Abstract
In utero smoke exposure has been shown to have detrimental effects on lung function and to be associated with persistent wheezing and asthma in children. One potential mechanism of IUS effects could be alterations in DNA methylation, which may have life-long implications. The goal of this study was to examine the association between DNA methylation and nicotine exposure in fetal lung and placental tissue in early development; nicotine exposure in this analysis represents a likely surrogate for in-utero smoke. We performed an epigenome-wide analysis of DNA methylation in fetal lung tissue (n = 85, 41 smoke exposed (48%), 44 controls) and the corresponding placental tissue samples (n = 80, 39 smoke exposed (49%), 41 controls) using the Illumina HumanMethylation450 BeadChip array. Differential methylation analyses were conducted to evaluate the variation associated with nicotine exposure. The most significant CpG sites in the fetal lung analysis mapped to the PKP3 (P = 2.94 × 10−03), ANKRD33B (P = 3.12 × 10−03), CNTD2 (P = 4.9 × 10−03) and DPP10 (P = 5.43 × 10−03) genes. In the placental methylome, the most significant CpG sites mapped to the GTF2H2C and GTF2H2D genes (P = 2.87 × 10−06 − 3.48 × 10−05). One hundred and one unique CpG sites with P-values < 0.05 were concordant between lung and placental tissue analyses. Gene Set Enrichment Analysis demonstrated enrichment of specific disorders, such as asthma and immune disorders. Our findings demonstrate an association between in utero nicotine exposure and variable DNA methylation in fetal lung and placental tissues, suggesting a role for DNA methylation variation in the fetal origins of chronic diseases.
Abbreviations
| CHR | = | chromosome |
| GTF2H2C | = | General transcription factor IIH, polypeptide 2C |
| GTF2H2D | = | General transcription factor IIH, polypeptide 2D |
| IUS | = | in utero smoke |
| JAK2 | = | Janus Kinase 2 |
| KEGG | = | Kyoto Encyclopedia of Genes and Genomes |
Introduction
Public health efforts have not succeeded in eliminating cigarette smoking in the developed world, as reflected by the fact that 19% of the adult population in the United States continues to smoke cigarettes, with the prevalence being 16.5% in women 18 years and older.Citation1 The 2008 Pregnancy Risk Assessment and Monitoring System (PRAMS) data from 29 states suggests that approximately 13% of women (i.e., 1 in every 8) reported smoking during the last 3 months of pregnancy.Citation2 In utero smoke (IUS) exposure has been linked to multiple detrimental effects on the developing fetus,Citation3-6 and a number of epidemiological studies have shown its aberrant influence on lung function.Citation7-9 In addition to its impact on lung function, there is evidence of increased wheezingCitation10-12 and asthma associated with prenatal smoke exposure.Citation11,Citation13-16 In asthmatic subjects, a history of IUS exposure has been associated with reduced efficacy of inhaled corticosteroids on decreasing airway responsiveness.Citation17 In addition to influencing fetal lung development, perinatal nicotine exposure has been shown to increase total airway resistance, and decrease dynamic compliance of the respiratory system in second generation offspring in animal models.Citation18
Nicotine exposure has also been related to abnormal placental structure and function. It has been shown to have an inhibitory effect on trophoblast migration, invasion and differentiation,Citation19,20 alter the release of vasoactive factors by trophoblastsCitation21 and also influence the transport of amino acids.Citation22 Despite the large amount of evidence supporting the effect of IUS on the developing lung and the placental tissue, the molecular mechanisms have not been completely understood. One potential mechanism is DNA methylation, and a number of studies have examined DNA methylation changes in cord bloodCitation23-26 and placental tissue as a result of smoke exposure.Citation27-29 Decreased DNA methyltransferase 1 (DNMT1) and increased DNA methyltransferase 3b (DNMT3b) expression have been shown to occur in human small airway smooth muscle cells exposed to cigarette smoke condensate.Citation30 In placental tissue, Suter et al. demonstrated the correlation between CYP1A1 expression and CpG methylation variation,Citation31 thus supporting the role of DNA methylation. In another study, the authors assessed DNA methylation and gene expression in tobacco smoke exposed and control tissue at the genome wide level and found that there was a correlation between the 2 at a significantly higher number of genes in smoke-exposed tissue.Citation28 Global and gene specific changes have also been associated with in utero exposure in the buccal mucosa of children.Citation32 Cord blood and placental samples obtained from pregnancies with gestational diabetes demonstrate mesoderm specific transcript (MEST) gene hypomethylation similar to that found in adults with morbid obesity,Citation33 thus suggesting that long-term disease programming occurring through DNA methylation may begin in utero. Studying these epigenetic patterns can allow us to understand and, potentially, interrupt disease development at an earlier stage.
