Abstract
Objective:
Multiple sclerosis (MS) causes significant disability globally and is especially prevalent in Canada. Delayed-release dimethyl fumarate (DMF; also known as gastro-resistant DMF) is an orally administered disease-modifying treatment (DMT) for patients with relapsing-remitting MS (RRMS) that is currently on the market in the US, Australia, Canada, and Europe. A budget impact model (BIM) was developed to assess the financial consequences of introducing DMF for treatment of RRMS in Canada.
Methods:
A BIM calculated the financial consequences of introducing DMF in Canada over 3 years based on RRMS prevalence, treatment market share, and clinical effects. RRMS prevalence in Canada was derived from published literature and natural relapse rates, and disease state distribution from clinical trial data. It was conservatively assumed that 100% of RRMS patients were treated with a DMT. DMF was assumed to absorb market share proportionally from the following current treatments: interferon beta-1a-IM, interferon beta-1a-SC, interferon beta-1b, and glatiramer acetate. Treatment efficacy, in terms of relapse rate reductions and treatment discontinuation rates, was determined from mixed treatment comparison. Treatment costs (including costs of acquisition, monitoring, and administration) and cost of relapse were considered. Deterministic one-way sensitivity analyses were conducted to assess the most sensitive input parameters.
Results:
Over 3 years, the introduction of DMF resulted in an average annual increase of CAD417 per treated patient per year, with reductions in costs associated with relapses (CAD192/patient/year) partially offsetting increased drug acquisition costs (CAD602/patient/year). On a population level, the average annual cost increase was CAD24,654,237, a CAD 0.68 increase per population covered by the Canadian healthcare system. The main drivers of budget impact were drop-out rates, proportion of RRMS patients treated, and market share assumptions.
Conclusions:
The acquisition costs of DMF for treatment of RRMS are predicted to be partially offset by reduced costs of relapses in the Canadian healthcare system.
Introduction
Multiple sclerosis (MS) is a chronic progressive immune-mediated demyelinating disease of the central nervous system. MS is the most common inflammatory disorder of the central nervous system, affecting ∼2–2.5 million people worldwide, and is a leading cause of disability among young adultsCitation1. In Canada in particular, where the average age of onset is in the 20–25 year range (falling well below the worldwide average of 29.2 years), the disease affects young adultsCitation2. The prevalence of MS in Canada is among the highest in the world. The 2008 World Health Organization (WHO) MS Atlas ranked Canada’s MS prevalence 5th highest out of 93 countries (132.5 per 100,000)Citation2. The prevalence of MS in Canada may be even higher than reported in the WHO Atlas; a cross-sectional survey conducted between 2000–2001 estimated that the prevalence of MS in Canada was 240 per 100,000, and varied across regionsCitation3.
The disease is characterized by relapses and remissions, and can generally be divided into four sub-types: relapsing-remitting multiple sclerosis (RRMS), primary progressive multiple sclerosis, secondary progressive multiple sclerosis (SPMS), and progressive relapsing multiple sclerosisCitation4. Most patients (85%) present with the RRMS sub-type, which is characterized by alternating periods of acute relapse (lasting days to months) and complete or partial remissionCitation4. Over time, in most patients, untreated RRMS becomes SPMS, with 90% of untreated RRMS patients converting to SPMS after 25 years of diseaseCitation1,Citation5. SPMS is characterized by steadily progressive disability and disuse damage, at times associated with occasional relapses, but less frequently than RRMSCitation4,Citation6. Patients with MS are further characterized by their disability progression. The Kurtzke Expanded Disability Status Scale (EDSS) is used to quantify the disability status of patients with MS according to neurological examCitation7,Citation8. The status of disability is assigned to one of 20 categories weighted on a scale of 0–10, with 0 being a normal neurological examination and 10 being death.
