Abstract
Objective:
To analyze medical costs and healthcare resource utilization (HRU) associated with everolimus-based therapy or chemotherapy among post-menopausal women with hormone-receptor-positive, human-epidermal-growth-factor-receptor-2-negative (HR+/HER2−) metastatic breast cancer (mBC).
Methods:
Patients with HR+/HER2− mBC who discontinued a non-steroidal aromatase inhibitor and began a new line of treatment with everolimus-based therapy or chemotherapy (index therapy/index date) between July 20, 2012 and April 30, 2014 were identified from two large claims databases. All-cause, BC-related, and adverse event (AE)-related medical costs (in 2014 USD) and all-cause HRU per patient per month (PPPM) were analyzed for both treatment groups across patients’ first four lines of therapies for mBC. Adjusted differences in costs and HRU between the everolimus and chemotherapy treatment group were estimated pooling all lines and using multivariable generalized linear models, accounting for difference in patient characteristics.
Results:
A total of 3298 patients were included: 902 everolimus-treated patients and 2636 chemotherapy-treated patients. Compared to chemotherapy, everolimus was associated with significantly lower all-cause (adjusted mean difference = $3455, p < 0.01) and BC-related ($2510, p < 0.01) total medical costs, with inpatient ($1344, p < 0.01) and outpatient costs ($1048, p < 0.01) as the main drivers for cost differences. Everolimus was also associated with significantly lower AE-related medical costs ($1730, p < 0.01), as well as significantly lower HRU (emergency room incidence rate ratio [IRR] = 0.83; inpatient IRR = 0.74; inpatient days IRR = 0.65; outpatient IRR = 0.71; BC-related outpatient IRR = 0.57; all p < 0.01).
Conclusions:
This retrospective claims database analysis of commercially-insured patients with HR+/HER2− mBC in the US showed that everolimus was associated with substantial all-cause, BC-related, and AE-related medical cost savings and less utilization of healthcare resources relative to chemotherapy.
Introduction
Breast cancer (BC) is the most common malignancy among women worldwide, accounting for 25% of all cancers, and is the second leading cause of cancer-related death for women in developed countriesCitation1. It is estimated that, in 2015, ∼232,000 new BC cases will be diagnosed and that BC-related deaths will account for 6.8% of all cancer-related deaths in the USCitation2. BC-related deaths are primarily due to the development of incurable metastatic diseaseCitation3. Approximately 5–10% of BC is metastatic (mBC) at the time of diagnosisCitation4, and 20–50% of patients who are first diagnosed with primary BC ultimately develop metastatic diseaseCitation3. The 5-year survival rates for patients with mBC are estimated to be 20–25%Citation4,Citation5. Furthermore, mBC is associated with substantial economic burden. Studies have reported average medical costs of ∼$10,000 per month per patientCitation6,Citation7, with medical services comprising about half of the total healthcare costs among patients with mBC receiving chemotherapy in the USCitation8.
As mBC is not curable, treatment aims to prolong survival by controlling tumor growth and to improve quality-of-life (QoL) for patientsCitation9. The preferred mBC treatments depend on the molecular sub-type of the patient’s neoplasm. The most prevalent molecular sub-type of BC is hormone-receptor-positive (HR+) human epidermal growth factor receptor-2-negative (HER2−), which comprises ∼70% of mBC diagnosesCitation10. HR+ disease is particularly common among post-menopausal women, as the relative incidence of HR positivity increases with ageCitation11. The National Comprehensive Cancer Network (NCCN) treatment guidelines recommend the use of endocrine therapy as preferred first-line treatment for post-menopausal women with HR+/HER2− mBCCitation4,Citation12. For patients who do not respond or develop resistance to the initial endocrine agent, NCCN guidelines recommend either additional endocrine therapy or chemotherapy, depending on the aggressiveness of the cancerCitation12. Chemotherapy is typically used for patients with rapidly progressive or symptomatic visceral diseaseCitation8. The targeted therapy everolimus, an inhibitor of mammalian target of rapamycin used in combination with exemestane, has also become a common treatment option for patients who previously failed a non-steroidal aromatase inhibitor (NSAI)Citation13.
