Abstract
Brain development diverges in males and females in response to androgen production by the fetal testis. This sexual differentiation of the brain occurs during a sensitive window and induces enduring neuroanatomical and physiological changes that profoundly impact behavior. What we know about the contribution of sex chromosomes is still emerging, highlighting the need to integrate multiple factors into understanding sex differences, including the importance of context. The cellular mechanisms are best modeled in rodents and have provided both unifying principles and surprising specifics. Markedly distinct signaling pathways direct differentiation in specific brain regions, resulting in mosaicism of relative maleness, femaleness, and sameness through-out the brain, while canalization both exaggerates and constrains sex differences. Non-neuronal cells and inflammatory mediators are found in greater number and at higher levels in parts of male brains. This higher baseline of inflammation is speculated to increase male vulnerability to developmental neuropsychiatric disorders that are triggered by inflammation.
El desarrollo cerebral difiere en hombres y mujeres en respuesta a la producción de andrógenos por los testículos fetales. La diferenciación sexual del cerebro ocurre durante una ventana sensible e induce cambios neuroanatómicos y fisiológicos duraderos que influyen profundamente en la conducta. Todavía está surgiendo el conocimiento acerca de la contribución de los cromosomas sexuales, por lo que es destacable la necesidad de integrar múltiples factores en la comprensión de las diferencias por sexo, incluyendo la importancia del contexto. Los mecanismos celulares están mejor modelados en roedores y han proporcionado tanto principios unificadores como supresores específicos. De manera muy diferente las vías de señales dirigen la diferenciación en regiones cerebrales específicas, resultando en un mosaicismo de masculinidad, feminidad e igualdad relativas a través del cerebro, mientras que la canalización exagera y restringe las diferencias por sexo. Las células no neuronales y los mediadores inflamatorios se encuentran en mayor número y en niveles más altos en zonas de los cerebros de los machos. Se especula que esta mayor basal de inflamación aumenta la vulnerabilidad en los machos para desarrollar trastornos neuropsiquiátricos que son desencadenados por la inflamación.
Les testicules du foetus mâle produisent des androgènes responsables d'un développement cérébral différent chez les hommes et chez les femmes. Cette différentiation cérébrale selon le sexe survient lors d'une fenêtre (sensitive ou délicate ?) et entraîne des changements neuroanatomiques et physiologiques durables qui influent profondément sur le comportement. Notre connaissance de l'implication des chromosomes sexuels est encore nouvelle, il faut donc intégrer de nombreux facteurs, dont l'importance du contexte, pour comprendre les différences selon le sexe. Les mécanismes cellulaires, mieux modélisés chez les rongeurs, ont fourni à la fois des principes communs et des spécificités surprenantes. Des voies de signalisation très distinctes orientent la différentiation dans des régions cérébrales spécifiques : il en résulte une mosaïque de masculinité, de féminité, de similarité relatives dans le cerveau, les différences selon le sexe étant à la fois exagérées et limitées par la canalisation. Les cellules non neuronales et les médiateurs inflammatoires sont plus nombreux et à des niveaux plus élevés dans des morceaux de cerveaux masculins. Ce plus haut degré d'inflammation initiale augmenterait la vulnérabilité des hommes aux troubles neuropsychiatriques du développement déclenchés par l'inflammation.
Introduction
Effective treatments for neuropsychiatric disorders with origins in development remain as elusive today as at the beginning of the “decade of the brain” in 1990. Our understanding of brain development and how genetic and environmental factors contribute to a dysregulation of that development has certainly accelerated, but we remain relatively stymied in the avenue of new therapeutics and treatments. This is probably a combination of the complexity of developmental disorders and our still-incomplete understanding of the key sources of vulnerability to the developing brain. One essential variable that has long been ignored is sex. Being male imparts a major risk for the development of a developmental neurological or neuropsychiatric disorder, whereas being female appears to afford some protection from the same. Identifying the biological origins of male vulnerability and female protection will generate novel targets for therapeutic intervention and prevention. Animal models are essential to this effort not only because of the experimental advantages but also because we can divorce sex and gender as only humans possess a gender, a combination of self and societal perception of one's sex. This review focuses largely on lessons learned from animal models in a historical context with the goal of informing the design and interpretation of clinical research.
Historical overview
What seems inherently obvious today—that neuroanatomical substrates contribute to, and at times direct, sex differences in brain and behavior—was not always the case. Pioneers in the burgeoning field of behavioral endocrinology in the 1950s contemplated the origins of sex differences in courtship, copulatory, and parental behaviors in animals and argued in favor of the view that it was somatic aspects of males and females that induced them to behave in particular ways. The brain was only there to execute the fixed -action pattern response that was dictated by the presence of primary and secondary sex characteristics such as genitalia and plumage coloration. This is not a particularly unreasonable view. The brain resides within a body after all, and that body is tremendously influenced by the sex of the individual. But the notion of the body directing behavior was gradually put to rest starting with an iconic publication in 1959 in which treatment of pregnant guinea pigs with testosterone resulted in daughters that showed the copulatory pattern of males as adults despite not having a penisCitation1 (but note that this required that the females be supplied with additional testosterone in adulthood to activate the preprogramming effects of the gestational exposure). The work of Phoenix and colleaguesCitation1 set the stage for concluding that the brain was the critical organ that had been modified by prenatal hormone exposure, but it did not close the case. Reports of subtle and limited sex differences in neuronal morphology in the rodent models in the 1960s hinted at the potential for neural substrates regulating sex differences in behavior, but it was not until the 1970s that irrefutable evidence convinced the world that male and female brains differed, at least in birds. Male canaries sing a complex and beautiful song, whereas females only twitter. Reasoning that this must have origins in the brain, Art Arnold and his mentor Fernando Nottebohm identified a nucleus of the songbird brain that was markedly larger in males than females.Citation2 Shortly thereafter, a much less impressive but nonetheless reliable sex difference was found in the size of a nucleus in the rodent (rat) brain and given the lofty name of the “sexually dimorphic nucleus of the preoptic area,” or SDN for short.Citation3 A star was born.
