Abstract
The newly adopted International Code of Nomenclature for algae, fungi and plants (ICN) demands that dimorphic fungi, in particular those with both sexual and asexual names, now bear a single name. Although priority is no longer associated with the mode of reproduction, the ICN requires justification for choosing an asexual name over an existing sexual one. The phylogenetic approach that made dual nomenclature for fungi obsolete can be used to help choose names for large groups of fungi that are best known by asexual names. Here we apply this approach to one of the largest and most diverse asexual genera, the genus Aspergillus. We find that existing sexual names may be given to well supported clades of fungi with distinct phenotypes, which include sexual morphology as well as physiological attributes associated with xerophily, thermophily and mycotoxin production. One group of species important to food production and food safety, Aspergillus subgen. Circumdati, lacks a well supported clade; here we propose that the name Aspergillus be retained for this group. Recognizing that nomenclature has economic and social implications, particularly for old, important genera, we discuss the consequences of various scenarios to implement the new “one name for one fungus” article in the ICN, showing that our approach requires the fewest appeals to the ICN while retaining the name Aspergillus for many of the most economically and socially important species.
Introduction
The International Code of Nomenclature for algae, fungi and plants (ICN, CitationMcNeill et al. 2012) adopted at the Melbourne International Botanical Congress abolished dual nomenclature for the species of Ascomycota and Basidiomycota that bear separate names for their different asexual (anamorph) and sexual (teleomorph) states. Among the changes described in the ICN are two aimed at guiding mycologists as they choose one name from among the several possible asexual and sexual names. These are reproduced below:
“Art. 59.1 Note 2. Previous editions of this Code provided for separate names for mitotic asexual morphs (anamorphs) of certain pleomorphic fungi and required that the name applicable to the whole fungus be typified by a meiotic sexual morph (teleomorph). Under the current Code, however, all legitimate fungal names are treated equally for the purposes of establishing priority, regardless of the life-history stage of the type (but see Art. 57.2; see also Art. 14.13).
“[Art.] 57.2. In pleomorphic fungi (including lichenicolous fungi, but excluding lichen-forming fungi and those fungi traditionally associated with them taxonomically, e.g. Mycocaliciaceae), in cases where, prior to 1 January 2013, both teleomorph-typified and anamorph-typified names were widely used for a taxon, an anamorph-typified name that has priority is not to displace the teleomorph name(s) unless and until a proposal to reject the former under Art. 56.1 or 56.3 or to deal with the latter under Art. 14.1 or 14.13 has been submitted and rejected.”
These provisions of the ICN make it clear that although sexual names no longer have priority over asexual names, mycologists wishing to choose a widely used asexual name over a widely used sexual name first must convince the ICN General Committee of the wisdom of their action. For the majority of fungi, this decision will be uncomplicated. However, for fungi that are economically and socially important, with sexual and asexual names that both are old and well established, this decision may be difficult. Here, we examine this problem as it relates to one of the oldest established and most economically and socially important groups of fungi, those in the asexual genus Aspergillus, and the sexual generic names validly associated with it.
The genus Aspergillus was illustrated first by Micheli in 1729 and then by Link in 1809 as a fungal genus with a distinctive mop-like, vesiculate conidiophore resembling an aspergillum. This feature was used as the criterion for Aspergillus by later authors, including CitationThom and Church (1926), CitationThom and Raper (1945) and CitationRaper and Fennell (1965). Aspergillus was more formally delimited from the closely related genus Penicillium by CitationPitt and Hocking (1985) who noted that “the simultaneous production of phialides from a single [solitary] head on a well defined stipe … and the presence of a foot-cell are absolute criteria for Aspergillus.” While no longer considered “absolute”, this characteristic, asexual structure still provides the basis for the morphological recognition of Aspergillus species in the broadest sense.
