Abstract
Objective:
To estimate cost-effectiveness of exenatide twice daily (BID) vs insulin glargine once daily (QD) as add-on therapy in Chinese type 2 diabetes patients not well controlled by oral anti-diabetic (OAD) agents.
Methods:
The Cardiff model was populated with data synthesized from three head-to-head randomized clinical trials of up to 30 weeks in China comparing exenatide BID vs insulin glargine as add-on therapies to oral therapies in the Chinese population. The Cardiff model generated outputs including macrovascular and microvascular complications, diabetes-specific mortality, costs, and quality-adjusted life years (QALYs). Cost and QALYs were estimated with a time horizon of 40 years at a discount rate of 3% from a societal perspective.
Results:
Compared with insulin glargine plus OAD treatments, patients on exenatide BID plus OAD gained 1.88 QALYs, at an incremental cost saving of Chinese Renminbi (RMB) 114,593 (i.e., cost saving of RMB 61078/QALY). The cost-effectiveness results were robust to various sensitivity analyses including probabilistic sensitivity analysis. The variables with the most impact on incremental cost-effectiveness ratio included HbA1c level at baseline, health utilities decrement, and BMI at baseline.
Conclusions:
Compared with insulin glargine QD, exenatide BID as add-on therapy to OAD is a cost-effective treatment in Chinese patients inadequately controlled by OAD treatments.
Introduction
Diabetes Mellitus, which is ranked at the top of the chronic conditions list in China, imposes an economic burden and great challenges for healthcare systemsCitation1,Citation2. The most recent investigation in 2010 estimated that 113.9 million adults in China may have diabetes and 493.4 million may have pre-diabetesCitation3. Glycosylated hemoglobin (HbA1c) is the gold standard that reflects the glycemic control level. Chinese Diabetes Society (CDS) and American Diabetes Association’s (ADA) guidelines, both of which are similar in treatment algorithm, have set the glycemic control goal as HbA1c <7.0% for most patients with type 2 diabetesCitation4,Citation5. The rescue therapy for CDS guideline is different from the ADA’s guideline: basal insulin therapy and neutral protamine hagedorn (NPH) insulin are widely adapted as rescue therapy in China when the current treatment fails to control HbA1c under the optimal level. While the disease burden of diabetes escalates in China, the majority of Chinese type 2 diabetes mellitus (T2DM) patients have poor glycemic control; only 25.8% of diabetes patients receive pharmacological treatment, and only 39.7% of those treated have adequate glycemic controlCitation3. The excessive costs and mortality associated with diabetes are largely due to diabetes complicationsCitation6 and metabolic risk-factors including obesityCitation7,Citation8. Seventy-two per cent of diabetes patients in China are reported to suffer from co-morbid hypertension, dyslipidemia, or bothCitation9. Weight control is essential in order to reduce the long-term health impact in T2DM patientsCitation8.
Exenatide twice daily (BID) and insulin glargine once daily (QD) are both recommended as third-line therapies for management of T2DM in ChinaCitation4. Exenatide BID significantly improves glycemic control with favorable effects on weight and cardiovascular risk markersCitation10, and it is recommended for patients to receive 5 μg subcutaneous (sc) twice daily (BID) for 4 weeks followed by 10 μg sc BID for the restCitation11. Insulin glargine is a long-acting basal insulin analog that works similarly to endogenous basal insulin. It is recommended for subcutaneous administration once daily at bedtime in adults with type 1 or 2 diabetesCitation12. Comparative efficacy studies of exenatide BID vs insulin glargine on T2DM patients have shown that exenatide BID have a similar effect to Insulin glargine on lowering the HbA1c levelCitation13, but fewer episodes of hypoglycemia were observedCitation14,Citation15. The risk of hypoglycemia is lower with exenatide BID than insulin glargine because it has glucose-dependent insulinotropic and glucagon protects against hypoglycemiaCitation16. Exenatide BID is associated with weight loss of ∼2–3 kg over 26 weeks compared to insulin glargine, although it causes more gastrointestinal adverse eventsCitation17. Since hypoglycemia and weight gain associated with anti-diabetic treatments have been observed to independently increase risk of cardiovascular diseases and treatment costsCitation18,Citation19, exenatide BID with superior hypoglycemia risk and weight profiles may be a cost-effective anti-diabetic treatment compared to insulin glargine as add-on therapies among patients who are not well controlled by oral anti-diabetic (OAD) agents.
There is a lack of cost-effectiveness evidence for the long-term benefit of exenatide BID in China, although a number of cost-effectiveness studies comparing exenatide BID vs insulin glargine as add-on therapies were carried out in different countries including SpainCitation20, GermanyCitation21, SwitzerlandCitation22, the UKCitation23,Citation24, PortugalCitation25, and TurkeyCitation26. Because head-to-head trials between exenatide BID and insulin glargine as add-on therapies to oral agents are emerging in China, our study from a societal perspective aimed to further examine the projected long-term cost-effectiveness of exenatide BID + OAD, compared with Insulin glargine + OAD in T2DM patients inadequately controlled by OADs in China.
