Abstract
Objective Patients with bone metastases or lesions secondary to solid tumors or multiple myeloma often experience bone complications (skeletal-related events [SREs]—radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression); however, recent data that can be used to assess the value of treatments to prevent SREs across European countries are limited. This study aimed to provide estimates of health resource utilization (HRU) and cost associated with all SRE types in Europe. HRU data were reported previously; cost data are reported herein.
Methods Eligible patients from 49 centers across Austria (n = 57), the Czech Republic (n = 59), Finland (n = 60), Greece (n = 59), Portugal (n = 59), and Sweden (n = 62) had bone metastases or lesions secondary to breast, lung, or prostate cancer, or multiple myeloma, and ≥1 index SRE (a SRE preceded by a SRE-free period of ≥ 6.5 months). SRE-related costs were estimated from a payer perspective using health resource utilization data from patient charts (before and after the index SRE diagnosis). Country-specific unit costs were from 2010 and local currencies were converted to 2010 euros.
Results The mean costs across countries were €7043, €5242, €11,101, and €11,509 per radiation to bone, pathologic fracture, surgery to bone, and spinal cord compression event, respectively. Purchasing power parity (PPP)-adjusted mean cost ratios were similar in most countries, with the exception of radiation to bone.
Limitations The overall burden of SREs may have been under-estimated owing to home visits and evaluations outside the hospital setting not being reported here.
Conclusions All SREs were associated with substantial costs. Variation in SRE-associated costs between countries was most likely driven by differences in treatment practices and unit costs.
Introduction
Bone metastases or lesions are found in a high proportion of patients with advanced cancer: 65–75% of patients with breast or prostate cancer; over 35% of patients with lung cancerCitation1; and almost all patients with multiple myelomaCitation2. Bone metastases are associated with bone complications known as skeletal-related events (SREs), which can cause pain, disability, and a reduction in functioning and health-related quality-of-lifeCitation1,Citation3,Citation4. Data summarizing outcomes in patients with advanced solid tumors and bone metastases receiving placebo in four clinical trials showed that more than half experienced at least one SRE within a 21–24 month timeframeCitation3.
Besides having a significant impact on patients, SREs are associated with high healthcare costs. Understanding these costs is important when evaluating the available treatments for preventing SREs, because cost-effectiveness is increasingly taken into consideration amid widespread resource constraintsCitation5. Additionally, a detailed cost analysis can provide valuable information to decision-makers on competing components of healthcare budgets and raise awareness of the economic burden of alternative modes of treatment. International cost comparisons, on the other hand, may suggest efficiency gains through price or volume adjustments in specific countriesCitation6.
Some studies have assessed the economic impact of bone metastases in detail, by examining the overall costs of metastatic bone diseaseCitation7–11, and the SRE-related costs in individual countries and tumor typesCitation12,Citation13. Data from a large study in Germany, Spain, Italy, and the UK showed that all types of SREs are associated with considerable health resource utilization (HRU) and costs of up to €12 082 per SRECitation14,Citation15; however, studies examining costs per SRE event across other European countries are scarce.
The objective of this study was to report detailed HRU cost data associated with SREs from six European countries. HRU data have been reported previouslyCitation16; cost data are reported herein.
Methods
Study design
We conducted a cost of illness study to estimate direct medical costs associated with each type of SRE across six European countries. Feasibility studies in each country identified and categorized centers by caseload and availability of electronic data. Measurement of costs was based on HRU data collected by a retrospective chart review study () that included patients from 49 centers in Austria, the Czech Republic, Finland, Greece, Poland, Portugal, Sweden, and Switzerland. For this cost analysis, Poland and Switzerland were excluded. In Poland, at the time of the investigation, substantial changes in healthcare reimbursement were ongoing (the Reimbursement Act) that affected healthcare pricing and reimbursementCitation17. In Switzerland, a different cost valuation model was used, using both a survey of physicians to identify the TARMED items (outpatient tariff codes used in Switzerland) and data from the Swiss national statistics office to calculate the cost of hospital staysCitation18; this rendered the results non-comparable. Cost valuation was based on applying unit costs of healthcare services obtained from published governmental data to the HRU obtained from the study and adopted a hospital perspective.
Ethics
Investigators obtained institutional and/or local or central ethics committee approval for this study.
