Abstract
Background:
Atypical long-acting injectable (LAI) antipsychotics are increasingly available for treating chronic schizophrenia in patients chronically non-adherent to prescribed regimens. Few economic studies have compared these products.
Purpose:
To determine the cost-effectiveness of aripiprazole (ARI-LAI), paliperidone (PP-LAI), olanzapine (OLZ-LAI), and risperidone (RIS-LAI) in patients with chronic schizophrenia in Finland.
Methods:
A 1-year decision tree model was adapted with guidance from an expert panel. Patients started hospitalized in relapse; those who responded continued treatment, others were switched to secondary drugs, then clozapine in the event of 2nd line failure. Rates of adherence, stable disease, relapse, and hospitalization were taken from pivotal trials, and utilities from published research. Included were direct costs paid by the Finnish Ministry of Health, in 2015 euros. Outcomes included quality-adjusted life-years (QALYs), hospitalization rates, and rates of relapse not requiring hospitalization. Model robustness was assessed using a series of 1-way and multivariate sensitivity analyses.
Results:
Expected costs were lowest for PP-LAI at 41,148€, followed by 41,543€ for ARI-LAI, 42,067€ for RIS-LAI and 45,406€ for OLZ-LAI. Respective QALYs were 0.683, 0.671, 0.666, and 0.672. Re-hospitalization rates and non-admitted relapses were 23.6% and 3.9% for PP-LAI, 28.5% and 4.1% for ARI-LAI, 28.8% and 5.0% for RIS-LAI, 28.3% and 5.2% for OLZ-LAI. PP-LAI treatment was associated with the most days with stable disease (132.0), followed by OLZ-LAI (125.5), ARI-LAI (122.6), and RIS-LAI (114.4). Sensitive inputs between PP-LAI and ARI-LAI included rates of adherence, dropouts, and relapses plus drug prices; dropout and relapse rates for RIS-LAI; OLZ-LAI results were insensitive. In probability sensitivity analyses, PP-LAI dominated ARI-LAI in 75.8% of the 10,000 iterations, RIS-LAI in 83.1% and OLZ-LAI in 95.7%.
Conclusions:
PP-LAI dominated the other atypicals. It appears to be the preferred option for treating chronic relapsing schizophrenia.
Introduction
Chronic schizophrenia remains a challenge for clinicians and healthcare providersCitation1. Treating schizophrenia is challenging and a costly disease to treat, impacting patients, families, and friends, as well as the healthcare systemCitation1–4. The largest proportion of the costs of care are due to relapses of symptoms, which result in hospital visitsCitation5–7. Non-adherence to medications has been identified as a major contributor to these relapsesCitation8,Citation9. Long-acting injectable (LAI) antipsychotics are becoming increasingly more available in clinical practice, as they help to decrease both intentional and unintentional non-adherence. At the same time, they have had a beneficial effect on clinical and economic outcomesCitation10. Bera et al.Citation11 also found that, along with other benefits, costs were lower with LAIs (44% of which were RIS-LAI and 56% typical depots) than with oral antipsychotics.
Presently, there are four atypical LAI antipsychotics available in Finland for use in patients requiring such products, including aripiprazole (ARI-LAI)Citation12, olanzapine (OLZ-LAI)Citation13, paliperidone (PP-LAI)Citation14, and risperidone (RIS-LAI)Citation15. The purpose of this research was to determine the cost-effectiveness of these atypical LAIs for treating chronic schizophrenia in Finland.
Methods
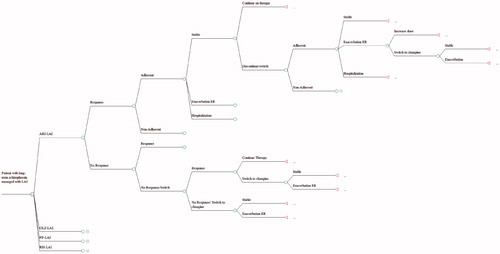
A 1-year decision tree previously developed for Finland was modified to reflect the current LAI landscapeCitation16. depicts the clinical pathway. One major difference between the original and modified model was that the original model started with patients in remission, while the modified model starts with patients in relapse. Another modification was the addition of ARI-LAI, which has recently been approvedCitation12. Treatment patterns and adaptations were determined in conjunction with a panel of clinical experts. The process was undertaken according to the Finnish Ministry of Social Affairs and Health guidelines for preparing a health economic evaluationCitation17.