Materials and methods
Cost-effectiveness model
The Cardiff T2DM model is a well-established cost-effectiveness modelCitation27–29, and was used for model adaption to compare exenatide BID with insulin glargine as an add-on therapy in China. The model predicted the number of cumulative diabetes-related complications (macrovascular and microvascular events), hypoglycemia events, diabetes mortality, non-diabetes mortality, and cost-effectiveness results. UKPDS 68 equationsCitation30 were used as the basis for simulation of macrovascular and microvascular events in the Chinese population. The simulation cohort consisted of 1000 individuals over 40 years of age (a life-time horizon, as the average entrance age of the cohort is 52 years of age)Citation31. Based on the WHO guidelines, the costs and benefits were discounted at the rate of 3% annually in China.
Patient population, treatment strategies, HbA1c threshold, and outcomes
The demographic and efficacy profiles of the patients were synthesized from three head-to-head randomized clinical trials in the Chinese populationCitation32–34. The study selection procedure is presented in Appendix 1. The cohort that entered the simulation was characterized by patients’ risk factors (e.g., HbA1c level, weight) profile ().
Table 1. Baseline demographics and risk factors.
Both exenatide BID and insulin glargine QD were modeled on patients with poor glycemic control at baseline. The HbA1c level progressed with time for patients on therapies. In the model, we used a 7.5% threshold for switching to pre-specified basal insulin therapy and neutral protamine hagedorn (NPH) insulin, which is a common rescue therapy in China. The HbA1c therapy escalation threshold of 8% was tested in the one-way sensitivity analysis.
Weight gain/loss
Weight changes affected the utility at different rates based on different weight levels. A weight increase had a larger negative impact on utility than the disutility caused by the weight decreaseCitation35–37. For this model, unit weight changes associated with health utility change were modeled linearly. Body-weight changes also had an impact on the long-term risk of cardiovascular complications and the cost of insulin, which is dosed by body weightCitation38.
Hypoglycemia
Most of the hypoglycemic cases reported during patient visits were of mild-to-moderate severity. Severe hypoglycemia was usually an episode requiring third party assistance due to severe impairment in consciousness according to the CDS GuidelinesCitation4. Each therapy in the model was assigned a fixed number of hypoglycemic events per patient in every cycle. The probability for severe hypoglycemia was assumed to be 2% of all the hypoglycemic cases reported, and the other 98% was assumed to be symptomatic hypoglycemia, based on an observational study of hypoglycemia in ChinaCitation39. Since severe hypoglycemia cannot be easily cured by self-treatment, it is an important driver of the disutility generated by fear of hypoglycemia. The utility decrements associated with fear of hypoglycemia are applied to the year of occurrence and are based on Hypoglycemia Fear Survey DataCitation40.
Other adverse events
The most common adverse events for exenatide BID treatment were gastro-intestinal reactions, of which the incidence rate is higher than that of the insulin glargine treatment. Gastrointestinal reactions were modeled to affect only quality-of-life in the Cardiff model.
Input data
Clinical trial data
The primary efficacy parameters for the model were the reduction in HbA1c level for the first 30 weeks (). The information of HbA1c reduction was created from meta-analysis of three trialsCitation32–34 after the literature search was completed using procedures as described in Appendix 1. Exenatide BID + OAD therapy was associated with weight loss in patients, while the treatment of insulin glargine + OAD was associated with weight gain. In the base case analysis, weight changes were assumed to be maintained for the entire modeled life cycle.
Table 2. Clinical efficacy and adverse events in 30 weeks based on literature review and meta-analysis.
Costs
The analysis included direct cost for medications, annual fatal and non-fatal cost at the inceptions of complications, followed by the direct cost for maintenance treatment. The data sources and calculations for exenatide BID and insulin glargine as well as OAD represented by metformin are shown in . For exenatide BID, the cost was calculated as twice per day (BID) based on the dosing information from three head-to-head studiesCitation32–34. The retail price of exenatide BID was obtained from the Zhejiang Provincial Price Bureau’s 2004–2012 government-regulated highest retail priceCitation41. For glargine, the retail price was obtained from the Kangdele Pharmacy retail priceCitation42. Both prices are for the original branded products. The cost of consumables for injections of both comparison therapies were not included (i.e., test strips, needles, lancets). Although glargine is once daily and exenatide is twice daily, we believe this will hardly influence our results, as the cost of consumables is only a small proportion of the overall cost.
Table 3. Annual treatment costs for study drugs.
From the OAD treatments in the three trials, only metformin dosage data were available for ascertainment and used for estimating OAD cost. The annual drug costs for metformin treatment was estimated from a cost-effectiveness study comparing two forms of metformin tabletsCitation43. The fatal, non-fatal costs, and maintenance costs were primarily based on a published study of Chinese diabetes patientsCitation44 (). The costs for severe hypoglycemia were primarily based on the Zheng et al.Citation39 study, which investigated the incidence and cost of hypoglycemia episodes in T2DM patients from hospitals in Beijing and Tianjin. We have also done a hospital field survey of one tertiary and one secondary hospital in Zhejiang province (Appendix 2). The hospital information system provided inpatient hospital records on the diagnosis, prescriptions, duration, and frequency of hospitalization, and a breakdown of medical costs. The total costs collected from the two hospitals were synthesized together to form an alternative cost profile, which was tested in the sensitivity analysis of cost estimates. All costs for complication events and hypoglycemia were converted to 2014 using the Chinese Consumer Price Index (National Statistics Bureau)Citation45.