Table I. Some phenotypes of the sexual genera associated with Aspergillus
Aspergillus has grown into a very large genus, currently comprising about 300 accepted species (CitationICPA, 2013). At the same time, the distinctive asexual fruiting structure characteristic of Aspergillus is known to be associated with no fewer than 11 sexual state genera reflecting variation in cleistothecial wall color, composition, decoration and the presence or absence of stroma of varying composition and colors. These sexual-state genera have been validly published at various times under the International Code of Botanical Nomenclature (CitationGeiser 2009; ). In addition to varying in sexual morphology, these genera differ widely in physiology and ecology (e.g. xerophily, thermophily and mycotoxin production). Names in the more important of these sexual genera, Eurotium, Neosartorya and Emericella, have been widely used over the past 40 or more years. We estimate that about one-third of the accepted Aspergillus species have been validly described in one of the 11 sexual genera.
CitationHoubraken and Samson (2011) used DNA sequences of four protein-coding genes (RPB1, RPB2, Tsr1, Cct8) to analyze phylogenetically many of the species in Aspergillus, Penicillium and other related genera, some as distant as Talaromyces and Trichocoma. Their analysis showed strong support for clades containing Aspergillus species with sexual names in the genera Eurotium, Neosartorya, Chaetosartorya and Emericella but weak support (< 50% ML bootstrap) for the clade embracing species in Aspergillus subgenus Circumdati, which includes species with the sexual names Petromyces, Neopetromyces and Fennellia. Their analysis also showed that the several clades of Aspergillus species were very closely related to species in Penicillium sensu stricto, such that the branch making Aspergillus monophyletic with respect to Penicillium sensu stricto had only 51% bootstrap support by maximum likelihood analysis.
Many phenotypic differences, both morphological and physiological, exist among the phylogenetically well supported clades that correspond to the sexual genera Eurotium, Neosartorya, Emericella and Chaetosartorya. Under Art. 57.2 of the ICN, acceptance of these genera is without complication and requires no further action. Aspergillus species without known sexual states but phylogenetically related to one of these genera are almost all well supported, so publication of lists of species to be included in each of these genera, either with valid sexual names or by recombination from Aspergillus, is a similarly straightforward exercise.
To preserve the old and important name, Aspergillus, a case could be made to the ICN to apply that name to the phylogenetic grade, that is, the non-monophyletic group that, in this case, comprises Aspergillus subgenus Circumdati, which does include a few species with sexual states in the less well known genera Petromyces, Neopetromyces and Fennellia.
In this paper, we re-examine the phylogenetic analysis conducted by CitationHoubraken and Samson (2011) by reducing the taxa to just those necessary to address support for clades of Aspergillus species and to address the monophyly of Aspergillus sensu lato compared to Penicillium sensu stricto.
Materials and methods
Phylogenetic analysis of DNA sequences rests on the assumption that the nucleotides in aligned positions are homologous, and this assumption is most likely to be true for closely related taxa where alignment of nucleotides is unambiguous. To maximize the proportion of homologous characters in a molecular phylogenetic analysis, the taxa in question should be reduced to the smallest group of closely related taxa needed to address the question. Therefore, to address the relationships among fungi with the asexual name Aspergillus we were guided by the phylogenetic analysis of CitationHoubraken and Samson (2011), with the one salient difference that we limited our analysis to clades containing fungi that bear the asexual name, Aspergillus, fungi in the very closely related genus Penicillium sensu stricto, and, as the closest outgroup, species of Thermoascus. We removed the more distantly related species classified in Talaromyces, Thermomyces, Sagenomella, Rasamsonia and Trichocoma. Information about the sequences and strains, including their status as type specimens, can be found in CitationHoubraken and Samson (2011).
