Abstract
Objective:
Tuberous sclerosis complex (TSC) is associated with non-malignant kidney lesions—angiomyolipomata—that may be associated with chronic kidney disease (CKD). This study investigated the relationship between renal angiomyolipomata and CKD in TSC, including the impact on healthcare resource utilization (HCRU) and costs.
Methods:
This was a retrospective, longitudinal cohort study based on medical record data spanning January 1990–April 2012 for 369 TSC patients treated at a specialty center in the Netherlands. Cohorts were established based on CKD stage and angiomyolipoma size. Rates of HCRU (physician visits, monitoring, and interventions) were compared across cohorts using rate ratios. Healthcare costs were compared across cohorts using cost differences. Regression models were used to identify predictive factors for HCRU and healthcare costs.
Results:
Sixteen per cent of patients reached CKD stage 3 or higher during follow-up. Patients at more advanced stages of CKD more frequently had either large or multiple small angiomyolipomata and higher HCRU rates and healthcare costs. In the multivariate analyses, male gender, CKD stage >1, angiomyolipoma size ≥3.5 cm, embolization, and the presence of moderate or severe lymphangioleiomyomatosis (LAM) were associated with greater HCRU (p ≤ 0.002 for all comparisons). Definite (vs suspected) TSC diagnosis, CKD stage 5 (vs CKD stage 1), angiomyolipoma size ≥3.5 cm, and moderate or severe LAM were associated with higher costs (p = 0.050 for TSC diagnosis, p ≤ 0.002 for other comparisons). Costs in CKD stage 5 were driven primarily by dialysis.
Conclusions:
A substantial proportion of patients with TSC developed moderate-to-severe CKD, which was associated with renal angiomyolipomata and increased HCRU and costs.
Introduction
Tuberous sclerosis complex (TSC) is a rare genetic disorder associated with cognitive and behavioral disorders and characterized by non-malignant lesions throughout the body, including the kidneys. Manifestations of TSC are linked to over-expression of the mammalian target of rapamycin (mTOR), which results from mutations in the TSC1 or TSC2 genes.
Complications associated with TSC impose a significant burden on patients and society. An estimated 80–90% of patients with TSC have epilepsy, the majority of which are refractory to anti-epileptic medicationCitation1. Nearly half of patients with TSC have below-normal IQ, and ∼30% of patients have profound intellectual disabilityCitation2,Citation3. TSC is also associated with pulmonary lymphangioleiomyomatosis (LAM), characterized by the proliferation of abnormal muscle cells in the lungs, airways, and blood and lymph vessels, primarily in females, that can lead to terminal respiratory insufficiencyCitation4. Pharmacologic treatments are often used to attempt to manage TSC manifestations, although hospitalization and surgery are commonCitation5–8.
Kidney manifestations of TSC include angiomyolipomata and cysts. Renal angiomyolipomata in TSC are characterized as multiple and commonly bilateral lesions that consist of blood vessels, smooth-muscle and fat cells that typically grow over time and, when large, present risk of acute, potentially life-threatening hemorrhageCitation9. While treatment approaches largely focus on mitigating the risk of bleeding presented by larger angiomyolipomata, renal involvement in TSC (including the presence of kidney cysts and smaller angiomyolipomata) is also associated with deterioration of kidney function. Following TSC diagnosis, consensus recommendations call for regular abdominal imaging as well as assessment of kidney function via blood tests and evaluation of blood pressure at least annuallyCitation10,Citation11.
Identification of the role of the mTOR cascade in TSC led to the development of mTOR inhibitors as a treatment option for TSC manifestationsCitation12,Citation13. While consensus recommendations now call for mTOR inhibitors as first-line therapy for growing, asymptomatic renal angiomyolipomata >3 cm in diameter, transcatheter embolization was the standard of care in the Netherlands (the setting for the current study) for such angiomyolipomata until recentlyCitation10. Embolization remains the recommended second-line treatment for asymptomatic angiomyolipomata and first-line treatment for angiomyolipomata presenting with acute hemorrhage. Although preventative embolization can help spare normal renal tissue, it often requires repeated treatments and may involve injury to the kidney which could further contribute to a decline in kidney functionCitation11.