The discovery of the SDN spawned a cottage industry of looking for and finding sex differences in the size (volume) of particular subregions of the brain, varying from small nuclei to entire hemispheres, and the size of major fiber tracks such as the corpus callosum. Early in the 1980s, the advent of the AIDS epidemic, combined with increasing interest in the potential biological origins of human homosexuality, created the opportunity to examine large numbers of postmortem brains from men, women, and homosexual men. A hypothalamic nucleus deemed analogous to the rodent SDN was found to be larger in heterosexual men than heterosexual women and homosexual men.Citation4 Interest in how the brain could impact complex behaviors differently in men and women was both at a fever pitch in some quarters and completely ignored or denigrated in others. The suggestion that women's brains were “different” was not a welcome message at a time when feminism was seriously taking hold.
And so a funny thing happened—two things actually. The first was that sex differences in the brain were relegated to the arena of reproduction and the discipline of neuroendocrinology, which became a poor cousin to the mothership of neuroscience. The second was a series of startling reports from the McEwen lab at Rockefeller University (the same place Arnold and Nottebohm had conducted their bird studies 20 years earlier) reporting that the dendritic synapse density of hippocampal pyramidal neurons varied by up to 30% across the female estrus cycle.Citation5 Initially met with incredulity but ultimately accepted in the face of overwhelming data, this finding had the effect of relegating females to the scientific bench as researchers sought to avoid variability, thus sealing the fate of the overwhelming majority of future studies on hippocampal physiology, which were conducted only in males. And so it has been for the past 25 years.Citation6
Today, a perfect storm of increased awareness, new policies, and novel findings—some discovered by accident and others on purpose—have generated renewed interest in sex differences in the brain. Equally compelling is the undeniable gender bias in most if not all neuropsychiatric and neurological disorders which demands our attention.
Sex differences in brain versus sex differences in behavior
A primary goal of neuroseience is to understand the governing principles by which the brain directs behavior. That this is a challenging goal is evident in the initial breakthroughs being those in the simplest organisms with simple behaviors, such as the gill withdrawal reflex of the marine snail Aplysia or swimming behavior of the flat worm Caenorhabditis elegans. But, ultimately, we seek to understand emotion, cognition, and motivation, which together encapsulate every essential behavior, ranging from the drive to eat to the ability to send a man to the moon. But causally connecting specific neuroanatomical or physiological traits in the brain to those behaviors remains an elusive goal. This is equally true in the arena of sex differences in brain and behavior, but is a truism often forgotten. Even the most minor findings of sex differences in the brain are often assumed to directly drive and determine sex differences in behavior. More appropriate is to assume that sex differences in the brain create predispositions or differentially weighted valences for responding to specific stimuli that can shift the probability in favor of a particular behavioral response in one sex versus the other. But all complex behaviors are influenced by current context and past experience, the influence of which can far outweigh that of an underlying neurophysiological sex difference at any given moment.
Brain sex differences in context
The more we learn, the more we don't know when it comes to sex differences in the brain. What is clear is that the types, origins, and impact of such differences are complex, multifactorial, and vary by species. It is useful to provide boundaries and operationally defined definitions of types of sex differences. In a recent “Circumspective” piece in Neuropsychopharmacology, Joel and McCarthy proposed four not-mutually exclusive dimensions along which sex differences can be defined.Citation7 These are: (i) persistent versus transient; (ii) context-independent versus -dependent; (iii) dimorphic versus continuous; and (iv) a direct versus indirect consequence of sex. These dimensions overlap in a classic Venn diagram fashion as they share some features but are unique in others ().
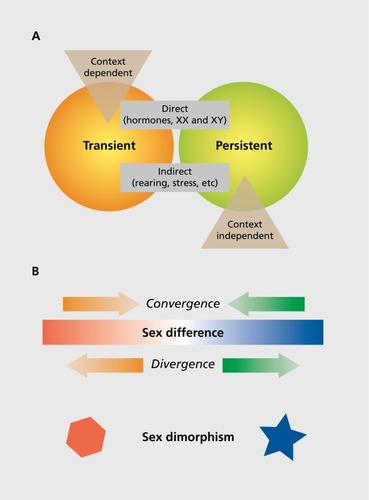
Sex-determined and sexually dimorphic differences
Sex-determined differences between males and females would be an example of a persistent difference that is established early in development by the programming effects of gonadal steroids and/or chromosome complement. Such end points are often sexually dimorphic, meaning there are two forms, one in males and one in females, and they may undergird behaviors that are highly sex-typic. For example, the neural circuitry controlling singing in songbirds is highly dimorphic in that some nuclei are only present in male brains, and only males of those species exhibit the complex songs of courtship. The song nuclei are differentiated early in development; however, they also show seasonal plasticity. So the sex difference is permanent, but there is a transience in the magnitude of that sex difference. As another example, most animals exhibit sex-typic forms of copulatory behavior that are determined by sexual differentiation of neural circuits. The circuit controlling mating differs between males and females in numbers of neurons, connectivity, neurochemistry, and synaptic profile. However, in mammals, there is no evidence that separate neural circuits regulate male mounting behavior versus female receptivity. Instead, there is a single circuit for sexual behavior, but it is differentially weighted in its response to olfactory, auditory, and somatosensory cues in males versus females (see ref 8 for review). Sex-determined neuroanatomical or neurophysiological end points such as this are not expected to change significantly across the life span or to shift dramatically in response to context or experience, although the behaviors they are associated with might. Sexual behavior is only engaged in when the moment is right, meaning the right season, the right phase of the female reproductive cycle, and the right age, but the underlying neural circuits for both sexual motivation and engagement are always at the ready.