Armstrong et al. recently studied DNA methylation at a global and gene specific level (7 genes) and found that placenta, cord blood and saliva varied in their DNA methylation patterns.Citation34 Similarly, other researchers have found that DNA methylation is tissue specific and that there are a small number of sites that share methylation patterns across tissues.Citation35 In this study, we investigated the variability of DNA methylation concurrently in lung tissue (which suffers the influence of this exposure) and the placenta (which may act as biomarker and mediator of exposure), to identify genes and disease pathways impacted by nicotine exposure.
Results
Demographic features of lung (n = 85, 41 smoke exposed, 44 controls) and placental samples (n = 80, 39 smoke exposed, 41 controls) that passed quality-control have been summarized (). We used placental cotinine as a marker of in utero nicotine exposure; the most likely source of nicotine is smoking and placental cotinine was previously shown to be a useful biomarker of IUS exposure. In a previous study, our group has demonstrated the relationship between placental cotinine levels and gene expression changes in fetal liver and lung.Citation36 Exposure to nicotine was dichotomized at a cotinine level of 7.5 ng/g placental tissue as this level has been shown to have a high sensitivity and specificity in identifying smoke exposure in pregnancy.Citation36
Table 1 Demographic Features of Lung and Placental Samples
In the lung tissue, we analyzed the association of DNA methylation with nicotine exposure and found 264 CpG sites (68 CpG sites type I probes and 196 CpG sites type II probes) with an unadjusted P-value < 0.05 (see Table S4 and S5 in the online data supplement). CpG sites that had an association with a P-value < 0.01 after removal of sites likely to be influenced by SNPs, as described in the methods, have been listed with their mean β values in nicotine exposed and control tissue (). After adjusting for post-conceptional age, 226 sites remained significant at a nominal P-value < 0.05. None of the CpG sites in the lung tissue analysis remained significant after adjustment for multiple comparisons. The top CpG sites demonstrate both relative higher or lower methylation in nicotine exposed versus unexposed tissue, depending upon the CpG site evaluated (–); thus, the directionality of association with nicotine varies from site to site.
Table 2 Differential DNA methylation for single CpG sites and nicotine exposure for lung samples (P< 0.01)
Figure 1. CpG sites Selected from the Lung Analyses. (Red in the scatter plots and 1 in the boxplots represents nicotine exposed tissue. Blue in the scatter plots and 0 in the boxplots represents unexposed tissue). The Y axis in both plots represents the β-values. The X axis in the scatter plots represents individual samples.
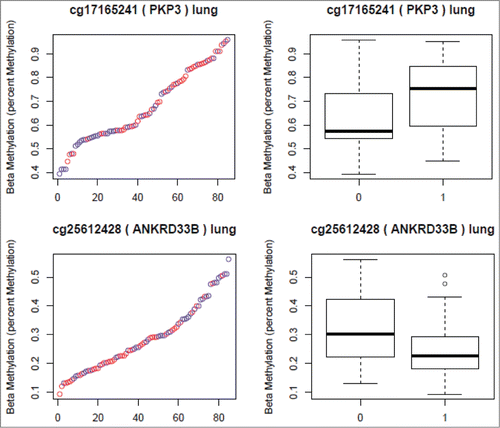
Figure 2. CpG sites Selected from the Lung Analyses. (Red in the scatter plots and 1 in the boxplots represents nicotine exposed tissue. Blue in the scatter plots and 0 in the boxplots represents unexposed tissue). The Y axis in both plots represents the β-values. The X axis in the scatter plots represents individual samples.