Literature exploring the relationship between renal angiomyolipomata and CKD is limited and to-date the related impact of kidney involvement in TSC on healthcare resource utilization (HCRU) and costs has not been studied. The objectives of this study were to document the association between angiomyolipomata and CKD in a longitudinal cohort of patients with TSC, and to assess the impact on HCRU and healthcare costs.
Patients and methods
This was a retrospective, longitudinal open cohort study utilizing medical record data from patients primarily treated at the University Medical Center – Utrecht (UMCU), a major specialty center in the Netherlands. Study approval was obtained from the UMCU institutional review board. Study subjects were eligible for inclusion based on a diagnosis of TSC according to the Revised 1998 Criteria (presence of either two major criteria or one major and two minor criteria)Citation14. All patients with TSC treated at the UMCU during January 1990–April 2012 were eligible, and only two opted out. The data combine demographic information with records from scans, specialist visits, medications, and other healthcare services.
The observation period for each patient started at the latest of January 1, 1990 or date of birth, and ended at the earliest of date of death, last follow-up date at the medical center, or April 1, 2012 (the end of medical record abstraction). Follow-up time was partitioned according to CKD stage and angiomyolipoma size.
CKD stage (1–5) was determined through estimation of the glomerular filtration rate (GFR) calculated from serum creatinine levels using the CKD-EPI formulaCitation15–17. Kidney function was measured on a routine basis and prior to any surgical procedures or embolization. GFR levels were carried forward until the next reported test value. Patients were assigned CKD stage 1 for the period of observation until the first kidney function measurement. Those without measurements were assigned to CKD stage 1 for the entire follow-up period. Patients on dialysis were assigned to CKD stage 5, regardless of GFR. Using these criteria, patients were assigned different CKD stages longitudinally using an open cohort approach.
Patients were further categorized based on the renal angiomyolipoma staging criteria described in . Computed Tomography (CT) was the primary imaging technique used to assess angiomyolipoma status, because MRI scanning under general anesthesia was not available on a regular basis throughout much of the sample period. CT scans were generally preformed every 2–3 years as part of routine follow-up. Typically abdominal CT scans were performed either with a single-detector row scanner (AVE; Philips, Cleveland, OH) or a 16-detector row scanner (Brilliance 16P or MX800 IDT; Philips). With the single-detector row scanner, spiral data acquisition with 5-mm collimation and 7-mm table feed per rotation was used. Images were reconstructed every 4 mm. With the 16-detector row scanners, a 16 × 1.5-mm collimation was applied, and images of 5-mm section thickness were reconstructed every 4 mm. Tube voltage was 140 kVp on the single-detector row scanner and 120 kVp on the 16-detector row scanners. A pre-contrast acquisition of the upper abdomen, including the kidneys, was followed by an infusion of iodinated contrast material. Kidney function was checked prior to and after the use of IV radiological contrast, and no cases of a rapid decline in kidney function to less than 30 mL/min after receiving IV radiological contrast were reported. Radiological reports were used when original scans were unavailable.
Table 1. Renal angiomyolipoma staging criteria.
Patients without a renal angiomyolipoma stage and follow-up time prior to a stage being determined were assigned to stage 0. The presence of a single angiomyolipoma ≥3.5 cm in longest diameter (angiomyolipoma stages 3–6) was used for comparison purposes as this was the size threshold used in determining whether or not to treat with embolization on an elective basis to prevent hemorrhage.