Base case scenario
Patients would be treated for the acute phase of the relapse in hospital as per recommendations and standard procedure in Finland. They would either respond to LAI antipsychotics after 13 weeks and receive maintenance therapy, or would continue for another 13 weeks with acute therapy. In cases of drug failure or intolerance, patients would be switched to alternative treatments. Those failing ARI-LAI, PP-LAI, or RIS-LAI would be switched to OLZ-LAI, while those on OLZ-LAI would be switched to RIS-LAI. Patients who failed second line treatment were assigned to clozapine, as per NICE guidelinesCitation18. Once stabilized, patients would proceed with treatment, with adherence rates taken into consideration. Further relapses would result in re-starting the drug or switching to another antipsychotic.
Clinical rates were obtained from published randomized controlled trials or from large databases with data from comparable patients. To avoid bias, we used all data currently available on a special group of patients; i.e., persons with chronic, relapsing schizophrenia, starting in relapse. These were patients who had difficulty with adherence to prescribed regimens of oral drugs and, therefore, required LAIs. summarizes the input rates and the sources of those valuesCitation19–63. Clinical states of interest included stable disease and two forms of relapse, which were those requiring hospitalization and those that could be treated in the emergency room (ER) as outpatients. Rates of response to acute treatment were determined from RCTs, all of which defined response as a ≥30% decrease in PANSS score. As placebo rates varied among studies, we first combined placebo rates across trials using a weighted average, then adjusted rates using the approach of Bucher et al.Citation24.
Table 1. Model inputs and their sources.
Only direct costs paid by the Finnish Ministry of Health were considered, including drugs, health professionals, and institutional care. lists the unit costs and sourcesCitation64–67. We did not include the costs associated with treating adverse events because they tend to be similar across drugs, as found in the CATIE trialCitation68. Also, the impact of discontinuing and switching drugs due to adverse events was incorporated into the model, thereby capturing most of the associated costs. All costs were calculated in 2014 euros; those from other years were adjusted to 2014 using the Consumer Price Index for FinlandCitation69. Because the model was 1 year in length, discounting was not applied to either costs or outcomes.
Table 2. Cost inputs used in the model (2015 euros).
Utilities were those used in our previous modelCitation16, which were derived from the literature. We assigned a utility of 0.890 to stable disease, 0.659 to an exacerbation that could be managed at the hospital emergency room or on an outpatient basis, and 0.490 to hospitalization. indicates the sources of these values.
We conducted both cost-utility and cost-effectiveness analyses. In the primary analysis, outcomes assessed included the expected cost per patient treated as well as quality-adjusted life-years (QALYs) associated with each treatment. The economic outcomes was the incremental cost per QALY gained. For the cost-effectiveness analyses, outcomes were hospitalization rate, rate of relapses that were treated in the emergency room (ER), and days with stable disease.
Sensitivity of the results was examined using 1-way (threshold and break-even) analyses on all important inputs. Also, we conducted pairwise multivariate probability sensitivity analyses, varying the input values of all parameters using standard distributions (i.e., gamma for costs and beta for clinical rates) and over plausible ranges for 10,000 simulations with each drug.
Results
presents the results from the base case analysis. PP-2LAI had the lowest overall expected cost per patent treated and the most QALYs. At the same time, it was associated with the highest number of days with stable disease and the lowest number of days in relapse, ER visits, and hospitalizations. Consequently, it dominated all other LAIs in the base case.
Table 3. Clinical and economic outcomes.
The cost driver was hospitalization, which accounted for 82–85% of the total cost. Drugs accounted for 10–11% and medical care 4–7%. In a sensitivity analysis, hospitalization was reduced to acute care only (14 days), which resulted in drastically reduced costs by 32–36%. Nonetheless, the rank order of costs remained the same, with PP-LAI dominating all other drugs.