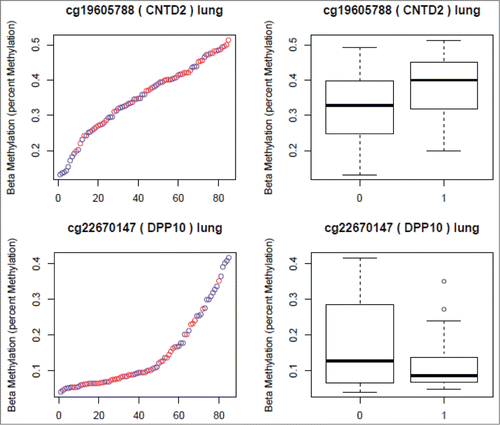
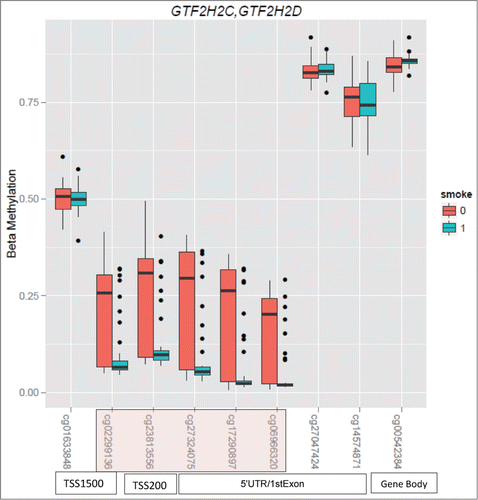
In the placental tissue, 657 CpG sites had unadjusted nominal P-values < 0.05 (377 CpG sites type I and 280 sites type II probes). After adjustment for multiple comparisons and post-conceptional age, 2 sites had a Benjamini-Hochberg (BH) P-value < 0.05 and 6 sites had a P-value < 0.1 (see Table S6 and S7 in the online data supplement). A total of 469 CpG sites remained significant at a nominal P-value < 0.05 after adjusting for post-conceptional age. CpG sites with nominal P < 0.01 after removal of sites with SNPs have been listed in . CpG sites that were most significant in the placental analyses mapped to the GTF2H2C and GTF2H2D genes on chromosome 5. Percent methylation in exposed and unexposed placental tissue for all CpG sites in the GTF2H2C and GTF2H2D gene suggest a region of relative hypomethylation associated with nicotine exposure ().
Table 3 Differential methylation for single CpG sites and nicotine exposure for placental samples (P< 0.01)
Figure 3. Boxplots of CpG sites in the GTF2H2C/GTF2H2D gene in placental tissue. Sites highlighted in pink represents sites that have a BH adjusted P-value < 0.1.
Nicotine likely has systemic molecular effects as well as tissue specific effects. To evaluate methylation differences that may not be tissue specific, we also assessed for sites that were concordant for the association with methylation in lung and placental analyses. One hundred and one CpG sites were found to overlap between the lung and placental analyses (see Table S8 in the online data supplement, ), and all these sites had the same directionality in both the tissues. shows a subset of these overlapping sites, which were obtained after assessment for SNPs, associated with nicotine exposure in lung and placental tissue.
Table 4 Overlapping CpG sites in the lung and placental analyses
Figure 4. (A). Boxplots of CpG sites in the PRR25 gene in lung tissue. Sites highlighted in blue represents sites that have a nominal P-value < 0.1. (B). Boxplots of CpG sites in the PRR25 gene in placental tissue. Sites highlighted in blue represent those that have a nominal P-value < 0.1.
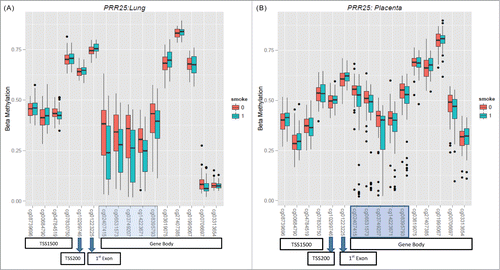
Pyrosequencing of selected sites from the lung and placental tissue was carried out on a smaller subset of samples and CpG sites, since we had limited DNA amounts remaining from the fetal tissues. For the lung samples, significant correlations between the illumina array percent methylation and pyrosequencing results were observed for CNTD2 cg19605788, DPP10 cg22670147, and KLHL35 cg05353869 (). For the placental tissue, we observed significant correlations for GTF2H2C (cg02299136 and cg23813556) and KLHL35 cg05353869 (). The pyrosequencing results for PPR25 cg02407415 had the lowest correlations for lung and placenta (Pearson: 0.34 and 0.57, respectively). The CpG in PPR25 site spans a region with a number of “A” homopolymers, which may partially account for these lower correlations.