Averages of GFRs reported for a reference group of Dutch Caucasian patients without TSC, broken out by age and gender, were utilized for comparison purposesCitation18,Citation19. The averages were calculated for each age and weighted to reflect the gender distribution of the TSC cohort of a given age. A linear regression of mean GFR on patient age was used to assess the age trend in mean GFR. Characteristics of patients with TSC (including age, TSC diagnosis based on genetic analysis [available for ∼55% of patients], gender, LAM presence and severity [mild, moderate, or severe], and angiomyolipoma stage), embolization status, and number of years in follow-up were assessed and compared across CKD and AML cohorts. LAM presence and severity were determined based on the extent of thin-walled cysts in the lung bases, as assessed from CT images in the original report from the attending radiologist.
Healthcare resource utilization was measured as event counts for scans, surgeries (broken out by any surgery and kidney-related surgery, including nephrectomy and transplant), non-surgical procedures, and specialist visits. For medications, HCRU was measured as the sum of patient-years of treatment with each medication. For dialysis, HCRU was measured as the number of patient-years on dialysis. Rates of HCRU per patient per year (PPPY) were calculated as the number of events or patient-years of treatment divided by the number of patient-years of observation and were reported and compared across cohorts using rate ratios (RR) estimated by Poisson regressions. Rate ratios for CKD stage were calculated relative to CKD stage 1 and for angiomyolipoma ≥3.5 cm were calculated relative to angiomyolipoma <3.5 cm. Ninety-five per cent confidence intervals and p values associated with RRs were estimated using the non-parametric bootstrap technique to account for potential correlation among observations from the same patient arising from the open cohort study design.
Healthcare costs, measured in 2012 Euro (€) PPPY, were estimated from unit costs based on Dutch tariffsCitation19,Citation20. Cost differences between cohorts were tested for statistical significance using the bootstrap technique due to the non-normality of the healthcare costs variable (which is truncated at zero and positively skewed) and open cohort study design.
Predictive factors for annualized HCRU and total healthcare costs (limited to subjects with more than 6 months of follow-up) were estimated using Poisson and linear regressions, respectively. The regression models, specified with random effects and repeated patient IDs to account for the open cohort study design and potential correlation among observations from the same patient, included indicators for gender, TSC diagnosis, patient age during follow-up, highest angiomyolipoma size, CKD, and LAM severity cohort, and embolization history (specified as an indicator for whether the patient had undergone embolization). Rate ratios were reported for potential HCRU predictive factors, and linear regression coefficient estimates (interpreted as the incremental cost in 2012 €) were reported for potential predictors of total healthcare costs. The statistical significance of the regression estimates was assessed using the bootstrap technique. All p values provided are nominal; hence, statistical interpretation should be made with caution.
Results
Patient characteristics
Three-hundred and sixty-nine patients were included, with 97% having a definitive TSC diagnosis and the remainder suspected of having TSC. Cognitive function, determined from observations by the treating physician, was low to very low in 60% of patients. Median follow-up time was 15.4 years (mean = 14.3 years). A total of 352 patients had serum creatinine measurements within the follow-up period. The remaining patients were assumed to be at CKD stage 1 throughout follow-up. Thirty-three patients did not have any recorded angiomyolipoma examinations and were assumed not to have angiomyolipomata throughout follow-up.
presents mean GFR by patient age for patients with TSC and the reference population of non-TSC patientsCitation18,Citation19. The decline in GFR with age was steeper for the TSC cohort compared with the non-TSC cohort (−1.53 vs −0.94 mL/min/1.73 m2 per year, respectively). At ages 50 and over, mean GFR for the TSC cohort frequently fell below the threshold for CKD stage 3Citation22.
Figure 1. GFR by patient age. Age range depicted includes ages for which there are at least 10 patients in the current study. GFR from UMCU cohort of patients with TSC: Mean GFR among patients at a given age, estimated from serum creatinine using the CKD-EPI formula. The same patient may contribute multiple observations to a given age and to multiple ages. Shading depicts 95% confidence interval for the mean. GFR from normal population: Based on GFR estimated from serum creatinine for Caucasian males and females using the CKD-EPI formula, reported in a 2007 population-based cross-sectional study conducted in the eastern part of the NetherlandsCitation19,Citation20. Plot depicts weighted averages of median GFR reported for males and females. Weights equal to the proportion of each gender within a given age cohort of the current study.