One-way sensitivity analyses indicated that results between PP-LAI and ARI-LAI were sensitive to changes in rates of adherence, dropouts, relapses, and possibly drug prices. Between PP-LAI and RIS-LAI, results were sensitive to changes in relapse rates; OLZ-LAI results were insensitive to all changes. Break-even points for dominance are presented in the Appendix. For PP-LAI to become not cost-effective, differences in inputs would have to be much greater. For example, PP-LAI would remain cost-effective over ARI-LAI, with monthly maintenance doses of up to 121–132 mg to match the NICE minimum (£20,000 ≈ 24,800€) and maximum thresholds (£30,000 ≈ 37,200€). In probability sensitivity analyses, PP-LAI dominated ARI-LAI in 75.8% of the 10,000 iterations, RIS-LAI in 83.1%, and OLZ-LAI in 95.7%. As well, it was cost-effective in 77.2%, 86.1%, and 96.3% of the 10,000 pairwise iterations when compared with ARI-LAI, RIS-LAI, and OLZ-LAI, respectively, when using the NICE threshold of £20,000/QALY gained (∼24,800€ using the average exchange rate for 2014)Citation70.
Discussion
This model is somewhat different from the original Finnish model, in that this model was initiated with patients in relapse. Several published economic analyses have started with patients in relapse, using a variety of analytical models including decision treesCitation71,Citation72, discrete event simulationsCitation73–76, and clinical trial modelsCitation77. As a result, outcomes would take into consideration the cost of hospitalization for all patients and lower QALY score for that initial time period. We hypothesized that the overall cost per patient would be considerably higher when compared to models that started with patients having stable disease, which was observed.
The results reported here generally validate what has been previously reported. A search of Medline identified 12 economic analyses involving comparisons between different atypical LAIs other than clozapineCitation16,Citation72,Citation78–87. In nine of those analyses, PP-LAI was compared with RIS-LAI (and other LAIs)Citation16,Citation78,Citation79,Citation81–86, eight compared PP-LAI with OLZ-LAI (and others)Citation16,Citation78,Citation80–82,Citation84–86, one examined OLZ-LAI against RIS-LAICitation72, and one studied ARI-LAI vs PP-LAICitation87. In eight out of nine analyses, PP-LAI had a lower cost and more QALYs than RIS-LAI. The same situation occurred in seven out of eight comparisons with OLZ-LAI, with PP-LAI dominating. In both cases, the discrepant study did not use data obtained from studies of PP-LAI, but assumed that PP-LAI and RIS-LAI were equal in every wayCitation82. Thus, the only difference was in the drug acquisition cost. In Norway, Carroll et al.Citation72 found that OLZ-LAI dominated RIS-LAI, having a lower cost and higher QALYs. Finally, Citrome et al.Citation87 recently published a pharmacoeconomic analysis comparing ARI-LAI with PP-LAI in the US. In their analysis, ARI-LAI was cost-effective, despite having a higher cost, due to a lower relapse rate for ARI-LAI. The present study found the same overall relapse rate for PP-LAI (27.5%), but a somewhat higher rate for ARI-LAI (32.6%). This latter rate is very close to the 33.8% relapse rate reported by Pigott et al.Citation88 after 26 weeks on oral aripiprazole. Since no real world data have been located for ARI-LAI, we must wait to see if these results can be validated.
Many economic analyses have used clinical rates derived from independent studies, which can introduce bias due to differences in patients, protocols, or environments. To produce comparable clinical rates, we adjusted them through placebo whenever possible using the method of Bucher et al.Citation24.