Table 5 Pyrosequencing results: Lung
Table 6 Pyrosequencing results: Placenta
KEGG pathway analysis was performed using gene set enrichment analysis (GSEA).Citation37 Two different pre-ranked lists of genes that were obtained using the absolute log fold change and the log fold change were used to assess enrichment. When using the first approach we found that the pathways obtained were similar in the lung and placental tissue, and included immune-related and asthma pathways (). When we used the second approach, none of the pathways were found to be significant at a false discovery rate (FDR) of 0.05. Pathways with a nominal P-value < 0.05 have been listed in the online data supplement (Table S9 and S10).
Table 7 GSEA Analysis
Variable DNA methylation at a number of different genes has previously been associated with IUS exposure. As such, we specifically assessed CpG sites in the FRMD4A, C11orf52, XPNPEP1, PPEF2, SMPD3, CRYGN,Citation38 TSLP,Citation26 IGF2,Citation23 PURA, GTF2H2, GCA, GPR135, HKR1,Citation28 AXL,Citation32, Citation39 BDNF,Citation40 PTPRO,Citation32 HSD11B,Citation29 NR3C1,Citation41, AHRR, CYP1A1, GFI1,Citation24 GSTM5, MAP2K3, and APOBCitation42 genes in lung and placental tissue. The only sites that were noted to be significant after adjusting for multiple comparisons mapped to the GTF2H2C, GTF2H2D region, which was added to the search, as no CpG sites mapped to the GTF2H2 gene (Table S11A, S11B, S12A and S12B).
Discussion
We identified significant pathway-related differential methylation associated with nicotine exposure, including evidence suggesting that asthma and other immune pathways may be perturbed by this exposure. To investigate the “molecular archive”Citation43 associated with in utero exposure to nicotine, we studied genome-wide methylation in matched lung and placental tissue. To our knowledge, this is the first time methylation in developing human fetal lung tissue and placental tissues have been assessed together. The overlap of pathways between the placenta and the lung is one more piece of evidence that the effects of in utero exposure are likely to be enduring and potentially systemic and have long-term effects on the developing fetus and subsequent risk for chronic diseases, including chronic lung diseases such as asthma.
One of the genes most significantly associated with DNA methylation changes in the lung was DPP10, which has been associated with asthma in a number of studies.Citation44-46 Thus, it is plausible that methylation variation as a result of smoke exposure predisposes an infant to develop lung disease later in life through epigenetic disruption of key developmental and homeostatic pathways; further research in human cohorts and animal models is warranted. Epidemiological data support the influence of in-utero exposures on long-term outcomes and alteration in DNA methylation is one potential mechanism through which environmental exposures can impact human health and disease. Experimental evidence suggests that methylation changes occurring during development are tissue specific and play a role in tissue differentiation.Citation47 A number of studies on placental tissue have demonstrated variable methylation in the setting of preeclampsia,Citation48,49 pregnancies conceived by assisted reproductive technologies,Citation50 gestational diabetes,Citation33 and maternal exposures such as smoking.Citation27-29 Variation in DNA methylation associated with cigarette smoking has also been demonstrated in studies of DNA from peripheral blood leukocytes,Citation51 peripheral blood,Citation52 lymphoblasts, and alveolar macrophagesCitation53 in adults. Studies in neonates have shown IUS exposure associated with variation in methylation of DNA in umbilical cord bloodCitation23-26 and placental tissue.Citation27-29 Other studies have shown an association of prenatal smoke exposure with DNA methylation in buccal source DNA in children,Citation32 blood in adolescents,Citation40 and peripheral blood granulocytes in adult women.Citation54 The potential health-related relevance of epigenetic variability in placental tissue on clinical outcomes has been suggested by a study which showed that methylation status of 11-β hydroxysteroid dehydrogenase was associated with infant quality of movement scores.Citation55 Similarly, birth weight has been associated with placental glucocorticoid receptor gene methylation.Citation56 Thus, investigating epigenetic variability in fetal tissues as a “molecular archive” of the prenatal environmentalCitation43 provides an opportunity to identify and potentially intervene at an early stage in disease trajectories.