Sex differences and effects of sex
The term “sex difference” is most appropriately used when an end point varies along a continuum, and the mean is significantly different for males versus females. Important points for consideration are the magnitude of the difference in the means and the variance associated with each mean. For some end points, there can be a great deal of overlap in the response or measurement in males and females. In this scenario, there might be a statistically significant difference between a group of males and a group of females, but the measure is not necessarily predictive of sex. There can also be a circumstance where a percentage of the population of males is closer to one end of a continuous spectrum, whereas a much smaller percentage of females is, resulting in an overall population mean that is different, but the end point would be identical in large numbers of males and females and therefore not a very accurate predictor of sex. Some would argue that in cases such as this there is an effect of sex, not a sex difference per se.Citation9,Citation10 The important distinction being that sex is just one of many variables that has an impact on a response, and that others such as age, disease state, genetics, etc, may be equally or more important than sex. Conversely, there are other end points that can have markedly different means in males and females with relatively little overlap, due to low variance around the mean for each sex. In this instance, there would be a sex difference, and the end point may be a relatively reliable predictor of sex.
Transience and context dependence, convergence, and divergence
Sometimes, sex differences emerge only under certain circumstances, or they may disappear under others. For instance, there is a robust and trans-species sex difference in the frequency and intensity of play behavior by juveniles.Citation11,Citation12 In what is referred to as rough-and-tumble play, young males will consistently engage more frequently and more intensely than females. But this sex difference is both transient and context-dependent in that once the Rubicon of puberty has been crossed, both males and females stop playing as new behavioral repertoires involving male-male competition, sexual solicitation, mate guarding, and territorial aggression emerge. Thus, the sex difference only exists during a brief period of life. The neural substrate of play remains poorly defined, and whether it changes postpuberty is not known, but it is clear that hormonal influences early in development are essential to the sex difference.Citation13 Nonetheless, the magnitude, and even the direction, of the sex difference in play is strongly influenced by context, ie, by prior social isolation, group size and composition, familiar partner versus stranger, and age.Citation14
Stress is one of the best examples of a contextual source for sex differences. There are hundreds of studies reporting sex differences in stress responses as evidenced by activation of the hypothalamic-pituitary-adrenal (HPA) axis, and an equal or greater number on anxious behavior as demonstrated by activity in an open field or elevated plus maze. But whether it's the males or the females that show a greater stress response or higher anxiety varies widely and appears to be strongly influenced by the age at which the stressor occurs, the nature of the stressor, and past experience.Citation15 Even the signal transduction pathways by which stress exerts its effects can differ in males and females.Citation16 Thus, it can never be assumed that males and females will respond to a stressful situation in the same way, but generalities about sex differences in the stress axis and anxious behavior also cannot be made.
Stress also provides us with one of the best examples of a divergence in responding between males and females during a particular context on a particular end point. In a series of elegant studies, Tracey Shors and colleagues have demonstrated that eye-blink conditioning, a learning and memory task, is strongly influenced by stress such that males improve at the task if stressed beforehand, whereas female performance deteriorates. Under nonstressful conditions, the two sexes perform at par. Shors further showed that a directional change in the density of dendritic spines on hippocampal neurons paralleled the change in performance, meaning males developed more dendritic spine synapses and learned better, whereas females did the opposite. But there is a caveat. The negative impact of stress on performance in females occurs only if they are under a particular hormonal state associated with the estrus cycle. If they are at another phase in the cycle or the ovaries are removed all together, the effect of stress, and therefore the sex difference, goes away.Citation17
The above is an example of divergence, meaning males and females start at the same baseline but fly apart in the face of a challenge, and thus an example of a contextual sex difference. Conversely, sometimes the two sexes converge on the same end point starting from different origins. This was most classically illustrated in the work of Geert de Vries in which he coined the term “compensation” to describe the phenomenon whereby males of a particular species of voles have a separate neural circuit from females that drives them to show parenting behavior toward their offspring.Citation18 The reasoning is that males do not experience pregnancy, parturition, or lactation and therefore lack the associated neural circuits that normally mediate maternal behavior. Biparental care must provide a fitness advantage in this species as males have evolved a distinct vasopressinergic neural network that drives them to behave just like newly parturient females when their young are born.Citation19
More recently, it has become evident that convergence is also found at the cellular level and control of synaptic function. For reasons that remain mysterious, the control of both synaptic inhibition and potentiation, via γ-aminobutyric acid (GABA) and glutamate respectively, is achieved via distinct cellular signaling pathways in the hippocampus of male and female rodents. Referred to as a “latent” sex difference, the end result on synaptic efficacy is the same, but the transduction pathway is different.Citation20,Citation21 Given that the physiological outcome is the same, one might say what does it matter? But as Woolley and colleagues point out, the significance is in the potential for markedly different effects of drugs, toxins, or nutrients that mod ulate specific components of signal transduction pathways in males versus females. The potential for sex differences in response is essentially hidden (ie, latent), as when unperturbed the end point is the same, and highlights the fact that we can never assume there is no difference in males versus females.