Since the approval of everolimus for HR+/HER2− mBC in 2012, several economic modelling studies have assessed the budget impact of everolimus for payers and cost-effectiveness of everolimus compared to endocrine therapy and chemotherapy. Budget impact models suggest that everolimus use is associated with medical cost savings for the treatment of HR+/HER2− mBC, which partially offset the increased pharmacy costs and result in modest total budget increase to the payersCitation14,Citation15. Cost-effectiveness models suggest that everolimus/exemestane combination therapy is a cost-effective option compared to other endocrine therapies benchmarked by the economic value of other novel cancer medicationsCitation16 and dominate chemotherapies including bevacizumab/capecitabine and bevacizumab/paclitaxel combination therapiesCitation17. Cost modeling results also suggest that costs for managing adverse events (AEs) are 33–50% less for everolimus/exemestane combination therapy as compared to commonly-used single-agent chemotherapy including capecitabine, docetaxel, and doxorubicinCitation18. However, no studies have yet directly assessed the economic outcomes associated with everolimus-based therapy among post-menopausal women with HR+/HER2− mBC in a real-world setting.
This study aims to fill the gap in the literature by retrospectively comparing medical costs and healthcare resource utilization for patients using everolimus-based therapy vs chemotherapy, and estimating the differences in these real-world economic outcomes. To that end, this retrospective claims database analysis compared all-cause, BC-related, and AE-related medical costs and healthcare resource utilization among patients with HR+/HER2− mBC who received everolimus-based therapy vs chemotherapy.
Methods
Data source
This study was conducted using combined administrative claims data from the Truven Health Analytics MarketScan Commercial and Medicare Supplemental (MarketScan) and IMS Health PharMetrics Plus (PharMetrics) databases spanning from January 1, 2002 to June 30, 2014. The MarketScan database captures the healthcare claims of ∼40 million annually covered lives insured by employer-sponsored private health plans from over 130 employers, as well as Medicare-eligible retirees with employer-provided Medicare supplemental plans. The PharMetrics database contains combined data from over 100 healthcare plans, representing over 42 million annually covered lives insured by private health plans, Medicare Advantage, and Medicare supplemental plans. These data are nationally representative and capture information on patient demographics, diagnoses, healthcare visits, clinical expenditures including detailed costs, and healthcare utilization data for healthcare services performed in inpatient and outpatient settings, and health insurance enrollment. The medical claims are linked to prescription drug claims and person-level health insurance enrollment data. The data were de-identified and comply with the patient confidentiality requirements of the Health Insurance Portability and Accountability Act.
Research design
A retrospective US claims database analysis was conducted to compare medical costs and healthcare resource utilization among post-menopausal women with HR+/HER2− mBC treated with everolimus-based therapy vs chemotherapy.
Sample selection and construction
Post-menopausal women with HR+/HER2− mBC who previously received a NSAI and initiated a new line of therapy for mBC were identified in the claims databases using an algorithm adapted from previous studiesCitation19,Citation20. Selected patients were required to have: (1) at least two diagnoses for BC (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code: 174.xx) on distinct medical claims, separated by at least 30 days; and (2) diagnoses for a secondary neoplasm (ICD-9-CM codes: 196.xx–197.xx, 198.0, 198.1, 198.3–198.7, 198.81, 198.89) on at least two medical claims, (1) no more than 30 days before, or (2) any time after the first BC diagnosis. Patients with HR+ BC were identified by at least one prescription fill for an endocrine therapy at any time, and patients with HER2− disease were identified as lacking any prescriptions for the treatment of HER2+ disease (trastuzumab, lapatinib, pertuzumab, or ado-trastuzumab). Patients were considered post-menopausal if they fulfilled any of the following criteria: (1) were at least 60 years old at mBC diagnosis; (2) had a prescription fill or medical claim for an aromatase inhibitor or fulvestrant; (3) had a prior bilateral oophorectomy; or (4) had at least one diagnosis related to post-menopausal status or menopause (e.g., unspecified menopausal and post-menopausal disorders: ICD-9-CM code 627.9).
Among post-menopausal women with HR+/HER2− mBC, patients with at least one eligible line of therapy among their first four lines of treatment for mBC were included in the analysis. Eligible lines of therapy must have been initiated between July 20, 2012 (the FDA approval date of everolimus for HR+/HER2− mBC) and March 31, 2014 (to ensure at least 3 months of potential follow-up). Patients must have received an NSAI prior to the date of initiation of index therapy; the beginning of the line of therapy was defined as index date. Additionally, patients were required to have at least 12 months of continuous health plan enrollment prior to (for studying patients’ baseline characteristics) and at least 4 weeks of continuous enrollment following the index date (to exclude patients with very short follow-up, among whom the costs and healthcare resource utilization information might not be complete).