The top CpG sites in placental tissue mapped to the general transcription factor IIH, polypeptide 2C (GTF2H2C ) and general transcription factor IIH, polypeptide 2D (GTF2H2D) genes. In an epigenome-wide analysis carried out on placental DNA from term pregnancies using the Illumina HumanMethylation27 BeadChip array, Suter et al.Citation28 reported a difference of more than 10% in placental methylation of GTF2H2 between smokers and non-smokers. Based on similarity, general transcription factor IIH subunit 2-like protein is believed to be a component of the core-TFIIH basal transcription factor, which plays a role in DNA nucleotide excision repair and transcription by RNA polymerase II.Citation57 Transcription factor IIH has been shown to have a significantly lower expression in alveolar macrophages in subjects with idiopathic pulmonary fibrosis.Citation58 Thus, altered methylation in GTF2H2C and GTF2H2D may occur in an attempt to repair the DNA damage resulting from exposure to cigarette smoke.
Among the overlapping sites in the lung and placental analyses was the Janus Kinase 2 (JAK2) gene, which encodes a protein tyrosine kinase that plays a role in a number of pathways, including cell growth, development, differentiation, and histone modifications.Citation59 JAK2 is also involved in erythropoietin signal transduction and mutations in this genes have been associated with polycythemia veraCitation60-63 and other myeloproliferative disorders.Citation63,64 Though it was not associated with a higher hematocrit, the JAK2 mutation was found more frequently in smokers than in non-smokers.Citation65 In a study of non-smokers with bladder carcinomas, increased DNA methylation of the JAK2 gene was associated with total and occupational smoke exposure,Citation66 but was not associated with childhood exposure. In our analysis, we found this locus to be hypermethylated in nicotine-exposed lung and placental tissue. Thus, variable methylation may be an intermediate state resulting from smoke exposure.
We acknowledge several potential limitations to our study, including the paucity of clinical/demographic data on our samples. We are considering the source of elevated placental cotinine to be IUS. However, as cotinine is a metabolite of nicotine metabolism, the mothers could have been exposed to nicotine in other forms besides active smoking, including passive smoking, e-cigarette use, and nicotine replacement therapy. As these are anonymized samples, we were unable to assess effects of racial distribution on variable methylation. We did apply the approach of Barfield et al.Citation67 to explore population substructure; however, given the relatively small size of our study, we present the adjusted P-values for the top sites in the supplemental data. It is not known if there were any underlying pathologies, nor could we adjust for other maternal prenatal behaviors, such as alcohol ingestion, use of other drugs or medications, or quality of routine prenatal care. We suggest that stringent quality control steps and removal of outlier samples would mitigate the influence of any such effect. Surrogate variable analysis also showed the absence of batch effects in our unadjusted model. The lack of CpG sites that were significantly altered in lung tissue analysis at an adjusted P-value of 0.05 is likely a function of our sample size. However, this observation is offset by the statistically robust and biologically plausible results of our pathway analysis. Lastly, we have been able to perform technical validation, using pyrosequencing, only on a small subset of CpG sites and samples due to the limited DNA availability from these precious fetal lung tissue samples. Future studies that focus on larger sample sizes could help further support the impact of IUS in lung and placental tissue, as well as address issues related to population substructure.
We conclude that variable methylation is a potential marker of injury in the developing lung exposed to nicotine in utero. In addition, a subset of methylation marks perturbed by nicotine exposure is recapitulated in the placenta. The enrichment in asthma and immune pathway genes observed in our analyses raises the possibility that variable methylation may be used as a biomarker of future risk of disease during childhood or adult life. Longitudinal studies and replication in other cohorts would add to our knowledge of lung diseases and other chronic illnesses resulting from nicotine/IUS exposure. Although the public health message remains that all individuals, especially pregnant women, should not smoke cigarettes (the most likely source of elevated placental cotinine), our research lays the groundwork for the identification of an epigenetic signature that could inform primary prevention and early diagnosis of chronic human diseases associated with IUS and in utero nicotine exposure.
