Abstract
The endocannabinoid system (ECS) is a highly versatile signaling system within the nervous system. Despite its widespread localization, its functions within the context of distinct neural processes are very well discernable and specific. This is remarkable, and the question remains as to how such specificity is achieved. One key player in the ECS is the cannabinoid type 1 receptor (CB1), a G protein–coupled receptor characterized by the complexity of its cell-specific expression, cellular and subcellular localization, and its adaptable regulation of intracellular signaling cascades. CB1 receptors are involved in different synaptic and cellular plasticity processes and in the brain’s bioenergetics in a context-specific manner. CB2 receptors are also important in several processes in neurons, glial cells, and immune cells of the brain. As polymorphisms in ECS components, as well as external impacts such as stress and metabolic challenges, can both lead to dysregulated ECS activity and subsequently to possible neuropsychiatric disorders, pharmacological intervention targeting the ECS is a promising therapeutic approach. Understanding the neurobiology of cannabinoid receptor signaling in depth will aid optimal design of therapeutic interventions, minimizing unwanted side effects.
El sistema endocannabinoide (SEC) apareció como un sistema de señalización muy versátil en el sistema nervioso. A pesar de su existencia amplia y ubicua, sus funciones están integradas en el contexto de distintos procesos neuronales y, en última instancia, son bastante bien discernibles y específicas. Esto es notable, y la pregunta sigue siendo ¿cómo puede surgir tal especificidad ? Un jugador clave del SEC es el receptor cannabinoide CB1; se trata de un receptor acoplado a proteína G, que se caracteriza por su complejidad de expresión específica del tipo celular, localización celular y subcelular y por su capacidad para la regulación adaptativa de las cascadas de señalización intracelular. El receptor CB1 participa en diferentes procesos de plasticidad sináptica y celular y en la bioenergética del cerebro de una manera contexto-específica. El receptor CB2 también se ha convertido en un actor importante en varios procesos en neuronas y células inmunes que residen en el cerebro. Las intervenciones farmacológicas dirigidas al SEC siguen siendo un enfoque terapéutico prometedor, dado que tanto los polimorfismos en los componentes del SEC, como los impactos externos (el estrés y las exigencias metabólicas) pueden conducir a una actividad desregulada del SEC y, posteriormente, a posibles trastornos neuropsiquiátricos. Una comprensión profunda de la neurobiología de la señalización de los receptores de cannabinoides ayudará a diseñar intervenciones terapéuticas de manera óptima, minimizando los efectos secundarios no deseados.
Le système endocannabinoïde (SEC) se comporte comme un système de signalisation très polyvalent au sein du système nerveux. Il est surprenant d’observer que ses fonctions, s'intégrant dans un cadre de processus neuronaux distincts, sont finalement très perceptibles et spécifiques malgré son étendue et son caractère ubiquitaire et l’on peut s’interroger sur l’origine d’une telle spécificité. Le récepteur cannabinoïde CB1, couplé à la protéine G, est au centre du SEC : il est caractérisé par sa complexité d'expression spécifique au type cellulaire, sa localisation cellulaire et sous-cellulaire et sa capacité de régulation flexible des cascades de signalisation intracellulaire. Le récepteur CB1 est impliqué dans différents processus de plasticité synaptique et cellulaire et il participe à la bioénergétique du cerveau selon le contexte. Le récepteur CB2 est également un acteur majeur dans plusieurs mécanismes neuronaux et des cellules immunitaires cérébrales. Le SEC pouvant être perturbé par des facteurs extérieurs comme le stress et les troubles métaboliques comme par ses composants polymorphes, générant par conséquent d'éventuels troubles neuropsychiatriques, les traitements médicamenteux le ciblant restent une approche thérapeutique prometteuse. Ces traitements seront d’autant plus efficaces et bien tolérés que nous comprendrons en détail la neurobiologie de la signalisation des récepteurs cannabinoïdes.
Introduction
The endocannabinoid system (ECS) comprises two cannabinoid receptors—CB 1 and CB 2 receptors—that belong to the family of seven transmembrane G protein-coupled receptors (GPCRs); the ligands for the cannabinoid receptors—the two major endocannabinoids (eCBs) anandamide (arachidonoylethanolamide, AEA) and 2-arachidonoylglycerol (2-AG), both being derivatives of the fatty acid arachidonic acid; and the machinery for the synthesis and degradation of eCBs. Citation1,Citation2 The ECS is evolutionarily well conserved in vertebrates, Citation3 is widely distributed in the body, and takes a central position in the regulation of a myriad of biological processes, both in neural and non-neural tissues. Citation4,Citation5 It is intertwined with many neurotransmitter and lipid signaling systems, thereby integrated into broad functional networks. Citation6 It appears that the ECS plays roles in the fine-tuning of physiological processes that keep the body in homeostatic set-points. Citation7-Citation10 In humans, several polymorphisms in ECS components with associated neuropathophysiological processes have been described, suggesting they promote susceptibility toward development of neuropsychiatric disorders. Citation11 ECS dysregulation can also be induced by particular life factors, such as living under chronic stress, Citation12 or by metabolic factors, such as with obesity. Citation8 Pharmacological interventions targeting ECS activity aim to normalize such pathophysiological processes, Citation13-Citation15 thereby rescuing the subject from unfavorable allostatic set-points.
Since the discovery of the ECS in the 1990s, this signaling system has attracted intense attention, especially as it aided understanding of the effects of phytocannabinoids. Furthermore, detailing the various components of the ECS uncovered the fascinating complexity of how this signaling system acts in the functional network of the entire organism, in particular in the brain. This article presents a general overview of the neurobiology of the ECS. However, the vast literature on this subject makes it nearly impossible to touch on all aspects, and gaps are inevitable. Furthermore, due to space constraints, the discussion here focuses on the ECS of the brain in rodents and human, in particular the CB 1 receptor. However, the ECS is also widely involved in the regulation of peripheral immune, cardiovascular, metabolic, gastrointestinal, muscular, and peripheral nervous system processes, Citation16-Citation19 which in turn can influence central nervous system (CNS) functions. Citation17,Citation20
Endocannabinoid mechanisms
The peculiarities of the cannabinoid receptors
CB 1 and CB 2 receptors Citation21 feature the many typical characteristics of GPCRs, making these receptors highly versatile and adaptable, for example, regarding ligand binding, intracellular signaling coupling, homo- and heterodimerization, and subcellular localization. Citation22 Here, the focus will be on the CB 1 receptor, the major cannabinoid receptor in the nervous system, but several aspects of CB 2 receptors will also be addressed. It is interesting to note that despite the ubiquitous occurrence of the CB 1 receptor in the nervous system, this signaling system appears to act in a highly specific manner in a given context. Several key features come into play and will be discussed below.
Receptor expression at the cellular level
Detailed expression analyses at the regional and cellular levels in the CNS revealed that the CB 1 receptor is present in virtually all brain regions and in all major cell types ( Figure 1 ), ie, in neurons, Citation2 glial cells (astrocytes, oligodendrocytes), Citation23,Citation24 and brain-resident immune cells (microglia). Citation25 The CB 1 receptor is also present in the neurons of the major neurotransmitter systems (glutamate, γ-aminobutyric acid [GABA], serotonin, noradrenalin, acetylcholine), Citation7 and possibly also in dopaminergic neurons. CB 1 receptor–expressing cells can also be classified according to the presence of peptidergic transmitters, such as corticotropin-releasing hormone, Citation26 cholecystokinin, Citation27,Citation28 and somatostatin. Citation29 The expression levels can vary greatly, depending on the brain region and cell type. For example, the central amygdala contains very low levels of CB 1 receptor in only a few of the presumably GABAergic neurons. Citation30,Citation31 On the other hand, very high levels of CB 1 receptor messenger RNA (mRNA) are detected in hippocampal and neocortical GABAergic interneurons. Citation27,Citation28 Glutamatergic neurons generally contain rather low levels of CB 1 receptor. Citation32,Citation33 Furthermore, the CB 1 receptor is barely detectable in astrocytes, Citation23,Citation34 oligodendrocytes, Citation24,Citation35,Citation36 oligodendrocyte precursor cells (OPCs), Citation36,Citation37 and adult neural stem cells (NSCs). Citation38,Citation39 Specific functions of CB 1 receptor in these different populations and in many brain areas have been described in mouse by using conditional gene inactivation of CB 1 receptor in the respective cell types and/or brain regions, together with local pharmacological interventions. Citation7,Citation17,Citation40-Citation42 Importantly, the relative abundance of CB 1 receptor does not indicate the importance of the receptor in a particular physiological process. Citation23,Citation32
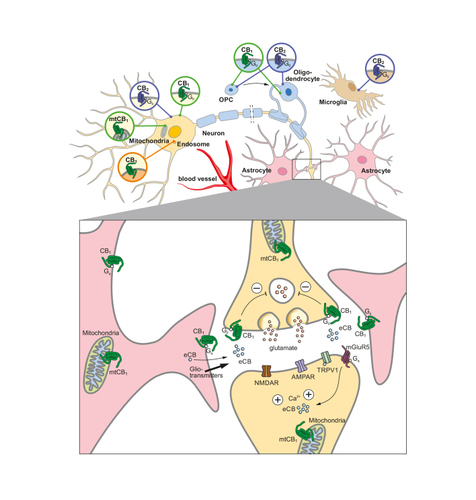
CB 2 receptor expression has been predominantly described in peripheral immune cells Citation16 and brain-resident immune cells, the macrophages, Citation43 but was eventually also detected in neurons, a circumstance that has fueled many investigations on CB 2 receptor in neural functions. Citation44 In the CNS, CB 2 receptor expression was reported in cells such as activated microglia, Citation43 brain stem neurons, Citation45 hippocampal glutamatergic neurons, Citation46 and dopaminergic neurons of the ventral tegmental area. Citation47,Citation48 CB 2 receptor transcripts are reported to be 100 to 200 times less abundant than CB 1 receptor mRNA, but are strongly upregulated in response to various insults, such as chronic pain, neuroinflammation, and stroke. Citation44,Citation49 Genetic approaches, together with pharmacology and very specific and sensitive cellular detection methods of mRNA, paved the way for the recognition of CB 2 receptor in many neural functions. Citation44
In summary, the mRNA encoding the two major receptors for eCBs is very widely expressed in the brain in many cell types, allowing the involvement in numerous physiological and pathophysiological processes. In the next paragraph, the subcellular location of the proteins will be discussed, preparing the ground for detailing the involvement of the receptors in particular cell-signaling processes.