Patient’s eligible line(s) of therapies were numbered chronologically from the date of mBC diagnosis up to the first four lines and classified into mutually exclusive regimen groups (everolimus-based therapy and chemotherapy). Each line of therapy started at the index date and ended at a treatment change (switch or add-on), a discontinuation, the end of insurance eligibility, or the end of data availability (June 30, 2014), whichever occurred first. Treatment discontinuation was defined as a treatment gap of at least 60 days. Everolimus-based therapy included everolimus monotherapy or a combination of everolimus with another therapy for mBC. Chemotherapy included chemotherapy monotherapy, combination therapy of chemotherapy agents, and combination therapy of chemotherapy and endocrine therapy.
Study variables
Patient baseline characteristics
Patients’ baseline characteristics were assessed at the beginning of each line of therapy, during the 12 months preceding the index date. Patient characteristics included health insurance plan type, mBC disease status (de novo, non-de novo, or type unknown), number of organ-level metastatic sites, Charlson Comorbidity Index (CCI)Citation21, prior use of chemotherapy for mBC, and time from initiation of last adjuvant endocrine therapy to first mBC diagnosis.
Cost and healthcare resource utilization
Cost outcomes analyzed in this study included all-cause, BC-related, and AE-related medical costs associated with inpatient, outpatient, emergency room (ER), and other medical services. Costs were measured from a combined payer and patient out-of-pocket cost perspective. The cost variables used in the study included the amount paid by the health plan, as well as the deductible, co-payment, co-insurance, and co-ordination of benefits amounts (where for Medicare Supplemental enrolled patients, it includes the portion reimbursed by Medicare). All-cause medical costs were defined as costs incurred by any medical services during the studied line of therapy. BC-related medical costs were assessed as costs incurred by medical services that were associated with a diagnosis of BC (ICD-9-CM code 174.xx) or a secondary neoplasm (ICD-9CM codes 196.xx–197.xx, 198.0, 198.1, 198.3–198.7, 198.81, or 198.89). AE-related medical costs were defined as costs of medical services that were associated with a diagnosis for an AE (Supplemental Table 1)Citation22. Costs were inflated to 2014 US dollars using the medical care component of the Consumer Price Index (CPI) and reported on a per patient per month (PPPM) basis to account for varying duration across lines of therapies.
Table 1. Baseline characteristics.
Healthcare resource utilization outcomes analyzed in this study included number of emergency care visits (defined as inpatient hospitalizations and emergency room [ER] visits), inpatient hospitalizations, days of inpatient hospitalization, ER visits, outpatient visits, BC-related outpatient visits (defined as the use of outpatient services associated with a diagnosis of BC or a secondary neoplasm), and other medical services visits (e.g., laboratory, home care, and hospice services) occurring during the studied line of therapy. AE-related utilization was additionally summarized as services that were associated with a diagnosis for an AE (Supplementary Table 1)Citation22. Healthcare resource utilization outcomes were calculated on a PPPM basis.
Statistical analysis
Patient baseline results were compared between everolimus and chemotherapy using Wilcoxon rank-sum tests for continuous variables and Chi-square tests for categorical variables.
Unadjusted costs were compared between everolimus and chemotherapy by line of therapy using Wilcoxon rank-sum tests. Adjusted cost differences were estimated across all lines using multivariable two-part models, where the first part was a logistic regression model and the second part a gamma generalized linear model (GLM). P-values were estimated using a non-parametric bootstrap re-sampling technique with 499 iterations. Multivariable models adjusted for differences in patient baseline characteristics, including health insurance plan type (private insurance only, Medicare Advantage, Medicare Supplemental, and other Medicare coverage), disease status (de novo, non-de novo, and type unknown), number of metastatic sites, CCI, prior use of chemotherapy for mBC, and time from initiation of last adjuvant endocrine therapy to mBC.
Unadjusted and adjusted healthcare resource utilization was compared between everolimus and chemotherapy by line of therapy (unadjusted) and across all eligible lines (adjusted) using GLMs with a log link and Poisson distribution. Results were reported as incidence rate ratios (IRRs). P-values were estimated using the bootstrapping technique discussed above. Multivariable models were adjusted for differences in patient baseline characteristics listed above for cost multivariable models.