On the other hand, we did not include the costs of adverse events because they comprise only a very small proportion of the costs and overall tend to be quite similar across drugs. As examples from other European countries, the Lindström study reported that those costs constituted 1.2–1.7% of the total costs for four different oral atypical antipsychoticsCitation89. Another analysis from Sweden from Mehnert et al.Citation85 found comparable rates of 0.2–1.4% for three atypical LAIs, including PP-LAI, RIS-LAI, and OLZ-LAI. In a study from SloveniaCitation90, adverse events associated with atypicals (including one depot, RIS-LAI) comprised 0.3–0.7% of the overall cost and, in GreeceCitation91, they constituted 0.3–0.4% of the total costs of six oral atypicals. Thus, adverse events exert a negligible impact on the final results and do not differ substantially between drugs.
We also found that QALYs were very similar between the drugs examined after 1 year. The difference between the highest (PP-LAI) and the lowest (RIS-LAI) was small, at 0.017 QALY (2.5%). However, even less variation was reported in a recent publication by Park and KuntzCitation92, who modeled the CATIE trial over 10 years. They compared 12 different strategies using four oral atypicals (olanzapine, quetiapine, risperidone, and ziprasidone), varying the second line treatment in the different scenarios. Over the 10-year time horizon, those authors recorded a difference of only 1% in QALYs between the highest (7.351 QALYs) and the lowest (7.275 QALYs)Citation92.
Limitations
As with all models, this one represents the average results for the average patient with chronic schizophrenia who is in relapse. They would not apply to other forms of the disease (e.g., first episode schizophrenia), other states (e.g., in remission), different forms of psychosis, or to persons with comorbidities. Also, results may not apply to other healthcare systems or jurisdictions due to differences in treatment patterns, availability of resources, or methods of financing. Results might vary in places where traditional depots and other approaches are still commonly used.
Also, the model had a 1-year time horizon; results could vary with a longer duration. Because of the duration, a large proportion of time was spent in hospital under highly supervised conditions; hence, there was very little opportunity for differences to emerge between drugs with respect to QALYs.
There was often a lack of specific input data for the analysis, especially for ARI-LAI, as it is a fairly new drug with very few published studies providing information. Also, little real world information has been published, as there has been limited clinical experience. In the absence of directly applicable data, assumptions had to be made. In all such cases, we took a conservative approach to avoid introducing bias. For example, we assumed that the adherence rate for ARI-LAI and OLZ-LAI would be comparable to that of PP-LAI as all can be dosed on a monthly basis. However, in three 52-week trials starting with patients in relapse, 57% of patients taking oral aripiprazole discontinued before the trials ended, suggesting that adherence rates in these patients may not be so highCitation93–95. More accurate estimates with the LAI formulation could alter results.
Conclusions
In this analysis, PP-LAI had the lowest overall cost per patient and was associated with the most QALYs and lowest rates of adverse outcomes (i.e., days in relapse, hospitalizations, and ER visits). In the base case analysis, it dominated the other alternatives; in sensitivity analyses, results were fairly robust across a wide range of possible values. It appears to be the preferred option for treating chronic relapsing schizophrenia in patients who require LAIs.
Transparency
Declaration of funding
TRE and BGB received funding for this study from Janssen-Cilag. HP, PG and KvI are employees of Janssen-Cilag.