Phylogenetic analysis of Aspergillus and related taxa was made with the DNA sequences of the four genes used by CitationHoubraken and Samson (2011) (): the RPB1 and RPB2 subunits of RNA polymerase II; Tsr1, a putative ribosome biogenesis protein; and Cct8, the theta subunit of the TCP-1 chaperonin complex. Sequences were aligned with MUSCLE (CitationGoujon et al. 2010) and trimmed to equal lengths. For Bayesian phylogenetic analysis the alignment was put in Nexus format for MrBayes 3.2 (CitationRonquist et al. 2012) using concatenated sequences, a generalized time-reversible evolutionary model allowing for invariant sites and providing a gamma distribution of variable sites (lset nst = 6 rates = invgamma), with Thermoascus aurantiacus (Th_aur_396.78) as the outgroup based on CitationHoubraken and Samson (2011). The alignment is deposited at TreeBASE (http://purl.org/phylo/treebase/phylows/study/TB2:S15611). Bayesian probabilities for each branch were calculated from samples taken every 1000 generations after discarding the first 25% as burn-in; after 106 generations the final average standard deviation of split frequencies was 0.007995. Maximum likelihood analysis of the same concatenated, aligned, trimmed sequences was by MEGA5 (CitationTamura et al. 2011) using converted fasta format and a discrete gamma distribution to model evolutionary rate differences among sites (five categories [+G, parameter = 0.5561]) while allowing for some sites to be evolutionarily invariable ([+I], 32.3809% sites). Maximum likelihood branch support is the percentage of 1000 bootstrap resampled datasets possessing the branch.
Results
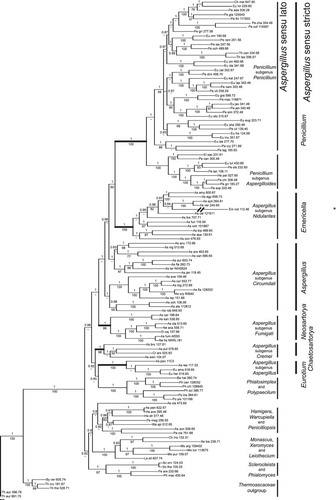
Our phylogenetic analysis () shows strong support in terms of Bayesian posterior probabilities and maximum likelihood bootstrap percentages for four clades of species with Aspergillus asexual morphology that are associated with the sexual names Eurotium, Neosartorya, Emericella and Chaetosartorya. It also shows strong support for the clade of Penicillium species and for the clade of Phialosimplex and Polypaecilum species. One group of species with Aspergillus asexual morphology does not form a well-supported clade, and this grade is coincident with Aspergillus subgenus Circumdati. Based on the topology of the phylogeny, the placement of the Thermoascus outgroup, and the Bayesian posterior probabilities, the genera Penicillium, Phialosimplex and Polypaecilum make it impossible to define a monophyletic clade of all analyzed fungi with Aspergillus asexual states (). That is, to apply Aspergillus to all of the fungi with Aspergillus asexual states and maintain a monophyletic genus Aspergillus, species in the genera Phialosimplex, Polypaecilum and Penicillium would have to be given the name Aspergillus.
In recognition of the phylogenetic equivalence of the well-supported clades of Penicillium, Eurotium, Neosartorya, Emericella and Chaetosartorya, and the obvious morphological and physiological differences among these clades, we advocate adopting the sexual names for the species with Aspergillus asexual states, in accordance with the ICN. However, in the case of the clades lacking strong support that comprise the grade Aspergillus subgenus Circumdati, we advocate applying Aspergillus over Petromyces. Although this action would require appealing to the ICN, it can be justified by its preservation of the old, important name Aspergillus. In the discussion, we address the merits and impact of our recommendation as compared to the extreme alternatives of abandoning the name Aspergillus entirely, or applying it broadly.