Results
A total of 3298 patients who used everolimus-based therapy or chemotherapy as their index treatment in the first four lines of therapy for mBC were selected. A total of 62.6% were from the MarketScan database and 37.4% were from the PharMetrics database. One patient could contribute more than one line of the same type of therapy (e.g., two lines of chemotherapy) or different types of therapy (e.g., one line of chemotherapy and one line of everolimus-based therapy). The sample included 902 patients who contributed to 940 lines of everolimus-based therapy and 2636 patients who contributed to 3410 lines of chemotherapy (). Among these patients, 240 patients contributed to both everolimus-based therapy and chemotherapy. Baseline characteristics are summarized in .
Figure 1. Sample selection. *Total lines of therapy may exceed the number of patients if patients receive more than one line of the same therapy type.
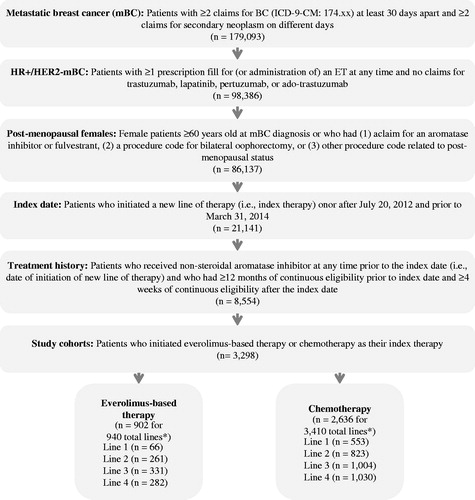
Across the first four lines of therapy, patients treated with everolimus and chemotherapy had generally similar characteristics. Patients were ∼60 years old and had similar mBC disease status, number of metastatic sites, and CCI across all lines. However, a lower proportion of patients treated with everolimus previously received chemotherapy for mBC (Line 2: 10.7% vs 37.1%; Line 3: 31.7% vs 60.0%; Line 4: 57.4% vs 75.4%, respectively; all p < 0.001; ) compared to patients treated with chemotherapy. In addition, in Line 1, patients treated with everolimus had a significantly shorter time from initiation of last adjuvant endocrine therapy to the first mBC diagnosis compared with patients treated with chemotherapy (18.9 vs 28.9 months, p < 0.01; ). Across all lines of therapy, 31.1% of patients treated with everolimus and 47.3% of patients treated with chemotherapy had less than 3 months of follow-up.
Across the first four lines of therapy, total unadjusted PPPM all-cause medical service costs () were lower for patients treated with everolimus than those treated with chemotherapy. All-cause medical costs ranged from $4790–$6158 for patients treated with everolimus and $8493–$8889 for patients treated with chemotherapy. Cost differences were between $2335–$3980 PPPM and were significantly different for all lines of therapy (p < 0.05). Patients treated with everolimus also had significantly lower costs associated with outpatient visits across all four lines of therapy than patients treated with chemotherapy (cost differences ranging from $967–$1699 PPPM, all p < 0.01; ).
Table 2. Comparison of medical services costsa between everolimus and chemotherapy.
Patients treated with everolimus also had lower PPPM BC-related medical service costs () compared with patients treated with chemotherapy. Across lines of therapy, patients treated with everolimus incurred BC-related medical costs ranging from $3355–$5155, while those treated with chemotherapy incurred costs ranging from $6090–$6929. Cost differences were significant (all p < 0.01) in lines 2–4 and ranged from $1692–$3574. Patients treated with everolimus also had significantly lower costs associated with BC-related outpatient visits across lines 2–4 than patients treated with chemotherapy (cost differences were $614, $737, and $1376 for lines 2–4, respectively; all p < 0.01; ).
Similar results were observed after pooling all lines and adjusting for patient baseline characteristics (). Everolimus was associated with significantly lower PPPM all-cause medical service costs than chemotherapy (adjusted mean difference = $3455, p < 0.01), with inpatient costs (difference = $1897, p < 0.01) and outpatient costs (difference = $1395, p < 0.01) as the main drivers for differences in all-cause medical costs. The same trend was seen in PPPM BC-related medical service costs (difference = $2510, p < 0.01). Inpatient (difference = $1344, p < 0.01) and outpatient (difference = $1048, p < 0.01) cost differences were the main drivers of differences in BC-related medical costs as well. There was a lack of differences in either all-cause or BC-related ER costs between everolimus and chemotherapy.