Brain sex differences begin in the womb
The importance of developmental processes for adult sex differences in brain and behavior cannot be overstated, but is often overlooked. As discussed above, the original report suggesting that the brain might be the source of adult copulatory behaviors involved treatment of pregnant guinea pigs and thereby established the principle of early organization (or imprinting or programming, depending on preference) of brain sex differences. Shortly after the studies of Phoenix and colleagues,Citation1 the same principle was established for adult fertility in females: a single exposure to male hormones during a perinatal critical period rendered them incapable of ovulation as adults by shifting the control of pituitary gonadotropin secretion to the male pattern.Citation22
So, in the realm of reproduction, it is very clear that early-life programming dictates adult responding. But this is not as strongly established for nonreproductive parameters that are of great interest in the adult brain. This may be for two reasons, as follows: (i) the rules and regulations of sexual differentiation of nonreproductive end points may be distinctly different; and/or (ii) there is reluctance on the part of many researchers comfortable working in adult brains to engage in the necessary studies to determine the role of developmental processes. Far easier is to determine if adult hormones are the source of any sex difference, and this is accomplished with a simple surgery to remove the gonads. If the sex difference goes away when either one or both sexes are deprived of steroids, then the source can be considered acute and due to hormones, that is as long as the behavior itself is not dependent upon steroids as in most reproductive behaviors. If, however, the sex difference doesn't go away, then there are really only two options—either it was programmed in development during the process of sexual differentiation or the sex chromosomes are the source.
Many brain sex differences are established during critical periods
Critical periods are defined as developmental windows during which a particular physiological or anatomical end point in the brain is established. Sensitive periods are related to critical periods in that they are times when development can be derailed by external or internal stimuli, such as toxic chemicals or inflammation. The establishment of many sex differences in the brain occurs during a critical period when the brain is sensitive to the internal signal of gonadal steroids that are endogenously generated in males from the fetal testis. Females are also sensitive to testosterone, equally to males, but they normally are not exposed to sufficient testosterone to induce masculinization. We define the onset of the critical period as the time when testosterone production surges in the male testis, which is around embryonic day 16 in mice, day 18 in rats, and at the end of the first and beginning of the second trimester in primates. The offset of the critical period is determined by finding the developmental stage at which females lose sensitivity to exogenous steroid treatment; in other words, they cannot be masculinized. The sensitivity of males to manipulations that deplete steroids or block their action is not a good marker of the end of the sensitive period because of the in utero exposure, which is difficult to cleanly manipulate. Thus, females are an excellent “tool” for determining how long the sensitive window stays open. Evidence suggests that the window closes at different times for different end points (ie, closes early for reproductive versus nonreproductive end points, for instance), although no coherent rules are established. Moreover, an unanswered question is how the critical period window closes. Recent evidence strongly implicates epigenetic changes to the DNA as a means for both closing off the ability to become masculinized and maintaining the masculinization phenotype for at least one neural phenotype and adult behavior.Citation23
Steroid hormones differentiate the male brain
As is obvious by now, most sex differences in the brain are either programmed by gonadal steroids during a developmental critical period or are induced acutely in the adult brain as a result of the different hormonal milieus of males and females. Developmentally, the brain is programmed by default to develop a female phenotype. A similar program exists for the ovary. The pivot point is expression of the sex-determining region Y (SRY) gene on the Y chromosome, which codes for a transcription factor called TDF or testis-determining factor.Citation24 If TDF is expressed during its own critical period, the bipotential gonad will divert from its path toward an ovary and develop into a testis. In this way, the gonadal sex of an individual will match the brain sex, meaning the physiology and behavior of reproduction that is controlled by the brain will support the gonad. In females, this means the brain will initiate the generation of a surge in luteinizing hormone as a component of the estrus or menstrual cycle to induce ovulation and will induce sexual receptivity tied to the timing of ovulation (with the exception of humans). Presumably, there are numerous other behavioral patterns invoked that are associated with the ultimate goal of careful choice of a mate, pregnancy, parturition, and postpartum care of the young, often without the help of the sire. In males, the brain will support regular but pulsatile secretion of luteinizing hormone to induce continuous production of testosterone and thereby continuous interest in mating, along with the attendant behaviors of territorial defense, male-to-male competition, and in some species, mate guarding.
It is tempting to assume that every sex difference in the brain is geared toward maximizing reproduction; indeed, as Theodosius Dobzhansky wrote, “nothing in biology makes sense except in the light of evolution.” But as noted above, sometimes the two sexes are attempting to converge in their physiology or behavior. There is a cost to continuously high testosterone, just as there is a cost to internal fertilization and extended parental care. In some species, there may be a benefit to males or females adapting behaviors or phenotypes that seem opposed to their presumed role. One of the most celebrated of these is the hyena, in which females have evolved a pseudopenis and develop strong social hierarchies that rival the dominance status seen in males of any other species.Citation25 Unfortunately, we know relatively little about the brains of this fascinating species.
Although the hormonal milieu of adult males and females is obviously different, it is often reduced to the simple characterization that males have high testosterone and females have fluctuating estrogens and progesterone. This is true, but lacks the nuance that males also make estrogens, particularly in the brain where the enzyme aromatase, which converts testosterone into estradiol, is found at very high levels. And females also make androgens, both as a necessary precursor to estradiol and from the adrenal glands. Even more nuanced, however, is the emerging realization that the brain itself is capable of de novo steroidogenesis, meaning from cholesterol all the way through to the finished product estradiol (which takes seven to 10 enzymatic conversions depending upon the route). This means that locally sourced steroid can have major impacts on neural functioning,Citation26 and this can differ in males and females.Citation27 Technically, it is challenging to measure local steroidogenesis and even harder sometimes to demonstrate its impact. To date, the best evidence exists for intrahippocampal steroidogenesis in the adultCitation28 and intracerebellar steroidogenesis in the neonate.Citation29
Every cell in the brain has a sex-specific chromosome complement
Despite the overwhelming importance of hormones for establishing, maintaining, and acutely inducing sex differences, the fact remains that every cell in the brain has a sex. Largely ignored for the first 40-plus years of research into sex differences in brain and behavior, the common sense notion that sex chromosomes matterCitation30 was forcefully brought to the fore by the efforts of one of the original pioneers of the field of hormonally induced sex differences, Art Arnold at the University of California Los Angeles (UCLA). Using a clever tool in which mice could be made to be gonadally male but genetically female, and vice versa,Citation31 he was able to independently explore the importance of each variable. He achieved this by removing Sry from the Y chromosome and replacing it on an autosome, which allowed for XX females to develop testes and XY males to develop ovaries. The initial forays found little to no impact of this dramatic phenotype/genotype swap.Citation31 But, the initial forays also focused on classic reproductive end points, such as sex behavior and parenting and the neural circuits that undergird them. Subsequent studies branched out into areas associated with emotion, cognition, and motivation; here, many effects were found. These include effects on habit formation, aggression, parenting, body weight gain, and more (see refs 30 and 32 for review). It was generally expected that the key genes of the X or Y chromosome would be identified shortly thereafter, but this has proven far more elusive. A surprise that is emerging is that it may not be specific genes per se but the number of X chromosomes that is key.
