER of £8600 per quality-adjusted life-year (QALY) compared with olanzapineCitation33. The longer time horizon of this study necessitated assumptions about the efficacy of subsequent lines of treatment and the use of QALYs as an end-point required estimates and assumptions around health utilities.
Several limitations of the present study should be noted. Due to a lack of available head-to-head clinical trials in bipolar disorder comparing lurasidone and quetiapine XR, the comparisons were indirect. In the quetiapine study, 19.5% of patients had bipolar II disorder, which may affect the comparability of the study populations. Whether the inclusion of the bipolar II population (compared to the lurasidone population with all bipolar I depression patients) affects the results in a positive or negative direction is unclear. Additional analyses would be warranted given the availability of head-to-head trials or additional trials in more similar patient populations. Additionally, the quetiapine XR study was an 8-week trial, while lurasidone was 6 weeks, which may comparatively under-estimate the remission rates for lurasidone. However, for the purposes of this analysis, the outcomes at the end of each study were used and no attempt was made to adjust for the difference in study duration. As such, the model represents the short-term cost-effectiveness of treating an episode of depression.
Healthcare decision-makers may seek information concerning outcomes over a longer time horizon to better capture real-world outcomes and costs, but extrapolation of the present findings to longer durations would likely result in greater uncertainties in the model estimates. Studies that evaluate response, remission, and relapse over a longer time horizon may show lower rates of remission, as some patients are unable to achieve sustained remission. If and when longer-term studies become available for quetiapine and lurasidone, additional analyses are warranted.
Adverse events were not included in the cost-effectiveness analysis. Differing adverse event profiles may affect the costs associated with each therapy. However, other pharmacoeconomic analyses have generally shown discontinuation and adverse event rates to have a minimal impact on the overall short-term costs of care in sensitivity analysesCitation33. Adverse events may be more likely to affect longer than shorter time estimates of comparative cost-effectiveness. Future iterations of a model comparing longer-term data should incorporate adverse event profiles in order to fully understand lurasidone and quetiapine XR cost-effectiveness.
It was assumed that patients achieving remission were fully adherent to therapy and patients not achieving remission were 80% adherent. These adherence rates likely over-estimate costs, as real-world adherence rates are probably lower. Because of this assumption, the model likely attributes higher-than-actual pharmacy costs, resulting in more conservative estimates of cost-effectiveness.
Resource utilization estimates for bipolar disease states were obtained from a study involving an expert panel on bipolar disease burden. To the extent that resource utilization varies from these estimates, the model results may be affected. However, these parameters had a negligible impact in sensitivity analyses. Resource costs were obtained from a large retrospective database analysis and drug costs were determined using mean drug utilization rates and published Red Book costs as of April 2014. To the extent that payer costs vary from these estimates, the model results may change. However, these parameters also had only a modest impact in the one-way sensitivity analyses.
Conclusions
Based on the model, lurasidone may be a cost-effective treatment option compared to quetiapine XR for the short-term treatment of bipolar depression, offering potentially higher remission rates at a modest additional cost. Further investigations of lurasidone’s cost-effectiveness over a longer duration and in real-world settings are warranted.
Transparency
Declaration of funding
This manuscript was sponsored by Sunovion Pharmaceuticals Inc.
Declaration of financial/other relationships
KR and AL are employees of Sunovion. KO, KM and MD are employees of Xcenda, a consulting firm that received funding from Sunovion to assist with this research. The authors are entirely responsible for the scientific content of the paper.
Acknowledgments
This manuscript was supported with copyediting assistance by Kylie Matthews, Xcenda. This manuscript was also reviewed by Daisy Ng-Mak, PhD, from Sunovion.