Receptor expression at the subcellular level
Presynaptic localization: The dominant site of CB 1 receptor protein location is the presynapse, where the activation of CB 1 receptor can suppress presynaptic neurotransmitter release. This mechanism is very well detailed for glutamatergic and GABAergic synapses, whereby the eCB 2-AG is generated in the postsynaptic site and travels retrogradely to the presynaptic site to stimulate the CB 1 receptor. This can result in a short-term decrease in Ca 2+ influx at the presynaptic terminal. Citation1,Citation2,Citation7,Citation50 This signaling can typically lead to processes called depolarization-induced suppression of excitation (DSE) at the glutamatergic synapse, and depolarization-induced suppression of inhibition (DSI) at the GABAergic synapse. Most of the investigations have substantiated 2-AG as the retrograde eCB, consistent with the neuroanatomical configuration with postsynaptic synthesizing and presynaptic degrading enzymes. Citation50 For AEA, the situation is far from being understood. First, AEA synthesis machinery seems to be mainly located at the presynaptic site, Citation51 whereas the degrading machinery is at the postsynaptic site. Citation52 Secondly, besides the cannabinoid receptors, the postsynaptically acting transient receptor potential cation channel subfamily V member 1 (TRPV1) has to be considered as an AEA receptor as well. Citation53 Altogether, DSE and DSI appear to be mediated by 2-AG but not by AEA, as evidenced via studies that used a genetically induced decrease in 2-AG and AEA signaling in hippocampal glutamatergic neurons by overexpression of 2-AG–degrading enzyme monoacylglycerol lipase (MAGL) Citation54 and the AEA-degrading enzyme fatty acid amide hydrolase (FAAH), Citation55 respectively.
Postsynaptic localization: Postsynaptic CB 1 receptor has been reported in electrophysiological experiments in neocortical GABAergic Citation56 and glutamatergic neurons, Citation57 and in hippocampal glutamatergic neurons involving potassium/sodium hyperpolarization-activated cyclic nucleotide-gated channel 1 (HCN1), possibly via a somatodendritic mechanism. Citation58 Based on immunohistological analysis, CB 1 receptor has been described to be intracellular, within somatodendritic endosomes, and until now not yet on the cell membrane. Citation59
In summary, the neuronal CB 1 receptor is dominantly expressed at the presynapse; however, functional postsynaptic CB 1 receptor has also been reported. CB 2 receptor has been reported to be localized in the soma, Citation48 but to date, no immunostaining has revealed a presynaptic or dendritic localization.
Intracellular localization: Receptor trafficking is central to activity regulation and function of GPCRs. Receptors are synthesized in the cell soma and transported to the destination—for CB 1 receptors, this is mainly the axonal terminal—and finally integrated into the cellular membrane. Distinct sequences of the CB 1 receptor are involved in axonal trafficking, Citation60,Citation61 whereby alterations in such sequences can lead to eCB-mediated electrophysiological and behavioral alterations. Citation62 Distribution of CB 1 receptor in the different neuronal compartments can be influenced by the activation state of the receptor. For example, pharmacological activation of CB 1 receptor increases its presence in the somatodendritic endosomes and decreases the receptor’s presence at the presynapse. This is explained by high internalization and retrograde transport of the CB 1 receptor. This process is very pronounced in hippocampus and neocortex, but absent in basal ganglia. On the other hand, treatment with CB 1 receptor antagonist leads to a decreased number of cell bodies with CB 1 receptor–containing endosomes. This observation suggests that under steady-state conditions, the CB 1 receptor is steadily activated and internalized into the soma. Citation59 Using high-resolution microscopy, long-term treatment with the psychoactive phytocannabinoid Δ 9 -tetrahydrocannabinol (THC) was shown to lead to a decrease in CB 1 receptors located in the presynaptic membrane of hippocampal GABAergic interneurons. This internalization persists for many days after termination of THC treatment. Citation63 Along these lines, genetic deletion of the 2-AG–degrading enzyme MAGL also leads to β-arrestin–mediated internalization and desensitization of the CB 1 receptor, Citation64 an effect that is not observed in FAAH-deficient mice containing increased AEA levels. Citation65
Apart from intracellular localization in the endosomal compartment in the context of receptor trafficking, CB 1 receptor was also detected at mitochondrial membranes of neurons and named mtCB 1 receptor. Citation66 Here it mediates the reduction in mitochondrial oxygen consumption upon stimulation by exogenous cannabinoids and eCBs, in the end decreasing the production of adenosine triphosphate (ATP). Subsequently, cannabinoids were shown to regulate complex I of the respiratory chain by modulation of mitochondrial protein kinase A (PKA) activity and downstream phosphorylation events. Citation41 Thus, the ECS directly regulates mitochondrial energy production via mtCB 1 receptor. Moreover, mtCB 1 receptor was shown in the same study to mediate the memory-impairing effects of cannabinoids. Recently, activation of astrocytic mtCB 1 was shown to inhibit glucose metabolism and lactate production, altering neuronal functions and behavioral responses in a social-interaction test. Citation67
In summary, considering that eCBs are synthesized locally and that these lipid-signaling molecules diffuse and occupy a restricted three-dimensional space, generating a microdomain of eCB signaling, the precise localization of the cannabinoid receptors determines the downstream signaling. Research in recent years has established that functional CB 1 receptor is present both in the plasma membrane as well as in intracellular compartments, particularly in the outer mitochondrial membrane. Duration and intensity of eCB signaling is determined by the dynamics of eCB synthesis and degradation. Furthermore, it is thought that eCB signaling contains both tonic (constitutive) and phasic (short-term, “on-demand”) components. Citation2,Citation68 Thus, important hallmarks of eCB signaling are its temporal and spatial restrictions, which of course is also a common feature of “classical” neurotransmitter receptor systems. Next, the diversity of the intracellular signal transduction upon cannabinoid receptor activation will be addressed.
Intracellular signaling: Among the GPCRs, the CB 1 receptor appears to be most highly expressed in the brain, with protein levels in the same range as for the major components of the excitatory and inhibitory neurotransmitter systems, ie, for N -methyl-d-aspartate (NMDA) and GABA A receptors, respectively. Citation21 Intrinsically, GPCRs contain a rich repertoire for regulation of cellular processes upon ligand binding. Citation22,Citation69 , Citation70 Many aspects of CB 1 receptor signaling have been reported Citation71,Citation72 ; however, we are far from understanding how CB 1 receptor signaling gains specificity depending on cellular context.
Dimerization: GPCRs contain the capacity to form homo- and heterodimers, ie, a particular GPCR interacts with the same type of receptor to form a homodimer, or a particular GPCR interacts with another type of receptor to form a heterodimer. Such dimerization processes are involved in signal integration upon receptor activation. Citation73 Moreover, dimerization processes are also implicated in the emergence of mental disorders, and this might also be the case for cannabinoid receptors. Citation74 Homodimers were reported for CB 1 receptors Citation75 and heterodimers reported for CB 1 receptor with several other GPCRs, including CB 2 receptor, Citation76,Citation77 dopamine D 2 receptor, Citation78,Citation79 and serotonin 5-HT 2A receptor. Citation80 Heterodimerization was reported to lead to alterations in signaling. For example, CB 1 receptor/dopamine D 2 –receptor heterodimers in cultured striatal neurons can change the intracellular signaling upon CB 1 receptor stimulation. Citation78 Along the same line, a recent investigation showed the heterodimerization of CB 1 receptor with the adenosine A 2A receptor in striatum, whereby the costimulation of both receptors reduces intracellular signaling. Citation81 CB 1 /CB 2 receptor heterodimers have also been reported to enhance ligand binding of the phytocannabinoid cannabigerol. Citation82 Furthermore, heterodimerization was shown between cannabinoid receptor with non-GPCR. For example, heterodimerization between CB 2 receptor and the tyrosine kinase receptor HER2 is involved in the antitumor action of THC, whereby THC interrupts the dimer. Citation83 Altogether, these features make up a powerful tool for cell-type–specific fine-tuning of intracellular signaling. Obviously, the two dimerizing receptors must be in tight proximity within the same cell compartment, providing a constraint for activation of this mechanism.