There is no cure for MS. Current treatment options include disease-modifying agents (DMTs). At the time of this study, DMTs available for first-line treatment of MS in Canada included interferon beta-1a IM (intramuscular), interferon beta-1a SC (subcutaneous: 22 mg and 44 mg doses), interferon beta-1b SC (Betaferon and Extavia), and glatiramer acetate (GA)Citation9–13. Tecfidera (delayed-release dimethyl fumarate [DMF; also known as gastro-resistant DMF]), a twice-daily orally administered capsule, was approved in Canada as a first-line treatment for adults with RRMS on April 9, 2013Citation1 Citation4. The efficacy of DMF has been studied in patients with RRMS in two Phase III clinical studies—Determination of the Efficacy and Safety of Oral Fumarate in Relapsing– Remitting MS (DEFINE) and Comparator and an Oral Fumarate in Relapsing–Remitting Multiple Sclerosis (CONFIRM). Six of DEFINE’s 160 and four of CONFIRM’s 199 study locations were located in Canada. The primary objective of DEFINE was the proportion of patients with relapse by 2 years; the primary end-point of CONFIRM was reduction in annualized relapse rate at 2 yearsCitation15,Citation16. DMF met the primary end-point in both studies. Twice-daily 240 mg DMF significantly reduced the annualized relapse rate compared with placebo by 53% (p < 0.0001) in the DEFINE trial and by 44% (p < 0.0001) in the CONFIRM trial. In both studies, DMF demonstrated an acceptable safety and tolerability profileCitation15,Citation16.
Treatment of RRMS involves numerous costs, including the cost of drug acquisition, administration, and monitoring, as well as costs associated with relapse. As an orally administered drug associated with reduced rates of relapse, DMF has the potential to provide cost offsets. A budget impact model (BIM) was developed to evaluate what impact the introduction of DMF for treatment of RRMS would have on the overall budget of the Canadian healthcare system.
Methods
Model structure
A BIM was developed in Microsoft ExcelCitation17 in which two scenarios were compared. The first scenario considered a treatment algorithm for RRMS in Canada in which DMF was not included. In the second scenario, the introduction of DMF was modeled in addition to the other currently available DMTs. Comparing the differences in costs (drug acquisition, administration, monitoring, and cost of relapse) between these two scenarios provided an estimate of the budgetary impact on the Canadian health system of introducing DMF for treatment of RRMS in Canada. The BIM took the perspective of the Canadian healthcare system and analyzed the costs and clinical outcomes of treatment over a 3-year time horizon (from 2014–2016).
Estimation of eligible population
The number of RRMS patients eligible for treatment was calculated by projecting the total population of Canada over the 3-year time horizon and multiplying each year’s population by the estimated prevalence of RRMS. Estimates of the total population of Canada by year were based on Canadian government population projections, which estimate an annual growth rate of roughly 1% from year 2014–2016, resulting in total population estimates of 35,767,773, 36,129,027, and 36,493,930 in years 2014, 2015, and 2016, respectivelyCitation18. The prevalence of MS in Canada was estimated to be 240 per 100,000, based on results of a large Canadian Community Health SurveyCitation3, of which 68% were estimated to have RRMSCitation19. This prevalence estimate includes both first- and subsequent-line MS patients, which would serve to inflate the number of patients treated in the BIM, and, therefore, DMF’s budget impact. The prevalence of RRMS was assumed to remain stable over the 3-year time horizon of the model. Incident cases of RRMS were not considered in the model. Given the relative low prevalence of RRMS on a population level, the number of incident cases would have very little impact on outcomes. It was conservatively assumed that 100% of RRMS patients were treated with DMTs; this assumption was tested in the one-way sensitivity analysis.
Treatment assumptions
Comparators were chosen based on the currently available first-line treatments for RRMS in Canada. These consisted of interferon beta-1a IM (intramuscular), interferon beta-1a SC (subcutaneous: 22 mg and 44 mg doses), interferon beta-1b SC (Betaferon and Extavia), and GA. In the scenario in which DMF was introduced, it was assumed to absorb 6.8%, 11.5%, and 20.4% of the market share for 2014, 2015, and 2016, respectively. DMF’s market uptake was assumed to be drawn proportionally from all other comparators in the market. presents the treatment mix for both scenarios.