References
- Silveira C, Marques-Teixeira J, de Bastos-Leite AJ. More than one century of schizophrenia: an evolving perspective. J Nerv Ment Dis 2012;200:1054-7
- Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull 2004;30:279-93
- Hansson L. Determinants of quality of life in people with severe mental illness. Acta Psychiatr Scand 2006;113(429 Suppl):46-50
- Foldemo A, Gullberg M, Ek A-C, et al. Quality of life and burden in parents of outpatients with schizophrenia. Soc Psychiatry Psychiatr Epidemiol 2005;40:133-8
- Lafeuille MH, Gravel J, Lefebvre P, et al. Patterns of relapse and associated cost burden in schizophrenia patients receiving atypical antipsychotics. J Med Econ 2013;16:1290-9
- Daltio CS, Mari JJ, Ferraz MB. Direct medical costs associated with schizophrenia relapses in health care services in the city of São Paulo. Rev Saude Publica 2011;45:14-23
- Hong J, Windmeijer F, Novick D, et al. The cost of relapse in patients with schizophrenia in the European SOHO (Schizophrenia Outpatient Health Outcomes) study. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:835-41
- Higashi K, Medic G, Littlewood KJ, et al. Medication adherence in schizophrenia: factors influencing adherence and consequences of non-adherence, a systematic literature review. Ther Adv Psychopharmacol 2013;3:200-18
- Olivares JM, Sermon J, Hemels M, et al. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry 2013;12:32
- Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence 2013;7:1171-80
- Bera R, Offord S, Zubek D, et al. Impact on healthcare resource usage and costs among Medicaid-insured schizophrenia patients after initiation of treatment with long-acting injectable antipsychotics. J Med Econ 2013;16:522-8
- Abilify Maintena, aripiprazole Summary of product characteristics. London, UK: European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002755/human_med_001711.jsp&mid=WC0b01ac058001d124. Accessed May 3, 2015
- Zypadhera, olanzapine Summary of product characteristics. London, UK: European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000890/human_med_001188.jsp&mid=WC0b01ac058001d124. Accessed May 3, 2015
- Xeplion, paliperidone Summary of product characteristics. London, UK: European Medicines Agency. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002105/human_med_001424.jsp&mid=WC0b01ac058001d124. Accessed May 3, 2015
- Risperdal Consta (risperidone) Summary of product characteristics. London, UK: European Medicines Agency, 2007. London, UK: European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Risperdal_Consta_30/WC500008168.pdf. Accessed May 3, 2015
- Einarson TR, Pudas H, Zilbershtein R, et al. Pharmacoeconomic analysis of atypical antipsychotic depots for chronic schizophrenia in Finland. J Med Econ 2013;16:1096-105
- Ministry of Social Affairs and Health, Finland. Guidelines for preparing a health economic evaluation. http://www.ispor.org/PEguidelines/source/GuidelinesinFinland_EnglishVersion.pdf. Helsinki, Finland, 2009. Accessed April 26, 2015
- National Collaborating Centre for Mental Health. Schizophrenia: The NICE guidelines on core interventions in the treatment and management of schizophrenia in adults in primary and secondary care. Updated edition. National Clinical Guideline Number 82. London, UK: National Institute for Health and Clinical Excellence, 2010.
- Lenert LA, Sturley AP, Rapaport MH, et al. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schiz Res 2004;71:155-75
- Revicki DA, Shakespeare A, Kind P. Preferences for schizophrenia-related health states: a comparison of patients, caregivers and psychiatrists. Int Clin Psychopharmacol 1996;11:101-8
- Abilify Maintena® summary of product characteristics. London, UK: European Medicines Agency, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002755/WC500156111.pdf. Accessed May 3, 2015
- Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. J Clin Psychiatry 2014;75:1254-60
- Kane JM, Osuntokun O, Kryzhanovskaya LA, et al. A 28-week, randomized, double-blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry 2009;70:572-81
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol 1997;50:683-91