G protein coupling: CB 1 receptor is typically coupled to G i/o proteins, leading to a decreased production of cyclic adenosine monophosphate (cAMP) and an inhibition of N- and P/Q-type Ca 2+ channels, resulting in decreased Ca 2+ influx upon stimulation. Citation1,Citation21 Under particular circumstances, G s coupling in striatal neurons was reported, in particular in concert with dopamine D 2 receptor signaling. Citation84-Citation86 G q coupling was reported in astrocytes. Citation87 G αz was found as a CB 1 receptor–interacting G protein, detected from a biochemical pull-down proteomic experiment using hippocampal synaptosomes expressing tagged CB 1 receptor. Citation88 Furthermore, biochemical analysis of G proteins interacting upon CB 1 receptor stimulation in neocortical extracts revealed the presence not only of G i/o , but also of G αz , G α12/13 , and G αq/11 . Citation89 The coupling was dependent on the CB 1 receptor agonist used. Differences in the G protein coupling has been proposed to be a possible mechanism to explain the observation that low levels of CB 1 receptor proteins in glutamatergic hippocampal neurons show higher GTPγ-binding activity than the high CB 1 receptor content in GABAergic interneurons. However, these experiments were performed with whole hippocampal extracts without subcellular fractionation and represent an average over the entire tissue. Citation90 Furthermore, the regulator of G protein–signaling (RGS) proteins constitute an important intracellular component in the control of GPCR signaling. Citation91 For example, RGS proteins have been implicated in the interaction of CB 1 receptor with dopamine D 2 receptor regarding the regulation of eCB-mediated retrograde synaptic signaling of striatal neurons. Citation92
Altogether, these observations suggest that CB 1 receptor signaling can depend on the availability of the intracellular G protein pool and on the specific ligand that activates the CB 1 receptor. The former parameter is obviously also influenced by the presence of other GPCRs in the same subcellular domain, by competing for the same G protein pool and RGSs. Furthermore, as dysregulation of ECS activity has been reported for pathophysiological processes, alterations in these different constituents (G proteins, RGS, other GPCRs), can also lead to altered CB 1 receptor signaling.
Signaling via β-arrestin: Binding of CB 1 receptor to β-arrestins is important for the internalization of the receptor upon ligand stimulation, Citation93 but β-arrestins are also signal transducers for GPCR intracellular pathways, such as extracellular-signal–regulated kinase (ERK), and c-jun terminal kinase (JNK). Citation94 So-called biased β-arrestin signaling upon ligand activation of GPCRs has been recognized as therapeutically relevant also for CB 1 receptor signaling. Citation93,Citation95 Deletion of the CB 1 receptor phosphorylation site that is involved in β-arrestin binding was reported to lead to resistance to cannabinoid tolerance and hypersensitivity to cannabinoids. Citation96 CB 1 receptor can also activate the mammalian target of rapamycin (mTOR) pathway, eg, to regulate presynaptic protein synthesis in the context of long-term synaptic plasticity. Citation97 On the other hand, mTOR mediates the amnesic effects of THC via the CB 1 receptor. Citation98
Receptor-interacting proteins: Furthermore, the presence of CB 1 receptor–interacting proteins can also contribute to the diversity of CB 1 receptor signaling. Here, the cannabinoid receptor interacting protein 1A (CRIP1A) is reported to influence CB 1 receptor agonist-induced regulation of excitatory neurotransmission, Citation99 to modulate which G i/o subtypes interact with CB 1 receptor, and to attenuate CB 1 receptor internalization via β-arrestin. Citation100
Splice variants and posttranslational modifications: Lastly, for both CB 1 and CB 2 receptors, splice variants have been reported in rodents and human. Citation44,Citation101 - Citation103 The in vivo significance of these variants has not yet been clarified, although differences in mRNA expression, receptor signaling, trafficking, and glycosylation have been reported. Citation101,Citation102 , Citation104,Citation105 Along these lines, posttranslational modifications, such as phosphorylation for the β-arrestin binding site Citation96 and N-linked glycosylation, also have to be considered in the regulation of receptor activity. For example, reduction in glycosylation of the CB 1 receptor reduces the cell membrane expression of the receptor, but not ligand-binding affinity. Citation106
In summary, in the CNS, the ECS constitutes an important mechanism for the fine-tuned regulation of synaptic transmission in numerous projections and local networks in most, if not all, brain regions. Its proposed function as a so-called circuit breaker, due to the retrograde mechanism of the suppression of neurotransmitter release, is certainly a central aspect of the ECS function, but the ECS is acting beyond this and might also be seen as an integrator of synaptic processes, enabling encoding of information.
Cellular plasticity in the adult brain
Generation of new neurons
Besides the involvement of the ECS in synaptic plasticity, eCB-regulated plasticity is also present at the cellular level ( Figure 2 ). Adult neurogenesis, ie, the generation of newly born neurons from NSCs, is an important physiological process and is implicated in neuropsychiatric disorders. Citation107-Citation109 In humans and rodents, the major sites of neurogenesis are the subgranular zone (SGZ) of the dentate gyrus of the hippocampus and the subventricular zone (SVZ) in the lateral ventricle. In the adult, neurogenesis occurs continuously, but the rate of proliferation and subsequent differentiation are tightly regulated and can be influenced by different intrinsic and extrinsic factors. Proliferation is increased, eg, by enriched environment and physical activity, and decreased, eg, upon stress, during depression, and over the course of aging. Citation107,Citation108 The role of the ECS in adult neurogenesis is well documented, as reviewed in detail elsewhere. Citation110-Citation112 Experiments performed in mice under home-cage conditions without behavioral challenges revealed that ubiquitous gene deficiency of CB 1 receptor Citation38 and impaired 2-AG signaling in mice with deficiency of the 2-AG synthesizing enzyme diacylglycerol lipase-α (DAGLα) Citation113,Citation114 lead to decreased NSC proliferation. On the contrary, under basal conditions, ubiquitous CB 2 receptor deficiency did not lead to impairments in NSC proliferation. Citation115 Reduced proliferation was also observed in rodents treated with specific CB 1 - and CB 2 receptor antagonists. Citation116 On the other hand, the enhancement of eCB signaling through the genetic inactivation of the AEA-degrading enzyme FAAH Citation38 and the pharmacological blockade of the 2-AG–degrading enzyme MAGL Citation117 led to increased NSC proliferation. Synthetic cannabinoid receptor agonist–stimulated neurogenesis appears to require both CB 1 and CB 2 receptors. Citation118 Furthermore, the phytocannabinoid THC can also stimulate neurogenesis. Citation119 Interestingly, the nonpsychotropic phytocannabinoid cannabidiol (CBD) was also reported to enhance neurogenesis, Citation120 possibly through facilitating eCB signaling. Citation121 Interestingly, CBD treatment was shown to increase AEA levels. Citation122
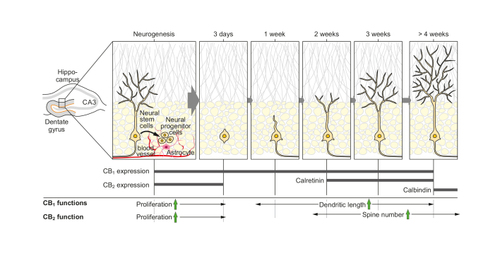
Exercise is an efficient intervention for increasing adult neurogenesis. Pharmacological blockade of the CB 1 receptor has been shown to blunt exercise-induced increase in proliferation in the SGZ. Citation123 However, in another study, using CB 1 receptor–deficient mice, no genotype differences were observed in neurogenesis over a 6-week running period, but the CB 1 receptor–deficient mice showed reduced motivation to run. Citation124 These divergent results might be explained by the differences in the pharmacological versus genetic blockade of CB 1 receptor. Results from an investigation in a mouse model of Down syndrome are very different. Here, interestingly, pharmacological blockade of CB 1 receptor led to the alleviation of impaired cognitive performance, synaptic plasticity, and neurogenesis, Citation125 an effect possibly explained by the pathology of enhanced hippocampal CB 1 receptor expression and concomitant increased receptor function at excitatory terminals.
NSCs contain a functional ECS, including the expression of CB 1 and CB 2 receptors, and eCB-synthesizing and -degrading enzymes. Citation110 In vitro experiments suggest the involvement of phosphoinositide 3-kinase/protein kinase B (PI3K/PKB), ERK, and mTOR complex 1 (mTORC) pathways in eCB-dependent stimulation of proliferation of NSCs. Citation110,Citation112,Citation116 For the CB 2 receptor, it has emerged that this receptor is mainly important under pathophysiological conditions, whereby reduction in neurogenesis induced by damaging conditions (epilepsy, alcohol, stroke, neurodegenerative processes) can be alleviated by CB 2 receptor activation. Citation112
It has to be recognized that the neurogenic niches are embedded into an environment that strongly affects proliferation and that also contains a functional ECS. Therefore, phenotypic outcomes after modulation of ECS activity depend on the cellular and temporal specificity of the targeting of the respective ECS components. A study using mice with ubiquitous loss of the CB 1 receptor and of FAAH revealed that eCB signaling controls neural progenitor differentiation in the adult brain by altering astroglial differentiation of newly born cells. The survival for the newly born cells was not changed. Citation38 The question arises whether CB 1 receptor expressed in NSCs is directly involved in the promotion of newly born neurons from NSCs. To this end, specific genetic loss of the CB 1 receptor in NSCs revealed decreased proliferation. Citation39 Furthermore, CB 1 receptor deficiency caused decreased dendritic branches and spine numbers in the differentiating neurons, reduced long-term potentiation (LTP) and short-term spatial memory, and increased depression-like behavior. Yet, no alteration in cell fate was observed, indicating that the effect on decreased astroglial differentiation observed in the ubiquitous CB 1 receptor–deficient mice was probably caused by the ECS changes around the NSC niche. The effect on dendritic branching observed on NSC-specific CB 1 receptor–deficient mice would also suggest a postsynaptic function of the CB 1 receptor that is required for maturation, but eventually, CB 1 receptor expression stops in terminally differentiated and integrated granule cells.