Table II. Fungi, culture numbers and genes sequence accession numbers
Table
Table
Table
Discussion
The strongly supported clades for which we use sexual names also were well supported in the analysis of CitationHoubraken and Samson (2011) (i.e. Eurotium = Aspergillus subgenus Aspergillus, Neosartorya = Aspergillus subgenus Fumigati, Emericella = Aspergillus subgenus Nidulantes and Chaetosartorya = Aspergillus section Cremei. Similar strong support was found in both studies for the Penicillium clade and the Phialosimplex and Polypaecilum clades. Weak support was found in both studies for the clades embracing the grade, Aspergillus subgenus Circumdati. Where the studies differ is in the relationship of Penicillium to the other clades. In the study by CitationHoubraken and Samson (2011), Penicillium was sister to Aspergillus sensu lato. In our study, Penicillium is sister to Eurotium, both of which are the most deeply nested clades among species with Aspergillus asexual states. In CitationHoubraken and Samson (2011), the one branch that unites Aspergillus clades with respect to Penicillium had insignificant maximum likelihood support (51%) and strong Bayesian support (1.0), while in our study the four branches that place Penicillium deeply within the clade of fungi having Aspergillus asexual morphology have strong Bayesian support (0.99–1.0) and weak (60% maximum) to insignificant maximum likelihood support. This difference in tree topology is likely due to the improved alignment obtained by omitting more distantly related taxa. Given that the deep branches are short and weakly supported, room exists for argument about the exact relationships among the clades named Penicillium, Eurotium, Neosartorya, Emericella and Chaetosartorya. However, no argument exists about their evolutionary equivalence in terms of age and variation in phenotypes of all sorts. Although the grade we name Aspergillus is not strongly supported phylogenetically, it is as old as the others and is as distinct as the others in sexual morphology and physiological phenotypes.
As noted in the Introduction, the abundant attention devoted to choosing names for species with Aspergillus asexual morphology is a consequence of their social and scientific importance. Here we consider the consequences of maintaining the sexual names. Eurotium species are xerophiles and are commercially important because many species are associated with spoilage, not only of food commodities but also of art and artefacts, paper, leather goods, textiles, timber, even lens of cameras, microscopes and binoculars. Eurotium is widely accepted among user groups, such that reintroduction of Aspergillus would cause widespread confusion, especially because many names would have to changed, as shown in CitationHubka et al. (2013).
Fig. 1. Bayesian and maximum likelihood phylogenetic analyses of Aspergillus and related taxa made with four genes: the RPB1 and RPB2 subunits of RNA polymerase II, Tsr1, a putative ribosome biogenesis protein and Cct8, the putative chaperonin complex component, TCP-1. Numbers above internal branches are Bayesian probabilities; numbers below are maximum likelihood bootstrap percentages. Taxon names correlate with those in . An asterisk marks the end of the exceptionally long terminal branch leading to Emericella nidulans 112.46. Clades are named for Aspergillus subgenera (left column) and for sexual genera (right column) as described in the text. Branch lengths are proportional to genetic distances among taxa. Details of phylogenetic analysis are in Materials and methods.
Neosartorya species are commercially and socially important because they are thermophiles. They affect commerce because their sexual spores resist pasteurization and initiate food spoilage and affect human and animal health because they grow at body temperatures. The food safety community is familiar with the name Neosartorya while the medical community is familiar with the name Aspergillus because A. fumigatus is the most important filamentous ascomycetous opportunistic human pathogen. The genus Neosartorya is more than 40 y old (CitationMalloch and Cain 1972) and discovery of the sexual state of this species, Neosartorya fumigata, has been widely reported (CitationO’Gorman et al. 2009). In our opinion, the medical mycology community is well organized with a strong history of continuing education, so Neosartorya will be accepted, particularly in light of the sound scientific basis for its usage. In fact, a large fraction of aspergillosis cases in neutorpenic patients are caused by species outside Neosartorya and these infections respond differently to treatment than N. fumigata. By emphasizing the different causes of aspergillosis, nomenclature proposed here may improve patient outcomes (CitationTorres et al. 2003). The genus Neocarpenteles nests within Neosartorya and should be synonymized.
Emericella species are of little commercial importance but are commonly isolated from soils. We estimate that about 90 species are accepted in Aspergillus subgenus Nidulantes and 35 (40%) of these already have valid names in Emericella. One species, E. nidulans, is scientifically important as a model in genetic studies on filamentous ascomycetes. It is better known as Aspergillus nidulans, but acceptance of the valid sexual name has no fundamental issues and the academic research community can be counted on to endorse nomenclature based on the best available science.