- Zypadhera Summary of product characteristics. London, UK: European Medicines Agency, 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000890/WC500054429.pdf. Accessed May 5, 2015
- Lauriello J, Lambert T, Andersen S, et al. An 8-week, double-blind, randomized, placebo controlled study of olanzapine long-acting injection in acutely ill patients with schizophrenia. J Clin Psychiatry 2008;69:790-9
- Xeplion Summary of product characteristics. London, UK: European Medicines Agency, 2013. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002105/WC500103317.pdf. Accessed May 6, 2015
- Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res 2010;116:107-17
- Gopal S, Hough DW, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled, dose-response study. Int Clin Psychopharmacol 2010;25:247-56
- Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol 2010;13:635-47
- Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology 2010;35:2072-82
- Pandina GJ, Lindenmayer J-P, Lull JM, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol 2010;30:235-44
- Takahashi N, Takahashi M, Saito T, et al. Randomized, placebo-controlled, double-blind study assessing the efficacy and safety of paliperidone palmitate in Asian patients with schizophrenia. Neuropsychiatr Dis Treat 2013;8:1889-98
- European Medicines Agency. Risperdal Consta_ summary of product characteristics. London, UK, 2008. characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Risperdal_Consta_30/WC500008170.pdf. Accessed May 5, 2015
- Pandina G, Lane R, Gopal S, Gassmann-Mayer C, Hough D, Remmerie B, Simpson G. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:218-26
- Meltzer HY, Lindenmayer JP, Kwentus J, et al. A six month randomized controlled trial of long acting injectable risperidone 50 and 100mg in treatment resistant schizophrenia. Schizophr Res 2014;154:14-22
- Fu DJ, Bossie CA, Sliwa JK, et al. Paliperidone palmitate versus oral risperidone and risperidone long-acting injection in patients with recently diagnosed schizophrenia: a tolerability and efficacy comparison. Int Clin Psychopharmacol 2014;29:45-55
- Li H, Rui Q, Ning X, et al. A comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2011;35:1002-8
- Briggs A, Wild D, Lees M, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes 2008;6:105
- Cummins C, Stevens A, Kisely S. The use of olanzapine as a first and second choice treatment in schizophrenia. A West Midlands Development and Evaluation Committee Report. Birmingham, UK: Department of Public Health & Epidemiology, University of Birmingham, 1998
- Hough D, Lindenmayer J-P, Gopal S, et al. Safety and tolerability of deltoid and gluteal injections of paliperidone palmitate in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2009;33:1022-31
- Oh PI, Lanctôt KL, Mittmann N, et al. Cost-utility of risperidone compared with standard conventional antipsychotics in chronic schizophrenia. J Med Econ 2001;4:137-56
- Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2012;73:617-24
- Ascher-Svanum H, Faries D, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry 2006;67:453-60
- Potkin SG, Saha AR, Kujawa MJ, et al. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry 2003;60:681-90
- McEvoy JP, Daniel DG, Carson WH, et al. A randomized, double-blind, placebo-controlled, study of the efficacy and safety of aripiprazole 10, 15 or 20 mg/day for the treatment of patients with acute exacerbations of schizophrenia. J Psychiatr Res 2007;41:895-905
- Kane JM, Carson WH, Saha AR, et al. Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2002;63:763-71
- Fleischhacker WW1, Sanchez R, Johnson B, et al. Long-term safety and tolerability of aripiprazole once-monthly in maintenance treatment of patients with schizophrenia. Int Clin Psychopharmacol 2013;28:171-6
- Detke HC, Lauriello J, Landry J, McDonnell DP. Within-drug benefit-risk evaluation of olanzapine long-acting injection at one and two years of treatment. Int J Methods Psychiatr Res 2014;23:439-50
- Kane J, Detke H, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry 2010;167:181-9
- Weiden PJ, Kozma C, Grogg A, et al. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv 2004;55:886-91