Generation of new oligodendrocytes
Another cell plasticity process is reported for the replenishment of oligodendrocytes from OPCs, which express the protein neural/glial antigen 2 (NG2). Citation126 Upon challenges of the adult brain, such as damaging of the myelin, OPCs are capable of proliferating and differentiating into mature oligodendrocytes, alleviating or even repairing the damage. Citation127 The ECS is functionally present in cultured OPCs and is required for maintaining proliferation, a process requiring PI3K/PKB/mTOR-signaling pathways, as inhibition of 2-AG synthesis and blockade of CB 1 /CB 2 receptors seem to induce cell-cycle arrest. Citation37 The ECS is also present in mature oligodendrocytes. Citation24,Citation35 , Citation36 CB 1 /CB 2 -agonist stimulation leads to increased myelin basic protein (MBP) expression. Citation128 Furthermore, in animal models of demyelination, stimulation of the ECS can alleviate various pathologies associated with demyelination. Citation129,Citation130 Altogether, the ECS regulates cellular plasticity in the adult brain. Here, we focused on proliferation, but the ECS can also regulate various aspects of apoptosis and autophagy, Citation131 two important cellular events for maintaining homeostasis in the body.
Neurogenesis in the embryo and early postnatal brain development
Finally, it is important to mention that the ECS has numerous functions during embryogenesis and at postnatal stages when the brain develops to its final maturation state. The understanding of these processes gives us essential mechanistic insights into the detrimental effects of cannabis use during these stages of development. Citation132-Citation134 As it is beyond the scope of this short presentation, the reader is referred to recent reviews on the ECS in neural development. Citation110,Citation135
Dysregulated endocannabinoid system
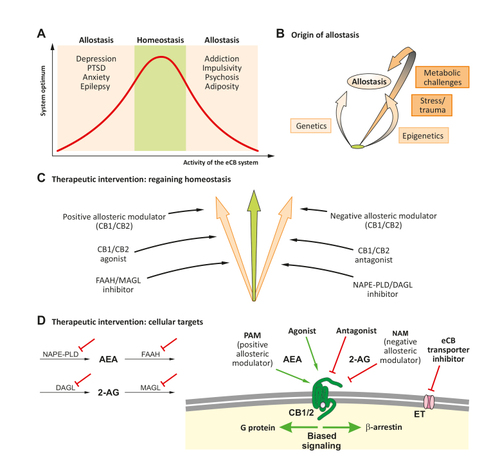
Functions of the ECS help to maintain homeostasis in the body ( Figure 3A ), for example, through regulation of the stress response, feeding, and energy metabolism, and for ensuring the excitatory/inhibitory balance in the nervous system. Considering the temporal and spatial activity of the ECS, it is no surprise that a dysregulated ECS can lead to new set-points, called allostasis, that might then be implicated in distinct neuropsychiatric disorders ( Figure 3A, B ). Human polymorphisms in genes of the ECS can be linked to particular phenotypes, as summarized in detail in a recent review, Citation11 suggesting a dysregulated ECS as origin of the observed altered behavior. A wealth of data is available for the phenotypes caused by the FAAH polymorphism rs324430 (C385A), Citation11 which leads to decreased protein stability of FAAH in the A allele, leading to increased AEA levels. Citation136 This point mutation was also introduced into the mouse genome, allowing for comparative studies in human and mouse. Citation137 These investigations indicate that the A allele promotes fear extinction, reduces anxiety, Citation137 and provides protection against stress-induced decreases in AEA. Citation138 On the other hand, the A allele of FAAH constitutes a risk factor for developing anxiety and depression from repetitive childhood trauma, Citation139 indicating the relevance of the developmental dynamics of ECS activity during childhood and adolescence. A recent case report describes a microdeletion in FAAH that led to increased AEA levels in blood and to insensitivity to pain. Citation140 Several polymorphisms in the CB 1 receptor gene ( CNR1 ) have been described. None of them lead to overt biochemical changes in CB 1 receptor protein functions. However, these polymorphisms might contribute to the susceptibility for development of certain neuropsychiatric disorders, Citation11 although the data are not as clear as for the FAAH C385A polymorphism; further studies are needed to substantiate previous observations with independent cohorts. Citation11 Investigations on a CB 2 receptor polymorphism ( CNR2 ) recently summarized in a meta-analysis suggested an association of a particular SNP (rn2501432) with depression. Citation141 Furthermore, a study revealed that the R allele of the CNR2 Q63R polymorphism together with the A allele of the FAAH C385A polymorphism are associated with enhanced vulnerability to childhood trauma and to a later anxious and depressive phenotype. Citation142
In humans, ECS activity can be monitored by measuring eCB levels in blood, saliva, hair, and cerebrospinal fluid. Expression of ECS genes can also be determined in the tissue post mortem. Numerous studies have reported alterations in eCB levels in patients suffering from various disorders, such as depression, posttraumatic stress disorder (PTSD), schizophrenia, anorexia nervosa, and Tourette syndrome. Citation11,Citation143,Citation144 Also, the course of therapy can be followed up by monitoring eCB levels, such as antidepressant treatment with electroconvulsive intervention. Citation145 In investigations with patients, drug treatments for the respective diseases constitute a serious confounding for eCB measurements. eCB can also be monitored in healthy subjects who are undergoing psychological tests and other challenges, and it is hoped that such monitoring, in combination with other biomarkers, would make possible the evaluation of potential predispositions toward development of distinct disorders.
In humans, as recently reviewed, Citation146 positron emission tomography (PET), a noninvasive method to determine ECS activity via radioactive tracers for cannabinoid receptors and ECS enzymes, has been applied to various disorders. Studies with alcohol-use disorders and schizophrenia are inconsistent, some reporting increased and others decreased CB 1 receptor binding. Data on aberrant CB 1 receptor binding in individuals with anorexia and PTSD are more coherent, but the data sets are still limited.
In summary, dysregulated ECS activity is reported in several neuropsychiatric disorders. This dysregulation may originate from genetic and/or epigenetic alterations in ECS components, or may be consequences of alterations of other signaling pathways, leading to changes in ECS activity.
The ECS as a therapeutic target
Given that dysregulated ECS activity can lead to allostatic set-points that could potentially represent pathological states, pharmacological treatment targeting ECS components is a promising strategy. A wealth of therapeutic applications has been investigated in preclinical animal models, but only in a few cases has translation to humans been achieved in clinical trials. The focus of the present discussion is on the modulation of the various components of the ECS ( Figure 3C, D ). Treatment options using phytocannabinoids are beyond the scope of this presentation, but information on this subject is found in other reviews. Citation147-Citation149
Complexity of eCB signaling molecules
Biosynthesis of eCBs occurs from membrane precursors, and eCB degradation products are precursors of eicosanoids. Citation1 Thus, eCB signaling is integrated into a lipid metabolism and signaling network. In consequence, modification of activity of eCB synthesizing and degrading enzymes may also alter other lipid signaling systems. Citation150 The synthesis and degradation machinery of AEA and 2-AG have different cellular and subcellular distributions, indicating differential functions. Citation50 Furthermore, AEA and 2-AG have different pharmacological profiles with regard to interaction with their receptors, CB 1 and CB 2 receptors, but can also activate other receptors, such as TRPV1 and GABA A receptors, respectively. Citation5,Citation21 Furthermore, endogenous peptides called pepcans or hemopressin were recently characterized Citation151-Citation153 ; these can act on CB 1 and CB 2 receptors, thereby modifying biological processes.
Cannabinoid receptors
For many years, the focus has been on CB 1 receptor antagonism/inverse agonism. However, the failure of rimonabant (Acomplia) because of CNS side effects Citation154 stopped clinical applications. Nevertheless, convincing alternative strategies, in particular peripherally acting CB 1 receptor antagonists, have been developed in recent years and shown to be active without appreciable CNS side effects. Citation13,Citation155 As peripheral organ systems interact with CNS functions, alleviation of dysregulated ECS activity in the periphery also has potential for beneficial therapeutic effects in dysregulated CNS functions. Citation17,Citation18,Citation20 As discussed above, the ECS is featured by its temporal and spatial specificity in signaling. Thus, both positive (PAM) and negative (NAM) allosteric receptor modulators constitute a promising alternative path compared with direct receptor agonism/antagonism. Citation156 Indeed, preclinical research has shown very promising efficacy using such compounds. Citation157,Citation158 Recently, pregnenolone and non-metabolizable derivatives thereof have emerged as a promising NAM of CB 1 receptor. Citation133,Citation159 The development of receptor ligands with bias toward distinct intracellular pathways, mainly G protein versus β-arrestin, also represents a very promising strategy to increase the therapeutic effects and diminish unwanted signaling side effects. Citation72 Pepcans (hemopressins) were reported to be a NAM for CB 1 receptor Citation151 and a PAM for CB 2 receptor. Citation160 A further strategy to enhance specificity is to generate dual-target drugs. Citation13 For example, a mainly peripherally acting hybrid CB 1 /inducible nitric oxide synthase (iNOS) antagonist is effective in the treatment of experimental liver fibrosis, Citation161 and the fusion peptide between hemopressin and neuropeptide VF shows potent antinociceptive effects with reduced cannabinoid-related side effects. Citation162 Comparable approaches have been pursued for interfering with CB 2 receptor activity, although with slower progress. Citation163 Particularly in the field of neuroinflammation and neurodegeneration, CB 2 receptor agonism seems to be promising. Citation44 CB 2 receptor as a target is particularly attractive because of the lack of psychotropic activity, which is present in the case of CB 1 receptor agonism.