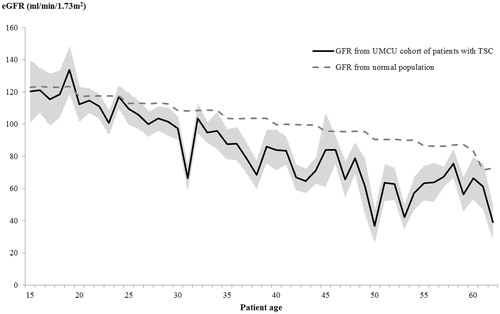
and present patient characteristics by CKD stage and AML size. Reflecting the results in , patients with more advanced CKD were on average older (). A similar age trend is present in patients with larger AML, reflecting the tendency for renal AML associated with TSC to grow over time: patients with renal AML ≥3.5 cm were on average older compared with those with renal AML <3.5 cm (mean age = 39.6 vs 32.8, p < 0.001; see )Citation9,Citation12. further shows that, while angiomyolipomata were prevalent among all CKD cohorts, patients with more advanced CKD tended to have a more advanced angiomyolipoma stage. Mean GFR among patients at angiomyolipoma stages 1 and 6 was 97.4 and 50.8 mL/min/1.73 m2, respectively ( provides the GFR ranges for each CKD stage). These results suggest a strong association between age, angiomyolipomata size, and CKD.
Table 2. Patient characteristics by CKD stage.a
Table 3. Patient characteristics by renal angiomyolipoma (AML) size.
In addition, CKD is associated with the presence of numerous small angiomyolipomata. Among subjects with only small angiomyolipomata, those with more advanced CKD tended to have more numerous angiomyolipomata (e.g., 6/11 [54.5%] subjects at CKD stage 3 had ≥5 angiomyolipomata vs 47/157 [29.9%] at CKD stage 1).
Among patients younger than 70 years old, 16% were at CKD stage 3 or higher at some point during follow-up (2% of patients entered follow-up at CKD stage 3 or higher, and 14% of patients evolved to CKD stage 3 or higher during follow-up). In contrast, among patients without TSC included in the Nijmegen Biomedical study, the proportion under age 70 with CKD stage 3 or higher was 3%Citation19.
Advanced CKD stages were also associated with female gender, TSC2 gene mutation, likelihood of having undergone renal embolization, and frequency and severity of LAM among patients in this study.
Healthcare resource utilization and costs by CKD stage
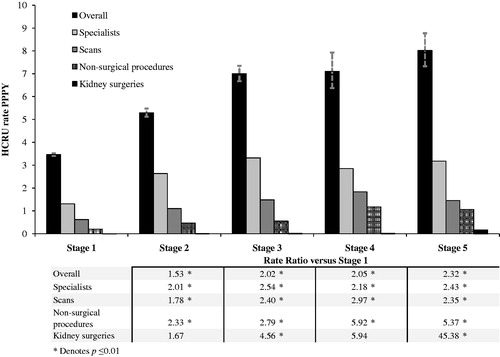
Rates of HCRU by CKD stage are presented in . Specialist visits, scans, and medications (not shown in figure) tended to have the highest utilization rates among patients at higher CKD stages. Compared with CKD stage 1, patients at CKD stage 3 had higher rates of scans (RR = 2.399, p < 0.001), kidney surgery (RR = 4.558, p = 0.007), non-surgical procedures (RR = 2.785, p < 0.001), specialist visits (RR = 2.535, p < 0.001), and medication use (RR = 1.234, p < 0.001).