There are two means by which the number of X chromosomes can matter. First, sexually reproducing species that have heterologous sex chromosomes (mammals, birds, some reptiles, and drosophila, to name a few), must solve the problem of dosage compensation. In mammals, females have two X chromosomes, a large and resource-rich partner to the Y, which is depauperate by comparison, with a modest number of genes mostly related to spermatogenesis. The X chromosome is also particularly enriched in genes associated with brain development and cognitive function.Citation33 Thus, to keep the dose of X chromosome genes relatively even between males and females, one is inactivated in females. But is it really? Not only is the X not completely inactivated, with upwards of 15% of genes capable of biallelic expression in humans,Citation34 but there is also an impact of having the largely inactive X hanging around the cell. The process of inactivation requires a coordinated recruitment of various enzymes that inhibit transcription by coating the associated chromatin and DNA with repressive markers.Citation35 This turns out to be an expensive process and creates a “heterochromatic sink” that sucks up valuable resources that could otherwise be employed regulating the gene expression of autosomes.Citation36 Understanding the consequences of this under normal circumstances is challenging, and so much is to be learned from the extremes, individuals with sex chromosome aneuploidies. The most common is XXY, commonly referred to as Kleinfelter syndrome, but there are also individuals that are XXXY and even XXXXY, although this is relatively rare. Equally rare are those with XYY, which allows for investigation of the opposite question—does the number of Y chromosomes matter? Imaging studies of humans with these sex chromosome aneuploidies confirmed that increasing numbers of X results in increasing distortion in the size and shape of striatum, pallidum, and thalamus, areas which are also different in XX versus XY women and men. Interestingly, and unexplainedly, increasing the number of Y chromosomes has similar effects.Citation37 These unexpected findings highlight that there is much research to be done but also demonstrate the power of sex chromosomes to impact brain structure.
Convergence of hormones, genetics, and epigenetics to induce and maintain sex differences
As scientists, our goal is to isolate variables and interrogate their relative importance to the whole. But in reality, there is always an interaction among variables. The frontier of research into sex differences in brain and behavior is to understand how all these variables interact to produce a coordinated whole. My research program has focused on the cellular mechanisms of steroid-mediated sexual differentiation using a regionby-region approach, meaning we interrogate the hypothalamus or the preoptic area or the amygdala and so on. In some cases, we have delved deep into the molecular and biochemical signaling pathway and identified specific genes, molecules, receptor subtypes, protein phosphorylation events, and so on to determine how steroids drive synaptic patterns, dendritic growth and branching, cell proliferation or differentiation, and naturally occurring cell death. Along the course of this systematic approach, several surprises and unifying principles have emerged. First is that when you understand how sex differences are established in one brain region, you understand how sex differences are established in only one region. There appear to be no generalities, no unifying principle that applies to brain sexual differentiation, with the exception of the initiating signal being steroids. Each brain region has its own unique style, its own set of necessary and sufficient players for achieving the differentiated end point. This has important implications for how the brain develops. With so many different systems engaged at the same time but in different regions, the potential for the degree of masculinization to vary across regions seems almost inevitable, likewise for feminization(). As a result, a single brain in a single individual will probably be a mosaic of relative “maleness” and “femaleness,” thus providing a nifty means by which nature ensures variability. There are no “Stepford wives” in nature. And although we don't know about regional variation in mechanism in humans, on the basis of image analyses, the principle of mosaicism appears to apply.Citation38
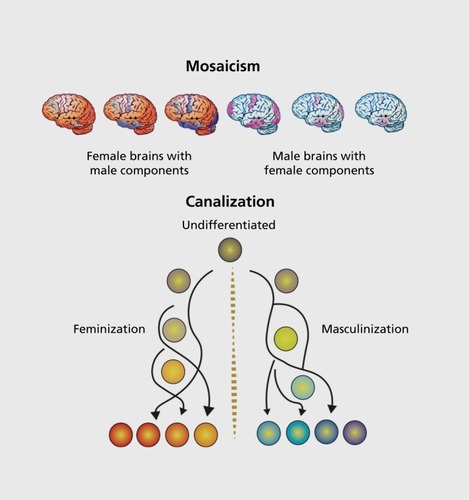
Despite regional variability in degree of “maleness” or “femaleness” within individuals, the brain still resides within a body that is diametrically either male or female (with rare exceptions). Thus, there are probably also forces that act to keep the brain within a narrow and uniform phenotype so that reproduction is not compromised. This is most evident in the role of Sry, which differentiates the testis, and then all hormonally mediated brain differentiation derives from that. An additional force above the genome is epigenetic changes induced by the gonadal hormones to ensure that the phenotype stays as intended. We discovered that the enzymes regulating DNA methylation, a canonical epigenetic regulator of gene expression, are downregulated by steroids in the male brain, thereby decreasing gene expression repression and leading to masculinization.Citation23 Importantly, in the female, these same genes must be repressed continuously to prevent masculinization from emerging. But epigenetics also provides a degree of malleability; unlike the genome, it can be undone. Under specific circumstances, including enduring exposure to high doses of steroid hormones typical of the opposite sex, behavior can be reversed in adults.Citation39 Might this be due to hormonal unraveling of epigenetic repression? The experiment hasn't been done but it seems likely.