Catabolic and metabolic eCB enzymes
Knowing that eCBs cannot be stored in vesicles, that ECS activity is spatially and temporally regulated, and that AEA and 2-AG have different functions in the brain, the ability to pharmacologically interfere with biosynthesis and degradation of AEA and 2-AG, respectively, is useful. It also allows a possible increase in specificity at the site of action and may reduce side effects. Therefore, the inhibition of FAAH and MAGL separately, and the inhibition of both FAAH and MAGL together are the most investigated approaches in preclinical research and in clinical trials for various applications, such as pain, inflammation, anxiety, and depression-like behavior. Citation164-Citation167 As for modulators of cannabinoid receptors, peripherally acting compounds may help avoid CNS-derived side effects. Citation167,Citation168 FAAH inhibitors also have preclinical applications. Citation169,Citation170 Whereas hopes are high that such compounds will be useful in humans, this has yet to be achieved. Clinical trials must be conducted with care so that setbacks can be avoided, a lesson learned, for example, in the clinical trial using the FAAH inhibitor BIA 10-2474, which contained nonspecific reactions toward serine hydrolases other than FAAH, revealed from incidences of clinical neurotoxicity. Citation171 Recently, new compounds inhibiting NAPE-PLD were reported. Citation172 Interestingly, CBD can alter levels of AEA and other N -acylethanolamines, Citation122 suggesting mechanisms via an indirect stimulation of the ECS, thereby possibly explaining the low level of side effects induced by CBD.
eCB membrane transporter
eCBs seem to be transported through a facilitated transporter across the plasma membrane. Despite the fact that to date no such protein has been cloned, drugs influencing the activity of such transporters have been found. The early generation of such compounds often did not have high efficacy and selectivity Citation173 ; however, recently, a series of compounds have been developed that lack activity on cannabinoid receptor, eCB-degrading enzymes, and binding to fatty-acid binding protein. Citation174,Citation175 Inhibition of such a transporter is expected to enhance the availability of extracellular eCBs, as eCBs cannot be transported into cells for degradation. Yet, it might also be argued that the export of eCBs is inhibited upon stimulated synthesis of eCBs, thereby increasing intracellular eCBs, leading, for example, to increased activation of mitochondrial CB 1 receptor (mtCB 1 ). Furthermore, because of the probable effect on both AEA and 2-AG levels, several different target receptors have to be considered (CB 1 /CB 2 receptor, TRPV1, peroxisome proliferator-activated receptor-γ [PPARγ]). Altogether, the inhibition of eCB membrane transporters would influence multiple cellular signaling systems, but represents a promising pharmacological target.
In summary, the diversity of ECS signaling molecules and their interactions with various receptors, together with signaling complexity of the receptor systems, makes pharmacological intervention of the ECS a challenging task, containing a considerable degree of unpredictability in the outcome of the biological effects in the whole organism.
Future directions and concluding remarks
Understanding of the brain’s ECS in its complexity at the mechanistic levels is very valuable for identifying promising disease states that can be optimally treated by modulating ECS activity. Given that eCB signaling is very widespread in the brain and intertwined with other signaling systems, the mechanistic insights will help minimize potentially unwanted side effects. Thus, it is important to understand ECS functions in the context of the entire organism. To this end, animal models, such as mice and rats, have been shown to be very suitable; owing to the evolutionary conservation of the ECS, these insights are expected to be transferrable to humans in many instances.
An important topic is the understanding of the integration of eCB signaling into the brain’s complex network. For example, excellent recent studies investigated pathways from amygdala to nucleus accumbens in the context of depressive-like behavior, Citation176 and from amygdala to cortex regarding stress effects. Citation177 Here, genetic manipulations of the CB 1 receptor functions were investigated in a pathway-specific manner, and in fact, very distinct functions of this receptor were uncovered. Thus, again, despite the very widespread presence of CB 1 receptor in the brain, its functions are amazingly specific. In further developments, high-resolution and specific genetic manipulations can be included, such as intersectional targeting of cells and circuits, Citation178-Citation180 and optogenetic-induced genetic manipulation. Citation181 Furthermore, with the advent of the clustered regularly interspaced short palindromic sequences (CRISPR)/CRISPR-associated protein (Cas) technology, Citation182,Citation183 the introduction of mutations into the mouse or rat genome, in particular point mutations that were characterized in in vitro structure-function analyses or are present in humans as small nucleotide polymorphisms (SNPs), Citation11 opens the path to novel insights into the analysis of ECS components in the context of the entire organism.
Furthermore, SNPs in ECS components Citation11 can also be investigated using human organoids generated from induced pluripotent stem cells, Citation184,Citation185 with the additional potential of genomic manipulation using CRISPR/Cas technology Citation186 and pharmacological interventions, for example, with cannabinoids, Citation187 together with state-of-the-art analyses, such as single-cell RNA-seq. Citation188 These approaches will further strengthen mechanistic insights into the roles of the ECS in psychiatric disorders.
There is no conflict of interest. This work was supported by the German Research Foundation DFG (CRC 1193 “Neurobiology of Resilience”; TRR-CRC 58 “Fear, Anxiety, Anxiety Disorders”). The author would like to thank Michael Plenikowski for his excellent work in the design of the illustrations.
REFERENCES
- PiomelliDThe molecular logic of endocannabinoid signallingNat Rev Neurosci2003487388414595399
- KanoMOhno-ShosakuTHashimotodaniYUchigashimaMWatanabeMEndocannabinoid-mediated control of synaptic transmissionPhysiol Rev20098930938019126760
- ElphickMRThe evolution and comparative neurobiology of endocannabinoid signallingPhilos Trans R Soc Lond B Biol Sci20123673201321523108540
- MechoulamRParkerLAThe endocannabinoid system and the brainAnnu Rev Psychol201364214722804774
- LigrestiADe PetrocellisLDi MarzoVFrom phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacologyPhysiol Rev2016961593165927630175
- CristinoLBisognoTDi MarzoVCannabinoids and the expanded endocannabinoid system in neurological disordersNat Rev Neurol20201692931831863
- LutzBMarsicanoGMaldonadoRHillardCJThe endocannabinoid system in guarding against fear, anxiety and stressNat Rev Neurosci20151670571826585799
- Ruiz de AzuaILutzBMultiple endocannabinoid-mediated mechanisms in the regulation of energy homeostasis in brain and peripheral tissuesCell Mol Life Sci2019761341136330599065
- MuzikODiwadkarVAHierarchical control systems for the regulation of physiological homeostasis and affect: can their interactions modulate mood and anhedonia?Neurosci Biobehav Rev201910525126131442518
- PiazzaPVCotaDMarsicanoGThe CB1 Receptor as the cornerstone of exostasisNeuron2017931252127428334603
- NavarreteFGarcia-GutierrezMSJurado-BarbaRet alEndocannabinoid system components as potential biomarkers in psychiatryFront Psychiatry20201131532395111
- RiebeCJWotjakCTEndocannabinoids and stressStress20111438439721663537
- CinarRIyerMRKunosGThe therapeutic potential of second and third generation CB1R antagonistsPharmacol Ther202020810747731926199
- ChiccaAArenaCManeraCBeyond the direct activation of cannabinoid receptors: new strategies to modulate the endocannabinoid system in CNS-related diseasesRecent Pat CNS Drug Discov20161012214127630088
- TomaselliGValleeMStress and drug abuse-related disorders: the promising therapeutic value of neurosteroids focus on pregnenolone-progesterone-allopregnanolone pathwayFront Neuroendocrinol20195510078931525393
- MaccarroneMBabIBiroTet alEndocannabinoid signaling at the periphery: 50 years after THCTrends Pharmacol Sci20153627729625796370
- Busquets-GarciaAGomis-GonzalezMSrivastavaRKet alPeripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidationProc Natl Acad Sci U S A20161139904990927528659
- Ruiz de AzuaIManciniGSrivastavaRKet alAdipocyte cannabinoid receptor CB1 regulates energy homeostasis and alternatively activated macrophagesJ Clin Invest20171274148416229035280
- BellocchioLSoria-GomezEQuartaCet alActivation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockadeProc Natl Acad Sci U S A20131104786479123487769
- SuarezJRiveraPAparisiRAet alAdipocyte cannabinoid CB1 receptor deficiency alleviates high fat diet-induced memory deficit, depressive-like behavior, neuroinflammation and impairment in adult neurogenesisPsychoneuroendocrinology201911010441831491589