Table 1. Treatment mix scenarios.
Treatment discontinuation was included in the model and allowed to differ by comparator. Probabilities of discontinuation were calculated through a mixed treatment comparison of published comparator trial dataCitation20. The baseline risk of discontinuation on placebo from the included studies, along with the relative risk of discontinuation on each treatment as compared to placebo, was used to calculate the risk of withdrawal on each treatment. Discontinuation rates were assumed to remain constant throughout the 3-year time horizon.
Rates of relapse
At baseline, patients in the model were stratified according to EDSS scores 0–9, based on the distribution of RRMS patients observed in the pooled data from the Phase III trials DEFINE and CONFIRM (see ). Patients were modeled to experience a baseline rate of relapse due to the natural history of disease as well as a rate of relapse specific to treatment. Progression or regression of disease was not modeled, but rather the EDSS distribution was assumed to remain constant over the 3-year time horizon.
Table 2. Population distribution and relapse rates according to EDSS distribution.
Annual rates of relapse due to natural history were modeled according to EDSS distribution (see ). Patients with an EDSS score from 0–5 were assigned relapse rates according to the pooled CONFIRM and DEFINE trial data, which documented the annual relapse rate in the 12 months before trial enrollment. Relapse was defined as ‘new or recurrent neurologic symptoms not associated with fever or infection, lasting at least 24 hours, and accompanied by new objective neurological findings upon examination by the examining neurologist’ (communication from Biogen Idec, 2011). Data with adequate sample size was available only up to EDSS 5. Therefore, relapse rates due to natural history for patients with EDSS scores from 6–9 were based on a survey of MS patients in the UKCitation21.
Comparator-specific relapse rates relative to placebo were calculated based on a mixed treatment comparison of published Phase III randomized controlled trialsCitation20. The relapse rate for DMF was based on pooled data from the CONFIRM and DEFINE trials. Relapse rates were adjusted to account for treatment-specific discontinuation by multiplying the non-relapse rate by one-half the treatment discontinuation rate of each comparator (see ).
Table 3. Discontinuation and relapse rates by treatment.Citation20
Costs
The model included costs of treatment (including costs of drug acquisition, administration, and monitoring) as well as the cost of relapse. All costs related to treatment were adjusted for treatment discontinuation. Costs are presented in 2013 Canadian dollars.
Annual drug acquisition costs were calculated by multiplying the cost per dose by the expected number of doses per year. The annual acquisition cost for DMF (based on a cost of $33.03 per 240 mg capsule), beta-1a IM, interferon beta-1a SC 22 mg, interferon beta-1a 44 mg, Betaferon, Extavia, and GA were calculated to be $24,131, $20,485, $18,909, $24,469, $20,075, $18,133, and $16,241.
DMF is an oral medication and, therefore, no administration costs were associated with it. Subcutaneous DMTs (interferon beta-1a SC, interferon beta-1b SC, and GA) and intramuscular interferon beta-1a were assumed to require a one-time, 3-h nurse visit during which a patient is instructed how to administer the drug, after which the drug is self-administered. Because these administration costs impact only the initial year of treatment and because this model dealt with a prevalent, not incident, RRMS population, administration costs were assumed to be $0.00 for all treatments over the course of the 3-year time horizon.
Monitoring costs associated with treatment of RRMS consist of healthcare visits to neurologists as well as laboratory tests, such as full blood count tests, liver function tests, metabolism tests, MRI scans, and optometrist visits. The frequency of use of each type of resource was treatment-dependent. A greater number of visits and monitoring tests are recommended in the first year of treatment compared to subsequent years of treatment. Again, because this model focused on a prevalent population, the model applied the monitoring costs of subsequent years for each modeled year. Treatment-specific resource use data were sourced from relevant prescription information and clinical expert opinion. Monitoring cost covers nursing and specialist hours, and lab tests accompanying the treatment. These data were obtained from the schedule of benefits of the Canadian health insurance systemCitation22. Based on this source, average annual administration cost for GA was estimated to be $185; the average annual monitoring cost for all other treatments was estimated to be $193.