Significant differences in PPPM AE-related medical service costs () were also observed in lines 2–4 when comparing everolimus and chemotherapy. Patients treated with everolimus had lower PPPM costs (ranging from $1474–$1637) than patients treated with chemotherapy (ranging from $2882–$3741). Cost differences ranged from $1370–$2233 (all p < 0.01). Adjusted results pooling all lines and adjusting for baseline characteristics confirmed this finding. Everolimus was associated with significantly lower PPPM AE-related medical service costs, with cost difference of $1730 (p < 0.01; ).
Consistent with the results of the cost analysis, patients treated with everolimus used significantly fewer healthcare resources () than patients treated with chemotherapy, in terms of numbers and days of inpatient hospitalizations (lines 1, 2, and 4), outpatient visits and BC-related outpatient visits (all lines), and other medical services (such as laboratory tests; lines 1, 2, and 4). Patients treated with everolimus were also associated with lower AE-related healthcare resource utilization, including inpatient admissions (lines 1, 2, and 4), outpatient (all lines), and other medical services related to AEs (lines 3 and 4).
Table 3. Comparison of healthcare resource utilization between everolimus and chemotherapya.
After pooling all lines and adjusting for baseline characteristics (), patients treated with everolimus had significantly fewer emergency care visits (IRR = 0.83; potentially driven by large differences in line 4), inpatient admissions (IRR = 0.74), inpatient days (IRR = 0.65), outpatient visits (IRR = 0.71), BC-related outpatient visits (IRR = 0.66), and other medical services (IRR = 0.67) (all p < 0.01). Patients treated with everolimus were also associated with lower AE-related healthcare resource use (IRR = 0.59), including inpatient admissions (IRR = 0.73), outpatient visits (IRR = 0.57), and other medical services (IRR = 0.59) (all p < 0.01).
To the best of our knowledge, this study is the first to examine healthcare costs and resource utilization among patients receiving everolimus vs chemotherapy in a real-world setting, using two large US commercial claims databases. This study found that everolimus was associated with significantly lower all-cause and BC-related total medical costs compared to chemotherapy, with inpatient and outpatient costs as the main drivers in cost savings. In addition, everolimus was also associated with significantly lower AE-related medical costs compared to chemotherapy. Healthcare resource utilization results were consistent with costs results, showing that everolimus was associated with significantly lower use of various healthcare resources compared to chemotherapy. Furthermore, AE-related resource use was a major driver of emergency care visits, including inpatient hospitalizations and emergency room visits, both of which were lower for patients using everolimus.
The real-world results of this study support the findings of recent economic modeling studies showing a medical cost advantage of everolimus-based therapy vs chemotherapy. Xie et al.Citation14 conducted a budget impact analysis to estimate costs of everolimus as the first- and second-line treatment option after failure of an NSAI for post-menopausal women with HR+/HER2− mBC in the US. They found that everolimus was associated with reduced medical services costs, such as those reported in the current study. A similar study by Lewis et al.Citation15 modeled 5-year costs for the same population in Central Asia and reached similar conclusions about reduced medical costs of everolimus due to improvement in disease management. In addition, a recent study by Kourlaba et al.Citation17 applied data collected from clinical trials to a Markov model that compared the costs-effectiveness of everolimus/exemestane combination therapy vs two bevacizumab-based chemotherapy regimens, including bevacizumab/paclitaxel combination therapy and bevacizumab/capecitabine combination therapy, from a third-party payer’s perspective over a lifetime horizon. The authors concluded that everolimus was associated with greater quality-adjusted life-years gained and lesser lifetime healthcare costs, suggesting that everolimus might be a dominant alternative compared to bevacizumab-based chemotherapies for the treatment of HR+/HER2− mBC after initial failure of NSAIs.