- PertweeRGHowlettACAboodMEet alInternational union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2Pharmacol Rev20106258863121079038
- WoottenDChristopoulosAMarti-SolanoMBabuMMSextonPMMechanisms of signalling and biased agonism in G protein-coupled receptorsNat Rev Mol Cell Biol20181963865330104700
- HanJKesnerPMetna-LaurentMet alAcute cannabinoids impair working memory through astroglial CB1 receptor modulation of hippocampal LTDCell20121481039105022385967
- IlyasovAAMilliganCEPharrEPHowlettACThe endocannabinoid system and oligodendrocytes in health and diseaseFront Neurosci20181273330416422
- AraujoDJTjoaKSaijoKThe endocannabinoid system as a window into microglial biology and its relationship to autismFront Cell Neurosci20191342431619967
- CotaDSteinerMAMarsicanoGet alRequirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis functionEndocrinology20071481574158117194743
- MarsicanoGLutzBExpression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrainEur J Neurosci1999114213422510594647
- KatonaISperlághBSíkAet alPresynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneuronsJ Neurosci1999194544455810341254
- ZouSKumarUColocalization of cannabinoid receptor 1 with somatostatin and neuronal nitric oxide synthase in rat brain hippocampusBrain Res2015162211412626115586
- KamprathKRomo-ParraHHäringMet alShort-term adaptation of conditioned fear responses through endocannabinoid signaling in the central amygdalaNeuropsychopharmacology20113665266320980994
- LangeMDDaldrupTRemmersFet alCannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threatMol Psychiatry2017221422143027698427
- MonoryKMassaFEgertovaMet alThe endocannabinoid system controls key epileptogenic circuits in the hippocampusNeuron20065145546616908411
- KatonaIUrbanGMWallaceMet alMolecular composition of the endocannabinoid system at glutamatergic synapsesJ Neurosci2006265628563716723519
- Gutierrez-RodriguezABonilla-DelRIPuenteNet alLocalization of the cannabinoid type-1 receptor in subcellular astrocyte compartments of mutant mouse hippocampusGlia2018661417143129480581
- BerrenderoFSepeNRamosJADi MarzoVFernandez-RuizJJAnalysis of cannabinoid receptor binding and mRNA expression and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal periodSynapse19993318119110420166
- Molina-HolgadoEVelaJMArevalo-MartinAet alCannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signalingJ Neurosci2002229742975312427829
- GomezOSanchez-RodriguezMAOrtega-GutierrezSet alA basal tone of 2-arachidonoylglycerol contributes to early oligodendrocyte progenitor proliferation by activating phosphatidylinositol 3-kinase (PI3K)/AKT and the mammalian target of rapamycin (MTOR) pathwaysJ Neuroimmune Pharmacol20151030931725900077
- AguadoTPalazuelosJMonoryKet alThe endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cellsJ Neurosci2006261551156116452678
- ZimmermannTMarosoMBeerAet alNeural stem cell lineage-specific cannabinoid type-1 receptor regulates neurogenesis and plasticity in the adult mouse hippocampusCereb Cortex2018284454447130307491
- LangeMDDaldrupTRemmersFet alCannabinoid CB1 receptors in distinct circuits of the extended amygdala determine fear responsiveness to unpredictable threatMol Psychiatry2017221422143027698427
- Hebert-ChatelainEDesprezTSerratRet alA cannabinoid link between mitochondria and memoryNature201653955555927828947
- RobinLMOliveira da CruzJFLanglaisVCet alAstroglial CB1 receptors determine synaptic D-serine availability to enable recognition memoryNeuron20189893594429779943
- TanakaMSackettSZhangYEndocannabinoid modulation of microglial phenotypes in neuropathologyFront Neurol2020118732117037
- JordanCJXiZXProgress in brain cannabinoid CB2 receptor research: from genes to behaviorNeurosci Biobehav Rev20199820822030611802
- Van SickleMDDuncanMKingsleyPJet alIdentification and functional characterization of brainstem cannabinoid CB2 receptorsScience200531032933216224028
- StempelAVStumpfAZhangHYet alCannabinoid type 2 receptors mediate a cell type-specific plasticity in the hippocampusNeuron20169079580927133464
- ZhangHYGaoMLiuQRet alCannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in miceProc Natl Acad Sci U S A201411146E5007E501525368177
- ZhangHYGaoMShenHet alExpression of functional cannabinoid CB2 receptor in VTA dopamine neurons in ratsAddict Biol20172275276526833913
- YuSJReinerDShenHWuKJLiuQRWangYTime-dependent protection of CB2 receptor agonist in strokePLoS One201510e013248726186541
- KatonaIFreundTFMultiple functions of endocannabinoid signaling in the brainAnnu Rev Neurosci20123552955822524785
- NyilasRDudokBUrbanGMet alEnzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminalsJ Neurosci2008281058106318234884
- EgertovaMCravattBFElphickMRComparative analysis of fatty acid amide hydrolase and CB1 cannabinoid receptor expression in the mouse brain: evidence of a widespread role for fatty acid amide hydrolase in regulation of endocannabinoid signalingNeuroscience200311948149612770562
- GibsonHEEdwardsJGPageRSVan HookMJKauerJATRPV1 channels mediate long-term depression at synapses on hippocampal interneuronsNeuron20085774675918341994
- GuggenhuberSRomo-ParraHBindilaLet alImpaired 2-AG signaling in hippocampal glutamatergic neurons: aggravation of anxiety-like behavior and unaltered seizure susceptibilityInt J Neuropsychopharmacol201519pyv09126232789
- ZimmermannTBartschJCBeerAet alImpaired anandamide/palmitoylethanolamide signaling in hippocampal glutamatergic neurons alters synaptic plasticity, learning, and emotional responsesNeuropsychopharmacology2019441377138830532004
- BacciAHuguenardJRPrinceDALong-lasting self-inhibition of neocortical interneurons mediated by endocannabinoidsNature200443131231615372034
- MarinelliSPacioniSCannichAMarsicanoGBacciASelf-modulation of neocortical pyramidal neurons by endocannabinoidsNat Neurosci2009121488149019915567
- MarosoMSzaboGGKimHKet alCannabinoid control of learning and memory through HCN channelsNeuron2016891059107326898775
- ThibaultKCarrelDBonnardDet alActivation-dependent subcellular distribution patterns of CB1 cannabinoid receptors in the rat forebrainCereb Cortex2013232581259122892424
- Fletcher-JonesAHildickKLEvansAJNakamuraYWilkinsonKAHenleyJMThe C-terminal helix 9 motif in rat cannabinoid receptor type 1 regulates axonal trafficking and surface expressionElife20198
- WickertMHildickKLBaillieGLet alThe F238L point mutation in the cannabinoid type 1 receptor enhances basal endocytosis via lipid raftsFront Mol Neurosci20181123030026687
- SchneiderMKasanetzFLynchDLet alEnhanced functional activity of the cannabinoid type-1 receptor mediates adolescent behaviorJ Neurosci201535139751398826468198
- DudokBBarnaLLedriMet alCell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signalingNat Neurosci201518758625485758
- ImperatoreRMorelloGLuongoLet alGenetic deletion of monoacylglycerol lipase leads to impaired cannabinoid receptor CB1R signaling and anxiety-like behaviorJ Neurochem201513579981326223500
- CravattBFDemarestKPatricelliMPet alSupersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolaseProc Natl Acad Sci U S A2001989371937611470906
- BenardGMassaFPuenteNet alMitochondrial CB1 receptors regulate neuronal energy metabolismNat Neurosci20121555856422388959
- Jimenez-BlascoDBusquets-GarciaAHebert-ChatelainEet alGlucose metabolism links astroglial mitochondria to cannabinoid effectsNature2020583781760360832641832
- AlgerBEKimJSupply and demand for endocannabinoidsTrends Neurosci20113430431521507493
- KenakinTBiased receptor signaling in drug discoveryPharmacol Rev20197126731530914442
- ZhaoPFurnessSGBThe nature of efficacy at G protein-coupled receptorsBiochem Pharmacol201917011364731585071
- LaprairieRBBagherAMDenovan-WrightEMCannabinoid receptor ligand bias: implications in the central nervous systemCurr Opin Pharmacol201732324327835801
- Al-ZoubiRMoralesPReggioPHStructural Insights into CB1 receptor biased signalingInt J Mol Sci20192081837
- Borroto-EscuelaDOFuxeKOligomeric receptor complexes and their allosteric receptor-receptor interactions in the plasma membrane represent a new biological principle for integration of signals in the CNSFront Mol Neurosci20191223031607863
- Borroto-EscuelaDOCarlssonJAmbroginiPet alUnderstanding the role of GPCR heteroreceptor complexes in modulating the brain networks in health and diseaseFront Cell Neurosci2017113728270751
- Wager-MillerJWestenbroekRMackieKDimerization of G protein-coupled receptors: CB1 cannabinoid receptors as an exampleChem Phys Lipids2002121838912505693
- CallenLMorenoEBarroso-ChineaPet alCannabinoid receptors CB1 and CB2 form functional heteromers in brainJ Biol Chem2012287208512086522532560
- NavarroGVaraniKReyes-ResinaIet alCannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexesFront Pharmacol2018963229977202
- BagherAMLaprairieRBKellyMEDenovan-WrightEMAntagonism of dopamine receptor 2 long affects cannabinoid receptor 1 signaling in a cell culture model of striatal medium spiny projection neuronsMol Pharmacol20168965266627053685