Figure 2. HCRU rates by CKD stage. Overall consists of specialists, scans, non-surgical procedures, and kidney surgeries, as well as non-kidney surgeries, medication, and dialysis (not shown). Dashed lines depict 95% confidence interval of utilization rates.
presents estimates of healthcare costs by CKD stage. Mirroring the HCRU results in , patients with more advanced CKD had significantly higher healthcare costs. Compared with patients at CKD stage 1, patients at CKD stage 3 had higher costs for scans, kidney surgeries, non-surgical procedures, specialist visits, and medications (p ≤ 0.026 for all). Overall healthcare costs among patients at CKD stage 5 were driven by costs associated with dialysis.
Table 4. Healthcare costs by CKD stage.
Healthcare resource utilization and costs by angiomyolipoma size
presents HCRU rates by angiomyolipoma size. Utilization rates for scans, non-surgical procedures, specialist visits, medications, and dialysis were higher among patients in the angiomyolipomata ≥3.5 cm group vs those in the <3.5 cm group (RRs = 1.716–4.177, p < 0.001). Patients with angiomyolipomata ≥3.5 cm also had a significantly higher rate of kidney-related surgeries (RR = 2.150, p = 0.035).
Table 5. Rates of HCRU by angiomyolipoma (AML) size.
presents estimates of healthcare costs by angiomyolipoma size. Compared with the <3.5 cm group, patients with angiomyolipomata ≥3.5 cm had higher costs of scans (difference = €157 PPPY, p < 0.001), non-surgical procedures (difference = €162 PPPY, p < 0.001), specialist visits (difference = €98 PPPY, p < 0.001), and medications (difference = €535 PPPY, p < 0.001).
Table 6. Healthcare costs by angiomyolipoma (AML) size.
and present multivariate regression estimates of predictive factors for HCRU and total healthcare costs. Male gender, CKD stage, angiomyolipoma size, renal embolization, and LAM are independent predictors of HCRU. Specifically, HCRU was significantly associated with angiomyolipoma size ≥3.5 cm (RR = 1.221, p < 0.001), higher CKD stages (RRs = 1.467–2.677, p < 0.001 for all), a history of embolization (RR = 1.393, p < 0.001), and the presence of moderate or severe LAM (RR = 1.518, p = 0.002).
Table 7. Predictive factors for HCRU.a
Table 8. Predictive factors for healthcare costs.a
CKD stage 5 was associated with significantly higher costs vs CKD stage 1 (incremental cost PPPY = €19 268, p < 0.001), a result explained primarily by dialysis costs. While the coefficients for other CKD stages did not reach statistical significance, all were positive, indicating they were associated with incremental costs PPPY = €388–€2152. In addition, angiomyolipoma size ≥3.5 cm was associated with higher costs (incremental cost PPPY = €771, p = 0.002), as was the presence of moderate or severe LAM (incremental cost PPPY = €3324, p = 0.002). Patients aged ≥60 years had lower costs compared with patients aged 0–19 years (incremental cost PPPY = −€1,757, p < 0.001). In contrast to HCRU, there was no clear pattern of association between embolization and healthcare costs.
Discussion
This long-term longitudinal study of a large cohort of patients with TSC demonstrates the presence of a strong association between angiomyolipomata and advanced CKD, both of which are predictors of HCRU and healthcare costs.
Two insights highlight the importance of routine kidney function monitoring and early intervention in patients with TSC. First, patients in this study developed kidney impairment more frequently, and at an earlier age, than patients without TSC, and more frequently than has been reported in other studies of patients with TSCCitation11,Citation18,Citation19,Citation24. A potential explanation of the latter is that patients in this study may have had a higher likelihood of being observed to develop severe TSC manifestations due to the longer length of follow-up and wider age range compared with previous studies.
Second, while current treatment approaches largely focus on preventing large angiomyolipomata from hemorrhaging, the presence of numerous small angiomyolipomata is associated with advanced CKD: substantial proportions of patients at CKD stages 2 and higher in this study had numerous small (and no large) angiomyolipomata.