Patients
Data were collected from the charts of eligible patients: aged ≥20 years; bone metastases or lesions secondary to breast, prostate, or lung cancer, or multiple myeloma; ≥1 index SRE (a SRE preceded by a SRE-free period of ≥6.5 months; between July 1, 2004 and July 1. 2009). A period of 6.5 months was deemed by an expert steering committee (comprising the authors of this paper) to be sufficient for any previous SREs to have ceased impacting on HRU. SREs were defined as radiation to bone, pathologic fracture (long bone/other), surgery to bone or spinal cord compression, and events were identified using specific hospital diagnostic and procedure codes. Target enrolment was 150 patients per country with 150 index SREs, split by: radiation to bone (n = 60); pathologic fracture (long bone [n = 30], other [n = 30]); surgery to bone (n = 20); and spinal cord compression (n = 10). Exclusion criteria included: current or past participation in a denosumab clinical trial; patients who died <2 weeks after the index SRE; patients whose chart data were of insufficient quality to extract comprehensive HRU data.
Identification
The costs associated with SREs were assumed to arise from requirements for HRU, comprising inpatient stays, outpatient visits, day care visits, emergency room visits, and procedures. Medication costs may also contribute to the cost of SREs, but were excluded because insufficient data were captured to estimate these costs precisely and because these costs were not exclusively attributed to bone involvement.
Measurement
HRU data collection
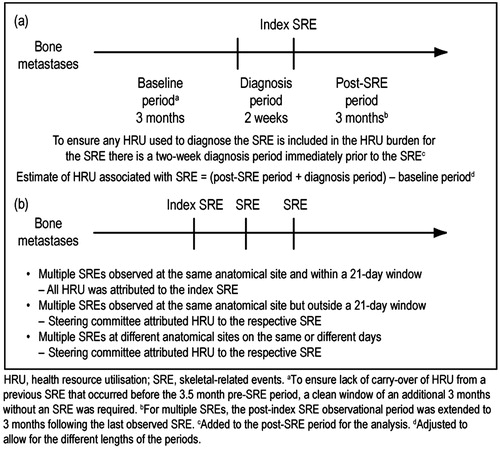
HRU data were captured from patient charts for single SREs for a time period of 3.5 months before the index SRE to 3 months after the index SRE (). These time periods were judged by the steering committee to be sufficient for capturing the majority of SRE-related costsCitation16. For patients with multiple SREs, data extraction was extended until 3 months after the last SRE ().
Attribution of HRU to SREs
For single SREs, HRU was objectively attributed to the index SRE as per the study design (). After adjustment for differences in the lengths of the baseline and post-baseline periods, the change from baseline was used to estimate HRU associated with the SRE. In case of multiple SREs at the same anatomical site and within a 21-day window, all HRU was attributed to the index SRE. In case of multiple SREs observed at the same anatomical site, but outside a 21-day window, or at different anatomical sites on the same or different days, the study steering committee subjectively attributed HRU to each respective SRE.
The HRU data used in this cost analysis have been described elsewhereCitation16. In brief, the mean number of inpatient stays per SRE increased from baseline by ∼0.5–1.5 stays per SRE, depending on SRE type, with increases in the total duration of inpatient stays of ∼6–37 days per event. Increases of 2.6–4.2 outpatient visits and 5.9–9.6 procedures per SRE were also observed (Supplementary Figure 1)Citation16.
Statistical analysis of HRU data
Statistical analyses were descriptive (n, mean, median, quartiles, standard deviation, minimum, maximum).
HRU data were calculated using means, rather than medians. This is because means better describe the total resources used at a population level: information that is required for healthcare policy decisionsCitation19. Means do not, however, illustrate the HRU and cost for individual patients.
Valuation
Cost valuation
Cost analyses of individual SREs for each country were conducted from the payer perspective.
Cost valuation was performed by multiplying the increase in HRU following an SRE by the respective governmental published, country-specific unit costs (using the 2010 price level) to estimate the overall cost per SRECitation20. Local currencies were converted to euros where appropriate (24.096 CZK; 9.200 SEK = 1€[2010 euros]). Diagnosis-related groups (DRGs) were used to estimate costs for countries where they were available (Austria, Finland, Portugal, Sweden); for the other countries, per diem costs were used (unit cost sources are shown in Supplementary Tables 1–6). Costs of procedures performed during an inpatient stay were assumed to be captured in the DRG or per diem costs. In detail, costs were calculated according to the formulae below.