- BagherAMLaprairieRBToguriJTKellyMEMDenovan-WrightEMBidirectional allosteric interactions between cannabinoid receptor 1 (CB1) and dopamine receptor 2 long (D2L) heterotetramersEur J Pharmacol2017813668328734930
- VinalsXMorenoELanfumeyLet alCognitive impairment induced by delta9-tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptorsPLoS Biol201513e100219426158621
- MorenoEChiarloneAMedranoMet alSingular location and signaling profile of adenosine A2A-cannabinoid CB1 receptor heteromers in the dorsal striatumNeuropsychopharmacology20184396497728102227
- NavarroGVaraniKReyes-ResinaIet alCannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexesFront Pharmacol2018963229977202
- Blasco-BenitoSMorenoESeijo-VilaMet alTherapeutic targeting of HER2-CB2R heteromers in HER2-positive breast cancerProc Natl Acad Sci U S A20191163863387230733293
- GlassMFelderCCConcurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptorJ Neurosci199717532753339204917
- KearnCSBlake-PalmerKDanielEMackieKGlassMConcurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor crosstalk?Mol Pharmacol2005671697170415710746
- Caballero-FloranRNConde-RojasIOviedoCAet alCannabinoid-induced depression of synaptic transmission is switched to stimulation when dopaminergic tone is increased in the globus pallidus of the rodentNeuropharmacology201611040741827506997
- NavarreteMAraqueAEndocannabinoids mediate neuron-astrocyte communicationNeuron20085788389318367089
- MattheusTKuklaKZimmermannTTenzerSLutzBCell type-specific tandem affinity purification of the mouse hippocampal CB1 receptor-associated proteomeJ Proteome Res2016153585360127596989
- Diez-AlarciaRIbarra-LecueILopez-CardonaAPet alBiased agonism of three different cannabinoid receptor agonists in mouse brain cortexFront Pharmacol2016741527867358
- SteindelFLernerRHäringMet alNeuron-type specific cannabinoid-mediated G protein signalling in mouse hippocampusJ Neurochem201312479580723289830
- O’BrienJBWilkinsonJCRomanDLRegulator of G-protein signaling (RGS) proteins as drug targets: progress and future potentialsJ Biol Chem2019294185711858531636120
- SongCAndersonGRSuttonLPDaoMMartemyanovKASelective role of RGS9-2 in regulating retrograde synaptic signaling of indirect pathway medium spiny neurons in dorsal striatumJ Neurosci2018387120713130006367
- RaehalKMBohnLMThe role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeuticsNeuropharmacology201160586520713067
- GurevichVVGurevichEVGPCR signaling regulation: the role of GRKs and arrestinsFront Pharmacol20191012530837883
- Delgado-PerazaFAhnKHNogueras-OrtizCet alMechanisms of biased b-arrestin-mediated signaling downstream from the cannabinoid 1 receptorMol Pharmacol20168961862927009233
- MorganDJDavisBJKearnCSet alMutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in miceJ Neurosci2014345152516324719095
- YountsTJMondayHRDudokBet alPresynaptic protein synthesis is required for long-term plasticity of GABA releaseNeuron20169247949227764673
- PuighermanalEMarsicanoGBusquets-GarciaALutzBMaldonadoROzaitaACannabinoid modulation of hippocampal long-term memory is mediated by mTOR signalingNat Neurosci2009121152115819648913
- GuggenhuberSAlparAChenRet alCannabinoid receptor-interacting protein Crip1a modulates CB1 receptor signaling in mouse hippocampusBrain Struct Funct20162212061207425772509
- BoothWTWalkerNBLowtherWTHowlettACCannabinoid receptor interacting protein 1a CRIP1a: function and structureMolecules2019243672
- RuehleSWager-MillerJStraikerAet alDiscovery and characterization of two novel CB1 receptor splice variants with modified N-termini in mouseJ Neurochem201714252153328608535
- LiuQRHuangNSQuHet alIdentification of novel mouse and rat CB1R isoforms and in silico modeling of human CB1R for peripheral cannabinoid therapeuticsActa Pharmacol Sin20194038739730202012
- RybergEVuHKLarssonNet alIdentification and characterisation of a novel splice variant of the human CB1 receptorFEBS Lett200557925926415620723
- StraikerAWager-MillerJHutchensJMackieKDifferential signalling in human cannabinoid CB1 receptors and their splice variants in autaptic hippocampal neuronesBr J Pharmacol20121652660267122014238
- BagherAMLaprairieRBKellyMEDenovan-WrightEMCo-expression of the human cannabinoid receptor coding region splice variants (hCB1) affects the function of hCB1 receptor complexesEur J Pharmacol201372134135424091169
- RapinoCCastellucciALizziARet alModulation of endocannabinoid-binding receptors in human neuroblastoma cells by tunicamycinMolecules2019241432
- BondAMMingGLSongHAdult mammalian neural stem cells and neurogenesis: five decades laterCell Stem Cell20151738539526431181
- TodaTParylakSLLinkerSBGageFHThe role of adult hippocampal neurogenesis in brain health and diseaseMol Psychiatry201924678729679070
- MillerSMSahayAFunctions of adult-born neurons in hippocampal memory interference and indexingNat Neurosci2019221565157531477897
- MaccarroneMGuzmanMMackieKDohertyPHarkanyTProgramming of neural cells by endocannabinoids: from physiological rules to emerging therapiesNat Rev Neurosci20141578680125409697
- PrendervilleJAKellyAMDownerEJThe role of cannabinoids in adult neurogenesisBr J Pharmacol20151723950396325951750
- OddiSScipioniLMaccarroneMEndocannabinoid system and adult neurogenesis: a focused reviewCurr Opin Pharmacol201950253231864101
- GaoYVasilyevDVGoncalvesMBet alLoss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out miceJ Neurosci2010302017202420147530
- JennichesITernesSAlbayramOet alAnxiety, stress, and fear response in mice with reduced endocannabinoid levelsBiol Psychiatry20167985886825981172
- MenschingLDjogoNKellerCRadingSKarsakMStable adult hippocampal neurogenesis in cannabinoid receptor CB2 deficient miceInt J Mol Sci201920153759
- PalazuelosJOrtegaZDiaz-AlonsoJGuzmanMGalve-RoperhICB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signalingJ Biol Chem20122871198120922102284
- ZhangZWangWZhongPet alBlockade of 2-arachidonoylglycerol hydrolysis produces antidepressant-like effects and enhances adult hippocampal neurogenesis and synaptic plasticityHippocampus201525162625131612
- RodriguesRSRibeiroFFFerreiraFVazSHSebastiaoAMXapelliSInteraction between cannabinoid type 1 and type 2 receptors in the modulation of subventricular zone and dentate gyrus neurogenesisFront Pharmacol2017851628848435
- JiangWZhangYXiaoLet alCannabinoids promote embryonic and adult hippocampus neurogenesis and produce anxiolytic- and antidepressant-like effectsJ Clin Invest20051153104311616224541
- WolfSABick-SanderAFabelKet alCannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesisCell Commun Signal201081220565726
- CamposACOrtegaZPalazuelosJet alThe anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: involvement of the endocannabinoid systemInt J Neuropsychopharmacol2013161407141923298518
- LeishmanEManchandaMThelenRMillerSMackieKBradshawHBCannabidiol’s upregulation of N-acyl ethanolamines in the central nervous system requires N-acyl phosphatidyl ethanolamine-specific phospholipase DCannabis Cannabinoid Res2018322824130515459
- HillMNTitternessAKMorrishACet alEndogenous cannabinoid signaling is required for voluntary exercise-induced enhancement of progenitor cell proliferation in the hippocampusHippocampus20102051352319489006
- DubreucqSKoehlMAbrousDNMarsicanoGChaouloffFCB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesisExp Neurol201022410611320138171
- Navarro-RomeroAVazquez-OliverAGomis-GonzalezMet alCannabinoid type-1 receptor blockade restores neurological phenotypes in two models for Down syndromeNeurobiol Dis20191259210630685352
- KirdajovaDAnderovaMNG2 cells and their neurogenic potentialCurr Opin Pharmacol201950536031877531
- KuhnSGrittiLCrooksDDombrowskiYOligodendrocytes in development, myelin generation and beyondCells201981424
- GomezOSanchez-RodriguezALeMSanchez-CaroCMolina-HolgadoFMolina-HolgadoECannabinoid receptor agonists modulate oligodendrocyte differentiation by activating PI3K/Akt and the mammalian target of rapamycin mTOR. pathwaysBr J Pharmacol20111631520153221480865
- Bernal-ChicoACanedoMManterolaAet alBlockade of monoacylglycerol lipase inhibits oligodendrocyte excitotoxicity and prevents demyelination in vivoGlia20156316317625130621
- Tomas-RoigJAgbemenyahHYCelarainNQuintanaERamio-TorrentaLHavemann-ReineckeUDose-dependent effect of cannabinoid WIN-55,212-2 on myelin repair following a demyelinating insultSci Rep20201059031953431
- Garcia-ArencibiaMMolina-HolgadoEMolina-HolgadoFEffect of endocannabinoid signalling on cell fate: life, death, differentiation and proliferation of brain cellsBr J Pharmacol20191761361136929797438
- HurdYLManzoniOJPletnikovMVLeeFSBhattacharyyaSMelisMCannabis and the developing brain: insights into its long-lasting effectsJ Neurosci2019398250825831619494
- FrauRMiczanVTraccisFet alPrenatal THC exposure produces a hyperdopaminergic phenotype rescued by pregnenoloneNat Neurosci2019221975198531611707
- de Salas-QuirogaAGarcia-RinconDGomez-DominguezDet alLong-term hippocampal interneuronopathy drives sex-dimorphic spatial memory impairment induced by prenatal THC exposureNeuropsychopharmacology20204587788631982904