- Ascher-Svanum H, Peng X, Montgomery W, et al. Assessing the infrequent oral supplementation of olanzapine long-acting injection in the treatment of schizophrenia. Eur Psychiatry 2011;26:313-19
- Asseburg C, Willis M, Löthgren M, et al. Hospitalisation utilisation and costs in schizophrenia patients in Finland before and after initiation of risperidone long-acting injection. Schizophr Res Treatment 2012;2012:791468
- Mehnert A, Diels J. Impact of administration interval on treatment retention with long-acting antipsychotics in schizophrenia. Presented at the Tenth Workshop on Costs and Assessment in Psychiatry -Mental Health Policy and Economics; Venice, Italy, 25-27 March 2011
- Gopal S, Vijapurkar U, Lim P, et al. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol 2010;25:685-97
- Fleischhacker WW, Gopal S, Lane R, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol 2012;15:107-18
- Olivares J, Peuskens J, Pecanek J, et al. Clinical and resource-use outcomes of risperidone long-acting injection in recent and long-term diagnosed schizophrenia patients: results from a multinational electronic registry. Curr Med Res Opin 2009;25:2197-206
- Ascher-Svanum H, Montgomery WS, McDonnell DP, et al. Treatment-completion rates with olanzapine long-acting injection versus risperidone long-acting injection in a 12-month, open-label treatment of schizophrenia: indirect, exploratory comparisons. Int J Gen Med 2012;5:391-8
- Kissling W, Heres S, Lloyd K, et al. Direct transition to long-acting risperidone - analysis of long-term efficacy. J Psychopharmacol 2005;19:15-21
- Lee M, Ko Y, Lee S, et al. Long-term treatment with long-acting risperidone in Korean patients with schizophrenia. Hum Psychopharmacol 2006;21:399-407
- Lindenmayer J-P, Khan A, Eerdekens M, et al. Long-term safety and tolerability of long-acting injectable risperidone in patients with schizophrenia or schizoaffective disorder. Eur Neuropsychopharmacol 2007;17:138-44
- Möller H-J, Llorca P-M, Sacchetti E, et al. Efficacy and safety of direct transition to risperidone long-acting injectable in patients treated with various antipsychotic therapies. Int Clin Psychopharmacol 2005;20:121-30
- Olivares JM, Rodrigues-Morales A, Diels J, et al. Long-term outcomes in patients with schizophrenia treated with risperidone long-acting injection or oral antipsychotics in Spain: Results from the electronic Schizophrenia Treatment Adherence Registry (e-STAR). Eur Psychiatry 2009;24:287-96
- IMS Health Oy SLD price. Finland: Suomen LääkeData Oy. http://www.sld.fi/finland.html. Accessed April 22, 2014
- Price list: Kliinisen Kemian Laboratoriotutkimusten Ja Verivalmisteiden. Hinnasto 2015. Seinäjoki, Finland: Etelä-Pohjanmaan sairaanhoitopiiri, January 1, 2015
- Kapiainen S, Väisänen A, Haula T. Terveyden- ja sosiaalihuolloin yksikkökustannukset Suomessa vuonna 2011. Raportti 3/2014. Terveyden ja hyvinvoinnin laitos. https://www.julkari.fi/bitstream/handle/10024/114683/THL_RAPO3_2014_web.pdf?sequence=1. Tampere, Finland 2014. Accessed May 3, 2015
- HUS Palveluhinnasto 2014. Osa 2 Suoriteperusteiset sairaanhoidolliset palvelut. http://www.hus.fi/hus-tietoa/talous/Hinnoittelu/Documents/HUS%20Palveluhinnasto%202014%20OSA%202.pdf. Helsinki, Finland 2014 Accessed May 3, 2015
- Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209-23
- Triami Media BV. Inflation.eu website. http://www.inflation.eu/inflation-rates/finland/historic-inflation/cpi-inflation-finland-2015.aspx. Accessed May 3, 2015
- European Central Bank. Euro foreign exchange reference rates. Frankfurt-am-Main, Germany, 2015. https://www.ecb.europa.eu/stats/exchange/eurofxref/html/eurofxref-graph-gbp.en.html. Accessed July 14, 2015
- Edwards NC, Pesa J, Meletiche DM, et al. One-year clinical and economic consequences of oral atypical antipsychotics in the treatment of schizophrenia. Curr Med Res Opin 2008;24:3341-55
- Carroll SM, Jemiai N, Suter B, et al. Cost-effectiveness analysis of olanzapine long-acting injection compared with risperidone long-acting injection in the treatment of schizophrenia in Norway. Abstract PMH42. Value Health 2009;12:A358
- Hensen M, Heeg B, Löthgren M, et al. Cost effectiveness of long-acting risperidone in Sweden. Appl Health Econ Health Policy 2010;8:327-41