Use of chemotherapy is associated with a high incidence of severe AEs, which can increase overall treatment costs. A claims database study of women under 65 with BC found those who received chemotherapy had significantly higher rates of hospitalization and number of ER visits related to AEs compared with those who did not receive chemotherapy, and subsequently incurred considerable costs for these AEs (∼$1271/patient/year)Citation23. Another claims database analysis of AE-related costs for patients with mBC found that AEs related to chemotherapy increased monthly costs from 9–70%, depending on the severity of the AEs, driven primarily by inpatient, outpatient, and pharmacy costsCitation22. The present study’s findings that, across the first four lines for HR+/HER2− mBC, AE-related medical costs for everolimus were 40–52% of costs for chemotherapy, are also supported by a recent economic modeling study on the costs of managing severe AEs (grades 3 or 4) during treatment with everolimus vs chemotherapy in Western EuropeCitation18. That study reported AE-related per patient costs were 33–50% less for treatment with everolimus/exemestane combination therapy compared with single-agent chemotherapy.
The findings from this study have important implications for the decision-making process of healthcare stakeholders, particularly payers. In addition to safety and efficacy, payers must also weigh the impact a therapy may have on patient QoL, productivity, and healthcare resource utilization and costs. As the projected cost of BC is expected to hit $36.5 billion per year by 2020 in the USCitation24, strategies to reduce this expense while maintaining optimal outcomes for patients with BC are highly important. The present study indicates that treatment with everolimus for HR+/HER2− mBC results in lower total medical costs than chemotherapy, driven by fewer outpatient and inpatient visits, as well as lower costs related to AEs. This may be attributed to everolimus’ superior effectiveness, with better overall survival, progression-free survival, and time on treatment compared with chemotherapy reported in real-world studiesCitation25, and consequently fewer healthcare resources are required.
QoL considerations are increasingly important to patients with BC as the survival rates continue to increase and survivors are living longerCitation26. The incidence and severity of AEs, which are higher during treatment with chemotherapy than with everolimus due to chemotherapy’s toxicity, directly impact QoL among patients with BCCitation27. A recent analysis of QoL data from the BOLERO-2 trial reported that, in contrast to chemotherapy, everolimus/exemestane combination therapy did not have a negative impact on health-related QoLCitation28. Thus, the lower AE-related medical costs for everolimus, bolstered by previous models which showed lower costs and higher quality-adjusted life-year gained in patients with HR+/HER2− mBC using everolimus vs chemotherapyCitation29, are valuable evidence for considering mBC treatments that can maintain QoL while reducing AEs as well as costs.
This study has some limitations inherent to the use of claims databases. First, the databases used did not contain direct information for identification of post-menopausal HR+/HER2− mBC or on lines of treatment, therefore patient identification and treatment line classification relied on an algorithm which is based on a combination of different proxies. Similarly, certain clinical factors that may impact treatment decisions (e.g., patients’ performance status) were not available in the database and cannot be adjusted for in multivariable models. Second, costs may vary across payer type, for example, commercial insurance, Medicare, and Medicaid. However, the current databases only include commercially-insured patients and those who have commercial insurance supplementing Medicare insurance; thus, the current results may not be generalizable across patient populations insured by other payers. Future studies using different claims databases, e.g., Medicare databases, can help provide additional real-world economic evidence between everolimus and chemotherapy. Finally, only direct medical costs were studied. Information to determine indirect costs, such as lost productivity and burden to caregivers, was not available.
Conclusion
This retrospective claims database analysis of patients with HR+/HER2− mBC in the US showed that everolimus was associated with substantial all-cause, BC-related, and AE-related medical cost savings and less utilization of healthcare resources relative to chemotherapy. In a commercially insured population, everolimus-based therapy was associated with adjusted total medical cost savings of $3455 PPPM compared to chemotherapy across lines of therapy.
Transparency
Declaration of funding
Funding for this research was provided by Novartis.
Declaration of financial/other relationships
YH is an employee of Novartis and owns stock/stock options. NL, VK, AF, AK, MP, AG, and EQW are employees of Analysis Group Inc., which has received consultancy fees from Novartis. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental_Material.docx
Download MS Word (16.8 KB)Acknowledgments
Manuscript drafts were prepared by the authors with editorial assistance from Shelley Batts, PhD, a professional medical writer for Analysis Group, Inc. ultimately paid by the sponsor, Novartis. A synopsis of the current research was submitted to the NCCN 2016 annual meeting, which will take place in Hollywood, FL, during March 31–April 2, 2016.
References
- World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevelance worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed May 21, 2015.
- National Cancer Institute. 2015. SEER Stat Fact Sheets: breast cancer. http://seer.cancer.gov/statfacts/html/breast.html.