Patients and Methods
Anonymized fetal lung and placental tissue samples were acquired through the NICHD-supported tissue retrieval program at the Laboratory of Developmental Biology, University of Washington, Seattle, WA. These tissues have been designated as non-human subjects by the Children's Mercy Hospital Pediatric Institutional Review Board. Phenotypic information on these samples is limited to post-conception age. Nicotine exposure was determined by measuring placental cotinine concentration in nanograms per gram of placental tissue. Exposure was treated as a dichotomous variable, with levels of cotinine < 7.5 ng/g being considered as unexposed (control group) and levels of cotinine > 7.5 ng/g as exposed. This level has been previously shown to have a sensitivity of 78.7% and a specificity of 100% for identifying mothers that smoked during pregnancy.Citation36 However, given the limited clinical history of the mothers, the actual source of positive nicotine levels is undetermined. Post-conceptional age was assessed based on the foot length of the fetus.
Placental and lung tissues were flash frozen at the time of procurement and stored at −80ºC. Lung tissue and placental DNA from 95 subjects were quantified in our laboratory using standard pico-green methods. Genome-wide methylation assay was performed with 750 ng of bisulfite-treated DNA per sample using the Illumina HumanMethylation450 BeadChip array, according to manufacturer's recommended protocol. Placental and lung tissue samples from the same subject were spotted on the same array. Results were extracted using GenomeStudio (Software v2011.1) and then read into Bioconductor (version 2.12) for further analyses. Percent methylation was reported as the Illumina β-value, which is the ratio between methylated signal intensity and total probe signal intensity.Citation68 Quality control steps performed for both lung and placental tissue DNA methylation marks have been described in the Supplemental data (see description and Table S3 in the online data supplement). The Illumina Infinium HumanMethylation450 Bead Chip array has 135501 type I probes and 350076 type II probes that differ in assay chemistry and, thus, in the distribution of methylation values. It has been shown that the β-values (percent methylation) obtained from the Infinium type II probes have a smaller range when compared with those from Infinium type I probes.Citation69 Currently, there is no consensus in the literature on the best approach for normalization of the data in the presence of this diversity. For this analysis, quantile normalization and differential methylation analysis were performed separately on the Infinium type I and type II probes, using the methylumi package.Citation70 Type I probes were color adjusted prior to normalization. A large number of CpG sites in the genome showed very little variance. Therefore, only sites with a variance in percent methylation higher than 1% (1986 and 4737 CpG sites for lung tissue and 6938 and 5444 for placental tissue, for type I and II probes, respectively) were carried forward for analysis. Surrogate variable analysis was also performed to assess for batch effects in both fetal lung and placental data sets.Citation71
Statistical analyses
Differential methylation analyses were carried out using linear models and empirical Bayes methods. These analyses were run using the Bioconductor package limmaCitation72 and results were considered together. For the initial analysis of the association of nicotine exposure—most likely a result of maternal cigarette smoke exposure—and methylation, we performed an unadjusted differential methylation analysis. The surrogate variable analysis for the unadjusted model was found to have zero surrogate variables in lung and placental tissue. Thereafter, P-values obtained for the type I and type II analyses were combined and adjusted for multiple comparisons using BH correction for multiple comparisons. We also carried out the limma analysis adjusting for post-conceptional age. CpG sites were mapped to genes using annotation provided by Illumina and, for instances in which a site mapped to more than one gene, the first gene name was randomly selected for pathway analysis. KEGG analysis of the pre-ranked list was performed using the gene set enrichment analysis (GSEA v2.0.14). Two different approaches that involved ranking genes based on their log fold change and the absolute log fold change were used for GSEA. When ranking genes by absolute log fold change, the highest value of this parameter was chosen to determine the rank of genes with multiple CpG sites. However, when using the log fold change, we used the lowest P-value to determine the rank of genes for GSEA. For the lung analysis, 2981 genes were added to the GSEA analysis; for the placental tissue analysis, 4229 genes were added. The manually curated KEGG gene set (c2.cp.kegg.v4.0.symbols.gmt) from the Molecular Signatures Database (v4.0 MSigDB) was used as a reference set for the pathway analysisCitation37,73.