Chaetosartorya species also are xerophiles but are not commercially important. Application of the name Chaetosartorya will not inconvenience any significant user group. The small sexual state genera Warcupiella and Sclerocleista include only species known by sexual names. Because their phylogenetic positions lie outside both Aspergillus and Penicillium sensu stricto, continued use will be consistent with the provisions of the ICN and not inconvenience any significant user group.
We emphasize that continued use of all of the sexual generic names described above is consistent with the provisions of the ICN and no nomenclatural issues need to be addressed other than preparation of lists of names with no known sexual state that must be recombined from Aspergillus.
The position of species currently classified in Aspergillus subgenus Circumdati is different. The name Petromyces could be applied to this grade, as encouraged by the ICN, and no nomenclatural argument exists against doing so. However, there are two arguments for using the old and important name, Aspergillus, in place of Petromyces for this grade. First, no more than five of the 100-plus species currently accepted in this grade possess a known sexual state in Petromyces. Second, several of these commonly occurring species are commercially important. Aspergillus oryzae has been in use for 1000 y in the production of Asian foods and, together with A. niger, is in widespread use as a source of food and industrial chemicals and enzymes. Equally, A. flavus, A. parasiticus, A ochraceus and A. carbonarius and a number of related species produce aflatoxins or ochratoxin A, mycotoxins of the greatest importance in human and animal health. The user base of the species that produce mycotoxins is both widespread and diffuse throughout both developed and developing countries. Applying the name Aspergillus to all fungi in this grade will be acceptable to almost all user groups with no inconvenience. Given that phylogenetic support for a Petromyces clade is wanting, we realize that additional research may resolve phylogenetic uncertainly in this grade and provide support for future nomenclatural activity. We think, however, that our answer to the problem posed by the new ICN is the one that most closely follows the ICN while also preserving the old and important name, Aspergillus. We chose Aspergillus subgenus Circumdati as the grade to bear the name Aspergillus based partly on its being a grade, rather than a well supported clade but also based on usage of names in scientific publications. CitationHawksworth (2012) has suggested that search engines may provide useful assistance in determining which of competing names are more widely used. To this end we searched major names of interest here in both Web of Science and Google Scholar. It can be seen () that A. niger is in much more frequent use in both databases than A. fumigatus. If we add figures for A. flavus and A. oryzae to those of A. niger—a reasonable process because both are in common use and both also are classified within Aspergillus subgenus Circumdati—we find 2.5 times as many citations for these three species than A. fumigatus in Web of Science and only slightly less than 2.5 times in Google Scholar. Therefore, usage in the scientific literature and, by inference, size of the user group, support our choice to retain Aspergillus for Aspergillus subgenus Circumdati rather than the other clade of socially-important species, Neosartorya. The same approach may be applied to the sexual names. Here Neosarotrya is the topic of far more articles than is Petromyces (i.e. 265–31 in Web of Science and 2820–488 in Google Scholar, that is 78–87% of the summed citations are for Neosartorya). The trend is increasing in favor of Neosartorya, which has seen a rise in citations from < 100 in 2004 to > 700 in 2013, whereas citations for Petromyces actually declined between 2012 and 2013, from 120 to 90. Again, usage in the scientific literature argues for retaining Neosartorya over Petromyces.
Fig. 2. Effect of the choice of teleomorph or anamorph names on appeals for exceptions to the ICN priority rule, non-monophyly of genera and information content of the generic names. Phylogenetic cartoon with strongly-supported branches thickened, based on the phylogeny presented in . A. A narrowly defined genus Aspergillus. The ICN must be asked for approval to apply the name Aspergillus to the non-monophyletic grade embracing Petromyces and Fennellia. The information content of the genera would be high because each name embraces one monphyletic clade or, in the case of Aspergillus, a small grade. B. Abandon the genus Aspergillus by applying the teleopmorph name to every clade. This approach is fully compliant with the ICN, and all names would be applied to monophyletic clades. The information content would be high. However, the name Aspergillus would be lost to mycology. C. Apply the name Aspergillus broadly to all clades having Aspergillus anamorphs. Six exceptions to the ICN would be needed. Aspergillus would be made non-monophyletic by the inclusion of Penicillium, Phialosimplex and Polypaecilum. The information content of the name Aspergillus would be low due to the large phenotypc variation found among the six clades bearing the name Aspergillus. D. Make Aspergillus broad by applying the name to species in the genera Penicillium, Phialosimplex and Polypaecilum. Nine exceptions to the ICN would be needed, but Aspergillus would be monophyletic. The information content of the name Aspergillus would be low, due to the extreme phenotypic variation in the broad genus. The name Penicillium would be lost to mycology.