The average cost per relapse was calculated to be $6615 using a method published in Karampampa et al.Citation19. This method calculated the annual excess cost attributable to relapses as the difference in the mean annual cost (excluding treatment-associated costs) between RRMS patients with EDSS ≤ 5 who experienced relapse and those who did not. The average cost per relapse was then estimated by dividing the annual cost attributable to relapses by the mean number of relapses reported by RRMS patients with EDDS ≤ 5. The cut-off of EDSS ≤ 5 was considered to be the most medically relevant, since most patients with EDSS > 5 have disability progression without experiencing disease exacerbationsCitation19. This method for calculating cost of relapse has been cited in a Canadian Agency for Drugs and Technologies in Health Therapeutic Review and captures the use of resources such as inpatient and outpatient care, consultations, investigations, and over-the-counter treatmentsCitation23.
AEs were assumed to present no additional costs to the Canadian healthcare system. While treatment of RRMS is associated with numerous AEs, some of which can be serious and incur costs, the incidence of most AEs is very low and would likely not have any significant cost impact given the 3-year time horizon of the model.
Model analyses
The total costs of introducing DMF to the Canadian healthcare system were analyzed as well as disaggregated costs (such as cost of drug acquisition and cost of relapse). Additionally, the cost per population was calculated by dividing the total cost to the healthcare system by the Canadian population. After base case analyses were performed, a one-way sensitivity analysis was conducted in which the values of key parameters were varied by ±25% to assess the robustness of model results and identify key drivers of budget impact. The following parameters were included in the sensitivity analysis: percentage of patients treated, market share assumptions, prevalence, and drop-out rate for DMF.
Results
Base case
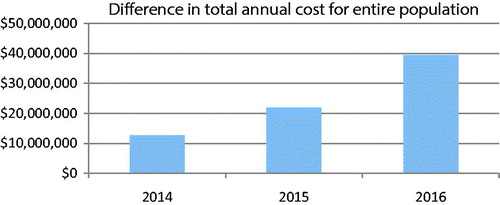
The introduction of DMF into the Canadian market resulted in an increase in cost per patient per year starting at $217 in 2014 and reaching $662 in 2016. On a population level, this amounts to an increase in total annual healthcare spending of $39,429,925 by 2016. These total cost outcomes are presented in . A graphic representation of the difference in total annual costs to the entire Canadian population is presented in . In , disaggregated cost outcomes are presented. Here, the increase in drug acquisition cost associated with DMF is partially offset by the cost savings realized by DMF’s lower rate of relapse compared to currently available treatments.
Table 4. Total cost outcomes.
Table 5. Disaggregated cost outcomes.
Sensitivity analysis
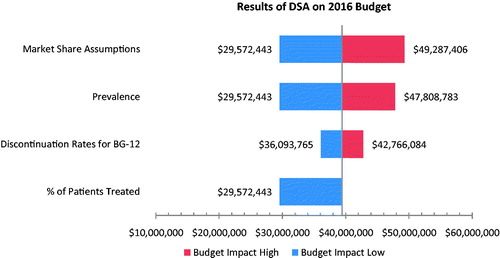
A deterministic sensitivity analysis was conducted in which DMF discontinuation rates and market share assumptions were varied by 25% in both directions. Additionally, a 25% lower MS prevalence estimate was tested as well as a higher prevalence estimate based on data from the 2013 WHO MS Atlas, which published an upper bound of MS prevalence of 291 per 100,000 in Canada. The percentage of patients treated was also varied, although only in one direction, given that the base case used the assumption that all RRMS patients received treatment. The results of the sensitivity analysis on the 2016 budget are presented in a tornado diagram in . Market share assumptions, prevalence of RRMS, and reduction of the percentage of patients treated were found to be the biggest drivers of budget impact. Discontinuation rates for DMF resulted in a smaller budget impact.