Cost of inpatient stays
Cost of inpatient stays per SRE = Mean number of inpatient stays * DRG tariffs associated with SREa
Cost of inpatient stays per SRE = Mean duration of inpatient stays * Per diem tariffb
Cost of outpatient visits
Cost of outpatient visits per SRE [for each specialisti = {Anesthetist; …; Urologist}] = ∑Mean number of visitsi * Outpatient visit cost tariff
Cost of day care visits
Cost of day care visits per SRE = Mean number of day care visits * DRG tariffs associated with SREa
Cost of day care visits per SRE = Mean duration of day care visits * Per diem tariffb
Cost of emergency room visits
Cost of emergency room visits per SRE [for each specialisti = {Anesthetist; …; Urologist}] = ∑Mean number of visitsi * Emergency room cost tariff
Cost of procedures
Cost of procedures per SRE [for each procedurej = {Bone mineral density; …; Vertebroplasty} = ∑Mean number of outpatient proceduresj * Outpatient procedure unit costjc
bWhere DRG data are not used by the health system (Greece, the Czech Republic).
cFor countries that utilize DRG data, only procedures not coinciding with an inpatient stay were included in the analysis.
Mean cost across countries (MCAC)
To show SRE costs across all the countries studied, the means of the country-specific costs per SRE were calculated for each SRE type.
Mean cost ratio (MCR)
To show SRE-associated cost differences across countries, the MCR was calculated by dividing the country-specific cost by the mean cost across countries for each SRE type.
Purchasing power parity (PPP)-adjusted MCR
To adjust for differences in purchasing power across countries, the MCR was adjusted using the EUROSTAT Organization for Economic Co-operation and Development methodological manual on PPPCitation21. Costs were expressed in euros.
Results
Study population
In total, 356 patients experienced 744 SREs (in Austria, 57 patients contributed 131 SREs; Czech Republic, 59 patients, 130 SREs; Finland, 60 patients, 117 SREs; Greece, 59 patients, 121 SREs; Portugal, 59 patients, 126 SREs; Sweden, 62 patients; 119 SREs); baseline and disease characteristics are described in . The SREs comprised 356 radiation to bone events, 255 pathologic fractures, 71 surgery to bone events, and 62 spinal cord compressions. For 95% of SREs, HRU was attributed as per the study design (); the steering committee was required to attribute HRU to a SRE in only ∼5% of casesCitation16.
Table 1. Patient baseline clinical and demographic characteristics.
MCAC by index SRE
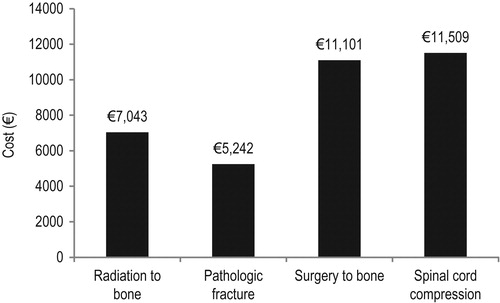
MCACs were substantial for all index SRE types, but were greatest for spinal cord compression (€11,509 per event) and surgery to bone (€11,101), followed by radiation to bone (€7034) and pathologic fracture (€5242) ().
Mean cost per index SRE by country
The mean costs per index SRE by country were considerable for all SRE types (), with the highest mean costs for each country generally associated with spinal cord compression and surgery to bone. For all countries except Sweden, pathologic fracture was associated with the lowest mean costs when compared with the other SREs (€1858–€10,305 vs €2258–€22,191 for other SREs). In Sweden, the cost per radiation to bone event (€3270) was lower than that for pathologic fracture (€5379). In contrast with Sweden, in Greece radiation to bone was the SRE associated with the highest mean costs (€9734) vs the other SRE types (€4478–€7943).