Table 4. Annual direct medical costs for diabetes-related complications and severe hypoglycemia (2014¥).
Health state utilities
Utility decrements associated with the T2DM complication events are presented in . Since no country-specific decrements exist for China, the disutilities were adopted from the UKPDS 62 studyCitation46. Disutility for hypoglycemia events were taken from Currie et al.Citation40. The change of utility per unit change in body mass index (BMI) were modeled from the study by Lane et al.Citation37. While the disutility associated with gastro-intestinal reaction was not available for the Chinese population either, the disutility scores from the population in England and ScotlandCitation35 were adopted in the model.
Table 5. Health utility decrements used in base case analysis.
Sensitivity analysis
The impact of uncertainty around model inputs were assessed in both one-way sensitivity analysis and probabilistic sensitivity analysis (PSA) performed as a second order Monte-Carlo simulation. The HbA1c profile weight changes followed a normal distribution. The probability of severe hypoglycemic event and utility decrements were modeled with beta distribution. The costs were modeled by gamma distribution. All those choices on distribution forms are default distribution settings in the Cardiff Model. Scatter plots of the ICER and a cost effectiveness acceptability curve (CEAC) were generated. In addition, sensitivity analyses were conducted to address the data variations such as selection of China’s local clinical trials vs multi-national clinical trial, and costs of diabetes complications from a field survey. The discount rate of 5% was used in the one-way sensitivity analysis.
Results
Base case analysis
presents the progression of HbA1c in both exenatide BID and Insulin glargine arms. Exenatide BID treatment was associated with weight loss over time, while the insulin glargine treatment was associated with weight gain over time (). In the base case analysis, the exenatide BID + OAD regime was associated with fewer macrovascular events and microvascular events. Consistent with the differences in cases of macrovascular and microvascular events, the costs for these events were all lower in the exenatide BID + OAD treatment arm compared to insulin glargine + OAD. For an individual patient, the total discounted cost accumulated over the lifetime on the treatment (exenatie BID + OAD) and control (insulin glargine QD + OAD) arm were ∼RMB 265,972 and RMB 380,565, respectively. The difference in cost (i.e., cost saving of exenatide BID + OAD arm) was RMB −114,593 over 40 years on average. The discounted QALYs for the exenatide BID and insulin glargine arms were 13.48 and 11.60, respectively. The difference in QALYs (i.e., the improvement in QALY associated with exenatide BID + OAD arm) was 1.88 QALYs gained. While the incremental cost-effectiveness ratio (ICER) was RMB −61,078/QALY, the results suggested that exenatide BID + OAD treatment was dominant over the glargine + OAD treatment ().
Figure 1. Simulated progression of HbA1c in exenatide BID + OAD (treatment arm) and insulin glargine QD + OAD (control arm) over the time horizon 40 years with a treatment escalation threshold of HbA1c ≥7.5%.
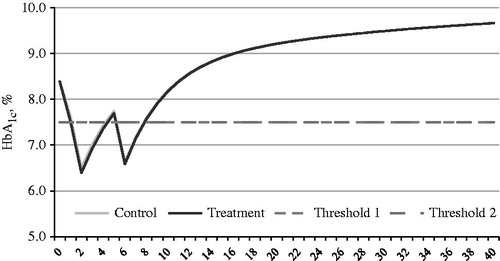
Figure 2. Simulated progression of weight in exenatide BID + OAD (treatment arm) and Insulin glargine QD + OAD (control arm) over the modeled time horizon.
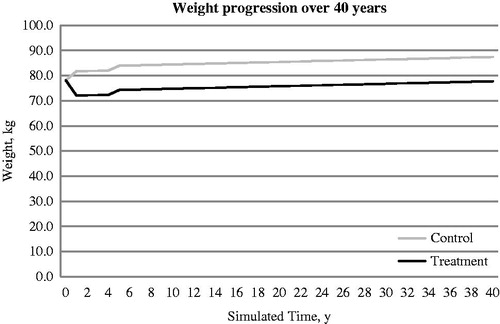
Table 6. Module output results for exenatide BID + OAD compared to insulin glargine QD + OAD.
The major difference between exenatide BID + OAD and insulin glargine + OAD was the medication treatment cost and the BMI-associated cost for a cohort of 1000 patients. The medication cost for the exenatide BID + OAD arm (RMB 77,245,179) was higher than the insulin glargine + OAD arm (RMB 66,230,332). However, the difference was offset by the difference in BMI-associated cost of the exenatide BID + OAD and insulin glargine + OAD treatments (RMB 124,837,331 vs RMB 250,169,538, respectively). In addition, episodes of symptomatic hypoglycemia were relative higher in the control arm (12,601 vs 12,545). As a result, the costs for hypoglycemia events were also higher in the control arm.