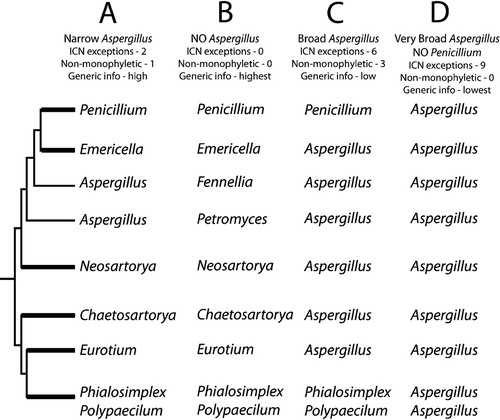
Table III. Results of a search of Web of Science and Google Scholar for the common species A. fumigatus, A. niger, A. flavus and A. oryzaea
Our suggestion that the several clades of fungi with Aspergillus asexual morphology be recognized as independent genera equal in age, in genetic and in phenotypic diversity to each other and the related genus Penicillium stands in contrast to the suggestion that these fungi be recognized as one genus. As described above, lumping these very different fungi into one genus would hide phenotypic variation of significant scientific and social importance, not to mention ignoring the principle of taxonomic monophyly. In both the analysis of CitationHoubraken and Samson (2011) and the analysis presented here the genera Phialosimplex and Polypaecilum make Aspergillus sensu lato non-monophyletic (). This situation could be rectified by renaming these genera as Aspergillus, but to do so would add two clades of fungi lacking the asexual spore producing aspergillum (Phialosimplex and Polypaecilum) to the already large phenotypic variation forced into Aspergillus sensu lato. In that case the name Aspergillus would not even carry the distinctive asexual morphology for which it was named. In the analysis presented here, the genus Penicillium also makes Aspergillus non-monophyletic. As noted above, the branches that enforce this non-monophyly are short and poorly supported by maximum likelihood analysis. However, any of the four branches strongly supported by Bayesian analysis would suffice to make Aspergillus non-monophyletic. As with the genera Phialosimplex and Polypaecilum, Penicillium could be renamed as Aspergillus to make Aspergillus sensu lato monophyletic. We think we are safe in assuming that renaming Penicillium to make an even larger, monophyletic Aspergillus would be rejected by the mycological community.
Proposal.
We advocate that sexual names be applied to the appropriate clades also known by subdivision of Aspergillus as follows: Eurotium = subgenus Aspergillus, Neosartorya = Aspergillus section Fumigati, Emericella = Aspergillus subgenus Nidulantes, and Chaetosartorya = Aspergillus section Cremei. Of the minor genera, Neocarpenteles is phylogenetically con-generic with Neosartorya while Neopetromyces and Fennellia nest within Aspergillus subgenus Circumdati and thus remain in Aspergillus. Sclerocleista, Warcupiella and Hemicarpenteles remain unaffected. We also advocate that Aspergillus be applied to the clade embracing species in Aspergillus subgenus Circumdati, a change that will require an application to the ICN and neotypification of the genus Aspergillus by A. niger in place of A. glaucus. The nomenclatural choices that we advocate are in accordance with the recommendations of the ICN, except for the use of Aspergillus in place of Petromyces. The genera Penicillium, Phialosimplex and Polypaecilum would remain unchanged. Our approach acknowledges the scientific reality of the distinctive morphological and physiological phenotypes associated with the several sexual genera and the evolutionary history of these fungi. Scientific advances based on genetic variation brought about the end of dual nomenclature for fungi (CitationTaylor 2011). The same scientific advances should be used in implementing the new fungal nomenclature. To do otherwise would be unscientific and anti-intellectual.