Other results in this study are consistent with and build upon those in the existing literature. Twenty-nine deaths were recorded during the observation period, and the mortality rate was nearly 5-times higher than that in the age- and gender-matched Dutch general population during the same time period (for further details on mortality among patients in our sample, see Eijkemans et al.Citation25). The finding that female patients were more likely to have kidney impairment is consistent with the Schillinger et al.Citation26 study based on a survey of 260 French dialysis centers. The association between advanced CKD and the TSC2 gene mutation is consistent with established knowledge that this mutation is associated with a more severe phenotypeCitation9,Citation11,Citation24. Finally, advanced CKD was associated with other adverse clinical outcomes (e.g., LAM) in addition to angiomyolipomata.
Patients at higher CKD stages tended to utilize more healthcare resources, of which non-surgical procedures (including renal embolization) and dialysis were important components. Although confounding by indication cannot be ruled out, this may suggest embolization is not able to prevent progressive CKD. Higher HCRU among patients with more severe kidney dysfunction was mirrored by higher HCRU among patients with larger angiomyolipomata. Scans, specialist visits, and medications comprised a large proportion of overall HCRU. Dialysis, and to a lesser extent surgeries and medications, accounted for substantial proportions of overall healthcare costs.
In multivariate regressions, female gender was a significant predictor of reduced HCRU and an insignificant predictor of healthcare costs after controlling for kidney impairment. Embolization history was significantly associated with HCRU, even after controlling for a dichotomous measure of renal angiomyolipomata size. Since embolization was used specifically to treat large renal angiomyolipomata in this patient population, this result suggests there is meaningful variation in angiomyolipoma size even within the ≥3.5 cm cohort. Finally, patients ≥60 years old tended to have lower costs compared with patients aged 0–19 years after controlling for CKD stage and other patient characteristics—a result that may be explained in part by survivor bias.
The average TSC severity of patients in our sample appears to be comparable to or greater than that in the population of all patients with TSC based on their estimated cognitive function and incidence of renal angiomyolipomata. For example, 60% of patients in our sample had below-normal cognitive function, compared with the 44% of patients with TSC with below-normal IQ reported for an epidemiological sample in England studied by Joinson et al.Citation2. Impaired cognitive function has, in turn, been associated with other symptoms of TSC such as seizures.
This study has a number of limitations common in retrospective analyses, including limited samples sizes for select sub-groups, and missing data. Sample sizes for some strata (e.g., CKD stages 4 and 5) were too small to obtain robust estimates. Data on angiomyolipoma status and other characteristics associated with TSC were not available prior to the observation period. These factors limit the precision and generalizability of the results and precluded more detailed analyses of angiomyolipomata onset. LAM, serum creatinine, and angiomyolipoma data were missing for some patients. Missing LAM data precluded a more comprehensive assessment of its role in the burden of TSC. In addition, the study database did not include information on PKD1 gene status or systematic data on renal cysts. Due to the proximity of the TSC2 and PKD1 genes on the same chromosome, some patients with TSC also have PKD1 gene mutations which can result in polycystic kidney disease and likely contribute to kidney function decline. Hence, the association between PKD1 gene status, renal cysts, and rate of kidney function decline in this population remains an important topic for future research. The inclusion of patients with no serum creatinine measurements in the CKD stage 1 cohort may cause this study to understate the true proportion of patients with TSC who advance to higher CKD stages as well as the healthcare resource utilization and costs associated with higher CKD stages (in absolute terms and in comparison with CKD stage 1), making this approach conservative.