Sensitivity analysis
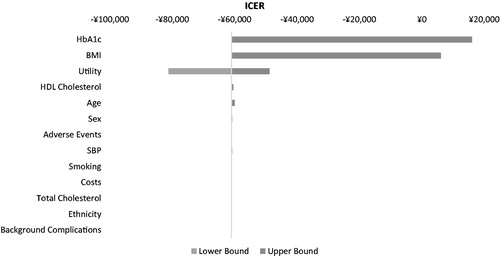
presents one-way sensitivity analyses as a Tornado diagram. The most influential factors for the ICER included HbA1c, health utilities, and BMI. Exenatide BID as add-on therapy was still dominant when baseline age changed from 40–70 years (ICER results available upon request). Further, we varied health utility decrement per unit BMI gain and BMI cost (). When the health utility decrement per unit BMI gain reduced to 75%, the incremental QALYs decreased from 1.88 to 1.50. As expected, when the BMI-associated cost was reduced by 50%, cost saving was shrunk by ∼75% (−27,677/QALY). When the BMI cost was reduced by 75%, the ICER changed from RMB −61,078/QALY to RMB −10,976/QALY. When the BMI cost was excluded from the model, the exenatide BID + OAD strategy cost more than insulin glargine + OAD. The ICER in this scenario (no BMI cost) was RMB 5724/QALY, which was still within the acceptable range of 2013 GDP per capita of China (RMB 43,320)Citation47. When the therapy escalation threshold increased to 8%, there was still a cost saving of RMB −12,811/QALY. The ICER was also not sensitive to cost of hypoglycemia, the cost of complication events, and the complication events’ cost profile estimated from the field survey (Appendix 2, 3).
Table 7. One-way sensitivity analysis on selected variables for exenatide BID + OAD vs glargine QD + OAD in China*.
In addition, considering the slightly different OAD profiles of the three head-to-head studies (two studies of metformin only as OAD, one study of unspecified OAD), we did extra sensitivity analyses. After excluding the study with unspecified OADCitation33, the ICER result was RMB −49,549/QALY with discounted incremental cost of RMB −131 676 and discounted incremental QALYs of 2.66. Further, the ICER was RMB 23,443/QALY after the BMI cost input was set to zero.
We have also replaced the key variables (age, female proportion, duration of diabetes, HbA1c, weight change) of the model with the estimates from the Heine et al.Citation48 exenatide BID vs insulin glargine multi-country trial. The results from this sensitivity analysis suggested that exenatide BID was either a dominant treatment strategy (i.e., cost saving of RMB −20,652/QALY and greater QALY gained) with a component of BMI-associated cost, or cost-effective (RMB 19,768/QALY) without cost impact of BMI (input data available upon request).
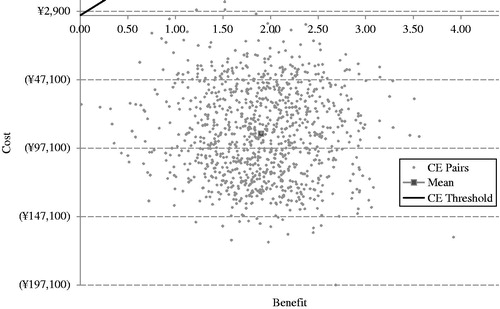
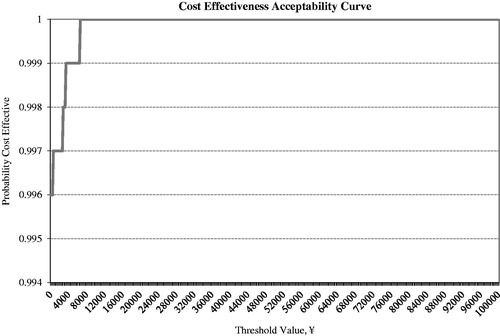
In the PSA, the average incremental quality adjusted life years remained approximately the same amount as the results of base case analysis (1.90 vs 1.88), while the average incremental cost was RMB −86,388, similar to the incremental cost in the base case analysis (RMB −114,593). As a result, the ICER of RMB −45,439/QALY in the PSA was also close to RMB −61,078/QALY in the base case analysis. shows the ICER scatter plot based on the PSA. All of the data points (100%) were found in the Southeast quadrant. This scatter plot suggested that exenatide BID compared to insulin glargine as add-on treatments had a greater improvement in quality adjustment life year while saving money at the same time. showed the CEAC for the base case analysis based on the PSA.
Discussion and conclusion
This is the first study using the Cardiff model comparing long-tern cost and health outcomes between exenatide BID and insulin glargine as add-on therapies to OAD treatments for Chinese patients who are not well controlled on OADs in China. The study simulated a cohort of patients who were inadequately controlled on OAD agents. The efficacy data were based on a meta-analysis of three head-to-head randomized clinical trials in China. Compared with insulin glargine + OAD, exenatide BID + OAD was a dominant therapy (cost-saving and better QALYs gained). The cost-effectiveness result was robust to a set of rigorous sensitivity analysis to address data variation (Chinese studies vs multi-national study), the cost of complication events in China (literature vs hospital field survey data), and treatment cost estimated by metformin only (i.e., excluding the article of unspecified OAD). The uncertainties in BMI cost, utility decrements associated with BMI increase, and hypoglycemic-case costs are all tested in the sensitivity analysis.