References
- National Institute of Mental Health. Bipolar disorder. Rockville, MD: U.S. Department of Health and Human Services, 2012. http://www.nimh.nih.gov/health/publications/bipolar-disorder-in-adults/Bipolar_Disorder_Adults_CL508_144295.pdf. Accessed May 2015
- Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the national comorbidity survey replication. Arch Gen Psychiatry 2007;64:543-52
- Fagiolini A, Frank E, Scott JA, et al. Metabolic syndrome in bipolar disorder, findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord 2005;7:424-30
- Vancampfort D, Vansteelandt K, Correl CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry 2013;170:265-74
- Huxley N, Baldessarini RJ. Disability and its treatment in bipolar disorder patients. Bipolar Disord 2007;9:183-96
- Gardner HH, Kleinman NL, Brook RA, et al. The economic impact of bipolar disorder in an employed population from an employer perspective. J Clin Psychiatry 2006;67:1209-18
- Kupka RW, Altshuler LL, Nolen WA, et al. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord 2007;9:531-5
- Judd L, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 2002;59:530-7
- Judd L, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry 2003;60:261-9
- Fu AZ, Krishnan AA, Harris SD. The burden of depression patients with bipolar disorder. Abstract NR556. Presented at: American Psychiatric Association 157th Annual Meeting; May 1-6, 2004; New York, NY
- Perlick DA, Rosenheck RA, Clarkin JF, et al. Symptoms predicting inpatient service use among patients with bipolar affective disorder. Psychiatr Serv 1999;50:806-12
- Altshuler LL, Gitlin MJ, Mintz J, et al. Subsyndromal depression is associated with functional impairment in patients with bipolar disorder. J Clin Psychiatry 2002;63:807-1
- Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States: 2009. J Affect Disord 2011;129:79-83
- National and regional estimates on hospital use for all patients from the HCUP Nationwide Inpatient Sample (NIS), 2011. Rockville, MD: AHRQ. http://hcupnet.ahrq.gov/HCUPnet.jsp. Accessed December 4, 2013
- Kleinman L, Lowin A, Flood E, et al. Costs of bipolar disorder. Pharmacoeconomics 2003;21:601-22
- Goetzel RZ, Hawkins K, Ozminkowski RJ, et al. The health and productivity cost burden of the “top 10” physical and mental health conditions affecting six large U.S. employers in 1999. J Occup Environ Med 2003;45:5-14
- Seroquel XR [package insert]. Wilmington, DE: AstraZeneca, 2013
- Latuda [package insert]. Marlborough, MA: Sunovion Pharmaceuticals Inc., 2013
- Begley CE, Annegers JF, Swann AC, et al. The lifetime cost of bipolar disorder in the US: an estimate for new cases in 1998. Pharmacoeconomics 2001;19:483-95
- Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry 2014;171:160-8
- Suppes T, Datto C, Minkwitz M, et al. Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression. J Affect Disord 2010;121:106-15
- Leclerc E, Mansur RB, Brietzke E. Determinants of adherence to treatment in bipolar disorder: a comprehensive review. J Affect Disord 2013;149:247-52
- Red Book Online®. Chicago, IL: Truven Health Analytics, 2014. http://www.micromedexsolutions.com. Accessed June 11, 2014
- Data on file. Truven Health MarketScan® Commercial and Medicare Supplemental Analysis for bipolar depression. Marlborough, MA: Sunovion Pharmaceuticals Inc., 2013
- McIntyre RS, Fallu A, Konarski JZ. Measureable outcomes in psychiatric disorders: remission as a marker of wellness. Clin Ther 2006;28:1882-91
- Unützer J, Patrick DL, Simon G, et al. Depressive symptoms and the cost of health services in HMO patients aged 65 years and older. A 4-year prospective study. JAMA 1997;277:1618-23
- Simon G, Ormel J, VonKorff M, et al. Health care costs associated with depressive and anxiety disorders in primary care. Am J Psychiatry 1995;152:352-7
- Fleurence FL, Chatterton ML, Dixon JM, et al. Economic outcomes associated with atypical antipsychotics in bipolar disorder: a systematic review. Prim Care Companion J Clin Psychiatry 2007;9:419-28
- Plosker GL. Quetiapine: a pharmacoeconomic review of its use in bipolar disorder. Pharmacoeconomics 2012;30:611-31
- Fajutrao L, Paulsson B, Liu S, et al. Cost-effectiveness of quetiapine plus mood stabilizers compared with mood stabilizers alone in the maintenance therapy of bipolar I disorder: results of a Markov model analysis. Clin Ther 2009;31:1456-68
- Woodward TC, Tafesse E, Quon P, et al. Cost-effectiveness of quetiapine with lithium of divalproex for maintenance treatment of bipolar I disorder. J Med Econ 2009;12:259-68
- Woodward TC, Tafesse E, Quon P, et al. Cost effectiveness of adjunctive quetiapine fumarate extended-release tablets with mood stabilizers in the maintenance treatment of bipolar I disorder. Pharmacoeconomics 2010;28:751-64
- Ekman M, Lindgren P, Miltenburger C, et al. Cost effectiveness of quetiapine in patients with acute bipolar depression and in maintenance treatment after an acute depressive episode. Pharmacoeconomics 2012;30:513-30