In the above-noted scenario there is a linear relationship between the gene determining gonadal phenotype, hormones, and epigenetics. But in other brain regions, we find there is a convergence such that hormones mediate an end point, in this case cell proliferation, but there is no sex difference in the endogenous level of hormone.Citation40 Instead, there is a divergent epigenetic regulation of cell proliferation that involves DNA methylation in males and histone acetylation in females. For this to be achieved, there must be an originating source from the X or Y chromosome. This work is still preliminary, but a sex difference in microRNAs, some of which originate on the X chromosome, is a potential source.Citation41 MicroRNAs might play an additional role, which is to both constrain and hone the consistency of sex differences by creating a threshold for gene expression.Citation42 This is a process analogous to canalization whereby phenotypic variation is constrained in order to protect species robustnessCitation43 (Figure 2B).
Lastly, although the mechanism for sexual differentiation is unique for each region so far examined, we have found a common theme in the requirement for multiple cell types, not just neurons, participating in the differentiation process. Of note is the importance of astrocytes and microglia,Citation41 the latter being the brain's innate immune system. We also find that the signaling molecules that mediate masculinization are usually identified with inflammation, eg, prostaglandins and some cytokines. Excitation is also a hallmark of male brain development, including the depolarizing actions of GABA, which are stronger and endure longer in developing males than females.Citation44,Citation45 We conclude that the developing male brain is naturally in a state of both higher excitation and inflammation and that this may be a contributing factor to the greater male vulnerability to neuropsychiatric and neurological disorders with origins in development ().
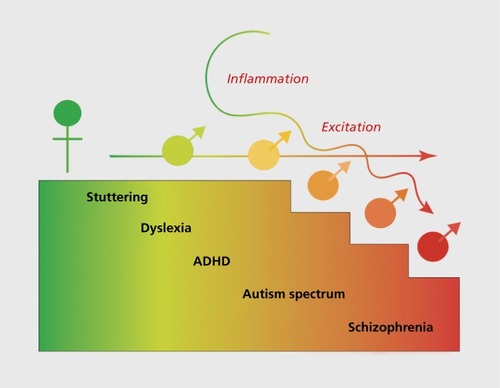
Gender-biased disorders with origins in development
One of the most robust and easily identifiable risk factors for an early onset disorder is being male, and yet it is relatively unexploited as an exploratory tool in basic research. This is beginning to change for a variety of reasons, including the inability to explain many of the more complex disorders and diseases by either genetic or environmental routes. Early onset disorders can be broadly divided into the neuropsychiatric (autism spectrum disorder, schizophrenia, and attention deficit/hyperactivity disorder being the most frequently modeled in rodents), the neurological (Tourette syndrome, stuttering, and dyslexia, all difficult or impossible at this time to model in animals), and insult or injury (prenatal infection and hypoxic/ischemic stroke, which can be well modeled with some limitations). All of these are either more frequently diagnosed or more severe in symptomology in males, both clinically and when modeled in rodents (see refs 46-50 for review). The most robust is the gender bias in diagnosis of autism, and this is also the area in which the most traction has been gained due to a recent emphasis on the heuristic value of comparing males and females. A brief overview follows.
Autism spectrum disorder (ASD) is among the most gender-biased of the early-onset syndromes, being diagnosed four to five times more frequently in boys.Citation50,Citation51 Acknowledgment of the importance of this bias was articulated in the Extreme Male Brain theory of autism put forth by Simon Baron-Cohen.Citation52 This theory proposes that autism is a continuation along a spectrum of femaleness to maleness but into a range of dysfunction as manifested by excessive systematizing, low empathy, and poor social skills, including bonding.Citation53 Attempts to support the theory have largely centered on measurement of steroid hormones in amniotic fluid with the assumption that autistic individuals would have higher androgen levels. Although there are some reported correlations in hormonal profile in the amniotic fluid and diagnosis of autism in boys,Citation54 as well has higher rates of androgen-related disorders in women with autistic traits,Citation55 overall the theory has fallen short in explaining the profound gender bias. An alternative view is that rather than males being more vulnerable, females are protected. This scenario predicts that females can tolerate a greater level of insult before tripping over into dysregulation. Focus has been on genetic insult, and there is evidence to support the contention that females are protected,Citation56 but again it does not explain the magnitude of the bias. More recently, extensive transcriptomic analyses of postmortem tissue from autistic and nonaffected individuals was used to distinguish between the two following hypotheses: (i) risk genes for ASD are expressed at higher levels in males; and (ii) genes involved in normal male brain development are more highly expressed in males with ASD.Citation57 The latter was concluded, that rather than ASD risk genes being higher in males, it is the genes normally involved in male brain development that were being overexpressed. More importantly, many of these genes were disproportionately associated with neuroinflammation. This is highly consistent with our observations of a key role for microglia and inflammatory signaling molecules in the sexual differentiation of the preoptic area in rodents.Citation41 But this is not consistent with another emerging approach, examination of genetic mouse models of ASD for sex differences. Both caspase 3 and neurexin 1 (Nrxnl) are candidate ASD genes and deletion of either one in mouse models produces deficits in males but not females.Citation58,Citation59 The latter was concluded; that rather than ASD risk genes being higher in males with ASD, it is the genes normally involved in male brain development that were being overexpressed in males with ASD. More importantly, many of these genes were disproportionately associated with neuroinflammation. This is highly consistent with our observations of a key role for microglia and inflammatory signaling molecules in the sexual differentiation of the preoptic area in rodents,Citation41 and could be considered consistent with the Extreme Male Brain theory since it is genes associated with normal male brain development that are higher in ASD males. However, there is another emerging approach which is not consistent with the Extreme Male Brain—the examination of genetic mouse models of ASD for sex differences. Both caspase 3 and neurexin 1 (Nrxnl) are candidate ASD genes and deletion of either one in mouse models produces deficits in males but not females.Citation58,Citation59 In this case there is a loss of a gene that is normally high in males and leads to the ASD-like phenotype. Lastly, the potential for differential diagnosis by physicians, as well as differential presentation of the disorder in boys versus girls, must also be incorporated into any consideration of how the male prevalence in ASD arises.Citation6
Conclusion
In summary, new advances in elucidating the biological foundations of sex differences in the brain shed light on the potential source of male vulnerability to neuropsychiatric and neurological disorders with origins in development. Heightened excitation and neuroinflammation may converge with environmental insult to increase risk of dysregulation in developing males versus females. But sex differences in physiology and behavior are found at every stage of the lifespan, and can vary profoundly with age, experience, and context. Not all differences between the sexes are manifested in the same way, and attention must be given to the contributing variables, the magnitude of the differences, and the relative importance to a particular condition or disease state. Ultimately, the study of sex differences provides a powerful heuristic tool for discovering novel regulatory processes and expands foundational principles of neurobiology to enhance the health of both males and females.