Although there is a lack of long-term clinical trials or observational studies in China for verification of our model outputs, one US studyCitation49 appears to provide similar results to our analysis. A retrospective analysis using a large managed care claims database in the US has reported that exenatide BID treatment has significantly lower total medical costs, despite higher total prescription costs. The results of greater improvement in QALY for the exenatide BID arm compared to the insulin glargine arm are also consistent with the results of other studies. While most of the studies have reported positive incremental QALYs over a time horizon of 10–35 yearsCitation20, the study by Woehl et al.Citation24 has shown a reverse relationship where glargine treatment has a better improvement in QALYs than exenatide BID treatment. The main difference between the Woehl et al. study and the others is the QALY estimation approach. The Woehl et al. study does not include utility estimates regarding weight changes and adverse events due to treatment; however, analysis of Health Outcome Data Repository (HODaR) data, which examine longitudinal weight and utility, suggests that utility gain becomes increasingly important when a patient gains or loses a great amount of weight (>5 kg)Citation50. Furthermore, the higher the initial weight of the patient, the more likely the patient will benefit from weight loss.
The cost-effectiveness results in our analysis are mainly the result of the differences in HbA1c control and weight progression between the two comparison therapies. The ICER is affected by costs associated with weight gain and utility decrements with increasing BMI; however, the ICER in the cost-effectiveness plane changed from dominant to the Northeast quadrant (positive value of ICER) after reducing the BMI associated cost to zero, and yet the positive value of ICER is still lower than the one-time GDP per capita in China (RMB 43,320). Hypoglycemic risk is not an important factor in the analysis as judged from the one-way sensitivity analysis results. Hypoglycemia may not affect the robustness of the conclusion because the case numbers or frequencies are close in the two arms.
Compared to the cost-effectiveness studies done by other countries via a multinational, randomized clinical trial conducted by Heine et al.Citation48 (clinical trial.gov identifier: NCT00082381), several features of our study contribute to more favorable results of exenatide BID, with or without the BMI cost being taken into account. First, it is based on the demographic and efficacy data generated from three head-to-head randomized clinical trials of exenatide BID vs insulin glargine as add-on therapies in the Chinese population. The reduction in HbA1c of exenatide BID (−1.42%) is larger than glargine (−1.25%) over 30 weeks, and these differences in HbA1c reduction effects are greater than the data reported by Heine et al.Citation48, which are −1.16% and −1.14% for exenatide BID and glargine over 26 weeks, respectively. Also, in studies of other Asian populations, the HbA1c reduction for exenatide BID was −1.13%Citation9. Second, the weight loss of exenatide BID (−7.95 kg) vs weight gain of insulin glargine (+1.71 kg) is also much larger than that observed in the population in the Heine et al.Citation48 trial, which were −2.3 kg and 1.8 kg for exenatide BID and glargine, respectively. Third, we have incorporated the BMI associated costs based on the Chinese diabetes patient data in our analysis. The inclusion of BMI associated costs tends to favor the cost-effectiveness of exenatide BID treatment, as measured by ICER; however, even without taking BMI cost into account, the ICERs under different input variables are still lower than the level of GDP per capita of 2013 in China. Lastly, all other pharmacoeconomic studies on exenatide BID vs insulin glargine were based on the IMS CORECitation51,Citation52 model, which does not have a component for BMI-related cost, compared with the Cardiff model (see Materials and method). suggests that most of the weight loss with exenatide BID is maintained over the lifetime horizon, with a small weight gain after 3–4 years. This small weight gain is likely caused by the assumed rescue therapy of using insulin formations. Weight profile assumptions may have generated a large QALY gain for exenatide BID vs insulin glargine in this analysis.
There are several limitations for this study. First, the clinical efficacy data are based on randomized clinical trials of up to 30 weeks. There is a lack of long-term observational studies of patients on GLP-1 treatment in China. Therefore, we need to adjust our results to reflect patients in real world settings. For example, patients’ medication adherence level affects treatment effectiveness, which in turn leads to changes both in treatment costs and health utilities. Second, all the utility data are adopted directly from studies of non-Chinese populations, because patient reported outcomes and health utilities for specific anti-diabetic drugs such as exenatide BID or insulin glargine are not established in China yet. Third, the risk equations in the model are all for a UK population; therefore, it may not fit the Chinese population very well. In addition, the cost of exenatide BID and insulin glargine QD were extracted from two different resources: Zhejiang retail price and Kangdele retail price. If the drug prices from these two resources are not comparable, it will introduce bias into our estimation; however, instead of the drug price after reimbursement, our study used the full price, which is not likely to be influenced by different drug policies for different regions. Also, the price of the brand-named product insulin, glargine, will not vary too much in different regions of China. In addition, the Zheijang province represents a high socio-economic region in China. Thus, even assuming the existent of an inconsistency of drug prices across regions, the price we used for exenatide BID should be a relative high price compared to other regions, thus the bias won’t change our research conclusion. Finally, the ICER results are robust to the cost of drugs according to the sensitivity analysis or the Tornado diagram. Therefore, the result will not be affected by the price sources, even if they will differ at around the 10% level.