Other limitations are inherent to the single country, single center design. This study under-estimates true HCRU rates and direct healthcare costs to the extent that patients utilized resources outside of the UMCU. However, this study captures the vast majority of direct healthcare costs. This study does not account for indirect costs associated with TSC (e.g., caregiver burden), and, hence, it under-estimates the true cost to society of this condition. Our results may not be fully generalizable outside of the Netherlands, since estimates of healthcare costs were based on unit costs specific to the Netherlands and since the characteristics, treatment patterns, resource utilization, and other outcomes of patients with TSC in the Netherlands might not reflect those of patients located elsewhere. Survivor bias may lead this study to understate the true extent to which increased age is associated with reduced kidney function. Despite this limitation, the multivariate regression approach used in this study helps minimize the extent to which survivor bias affects the other regression results. Finally, as with all observational studies, confounding by indication may be at play, since patients who received more diagnostic tests and procedures may have done so for reasons related to the severity of their TSC.
This study contributes to the growing body of evidence pointing to a strong relationship between kidney lesions and CKD in patients with TSC. Impaired kidney function associated with angiomyolipomata remains a key concern in patients with TSC, despite the use of preventive embolization to mitigate the risk of hemorrhage. The study also demonstrates the significant burden in terms of HCRU and healthcare costs of kidney involvement associated with TSC, building upon the existing literature suggesting that TSC imposes substantial humanistic and economic burdensCitation5,Citation7,Citation9,Citation13. These findings highlight the need for routine monitoring of kidney function and alternative TSC treatment approaches that preserve kidney function. The data suggest that such a treatment approach may substantially reduce HCRU and healthcare costs associated with CKD and renal angiomyolipomata.
Conclusions
This retrospective, longitudinal cohort study was based on medical record data for 369 patients with TSC treated at a specialty center in the Netherlands. A substantial proportion of patients with TSC developed moderate-to-severe CKD, which was associated with renal angiomyolipomata and increased HCRU and costs. These findings highlight the need for routine monitoring of kidney function and exploration of alternative TSC treatment approaches that preserve kidney function.
Transparency
Declaration of funding
This study was supported by funding from Novartis Pharmaceuticals Corporation (Novartis). Funding provided by Novartis was not contingent upon the study results.
Declaration of financial/other relationships
Research support was provided to Analysis Group, Inc. by Novartis. FV, PK, and MSD are employees of Analysis Group, Inc. TN was an employee of Analysis Group, Inc. at the time of the study. MM is an employee of Novartis, and SB van W van DK was an employee of Novartis at the time of the study. BZ is an employee of the University Medical Center Utrecht, which received research funding from Novartis for this study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Research idea and study design: BZ, MM, MD; data analysis/interpretation and statistical analysis: FV, PK, TN, MD Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. The authors thank Casey Jones of Analysis Group, Inc. for research assistance. All authors take the responsibility that this study has been reported honestly, accurately, and transparently, and that no important aspects of the study have been omitted.
References
- Evans LT, Morse R, Roberts DW. Epilepsy surgery in tuberous sclerosis: a review. Neurosurg Focus 2012;32:E5
- Joinson C, O’Callaghan FJ, Osborne JP, et al. Learning disability and epilepsy in an epidemiological sample of individuals with tuberous sclerosis complex. Psychol Med 2003;33:335-44
- Tuberous Sclerosis Alliance. Mental retardation in tuberous sclerosis complex. Silver Spring, MD: Tuberous Sclerosis Alliance, 2006. http://www.tsalliance.org/documents/Mental%20Retardation%20in%20TSC.pdf. Accessed November 5, 2014