Discussion
Based on this model, the acquisition costs of DMF are predicted to be partially offset by reduced costs of relapses for patients in the Canadian healthcare system. The introduction of DMF for treatment of RRMS resulted in a modest impact on the Canadian healthcare budget. The projected impact to the 2016 budget is an increase in per patient cost of $662, or a 2.7% increase in per patient cost by 2016. This translates into an annual per population healthcare budget increase of $1.08 by 2016. Budget impact analyses do not take into account the impact to quality-of-life for individuals with disease and their caretakers that may follow from DMF’s improved relapse rates.
Results were shown to be quite robust to variations in model inputs, as shown by the results of the one-way sensitivity analysis. When market share assumptions were varied in both directions by 25%, the 2016 budget impact varied from a high of $49,287,406 (or $828 per patient cost) to a low of $29,572,443 (or $497 per patient cost). Similarly, alternative high and low prevalence estimates resulted in a 2016 budget impact that varied from a high of $47,808,783 to a low of $29,572,443. If only 75% of RRMS patients are modeled to receive treatment (in place of the base assumption that 100% of patients are treated), the budget impact drops to a 2016 cost of $29,572,443. Variation in discontinuation rates for DMF resulted in even smaller impacts to the 2016 budget, with a per patient cost of $606 when the DMF discontinuation rate is reduced by 25%, and a per patient cost of $718 when the DMF discontinuation cost is increased by 25%.
No other budget impact studies have been published comparing DMF to other DMTs in the RRMS population, which limits the comparisons that can be made using the present model results. However, the structure and assumptions of the present model are similar to other published budget impact models for the treatment of RRMS. For example, in a budget impact analysis of natalizumab for treatment of RRMS in the US setting, the costs of AEs were excluded (as they were in the current analysis) due to the fact that severe AEs in this population are rareCitation24. A similar study evaluating the budget impact of natalizumab in Ireland did include cost of AEs, specifically the cost of monitoring for progressive multi-focal leukoencephalopathy (PML), a severe and rare neurological condition that has been linked to treatment with natalizumabCitation25. However, as the current analysis did not include natalizumab as a comparator, monitoring for PML was not included in monitoring costs. Similar to the current analysis, an average cost per relapse was used in cost calculationsCitation25.
The current analysis had a number of strengths. These results should be highly relevant to the Canadian healthcare system in that the analysis was based on Canadian-specific population and prevalence data. That administration, monitoring costs, and relapse rates were adjusted for discontinuation from treatment ensures that the results of this analysis reflect adherence to treatment as captured in clinical trials. The current analysis is also conservative in a number of respects. The model did not include any treatment effects on EDSS state, although any improvement in EDSS progression with DMF treatment may further moderate the overall budget impact. The assumption that 100% of RRMS patients would receive DMT treatment was also conservative from a cost perspective. Finally, the estimate of prevalence used in the modelCitation3 was one of the highest reported; using the 2008 WHOCitation2 lower estimate of MS prevalence in Canada would have resulted in a lower budget impact.
This study is not without limitations. The prevalence of RRMS was assumed to remain stable over the 3-year time horizon of the model. Including incident cases of RRMS may have provided a more accurate estimate of the population in need in Canada. However, due to the short timeline of this model and relatively low incidence rate of RRMS, the assumption of stable prevalence should not have impacted the analysis greatly. The DMF clinical trials may have included more patients with lower EDSS scores than the Canadian patient populationCitation26. This would correspond with fewer relapses and potentially lead to an under-estimate of the cost-offsets in this analysis. The model also excluded progression or regression of disease, assuming that EDSS distribution remained constant over the 3-year timeline. Finally, the cost of AEs was not included in the current analysis.
In summary, the results of this analysis indicate that introducing DMF for treatment of RRMS in Canada would have a modest annual budget impact to the Canadian healthcare system of ∼2.7% per patient treated or $1.08 per population. The reduction in relapse rate seen with DMF should provide cost savings that partially offset its acquisition cost.
Transparency
Declaration of funding
Funding for this study was provided by Biogen Idec.