All these limitations indicate gaps in observational studies and modeling research in diabetes in China. Finally, there were other limitations in some implicit assumptions; for example, cost of glucose monitoring was not included and cost of additional treatment intensification (e.g., exenatide BID with insulin) was not considered.
To conclude, adding exenatide BID to oral anti-diabetic agents is a cost-effective treatment alternative for Chinese type 2 diabetics who are inadequately controlled by oral anti-diabetic agents. Exenatide BID may also provide a treatment option for those patients who may also suffer from other treatment challenges such as hypoglycemia, obesity, and elevated risk factors for cardiovascular disease.
Transparency
Declaration of funding
There are no funding sources reported for this manuscript.
Declaration of financial/other relationships
No potential conflicts of interest relevant to this article were reported. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors thank Xuemei Zhen and Yuhang Zeng (Zhejiang University School of Public Health, Hangzhou, China) for their help in collecting data.
References
- Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2013;381:1987-2015
- (WHO) WHO. Noncommunicable Disease (NCD) Country Profile China 2014. Geneva, Switzerland, 2015 http://www.who.int/nmh/countries/chn_en.pdf. Accessed on May 24, 2015
- Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948-59
- CDS. China Guideline for Type 2 Diabetes. 2010. Beijing, China, 2010 http://www.cdschina.org/x_uploadfiles/dm201otaolungao.pdf. Accessed on May 24, 2015
- Association AD. Standards of medical care in diabetes—2013. Diabetes Care 2013;36(1 Suppl):S11
- Wang W, McGreevey WP, Fu C, et al. Type 2 diabetes mellitus in China: a preventable economic burden. Am J Manag Care 2009;15:593-601
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013;380:2224-60
- James WPT, Jackson-Leach R, Mhurchu CN, et al. Overweight and obesity (high body mass index). Comparative quantification of health risks: global and regional burden of disease attribution to selected major risk factors. WHO 2004;1:497-596
- Ji L, Onishi Y, Ahn CW, et al. Efficacy and safety of exenatide once-weekly vs exenatide twice-daily in Asian patients with type 2 diabetes mellitus. J Diabetes Invest 2013;4:53-61
- Chiquette E, Toth PP, Ramirez G, et al. Treatment with exenatide once weekly or twice daily for 30 weeks is associated with changes in several cardiovascular risk markers. Vasc Health Risk Manag 2012;8:621
- Inc AP. exenatide injection [prescribing information]. San Diego, CA: Amylin Pharmaceuticals Inc; 2009 BYETTA
- Du Y-J. Controlled clinical research of poor glycemic control in type 2 diabetes treating with exenatide and insulin glargine. Pract Pharm Clin Remed 2014;17:583-7
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559-69
- Karagianni P, Polyzos S, Kartali N, et al. Comparative efficacy of exenatide versus insulin glargine on glycemic control in type 2 diabetes mellitus patients inadequately treated with metformin monotherapy. Adv Med Sci 2013;58:38-43
- Barnett AH, Burger J, Johns D, et al. Tolerability and efficacy of exenatide and titrated insulin glargine in adult patients with type 2 diabetes previously uncontrolled with metformin or a sulfonylurea: a multinational, randomized, open-label, two-period, crossover noninferiority trial. Clin Therapeut 2007;29:2333-48
- Lund A, Knop FK, Vilsbøll T. Glucagon-like peptide-1 receptor agonists for the treatment of type 2 diabetes: Differences and similarities. Eur J Intern Med 2014;25:407-14
- Li W-X, Gou J-F, Tian J-H, et al. Glucagon-like peptide-1 receptor agonists versus insulin glargine for type 2 diabetes mellitus: a systematic review and meta-analysis of randomized controlled trials. Curr Therapeut Res 2010;71:211-38
- Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetic agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin 2007;23:2157-69
- Liu S, Zhao Y, Hempe JM, et al. Economic burden of hypoglycemia in patients with Type 2 diabetes. Exp Rev Pharmacoecon Outcomes Res 2012;12:47-51
- Goodall G, Costi M, Timlin L, et al. Cost-effectiveness of exenatide versus insulin glargine in Spanish patients with obesity and type 2 diabetes mellitus. Endocrinol Nutr (English Edition) 2011;58:331-40
- Mittendorf T, Smith-Palmer J, Timlin L, et al. Evaluation of exenatide vs. insulin glargine in type 2 diabetes: cost-effectiveness analysis in the German setting. Diabetes Obes Metab 2009;11:1068-79
- Brändle M, Erny-Albrecht K, Goodall G, et al. Exenatide versus insulin glargine: a cost-effectiveness evaluation in patients with Type 2 diabetes in Switzerland. Int j clin pharmacol therapeut 2009;47:501-15
- Ray JA, Boye KS, Yurgin N, et al. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin 2007;23:609-22
- Woehl A, Evans M, Tetlow AP, et al. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled type 2 diabetes in the United Kingdom. Cardiovasc Diabetol 2008;7:24
- Palmer J, Pinto C, Duarte R, et al. PDB51 cost-effectiveness of exenatide versus insulin glargine and versus biphasic insulin aspart for the treatment of type 2 diabetes in Portugal: a long-term health economic analysis. Value Health 2010;13:A293
- Bruhn D, Malhan S, Kavuncubasi S, et al. PDB27 cost-effectiveness of exenatide versus insulin glargine for the treatment of type-2 diabetes in Turkey: a long-term health economic analysis. Value Health 2009;12:A406
- Eastman RC, Javitt JC, Herman WH, et al. Model of complications of NIDDM: II. Analysis of the health benefits and cost-effectiveness of treating NIDDM with the goal of normoglycemia. Diabetes Care 1997;20:735-44