- RodriguesRSLourencoDMPauloSLet alCannabinoid actions on neural stem cells: implications for pathophysiologyMolecules2019241350
- SipeJCChiangKGerberALBeutlerECravattBFA missense mutation in human fatty acid amide hydrolase associated with problem drug useProc Natl Acad Sci U S A2002998394839912060782
- DinchevaIDrysdaleATHartleyCAet alFAAH genetic variation enhances fronto-amygdala function in mouse and humanNat Commun20156639525731744
- MayoLMAsratianALindeJet alProtective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and miceMol Psychiatry202025993100530120421
- GeeDGFetchoRNJingDet alIndividual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across speciesProc Natl Acad Sci U S A20161134500450527001846
- HabibAMOkorokovALHillMNet alMicrodeletion in a FAAH pseudogene identified in a patient with high anandamide concentrations and pain insensitivityBr J Anaesth2019123e249e25330929760
- KongXMiaoQLuXet alThe association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression: a meta-analysisMedicine (Baltimore)201998e1740331725603
- LazaryJEszlariNJuhaszGBagdyGA functional variant of CB2 receptor gene interacts with childhood trauma and FAAH gene on anxious and depressive phenotypesJ Affect Disord201925771672231382124
- HillardCJCirculating endocannabinoids: from whence do they come and where are they going?Neuropsychopharmacology20184315517228653665
- Muller-VahlKRBindilaLLutzBet alCerebrospinal fluid endocannabinoid levels in Gilles de la Tourette syndromeNeuropsychopharmacology2020451323132932272483
- KranasterLHoyerCMindtSet alThe novel seizure quality index for the antidepressant outcome prediction in electroconvulsive therapy: association with biomarkers in the cerebrospinal fluidEur Arch Psychiatry Clin Neurosci2019 Nov 23 Epub ahead of print https://doi.org/10.1007/s00406-019-01086-x
- SloanMEGrantCWGowinJLRamchandaniVALe FollBEndocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studiesActa Pharmacol Sin20194034235030166624
- RussoEBCannabis therapeutics and the future of neurologyFront Integr Neurosci2018125130405366
- SarrisJSinclairJKaramacoskaDDavidsonMFirthJMedicinal cannabis for psychiatric disorders: a clinically-focused systematic reviewBMC Psychiatry2020202431948424
- BlackNStockingsECampbellGet alCannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysisLancet Psychiatry20196995101031672337
- NomuraDKMorrisonBEBlankmanJLet alEndocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammationScience201133480981322021672
- GomesIGrushkoJSGolebiewskaUet alNovel endogenous peptide agonists of cannabinoid receptorsFASEB J2009233020302919380512
- BauerMChiccaATamborriniMet alIdentification and quantification of a new family of peptide endocannabinoids (Pepcans) showing negative allosteric modulation at CB1 receptorsJ Biol Chem2012287369443696722952224
- WeiFZhaoLJingYSignaling molecules targeting cannabinoid receptors: hemopressin and related peptidesNeuropeptides20207910199831831183
- ChristensenRKristensenPKBartelsEMBliddalHAstrupAEfficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trialsLancet20073701706171318022033
- AmatoGKhanNSMaitraRA patent update on cannabinoid receptor 1 antagonists (2015-2018)Expert Opin Ther Pat20192926126930889997
- KhuranaLMackieKPiomelliDKendallDAModulation of CB1 cannabinoid receptor by allosteric ligands: pharmacology and therapeutic opportunitiesNeuropharmacology201712431228527758
- SlivickiRAXuZKulkarniPMet alPositive allosteric modulation of cannabinoid receptor type 1 suppresses pathological pain without producing tolerance or dependenceBiol Psychiatry20188472273328823711
- SlivickiRAIyerVMaliSSet alPositive allosteric modulation of CB1 cannabinoid receptor signaling enhances morphine antinociception and attenuates morphine tolerance without enhancing morphine-induced dependence or rewardFront Mol Neurosci2020135432410959
- TomaselliGValleeMStress and drug abuse-related disorders: the promising therapeutic value of neurosteroids focus on pregnenolone-progesterone-allopregnanolone pathwayFront Neuroendocrinol20195510078931525393
- PetrucciVChiccaAGlasmacherSet alPepcan-12 (RVD-hemopressin) is a CB2 receptor positive allosteric modulator constitutively secreted by adrenals and in liver upon tissue damageSci Rep20177956028842619
- IyerMRCinarRKatzAet alDesign, synthesis, and biological evaluation of novel, non-brain-penetrant, hybrid cannabinoid CB1R inverse agonist/inducible nitric oxide synthase (iNOS) inhibitors for the treatment of liver fibrosisJ Med Chem2017601126114128085283
- XuBXiaoJXuKet alVF-13, a chimeric peptide of VD-hemopressin(α) and neuropeptide VF, produces potent antinociception with reduced cannabinoid-related side effectsNeuropharmacology202010817832544481
- MoralesPGoyaPJagerovicNEmerging strategies targeting CB2 cannabinoid receptor: biased agonism and allosterismBiochem Pharmacol201815781730055149
- FazioDCriscuoloEPiccoliABarboniBFezzaFMaccarroneMAdvances in the discovery of fatty acid amide hydrolase inhibitors: what does the future hold?Expert Opin Drug Discov2020114
- Gil-OrdonezAMartin-FontechaMOrtega- GutierrezSLopez-RodriguezMLMonoacylglycerol lipase (MAGL) as a promising therapeutic targetBiochem Pharmacol2018157183230059673
- BedseGHillMNPatelS2-Arachidonoylglycerol modulation of anxiety and stress adaptation: from grass roots to novel therapeuticsBiol Psychiatry2020S0006-322320300494
- RenSYWangZZZhangYChenNHPotential application of endocannabinoid system agents in neuropsychiatric and neurodegenerative diseases-focusing on FAAH/MAGL inhibitorsActa Pharmacol Sin2020 Mar 18 Epub ahead of print https://doi.org/10.1038/s41401-020-0385-7
- VozellaVAhmedFChoobchianPet alPharmacokinetics, pharmacodynamics and safety studies on URB937, a peripherally restricted fatty acid amide hydrolase inhibitor, in ratsJ Pharm Pharmacol2019711762177331579946
- D’SouzaDCCortes-BrionesJCreaturaGet alEfficacy and safety of a fatty acid amide hydrolase inhibitor (PF-04457845) in the treatment of cannabis withdrawal and dependence in men: a double-blind, placebo-controlled, parallel group, phase 2a single-site randomised controlled trialLancet Psychiatry20196354530528676
- MayoLMAsratianALindeJet alElevated anandamide, enhanced recall of fear extinction, and attenuated stress responses following inhibition of fatty acid amide hydrolase: a randomized, controlled experimental medicine trialBiol Psychiatry20208753854731590924
- van EsbroeckACMJanssenAPACognettaAB IIIet alActivity-based protein profiling reveals off-target proteins of the FAAH inhibitor BIA 10-2474Science20173561084108728596366
- MockEDMustafaMGunduz-CinarOet alDiscovery of a NAPE-PLD inhibitor that modulates emotional behavior in miceNat Chem Biol20201666767532393901
- NicolussiSGertschJEndocannabinoid transport revisitedVitam Horm20159844148525817877
- ChiccaANicolussiSBartholomausRet alChemical probes to potently and selectively inhibit endocannabinoid cellular reuptakeProc Natl Acad Sci U S A2017114E5006E501528584105
- Reynoso-MorenoIChiccaAFlores-SotoMEViveros-ParedesJMGertschJThe endocannabinoid reuptake inhibitor WOBE437 is orally bioavailable and exerts indirect polypharmacological effects via different endocannabinoid receptorsFront Mol Neurosci20181118029910713
- ShenCJZhengDLiKXet alCannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behaviorNat Med20192533734930643290
- MarcusDJBedseGGauldenADet alEndocannabinoid signaling collapse mediates stress-induced amygdalo-cortical strengtheningNeuron20201051062107631948734
- HermannMStillhardPWildnerHet alBinary recombinase systems for high-resolution conditional mutagenesisNucleic Acids Res2014423894390724413561
- MadisenLGarnerARShimaokaDet alTransgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performanceNeuron20158594295825741722
- KarimovaMBakerOCamgozANaumannRBuchholzFAnastassiadisKA single reporter mouse line for Vika, Flp, Dre, and Cre-recombinationSci Rep201881445330262904
- YaoSYuanPOuelletteBet alRecV recombinase system for in vivo targeted optogenomic modifications of single cells or cell populationsNat Methods20201742242932203389
- ShalemOSanjanaNEZhangFHigh-throughput functional genomics using CRISPR-Cas9Nat Rev Genet20151629931125854182
- NavabpourSKwapisJLJaromeTJA neuroscientist’s guide to transgenic mice and other genetic toolsNeurosci Biobehav Rev202010873274831843544
- ArlottaPPascaSPCell diversity in the human cerebral cortex: from the embryo to brain organoidsCurr Opin Neurobiol20195619419831051421
- SidhayeJKnoblichJABrain organoids: an ensemble of bioassays to investigate human neurodevelopment and diseaseCell Death Differ2020 Jun 1 Epub ahead of print https://doi.org/10.1038/s41418-020-0566-4
- FischerJHeideMHuttnerWBGenetic modification of brain organoids. Front Cell Neurosci20191355831920558
- AoZCaiHHavertDJet alOne-stop microfluidic assembly of human brain organoids to model prenatal cannabis exposureAnal Chemi20209246304638
- VelascoSKedaigleAJSimmonsSKet alIndividual brain organoids reproducibly form cell diversity of the human cerebral cortexNature201957052352731168097