Declaration of financial/other relationships
ED and AK were employed at Evidera, a company that received funding to assist with the conduct of this analysis and the preparation of this manuscript. SS was employed at Biogen Idec, the financial sponsor of this study. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
The authors gratefully acknowledge the contributions of Parexel (formerly Heron) to the model development.
References
- Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev 2010;9:A387-94
- World Health Organization. Atlas multiple sclerosis resources in the world. 2008. Availabel online at: http://www.who.int/mental_health/neurology/Atlas_MS_WEB.pdf. Accessed September 16, 2013
- Beck CA, Metz LM, Svenson LW, Patten SB. Regional variation of multiple sclerosis prevalence in Canada. Multiple Sclerosis 2005;11:516-19
- Hurwitz BJ. The diagnosis of multiple sclerosis and the clinical subtypes. Ann Ind Acad Neurol 2009;12:226-30
- Ebers GC. Natural history of multiple sclerosis. J Neurol Neurosurg Psychiatry 2001;71(Suppl 2):ii16-19
- Zuvich RL, McCauley JL, Pericak-Vance MA, et al. Genetics and pathogenesis of multiple sclerosis. Semin Immunol 2009;21:328-33
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983;33:1444-52
- Multiple Sclerosis Trust. A to Z of MS: Expanded Disability Status Scale (EDSS). 2013. Available online at: http://www.mstrust.org.uk/atoz/edss.jsp, Accessed November 2013
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 1993;43:655-61
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 1996;39:285-94
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology 1995;45:1268-76
- PRISMS Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352:1498-504
- Goldenberg MM. Multiple sclerosis review. P & T 2012;37:175-84
- Biogen Idec Canada. TECFIDERA™, formerly BG-12, provides a combination of proven efficacy and favourable safety profile. 2013. Available online at: http://www.newswire.ca/en/story/1142785/health-canada-approves-tecfideratm-as-a-first-line-oral-treatment-for-multiple-sclerosis. Accessed November 2013
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med 2012;367:1087-97
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med 2012;367:1098-107
- Microsoft Excel [computer software]. Redmond, WA; 2007. Available online at: https://products.office.com/en-us/excel. Accessed November 2013
- Statistics Canada. Projected population by age group and sex according to three projection scenarios for 2010, 2011, 2016, 2021, 2026, 2031 and 2036, at July 1 (2016). 2010. Available online at: http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/demo23c-eng.htm. Accessed November 2013
- Karampampa K, Gustavsson A, Miltenburger C, et al. Treatment experience, burden, and unmet needs (TRIBUNE) in multiple sclerosis: the costs and utilities of MS patients in Canada. J Populat Ther Clin Pharmacol 2012;19:e11-25
- Hutchinson M, Fox RJ, Havrdova E, et al. Efficacy and safety of BG-12 (dimethyl fumarate) and other disease-modifying therapies for the treatment of relapsing-remitting multiple sclerosis: a systematic review and mixed treatment comparison. Curr Med Res Opin 2014;30:613-27
- Orme M, Kerrigan J, Tyas D, et al. The effect of disease, functional status, and relapses on the utility of people with multiple sclerosis in the UK. Value in Health: J Int Soc Pharmacoeconom Outcomes Res 2007;10:54-60
- Gani R, Giovannoni G, Bates D, et al. Cost-effectiveness analyses of natalizumab (Tysabri) compared with other disease-modifying therapies for people with highly active relapsing-remitting multiple sclerosis in the UK. PharmacoEconomics 2008;26:617-27
- CADTH Therapeutic Review. Comparative Clinical and Cost-Effectiveness of Drug Therapies for Relapsing-Remitting Multiple Sclerosis. Vol. 1. Ottawa, ON: CADTH; 2013
- Chiao E, Meyer K. Cost effectiveness and budget impact of natalizumab in patients with relapsing multiple sclerosis. Curr Med Res Opin 2009;25:1445-54
- Dee A, Hutchinson M, De La Harpe D. A budget impact analysis of natalizumab use in Ireland. Irish J Med Sci 2012;181:199-204
- Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain J Neurol 1989;112:133-46