Figure 3. Mean cost per index SRE by country for radiation to bone (a), pathologic fracture (b), surgery to bone (c) and spinal cord compression (d)
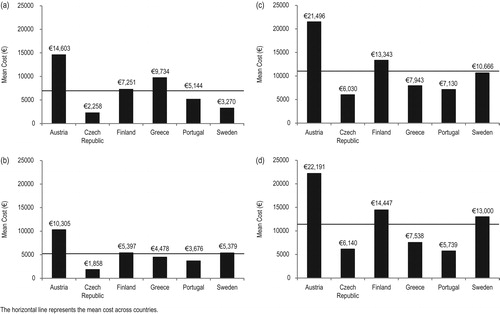
Country-specific differences in mean costs were evident for each index SRE. Mean costs per index SRE were highest in Austria for all SREs (radiation to bone, €14,603; pathologic fracture, €10,305; surgery to bone, €21,496; and spinal cord compression, €22,191). In contrast, mean costs per SRE were generally lower in the Czech Republic and, to a lesser extent, in Portugal than in the other countries. In Finland, mean costs per SRE were above the MCAC for all SREs, but were particularly high for surgery to bone (€13,343) and spinal cord compression (€14,447). In Sweden, mean costs were generally close to the level of the MCAC, with the exception of radiation to bone, which was considerably lower.
MCR by country and index SRE
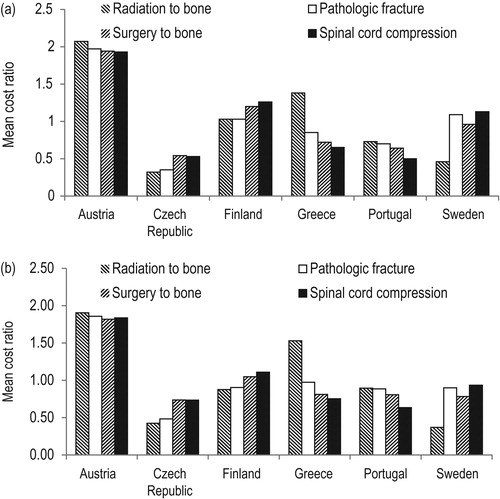
The MCR was highest in Austria for all SRE types () and was approximately double the mean cost of all SREs (1.93–2.07). For Finland, MCRs were greater than one for all SREs (1.03–1.26), particularly for surgery to bone (1.20) and spinal cord compression (1.26). Compared with other countries, the Czech Republic had the lowest MCR for the majority of SRE types, including the lowest recorded MCR (radiation to bone = 0.32). Both the Czech Republic and Portugal had MCRs below the mean for all SREs types (0.32–0.54 and 0.50–0.73, respectively). For Greece, most MCRs were below 1 (0.65–0.85), except radiation to bone (1.38); for Sweden, most MCRs were close to 1 (0.96–1.13), except radiation to bone, for which costs were less than half the mean cost across countries (0.46).
Purchasing power parity-adjusted MCR by country and index SRE
Compared with unadjusted MCRs, purchasing power parity (PPP)-adjusted MCRs increased in those countries with lower MCRs and decreased for countries that recorded higher MCRs (). As a result, PPP-adjusted MCRs were generally similar across countries, with the exception of Austria, where MCRs remained almost double the MCAC (1.82–1.90), and the Czech Republic, which remained relatively low (0.43–0.74). In addition, the cost of radiation to bone remained comparatively high in Greece (1.53) and comparatively low in Sweden (0.37).
Discussion
This study demonstrated that all SREs are associated with substantial costs. In general, spinal cord compression and surgery to bone were linked with the highest costs.
Although the study was not powered to compare results across countries, substantial variations in SRE-associated costs were observed. These were driven in part by differences in unit costs across countries, but were also likely to have been driven by differences in treatment practices, e.g., costs associated with radiation to bone reflected whether single- or multiple-fraction radiotherapy was used. Indeed, for radiation to bone, Austria and Greece (where costs per event for this SRE were highest, and were above the mean even after PPP adjustment) also used the highest number of procedures per radiation to bone eventCitation16. Similarly, Austria also had the highest cost per surgery to bone, in line with having a high mean number of inpatient stays (2 per SRE), a long duration of inpatient stays (28 days per SRE)Citation16, and a high DRG tariff for this event. Such variations should, however, be interpreted with caution because of differences across countries in patient characteristics, such as tumor type. For example, costs per SRE may differ across tumor types, as a result of variations in patient age and comorbidities. Although a regression analysis of how these factors affect HRU was beyond the scope of this study, it may be of interest in the future. However, data from a study of patients with SREs secondary to breast, prostate, or lung cancer in Germany, Spain, Italy, and the UK, and from a study of patients with breast or prostate cancer in Portugal, suggest that patterns of HRU are consistent across tumor typesCitation22–25.