The author declares no conflict of interest.
REFERENCES
- PhoenixCH.GoyRW.GerallAA.YoungWC.Organizing action of prenatally administered testosterone proprionate on the tissues mediating mating behavior in the female guinea pig.Endocrinology.19596536938214432658
- NottebohmF.ArnoldAP.Sexual dimorphism in vocal control areas of the songbird brain.Science.19761944261211213959852
- GorskiRA.GordonJH.ShryneJE.SouthamAM.Evidence for a morphological sex difference within the medial preoptic area of the rat brain.Brain Res.19781482333346656937
- LeVayS.A difference in hypothalamic structure between heterosexual and homosexual men.Science.19912535023103410371887219
- WoolleyCS.GouldE.FrankfurtM.McEwenBS.Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons.J Neurosci.19901012403540392269895
- ZuckerI.BeeryAK.Males still dominate animal studies.Nature.2010465729969020535186
- JoelD.McCarthyMM.Incorporating sex as a biological variable in neuropsychiatric research: Where are we now and where should we be?Neuropsychopharmacology. 2016 Jun 22. Epub ahead of print. doi:10.1038/ npp. 2016.79.
- McCarthyM.De VriesG.ForgerN.Sexual differentiation of the brain: mode, mechanisms and meaning. In: Pfaff D, Arnold AP, Etgen AM, Fahrbach SE, and Rubin RT, eds.Hormones, Brain and Behavior. Vol 3. San Diego, CA: Academic Press200917071744
- McCulloughLD.de VriesGJ.MillerVM.BeckerJB.SandbergK.McCarthyMM.NIH initiative to balance sex of animals in preclinical studies: generative questions to guide policy, implementation, and metrics.Biol Sex Differ.201451525780556
- SandbergK.UmansJG.Georgetown Consensus Conference Work Group. Recommendations concerning the new U.S. National Institutes of Health initiative to balance the sex of cells and animals in preclinical research.FASEB J.20152951646165225713032
- AugerAP.OlesenKM.Brain sex differences and the organisation of juvenile social play behaviour.J Neuroendocrino!.2009216519525
- VanderschurenU.TrezzaV.What the laboratory rat has taught us about social play behavior: role in behavioral development and neural mechanisms.Curr Top Behav Neurosci.20141618921224338663
- MeaneyMJ.McEwenBS.Testosterone implants into the amygdala during the neonatal period masculinize the social play of juvenile female rats.Brain Res.198639823243283801906
- ArgueKJ.McCarthyMM.Characterization of juvenile play in rats: importance of sex of self and sex of partner.Biol Sex Differ.201561626361539
- BaleTL.EppersonCN.Sex differences and stress across the lifespan.Nat Neurosci.201518101413142026404716
- ValentinoRJ.Van BockstaeleE.BangasserD.Sex-specific cell signaling: the corticotropin-releasing factor receptor model.Trends Pharmacol Sci.201334843744423849813
- ShorsTJ.A trip down memory lane about sex differences in the brain.Philos Trans R Soc Lond B Biol Sci.201637116882015012426833842
- De VriesGJ.Minireview: sex differences in adult and developing brains: compensation, compensation, compensation.Endocrinology.200414531063106814670982
- De VriesGJ.BoylePA.Double duty for sex differences in the brain.Behav Brain Res.19989222052139638962
- TabatadzeN.HuangG.MayRM.JainA.WoolleyCS.Sex differences in molecular signaling at inhibitory synapses in the hippocampus.J Neurosci.20153532112521126526269634
- OberlanderJG.WoolleyCS.17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females.J Neurosci.20163692677269026937008
- BarracloughCA.GorskiRA.Evidence that the hypothalamus is responsible for androgen-induced sterility in the female rat.Endocrinology.196168687913687240
- NugentBM.WrightCL.ShettyAC.et alBrain feminization requires active repression of masculinization via DNA methylation.Nat Neurosci.201518569069725821913
- GoodfellowPN.Lovell-BadgeR.SRY and sex determination in mammals.Annu Rev Genet.19932771928122913
- PlaceNJ.GlickmanSE.Masculinization of female mammals: lessons from nature.Adv Exp Med Biol.200454524325315086031
- BalthazartJ.BallGF.New insights into the regulation and function of brain estrogen synthase (aromatase).Trends Neurosci.19982162432499641536
- VierkR.GlassmeierG.ZhouL.et alAromatase inhibition abolishes LTP generation in female but not in male mice.J Neurosci.201232248116812622699893
- HojoY.HattoriTA.EnamiT.et alAdult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017cxand P450 aromatase localized in neurons.Proc Natl Acad Sci USA.2004101386587014694190
- DeanSL.WrightCL.HoffmanJF.WangM.AlgerBE.McCarthyMM.Prostaglandin E2 stimulates estradiol synthesis in the cerebellum postnatally with associated effects on purkinje neuron dendritic arbor and electrophysiological properties.Endocrinology.2012153115415542723054057