- Mount H. Computer modeling of diabetes and its complications: a report on the Fourth Mount Hood Challenge Meeting. Diabetes Care 2007;30:1638
- Palmer AJ. Computer modeling of diabetes and its complications: a report onthe fifth Mount Hood challenge meeting. Value Health 2013;16:670-85
- Clarke P, Gray A, Briggs A, et al. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747-59
- China Minister of Health. Chinese Health Statistics Yearbook. Beijing: Chin Minister of Health, 2013
- Xian S, Ting-qi Z. Effects of exenatide on glycemic control over 52 weeks in patients with type 2 diabetes. Chin Pharm J 2014;49:941-3
- Han W. Initial observation of exenatide for treatment of type 2 diabetes. J Qinghai Univ 2011;10:22-3
- Xu H, Lu L, He Y, et al. Clinical efficacy of exenatide treating type 2 diabetes patients who are not well controlled by oral antiglycemia drugs. Chin J Prim Med Pharm 2014;1:1378-9
- Matza LS, Boye KS, Yurgin N, et al. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual life Res 2007;16:1251-65
- Dennett SL, Boye KS, Yurgin NR. The impact of body weight on patient utilities with or without type 2 diabetes: a review of the medical literature. Value Health 2008;11:478-86
- Lane S, Levy A, Mukherjee J, et al. The impact on utilities of differences in body weight among Canadian patients with type 2 diabetes. Curr Med Res Opin 2014;30:1267-73
- Hui G, Jun L, Zhongli J. Follow-up effects of the increased physical activity on the glucolipid metabolic factors and medicla costs in type 2 diabetes patients. Chin J Rehab Med 2007;22:395-8
- Ya-ming Z, Jing W, Kun X. Incidence and cost of hypoglycemia episode in patients with type 2 diabetes mellitus (T2DM). Chinese Rural Health Service Administration 2012;32:1195-8
- Currie CJ, Morgan CL, Poole CD, et al. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 2006;22:1523-34
- Bureau of Zhejiang Province Pharmacy. Government Regulated Highest Retail Price. 2004-2013. Zhejiang Provincial Government, Zhejiang Province, 2013. http://www.zjpi.gov.cn/main/html/2013/CT10138/4c69032c8e3344f98c29388cdd8390fe.html. Accessed on September 15, 2014
- Kangdele Pharmacy. Kangdele Pharmacy Retail Price. 2014. Available from: http://www.baiji.com.cn/goods-6710.html. Accessed on September 15, 2014
- Xing-yun H, Zhun Z, Xia T, et al. Cost-effetiveness analysis of 2 dosage forms of metformin hydrochloride in the treatment of type 2 diabetes. China Pharm 2014;25:1844-7
- Gao L, Zhao F-L, Li S-C. Cost-utility analysis of liraglutide versus glimepiride as add-on to metformin in type 2 diabetes patients in China. Int J Technol Assess Health Care 2012;28:436-44
- China NBoSo. General CPI for all the years. Beijing, 2015. http://data.stats.gov.cn/workspace/index?a=q&type=global&dbcode=hgnd&m=hgnd&dimension=zb&code=A090101®ion=000000&time. Accessed on May 24, 2015
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9
- China NBoSo. GDP per capita National Data. Beijing, 2013
- Heine RJ, Van Gaal LF, Johns D, et al. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes A randomized trial. Ann Intern Med 2005;143:559-69
- Pawaskar M, Anderson J, Zagar A. PDB18 cost offsets associated with use of exenatide compared to glargine for the treatment of patients with type 2 diabetes. Value Health 2009;12:A404
- Currie CJ, McEwan P, Peters JR, et al. The routine collation of health outcomes data from hospital treated subjects in the Health Outcomes Data Repository (HODaR): descriptive analysis from the first 20,000 subjects. Value Health 2005;8:581-90
- Palmer AJ, Roze S, Valentine WJ, et al. The CORE Diabetes Model: projecting long-term clinical outcomes, costs and costeffectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin 2004;20(1 Suppl):S5-26
- Palmera AJ, Rozea S, Valentinea WJ, et al. Validation of the CORE Diabetes Model against epidemiological and clinical studies. Curr Med Res Opin 2004;20(1 Suppl):S27-40
- Ji L, Hu D, Pan C, et al. Primacy of the 3B approach to control risk factors for cardiovascular disease in type 2 diabetes patients. Am J Med 2013;126:925. e11-. e22
- China GAoSo. 2010 National Physical Condition Monitoring Report. Beijing, 2010. http://www.sport.gov.cn/n16/n1077/n297454/2052709.html. Accessed on May 24, 2015
- Li H, Xu F, Wang F. Cost-effectiveness of biphasic insulin aspart 30 combined with metformin in patients with type 2 diabetes mellitus. Chin J New Drugs 2011;1:2163-70
- Palmer JL, Gibbs M, Scheijbeler HW, et al. Cost-effectiveness of switching to biphasic insulin aspart in poorly-controlled type 2 diabetes patients in China. Adv Ther 2008;25:752-74
- Zheng Y, Wu J, Xie K. Incidence and cost of hypoglycemia episode in patients with type 2 diabetes mellitus. Hefei, China: Chinese Rural Health Service Administration, 2012. pp. 1195-8
- Jaime Caro J, Ozer Stillman I, Danel A, et al. Cost effectiveness of rimonabant use in patients at increased cardiometabolic risk: estimates from a Markov model. J Med Econ 2007;10:239-54
Appendix 1: Literature review and meta-analysis procedures
The objective of the literature review was to find studies carried out in China. English databases including PubMed and Web of Science and Chinese databases including China National Knowledge Infrastructure (CNKI), Wanfang, and Weipu databases were systematically searched for eligible studies/reports from English and Chinese literature using the terms: exenatide, glargine, type 2 diabetes mellitus, and Chinese. Both randomized clinical trials and observational studies were extracted. The searching period started from 1995–2014. These articles were only extracted for later years based on the drugs’ respective Chinese FDA (CFDA) approval time (Exenatide 2009).