PPP-adjusted MCRs were generally similar for Finland, Greece, Portugal, and Sweden, but remained noticeably lower for the Czech Republic and higher for Austria. Contrary to what may have been expected, costs associated with SREs were not lower in DRG countries compared with non-DRG countries. However, a direct comparison of SRE costs across DRG and non-DRG countries is of limited value, owing to differences in the reimbursement systems.
A different observational study (conducted in Germany, Italy, Spain, and the UK) that estimated SRE-associated costs in patients with bone metastases from solid tumors also reported substantial SRE-associated costs for all SRE typesCitation14. Data were collected prospectively, HRU was directly attributed by physicians and only patients with a life expectancy >6 months were included. Findings from this study were similar to those from the current study; spinal cord compression accounted for the highest SRE-associated costs in the UK (€12,082), Italy (€4884), and Spain (€7903), whereas in Germany, surgery to bone was associated with the highest costs (€9407)Citation14.
In a French prospective study in patients with lung cancer and metastatic bone disease, the reported SRE-associated costs were lower than those in the present analysis, estimating an average first-year management cost of €3999, with approximately half of this cost attributable to patients with a SRECitation12. However, median survival time in the French study was only 5.8 months, which could account for the seemingly low modelled annual costs. In contrast, our study included patients with tumor types associated with longer life expectancies, and excluded patients who died within 2 weeks of their index SRE. Furthermore, the sample size in the French study was small; although 554 patients were recruited, only 73 experienced a SRE. It is also possible that the Markov model used did not capture all SRE-associated costs. Although the assumptions made in the French study are not described in detail, it appears that palliative radiotherapy and surgery for bone metastases were not included in the model’s definition of a SRE.
A previous study in Portugal found costs associated with radiation to bone were lower than in our study (€1485 vs €7043 in our study), whereas pathologic fracture costs were higher (€8730 vs €5242), as were spinal cord compression costs (€13,203 vs €11,509)Citation22. These differences are likely to reflect the source of the cost data: Felix et al.Citation22 used the Portuguese National Health System price list. Their follow-up period in the study also differed from that in ours: resource utilization data were collected from the occurrence of the SRE until the end of the 2-year observation period, regardless of when the SRE occurred during that period.
Another retrospective cost analysis from the Netherlands involving patients with prostate cancer and bone metastases (n = 28) and including data collected over a 24-month period reported a per-patient cost to treat SREs of €6973 (range = €1187–€40,948)Citation09; SREs doubled the total cost of treating patients in this setting. Similarly, a study in Spain that followed patients for 18–21 months has estimated costs ranging from €2378 for radiation to bone to €7903 for spinal cord compressionCitation26. Finally, a study from the perspective of the UK’s National Health Service used modeling techniques to simulate costs in patients with breast cancer and bone metastasesCitation7; here, costs were in the range €14 029–€23,710 (£11,314–£19 121; 1 GBP = 1.24 EUR). Although it is difficult to draw direct comparisons between these cost analyses and the present study, the results from these five studies appear to be in line with the findings reported here and those previously reported by Hechmati et al.Citation14.
Our study has some limitations. It is possible that the overall burden of SREs may have been under-estimated because data were limited to those from a hospital perspective. Patient visits and evaluations outside of the hospital setting, including home visits and additional private medical care, were not reported. These costs usually fall directly on patients and may, in some instances, be significant. Furthermore, over the natural course of disease, metastatic bone disease continues to spread, potentially resulting in progressively more resource utilization as the frequency and severity of bone complications increasesCitation1,Citation27. As such, the 3-month follow-up period may not be sufficient to capture all SRE-associated costs. As with all retrospective studies, there is also the concern that not all information will be captured owing to incomplete data in the original patient charts; however, to limit this concern, quality audits were performed on a proportion of the chart abstractions to test for consistency and completeness against source data.
It should be noted that the costs represent the total cost of treating a patient during the post-SRE period, as was defined by the study methodology. In Finland, patients were not hospitalized for radiation therapy alone, but received this in combination with other therapies, such as chemotherapy, and these costs were also captured. As such, costs associated with radiation to bone therapy in Finland should be interpreted with care. Despite a thorough sample selection procedure, some bias may have been observed as patients receiving radiation to bone who were enrolled at the participating Finnish center were referred only from palliative wards, thus the associated costs may have been at their highest owing to the increasing levels of care required in this setting. No selection bias was observed for patients with other SREs enrolled in Finland.