- Tuberous Sclerosis Alliance. Lung involvement in TSC. Silver Spring, MD: Tuberous Sclerosis Alliance, 2013. http://www.tsalliance.org/documents/LUNG%20INVOLVEMENT%20IN%20TSC%20(updated%20Nov%202013).pdf. Accessed July 2, 2015
- Hallett L, Foster T, Liu Z, et al. Burden of disease and unmet needs in tuberous sclerosis complex with neurological manifestations: systematic review. Curr Med Res Opin 2011;27:1571-83
- Capurro S, Fiallo P. Timed surgery for treatment of angiofibromas in tuberous sclerosis. Dermatol Surg 2001;27:486-8
- Lennert B, Farrelly E, Sacco P, et al. Resource utilization in children with tuberous sclerosis complex and associated seizures: a retrospective chart review study. J Child Neurol 2013;28:461-9
- Mayo Clinic. Tuberous sclerosis. Rochester, MN: Mayo Clinic, 2014. http://www.mayoclinic.com/health/tuberous-sclerosis/DS01032/DSECTION=treatments-and-drugs. Accessed July 31, 2015
- Pirson Y. Tuberous Sclerosis Complex-associated kidney angiomyolipoma. Nephrol Dial Transplant 2013;28:1680-5
- Krueger DA, Northrup H, on behalf of the International Tuberous Sclerosis Complex Consensus Group. Tuberous Sclerosis Complex Surveillance and Management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol 2013;49:255-65
- Rouvière O, Nivet H, Grenier N, et al. Kidney damage due to tuberous sclerosis complex: Management recommendations. Diagn Interv Imaging 2013;94:225-37
- Franz DN, Belousova E, Sparagana S. Efficacy and safety of everolimus for subependymal giant cell astrocytomas associated with tuberous sclerosis complex (EXIST-1): a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2013;381:125-32
- Bissler J, Kingswood JC, Radzikowska E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis comples or sporadic lymphangioleiomyomatosis (EXIST-2): a multicenter, randomized, double-blind, placebo-controlled trial. Lancet 2013;381:817-24
- Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol 1998;13:624-8
- Eckardt KU, Berns JS, Rocco MV, et al. Definition and classification of CKD: the debate should be about patient prognosis – A position statement from KDOQI and KDIGO. Am J Kidney Dis 2009;53:915-20
- National Kidney Foundation. Calculators for health care professionals. New York, NY: National Kidney Foundation, 2013. http://www.kidney.org/professionals/kdoqi/gfr_calculator.cfm. Accessed June 10, 2014
- Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604-12
- Wetzels JFM, Kiemeney LALM, Swinkels DW, et al. Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 2007;72:632-7
- Van den Brand JAJG, van Boekel GAJ, Willems HL, et al. Introduction of the CKD-EPI equation to estimate glomerular filtration rate in a Caucasian population. Nephrol Dial Transplant 2011;26:3176-81
- Zorginstituut Nederland. Medication Costs. Amsterdam: Zorginstituut Nederland, 2012. http://www.medicijnkosten.nl/. Accessed 2012
- Nederlandse Zorgautoriteit. DBC zorgproducten tariefapplicatie. Utrecht: Nederlandse Zorgautoriteit, 2014. http://dbc-zorgproducten-tarieven.nza.nl/nzaZpTarief/ZoekfunctieDbc.aspx. Accessed October 31, 2014
- The Renal Association. CKD stages. Petersfield: The Renal Association, 2013. http://www.renal.org/information-resources/the-uk-eckd-guide/ckd-stages#sthash.hy4VyJRL.ylqkO4Lq.dpbs. Accessed September 2, 2014
- American Heart Association. Understanding blood pressure readings. Dallas, TX: American Heart Association, 2014. http://www.heart.org/HEARTORG/Conditions/HighBloodPressure/AboutHighBloodPressure/Understanding-Blood-Pressure-Readings_UCM_301764_Article.jsp. Accessed September 2, 2014
- Rakowski SK, Winterkorn EB, Paul E, et al. Renal manifestations of tuberous sclerosis complex: Incidence, prognosis, and predictive factors. Kidney Int 2006;70:1777-82
- Eijkemans MJC, van der Wal W, Reijnders LJ, et al. Long-term follow-up assessing renal angiomyolipoma treatment patterns, morbidity and mortality: an observational study in tuberous sclerosis complex patients in the Netherlands. Am J Kidney Dis 2015;S0272-6386:00845-8 (Epub ahead of print)
- Schillinger F, Montagnac R. Chronic renal failure and its treatment in tuberous sclerosis. Nephrol Dial Transpl 1996;11:481-5