- Lu J, Steeg PS, Price JE, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res 2009;69:4951-3
- Cardoso F, Harbeck N, Fallowfield L, et al. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J ESMO 2012;23(7 Suppl):vii11-9
- National Cancer Institute. SEER Stat Fact Sheets: Breast Cancer 2014. 2014. http://seer.cancer.gov/statfacts/html/breast.html. Accessed September 15, 2014
- Montero AJ, Eapen S, Gorin B, et al. The economic burden of metastatic breast cancer: a U.S. managed care perspective. Breast Cancer Res Treat 2012;134:815-22
- Chitre M, Reimers KM. Considerations for payers in managing hormone receptor-positive advanced breast cancer. Clinicoecon Outcomes Res 2014;6:331-9
- Vera-Llonch M, Weycker D, Glass A, et al. Healthcare costs in women with metastatic breast cancer receiving chemotherapy as their principal treatment modality. BMC Cancer 2011;11:250
- Susan G. Komen Foundation. http://ww5.komen.org/. Accessed
- Howlader N, Altekruse SF, Li CI, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014;106:
- Cadoo KA, Fornier MN, Morris PG. Biological subtypes of breast cancer: current concepts and implications for recurrence patterns. Q J Nucl Med Mol Imag 2013;57:312-21
- National Comprehensive Cancer Network. Breast Cancer, Version 3.2015. 2015. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed May 21, 2015
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012;366:520-9
- Xie J, Diener M, De G, et al. Budget impact analysis of everolimus for the treatment of hormone receptor positive, human epidermal growth factor receptor-2 negative (HER2-) advanced breast cancer in the United States. J Med Econ 2013;16:278-88
- Lewis L, Taylor M, Suriya Ertugyrovna Y, et al. Budget impact analysis of everolimus for the treatment of hormone receptor positive, human epidermal growth factor receptor-2 negative (HER2-) advanced breast cancer in Kazakhstan. J Med Econ 2015;18:189-99
- Xie J, Hao Y, Zhou ZY, et al. Economic evaluations of everolimus versus other hormonal therapies in the treatment of HR/HER2 advanced breast cancer from a US payer perspective. Clin Breast Cancer 2015; 15:e263-76;
- Kourlaba G, Rapti V, Alexopoulos A, et al. Everolimus plus exemestane versus bevacizumab-based chemotherapy for second-line treatment of hormone receptor-positive metastatic breast cancer in Greece: an economic evaluation study. BMC Health Serv Res 2015;15:307
- Campone M, Yang H, Faust E, et al. Cost of adverse events during treatment with everolimus plus exemestane or single-agent chemotherapy in patients with advanced breast cancer in Western Europe. J Med Econ 2014;17:837-45
- Swallow E, Zhang J, Thomason D, et al. Real-world patterns of endocrine therapy for metastatic hormone-receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) breast cancer patients in the United States: 2002–2012. Curr Med Res Opin 2014;30:1537-45
- Whyte JL, Engel-Nitz NM, Teitelbaum A, et al. An evaluation of algorithms for identifying metastatic breast, lung, or colorectal cancer in administrative claims data. Medl Care 2015;53:e49-57
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9
- Hurvitz S, Guerin A, Brammer M, et al. Investigation of adverse-event-related costs for patients with metastatic breast cancer in a real-world setting. Oncologist 2014;19:901-8
- Hassett MJ, O'Malley AJ, Pakes JR, et al. Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 2006;98:1108-17
- Mariotto AB, Yabroff KR, Shao Y, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst 2011;103:117-28
- Li N, Hao Y, Xie J, et al. Everolimus-based therapy versus chemotherapy among patients with HR+/HER2− Metastatic breast cancer: comparative effectiveness from a chart review study. Int J Breast Cancer 2015;2015:1-9
- Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin 2012;62:220-41
- Brem S, Kumar NB. Management of treatment-related symptoms in patients with breast cancer. Clin J Oncol Nurs 2011;15:63-71
- Burris HA, 3rd, Lebrun F, Rugo HS, et al. Health-related quality of life of patients with advanced breast cancer treated with everolimus plus exemestane versus placebo plus exemestane in the phase 3, randomized, controlled, BOLERO-2 trial. Cancer 2013;119:1908-15
- Xie J, Hao Y, Zhou ZY, et al. Economic evaluations of everolimus versus other hormonal therapies in the treatment of HR(+)/HER2(−) Advanced breast cancer from a US payer perspective. Clin Breast Cancer 2015;15:e263-76