Although the costs used in the study are from 2010, inflation since then has been low (0.55–3.09%) and is, therefore, expected to have little impact on the cost patterns reported hereCitation28.
The main strength of this study was that HRU was captured in detail. In addition, the study was objective by nature of its design and the steering committee was required to attribute HRU subjectively in only ∼5% of all SRE cases. As a result, the study design limits the influence of the investigators on the results. In contrast to clinical trials, which can be selective and less representative of clinical practice, this study provided real world data that will help to inform the treatment of patients with bone metastases. Future analyses of how costs are incurred over a patient cohort’s lifetime would complement these data and help us understand how this analysis of per-event costs fits within the clinical landscape in which not all patients will experience a SRE. Whereas in clinical trials asymptomatic SREs are often detected owing to regular radiographic screeningCitation11, another strength of our study is that it included only SREs that were detected as part of routine practice.
Conclusions
In conclusion, further preventing SREs with bone-targeted agents should help to alleviate the economic burden on European healthcare systems. With budget restrictions and a growing debt crisis, cost analyses are becoming crucial; data collected via analyses like those presented here will be helpful for determining resource allocation in the future. Such data may also be used in formal cost-effectiveness analyses and budget impact studies or more generally to inform policy-makers of the potential impact of prevention and treatment strategies.
Transparency
Declaration of funding
Funding for this study was provided by Amgen (Europe) GmbH.
Declaration of financial/other relationships
JJB has received sponsorship from Amgen, is a consultant for Amgen and Bayer, and lectures for Amgen, Bayer and GSK. GH is employed by Amgen (Europe) GmbH and holds stock. JP has received unrestricted research grants and speaker honoraria from Amgen. ET has received honoraria and research grants from Amgen. JF is a consultant for GSK, Amgen, Bayer, Novartis, and Roche, and lectures for Amgen, Novartis, Roche, and Pfizer. OG was employed by Amgen (Europe) GmbH at the time of the study. RvM has participated in advisory boards for Amgen, Roche, and Novartis, has received unrestricted research grants from Amgen and Novartis, and speaker honoraria from Amgen and Roche. JME peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
Medical writing support was provided by Kim Croskery from Watermeadow Medical (funded by Amgen (Europe) GmbH) and Emma Booth of Amgen (Europe) GmbH. We would like to thank all of the investigators and co-ordinators who participated in this study: Austria: Heinz Ludwig, Michael Krainer, Rainer Kolb, Richard Greil, Günther Gastl, Paul Sevelda, Christian Singer; Czech Republic: Jitka Abrahamova, Jindrich Finek, Rostislav Vyzula, Lubomir Slavicek, Jana Katolicka; Finland: Tiina Saarto; Greece: Anastasios Thanos, Meletios Dimopoulos, Vassilios Georgoulias, George Fountzilas, Konstantinos Syrigos; Portugal: Francisco Pina, Margarida Damasceno, Henrique Queiroga, Luís Costa, Tomé Lopes, Encarnação Teixeira, Noémia Afonso, Isabel Sousa, Francisco Gomes; Sweden: Hareth Nahi, Jan-Erik Damber, Johan Ahlgren. Professor Carl Blomqvist provided critical review and comments relating to the Finnish data. Statistical support was provided by Tony Mossman, who was a Consultant employed by Amgen Ltd and Susan Shepherd of Amgen Ltd.