- Heeg B, Buskens E, Botteman M, et al. The cost-effectiveness of atypicals in the UK. Value Health 2008;11:1007-21
- Heeg BM, Antunes J, Figueira ML, et al. Cost-effectiveness and budget impact of long-acting risperidone in Portugal: a modeling exercise. Curr Med Res Opin 2008;24:349-58
- Laux G, Heeg B, van Hout BA, et al. Costs and effects of long-acting risperidone compared with oral atypical and conventional depot formulations in Germany. Pharmacoeconomics 2005;23(1 Suppl):49-61
- Edgell ET, Andersen SW, Johnstone BM, et al. Olanzapine versus risperidone. A prospective comparison of clinical and economic outcomes in schizophrenia. Pharmacoeconomics 2000;18:567-79
- Einarson TR, Zilbershtein R, Skoupá J, et al. Economic and clinical comparison of atypical depot antipsychotic drugs for treatment of chronic schizophrenia in the Czech Republic. J Med Econ 2013;16:1089-95
- Einarson TR, Geitona M, Chaidemenos A, et al. Pharmacoeconomic analysis of paliperidone palmitate for treating schizophrenia in Greece. Ann Gen Psychiatry 2012;11:18
- Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomic analysis of paliperidone palmitate versus olanzapine pamoate for chronic schizophrenia in Norway. Acta Neuropsychiatr 2013;25:85-94
- Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomics of depot antipsychotics for treating chronic schizophrenia in Sweden. Nord J Psychiatry 2014;68:416-27
- Furiak NM, Ascher-Svanum H, Klein RW, et al. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision model. Curr Med Res Opin 2011;27:713-30
- Hemels MEH, Einarson TR, Zilbershtein R, et al. Cost-effectiveness of injectable atypical long-acting antipsychotics for chronic schizophrenia in Poland. J Health Policy Outcomes Res 2013;2:50-55
- Jukic V, Jakovljevic M, Filipcic I, et al. Cost-utility analysis of depot antipsychotics for chronic schizophrenia in Croatia. Value Health Reg J Cent East Eur 2013;2:181-8
- Mehnert A, Nicholl D, Pudas H, et al. Cost effectiveness of paliperidone palmitate versus risperidone long-acting injectable and olanzapine pamoate for the treatment of patients with schizophrenia in Sweden. J Med Econ 2012;15:844-61
- Zeidler J, Mahlich J, Greiner W, et al. Cost effectiveness of paliperidone palmitate for the treatment of schizophrenia in Germany. Appl Health Econ Health Policy 2013;11:509-21
- Citrome L, Kamat SA, Sapin C, et al. Cost effectiveness of aripiprazole once-monthly compared with paliperidone palmitate once-monthly injectable for the treatment of schizophrenia in the United States. J Med Econ 2014;17:567-76
- Pigott TA, Carson WH, Saha AR; Aripiprazole Study Group. Aripiprazole for the prevention of relapse in stabilized patients with chronic schizophrenia: a placebo-controlled 26-week study. J Clin Psychiatry 2003;64:1048-56
- Lindström E, Eberhard J, Fors BM, et al. A pharmacoeconomic analysis of sertindole in the treatment of schizophrenia in Sweden. Nord J Psychiatry 2011;65:403-13
- Obradovic M, Mrhar A, Kos M. Cost-effectiveness of antipsychotics for outpatients with chronic schizophrenia. Int J Clin Pract 2007;61:1979-88
- Geitona M, Kousoulakou H, Ollandezos M, et al. Costs and effects of paliperidone extended release compared with alternative oral antipsychotic agents in patients with schizophrenia in Greece: a cost effectiveness study. Ann Gen Psychiatry 2008;7:16
- Park T, Kuntz KM. Cost-effectiveness of second-generation antipsychotics for the treatment of schizophrenia. Value Health 2014;17:310-19
- Fleischhacker WW, McQuade RD, Marcus RN, et al. A double-blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophrenia. Biol Psychiatry 2009;65:510-17
- Hsieh MH, Lin WW, Chen ST, et al. A 64-week, multicenter, open-label study of aripiprazole effectiveness in the management of patients with schizophrenia or schizoaffective disorder in a general psychiatric outpatient setting. Ann Gen Psychiatry 2010;9:35
- Kasper S, Lerman MN, McQuade RD, et al. Efficacy and safety of aripiprazole vs. haloperidol for long-term maintenance treatment following acute relapse of schizophrenia. Int J Neuropsychopharmacol 2003;6:325-37