The inclusion criteria were as follows: (1) The target population was T2DM patients who were older than 18 years old. (2) The study duration was at least 12 weeks. (3) The randomized control studies or the observational studies were eligible. (4) The intervention was our target drugs treatment alone or combination treatment with metformin and other oral anti-diabetic agents. No other treatments at the same time except for diet and physical exercise. (5) Literature published in both Chinese and English databases were searched. (6) The literature should contain the clinical efficacy data, as is listed in Table A1.
The exclusion criteria were as follows: (1) The target population were not T2DM patients, for example T1DM or gestational diabetes; (2) The study did not include the target drugs as comparisons; (3) Studies other than our target types, such as epidemiology study, drug utilization, drug research progression, case study, and methodology articles were excluded; (4) The study target was not human, such as cell lines or animal research; (5) The drug combinations were not OAD agents, such as exenatide + insulin; (6) Equal to or more than three drugs combination treatment trial; (7) The article that did not report the major efficacy variables (i.e., glycosylated hemoglobin: HbA1c).
Data extraction were performed by recording the study characteristics, interventions, and results in Table A1. Study characteristics included the first author’s name, sample size, publication date, study period, setting, patient baseline characteristics, and other details of the study design (e.g., randomization, use of blinding, and dropout rates). Extracted results included the means and standard deviations (SDs) or standard errors (SEs) of all the demographic and clinical efficacy variables, for example, HbA1c levels in the study groups. Two reviewers independently extracted data from each study retrieved, any discrepancy between the two reviewers’ records were addressed by a mutual check and resolved by consensus with a third reviewer.
For the second round search, only the head-to-head studies that are comparing the clinical efficacy of exenatide BID vs insulin glargine QD as add-on therapy to the patients who are not adequately controlled by metformin or other OADs alone were included. Also, the exenatide BID treatment regime was administered twice per day or BID, studies using any other formulations were excluded.
Clinical effects of each individual anti-diabetic agent
The clinical effects included the changes of HbA1C, weight, hypoglycemia, TC, HDL-c, and SBP after the intervention. Because the clinical effects in this study are continuous variables, we used the weighted mean difference (WMD) to describe the data, and calculated SEs from SEs presented in the reports. The effects were measured by calculating the difference between baseline and endpoint values (equation Equation1(1) ):
(1)
Where
is the mean post-treatment value and
is the mean pre-treatment value. When not reported in the original document, the SD of the treatment effect was calculated using equation (Equation2
(2) ):
(2)
Where SD1 is the SD of the pre-treatment value, SD2 is the SD of the post-treatment value, and r is the correlation coefficient, with 0.4 taken as a conservative estimate.
Results
There were three studies which met all inclusion and exclusion criteria in China. For each study, we extracted information (demographics, clinical efficacy, and adverse effects). The results of meta-analysis were used for the cost-effectiveness model.
Table A1. Detailed parameter values for the three extracted clinical trials.
Table A2. Meta-analysis results used for cost-effectiveness model.
Table A3. Final cost-effective inputs of study population.
Table A4. Final cost-effective inputs of comparison therapies.
Appendix 2: Field survey
The field survey collected current cost data and served as a compliment/alternative to the cost data from the literature review. One secondary hospital (Longyou Renmin Hospital) and one tertiary hospital (Taizhou Hospital) were selected for the field survey. All related direct costs in hospital were collected.
Our sampling procedure was as follows: (1) Collect inpatient data for patients with T2DM as the primary diagnosis. (2) Based on the disease diagnosis and transition conditions and the sample size for the diabetes patients, if the cost for complications before and after the transition can be separated and the sample size of at least 100. (3) The time period for search was 2009–2014. (4) At least 20 patients should be collected for either fatal or non-fatal complications. For cost information collected from hospitals, arithmetic mean and SE were calculated for the average cost of inpatient care and were converted to 2014 price.
Appendix 3: BMI related cost estimation