Supplemental_tables.zip
Download Zip (66.3 KB)References
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006;12:6243s-9s
- Coleman RE. Skeletal complications of malignancy. Cancer 1997;80:1588-94
- Costa L, Badia X, Chow E, et al. Impact of skeletal complications on patients' quality of life, mobility, and functional independence. Support Care Cancer 2008;16:879-89
- Weinfurt KP, Li Y, Castel LD, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol 2005;16:579-84
- Wilsdon T, Fiz E and Haderi A. A comparative analysis of the role and impact of Health Technology Assessment: 2013. Charles River Associates, 2014. Brussels, Belgium http://www.efpia.eu/uploads/documents/cra-comparative-analysis.pdf. Accessed October 2015
- Drummond M. Cost-of-illness studies: a major headache? Pharmacoeconomics 1992;2:1-4
- Botteman M, Barghout V, Stephens J, et al. Cost effectiveness of bisphosphonates in the management of breast cancer patients with bone metastases. Ann Oncol 2006;17:1072-82
- Delea T, McKiernan J, Brandman J, et al. Retrospective study of the effect of skeletal complications on total medical care costs in patients with bone metastases of breast cancer seen in typical clinical practice. J Support Oncol 2006;4:341-7
- Groot MT, Boeken Kruger CG, Pelger RC, et al. Costs of prostate cancer, metastatic to the bone, in the Netherlands. Eur Urol 2003;43:226-32
- Hillner BE, Weeks JC, Desch CE, et al. Pamidronate in prevention of bone complications in metastatic breast cancer: a cost-effectiveness analysis. J Clin Oncol 2000;18:72-9
- Lage MJ, Barber BL, Harrison DJ, et al. The cost of treating skeletal-related events in patients with prostate cancer. Am J Manag Care 2008;14:317-22
- Decroisette C, Monnet I, Berard H, et al. Epidemiology and treatment costs of bone metastases from lung cancer: a French prospective, observational, multicenter study (GFPC 0601). J Thorac Oncol 2011;6:576-82
- Delea T, Langer C, McKiernan J, et al. The cost of treatment of skeletal-related events in patients with bone metastases from lung cancer. Oncology 2004;67:390-6
- Hechmati G, Cure S, Gouepo A, et al. Cost of skeletal-related events in European patients with solid tumours and bone metastases: data from a prospective multinational observational study. J Med Econ 2013;16:691-700
- Hoefeler H, Duran I, Hechmati G, et al. Health resource utilization associated with skeletal-related events in patients with bone metastases: results from a multinational retrospective – prospective observational study – a cohort from 4 European countries. J Bone Oncol 2014;3:40-8
- Body JJ, Pereira J, Sleeboom H, et al. Health resource utilization associated with skeletal-related events: results from a retrospective European study. Eur J Health Econ 2015; DOI: 10.1007/s10198-10015-10716-10197.
- Giermaziak W. [Impact Reimbursement Act on the pharmaceutical market in Poland]. Pol Merkur Lekarski 2014;36:270-3
- Delmore G, Biteeva I, Steinmann K, et al. Direct medical costs associated with skeletal-related events in Switzerland. Presented at the European Conference on Health Economics 2012. Zurich, Switzerland: 2012
- Thompson SG, Barber JA. How should cost data in pragmatic randomised trials be analysed? BMJ 2000;320:1197-200
- European Commission, Eurostat. Consumer prices - inflation and comparative price levels. Eurostat, 2012. Luxembourg http://epp.eurostat.ec.europa.eu/statistics_explained/index.php/Consumer_prices_-_inflation_and_comparative_price_levels. Accessed November 2015
- Eurostat and OECD. Eurostat-OECD methodological manual on purchasing power parities. Eurostat, 2012. Luxembourg http://www.oecd.org/std/pricesandpurchasingpowerparitiesppp/eurostat-oecdmethodologicalmanualonpurchasingpowerparitiesppps.htm Accessed November 2015
- Felix J, Andreozzi V, Soares M, et al. Hospital resource utilization and treatment cost of skeletal-related events in patients with metastatic breast or prostate cancer: estimation for the Portuguese National Health System. Value Health 2011;14:499-505
- Bahl A, Hoefeler H, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced prostate cancer: a European subgroup analysis from an observational, multinational study. J Clin Med 2014;3:883-96
- Lorusso V, Duran I, Garzon-Rodriguez C, et al. Health resource utilisation associated with skeletal-related events in European patients with lung cancer: Alpha subgroup analysis from a prospective multinational study. Mol Clin Oncol 2014;2:701-8
- Luftner D, Lorusso V, Duran I, et al. Health resource utilization associated with skeletal-related events in patients with advanced breast cancer: results from a prospective, multinational observational study. Springerplus 2014;3:328
- Duran I, Garzon C, Sanchez A, et al. Cost analysis of skeletal-related events in Spanish patients with bone metastases from solid tumours. Clin Transl Oncol 2014;16:322-9
- Pockett RD, Castellano D, McEwan P, et al. The hospital burden of disease associated with bone metastases and skeletal-related events in patients with breast cancer, lung cancer, or prostate cancer in Spain. Eur J Cancer Care (Engl) Engl2010;19:755-60
- OECD, Inflation (CPI) (indicator). OECD, 2015. Paris, France https://data.oecd.org/price/inflation-cpi.htm Accessed October 2015