Acknowledgments
We acknowledge the expert assistance of Dr Emily Whiston with phylogenetic analysis and the support of NIH U54 AI065359 to JWT.
Literature cited
- GeiserDM. 2009. Sexual structures in Aspergillus: morphology, importance and genomics. Med Mycol 47:S21–S26, doi:10.1080/13693780802139859
- GoujonMMcWilliamHLiWZValentinFSquizzatoSPaernJLopezR. 2010. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res 38: W695–W699, doi:10.1093/nar/gkq313
- HawksworthDL. 2012. Managing and coping with names of pleomorphic fungi in a period of transition. IMA Fungus 3:15–24, doi:10.5598/imafungus.2012.03.01.03
- HornBWMooreGGCarboneI. 2009a. Sexual reproduction in Aspergillus flavus. Mycologia 101:423–429, doi:10.3852/09-011
- HornBWRamirez-PradoJHCarboneI. 2009b. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet Biol 46:169–175, doi:10.1016/j.fgb.2008.11.004
- HoubrakenJSamsonRA. 2011. Phylogeny of Penicillium and the segregation of Trichocomaceae into three families. Stud Mycol, 1–51, 10.3114/sim.2011.70.01
- HubkaVKolaříkMKubátováAPetersonSW. 2013. Taxonomic revision of Eurotium and transfer of species to Aspergillus. Mycologia 105:912–937, doi:10.3852/12-151
- ICPA (International Commission on Penicillium & Aspergillus). 2013. www.AspergillusPenicillium.org.
- MallochDCainRF. 1972. The Trichocomataceae: Ascomycetes with Aspergillus, Paecilomyces and Penicillium imperfect states. Can J Bot 50:2613–2628, doi:10.1139/b72-335
- McNeillJBarrieFRBuckWRDemoulinVGreuterWHawksworthDLHerendeenPSKnappSMarholdKPradoJet al. 2012. International code of nomenclature for algae, fungi and plants (Melbourne Code) adopted by the 18th International Botanical Congress Melbourne, Australia, Jul 2011. Regnum Vegetabile 154. Koenigstein: Koeltz Scientific Books. ISBN 978-3-87429-425-6
- O’GormanCMFullerHTDyerPS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474, doi:10.1038/nature07528
- PittJIHockingAD. 1985. Interfaces among genera related to Aspergillus and Penicillium. Mycologia 77:810–824, doi:10.2307/3793288
- RaperKBFennellDI. 1965. The genus Aspergillus. Baltimore, Maryland: Williams & Wilkins. 686 p.
- RonquistFTeslenkoMvan der MarkPAyresDLDarlingAHöhnaSLargetBLiuLSuchardMAHuelsenbeckJP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542, doi:10.1093/sysbio/sys029
- TamuraKPetersonDPetersonNStecherGNeiMKumarS. 2011. MEGA 5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28:2731–2739, doi:10.1093/molbev/msr121
- TaylorJW. 2011. One fungus = one name: DNA and fungal nomenclature 20 years after PCR. IMA Fungus 2:113–120, doi:10.5598/imafungus.2011.02.02.01
- ThomCChurchMB. 1926. The Aspergilli. Baltimore, Maryland: Williams & Wilkins. 272 p.
- ThomCRaperKB. 1945. A manual of the Aspergilli. Baltimore, Maryland: Williams & Wilkins. 153 p.
- TorresHARiveroGALewisREHachemRRaadIIKontoyiannisDP. 2003. Aspergillosis caused by non-fumigatus Aspergillus species: risk factors and in vitro susceptibility compared with Aspergillus fumigatus. Diagn Microbiol Infect Dis 46:25–28, doi:10.1016/S0732-8893(03)00013-0