- ArnoldAP.XuJ.GrishamW.ChenX.KimYH.ItohY.Minireview: sex chromosomes and brain sexual differentiation.Endocrinology.200414531057106214670983
- De VriesGJ.RissmanEF.SimerlyRB.et alA model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits.J Neurosci.200222209005901412388607
- ArnoldAP.The end of gonad-centric sex determination in mammals.Trends Genet.2012282556122078126
- ZechnerU.WildaM.Kehrer-SawatzkiH.VogelW.FundeleR.HameisterH.A high density of X-linked genes for general cognitive ability: a runaway process shaping human evolution?Trends Genet.2001171269770111718922
- BerletchJB.YangF.DistecheCM.Escape from X inactivation in mice and humans. GenomeBiol.2010116213
- ChangSC.TuckerT.ThorogoodNP.BrownCJ.Mechanisms of X-chromosome inactivation.Front Biosci.20061185286616146776
- ArnoldAP.ReueK.EghbaliM.et alThe importance of having two X chromosomes.Philos Trans R Soc Lond B Biol Sci.201637116882015011326833834
- ReardonPK.ClasenL.GieddJN.et alAn allometric analysis of sex and sex chromosome dosage effects on subcortical anatomy in humans.J Neurosci.20163682438244826911691
- JoelD.Fausto-SterlingA.Beyond sex differences: new approaches for thinking about variation in brain structure and function.Philos Trans R Soc Lond B Biol Sci.201637116882015045126833844
- De VriesGJ.SoderstenP.Sex differences in the brain: the relation between structure and function.Horm Behav.200955558959619446075
- BowersJM.WaddellJ.McCarthyMM.A developmental sex difference in hippocampal neurogenesis is mediated by endogenous oestradiol.Biol Sex Differ.201011821208470
- McCarthyMM.PickettLA.VanRyzinJW.KightKE.Surprising origins of sex differences in the brain.Horm Behav.20157631025917865
- McCarthyMM.Multifaceted origins of sex differences in the brain.Philos Trans R Soc Lond B Biol Sci.201637116882015010626833829
- RohnerN.JaroszDF.KowalkoJE.et alCryptic variation in morphological evolution: HSP90 as a capacitor for loss of eyes in cavefish.Science.201334261641372137524337296
- AugerAP.Perrot-SinalTS.McCarthyMM.Excitatory versus inhibitory GABA as a divergence point in steroid-mediated sexual differentiation of the brain.Proc Natl Acad Sci U S A.200198148059806411427701
- McCarthyMM.AugerAP.Perrot-SinalTS.Getting excited about GABA and sex differences in the brain.Trends Neurosci.200225630731212086749
- CosgroveKP.MazureCM.StaleyJK.Evolving knowledge of sex differences in brain structure, function, and chemistry.Biol Psychiatry.200762884785517544382
- BaoAM.SwaabDF.Sex differences in the brain, behavior, and neuropsychiatric disorders.Neuroscientist.201016555056520889965
- AbelKM.DrakeR.GoldsteinJM.Sex differences in schizophrenia.Int Rev Psychiatry.201022541742821047156
- BaleTL.BaramTZ.BrownAS.et alEarly life programming and neurodevelopmental disorders.Biol Psychiatry.201068431431920674602
- HalladayAK.BishopS.ConstantinoJN.et alSex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging areas of priority.Mol Autism.201563626075049
- WerlingDM.GeschwindDH.Sex differences in autism spectrum disorders.Curr Opin Neurol.201326214615323406909
- Baron-CohenS.Empathizing, systemizing, and the extreme male brain theory of autism.Prog Brain Res.201018616717521094892
- AuyeungB.WheelwrightS.AllisonC.AtkinsonM.SamarawickremaN.Baron-CohenS.The children's Empathy Quotient and Systemizing Quotient: sex differences in typical development and in autism spectrum conditions.J Autism Dev Disord.200939111509152119533317
- AuyeungB.Baron-CohenS.AshwinE.KnickmeyerR.TaylorK.HackettG.Fetal testosterone and autistic traits.Br J Psychol.2009100pt 1122
- IngudomnukulE.Baron-CohenS.WheelwrightS.KnickmeyerR.Elevated rates of testosterone-related disorders in women with autism spectrum conditions.Horm Behav.200751559760417462645
- GockleyJ.WillseyAJ.DongS.DoughertyJD.ConstantinoJN.SandersSJ.The female protective effect in autism spectrum disorder is not mediated by a single genetic locus.Mol Autism.201562525973162
- WerlingDM.ParikshakNN.GeschwindDH.Gene expression in human brain implicates sexually dimorphic pathways in autism spectrum disorders.Nat Commun.201671071726892004
- EsclassanF.FrancoisJ.PhillipsKG.LoomisS.GilmourG.Phenotypic characterization of nonsocial behavioral impairment in neurexin 1a knockout rats.Behav Neurosci.20151291748525420124
- LoSC.Scearce-LevieK.ShengM.Characterization of social behaviors in caspase-3 deficient mice.Sci Rep.201661833526783106
- LaiMC.Baron-CohenS.BuxbaumJD.Understanding autism in the light of sex/gender,Mol Autism.201562425973161