For CpG sites that were found to be significant in the limma analysis, we removed results in which a SNP was at a single base extension site or at the proximal CpG on the probe, using data obtained from the Bioconductor package minfi.Citation74 We also assessed scatter plots of methylation and removed sites with apparent SNP effects based on clustering of methylation values. Pyrosequencing of a limited number of sites that were selected based on significance in our analysis, absence of SNPs, having more than one significant CpG sites in the same genomic region, and biological plausibility was performed using a subset of lung and placental tissues (for which we had adequate DNA), as detailed in the Supplemental Methods. All probes used for pyrosequencing have been listed in the Supplemental Methods. After removal of failed samples and outliers, Pearson and Spearman correlations were assessed between the pyrosequencing results and the β-values used in our analysis. Literature review was carried out to obtain a list of genes for which DNA methylation had been previously observed as a result of IUS exposure. These sites were assessed in lung and placental tissue to determine if they were differentially methylated.
Disclosure of Potential Conflicts of Interest:
No conflicts of interest were disclosed.
Contributor's Statement
Divya Chhabra: analysis and interpretation of data, Manuscript preparation and approval of the final version of the manuscript.
Sunita Sharma: acquisition of data, analysis and interpretation of data, review of manuscript; and approval of final version of the manuscript.
Alvin T Kho: analysis and interpretation of data; approval of final version of the manuscript.
Roger Gaedigk, Carrie A. Vyhlidal, J. Steven Leeder: conception and design, acquisition of data, review of manuscript and approval of the final version of manuscript.
Kelan G Tantisira: conception and design, acquisition of data, analysis and interpretation of data, review of manuscript and approval of the final version of the manuscript.
Scott T Weiss: conception and design, acquisition of data, analysis and interpretation of data, review and approval of final version of manuscript;
Jarrett Morrow, Vincent J Carey: analysis and interpretation of data, review of manuscript and approval of the final version of the manuscript.
Dawn L DeMeo: conception and design, acquisition of data, analysis and interpretation of data, preparation, review and approval of the final version of the manuscript.
Supplement_Part_3.docx
Download MS Word (49 KB)Supplement_Part_2.doc
Download MS Word (9.4 MB)Supplement_Part_1.docx
Download MS Word (577.7 KB)Funding
The project entitled “Laboratory of Developmental Biology” was supported by National Institutes of Health Award Number 5R24HD0008836 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
The following are the sources for funding: NIH R21 HL107927, NIH R01 HL089438, NIH R01 HL097144, NIH P01 HL083069, NIH T32 HL007427, NIH K08 HL096833, NIH K25 HL091124, NLM T15LM011271
This manuscript is subject to the NIH public access policy
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website.
References
- Centers for Disease Control and Prevention (CDC). Current cigarette smoking among adults - United States, 2011. MMWR Morb Mortal Wkly Rep 2012; 61:889-94; PMID:23134971
- Centers for Disease Control and Prevention(2012). PRAMS and Smoking. Retrieved from http://www.cdc.gov/prams/TobaccoandPrams.htm Dec 1.
- Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update 2011; 17:589-604; PMID:21747128; http://dx.doi.org/10.1093/humupd/dmr022
- Wisborg K, Kesmodel U, Henriksen TB, Olsen SF, Secher NJ. Exposure to tobacco smoke in utero and the risk of stillbirth and death in the first year of life. Am J Epidemiol 2001; 154:322-7; PMID:11495855; http://dx.doi.org/10.1093/aje/154.4.322
- Naeye RL. Effects of maternal cigarette smoking on the fetus and placenta. Br J Obstet Gynaecol 1978; 85:732-7; PMID:708656; http://dx.doi.org/10.1111/j.1471-0528.1978.tb15593.x
- Bernstein IM, Plociennik K, Stahle S, Badger GJ, Secker-Walker R. Impact of maternal cigarette smoking on fetal growth and body composition. Am J Obstet Gynecol 2000; 183:883-6; PMID:11035331; http://dx.doi.org/10.1067/mob.2000.109103
- Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis 1992; 145:1129-35; PMID:1586058; http://dx.doi.org/10.1164/ajrccm/145.5.1129
- Stick SM, Burton PR, Gurrin L, Sly PD, LeSouëf PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet 1996; 348:1060-4; PMID:8874457; http://dx.doi.org/10.1016/S0140-6736(96)04446-7
- Lodrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J 1997; 10:1774-9; PMID:9272918; http://dx.doi.org/10.1183/09031936.97.10081774
- Stein RT, Holberg CJ, Sherrill D, Wright AL, Morgan WJ, Taussig L, et al. Influence of parental smoking on respiratory symptoms during the first decade of life: the Tucson Children's Respiratory Study. Am J Epidemiol 1999; 149:1030-7; PMID:10355379; http://dx.doi.org/10.1093/oxfordjournals.aje.a009748
- Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 2012; 129:735-44; PMID:22430451; http://dx.doi.org/10.1542/peds.2011-2196
- Wang C, Salam MT, Islam T, Wenten M, Gauderman WJ, Gilliland FD. Effects of in utero and childhood tobacco smoke exposure and beta2-adrenergic receptor genotype on childhood asthma and wheezing. Pediatrics 2008; 122:e107-14; PMID:18558635; http://dx.doi.org/10.1542/peds.2007-3370
- Neuman A, Hohmann C, Orsini N, Pershagen G, Eller E, Kjaer HF, et al. Maternal smoking in pregnancy and asthma in preschool children: a pooled analysis of eight birth cohorts. Am J Respir Crit Care Med 2012; 186:1037-43; PMID:22952297; http://dx.doi.org/10.1164/rccm.201203-0501OC
- Lannerö E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE). Respir Res 2006; 7:3; PMID:16396689; http://dx.doi.org/10.1186/1465-9921-7-3
- Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med 2001; 163:429-36; PMID:11179118; http://dx.doi.org/10.1164/ajrccm.163.2.2006009
- Midodzi WK, Rowe BH, Majaesic CM, Saunders LD, Senthilselvan A. Early life factors associated with incidence of physician-diagnosed asthma in preschool children: results from the Canadian Early Childhood Development cohort study. J Asthma 2010; 47:7-13; PMID:20100014; http://dx.doi.org/10.3109/02770900903380996
- Cohen RT, Raby BA, Van Steen K, Fuhlbrigge AL, Celedón JC, Rosner BA, et al. In utero smoke exposure and impaired response to inhaled corticosteroids in children with asthma. J Allergy Clin Immunol 2010; 126:491-7; PMID:20673983; http://dx.doi.org/10.1016/j.jaci.2010.06.016
- Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, et al. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med 2012; 10:129; PMID:23106849; http://dx.doi.org/10.1186/1741-7015-10-129
- Holloway AC, Salomon A, Soares MJ, Garnier V, Raha S, Sergent F, et al. Characterization of the adverse effects of nicotine on placental development: in vivo and in vitro studies. Am J Physiol Endocrinol Metab 2014; 306:E443-56; PMID:24368670; http://dx.doi.org/10.1152/ajpendo.00478.2013
- Genbacev O, Bass KE, Joslin RJ, Fisher SJ. Maternal smoking inhibits early human cytotrophoblast differentiation. Reprod Toxicol 1995; 9:245-55; PMID:7579909; http://dx.doi.org/10.1016/0890-6238(95)00006-V
- Romani F, Lanzone A, Tropea A, Tiberi F, Catino S, Apa R. Nicotine and cotinine affect the release of vasoactive factors by trophoblast cells and human umbilical vein endothelial cells. Placenta 2011; 32:153-60; PMID:21145589; http://dx.doi.org/10.1016/j.placenta.2010.11.010
- Pastrakuljic A, Derewlany LO, Knie B, Koren G. The effects of cocaine and nicotine on amino acid transport across the human placental cotyledon perfused in vitro. J Pharmacol Exp Ther 2000; 294:141-6; PMID:10871305
- Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, et al. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene 2012; 494:36-43; PMID:22202639; http://dx.doi.org/10.1016/j.gene.2011.11.062
- Joubert BR, Haberg SE, Nilsen RM, Wang X, Vollset SE, Murphy SK, et al. 450K epigenome-wide scan identifies differential DNA methylation in newborns related to maternal smoking during pregnancy. Environ Health Perspect 2012; 120:1425-31; PMID:22851337; http://dx.doi.org/10.1289/ehp.1205412
- Guerrero-Preston R, Goldman LR, Brebi-Mieville P, Ili-Gangas C, Lebron C, Witter FR, et al. Global DNA hypomethylation is associated with in utero exposure to cotinine and perfluorinated alkyl compounds. Epigenetics 2010; 5:539-46; PMID:20523118; http://dx.doi.org/10.4161/epi.5.6.12378
- Wang IJ, Chen SL, Lu TP, Chuang EY, Chen PC. Prenatal smoke exposure, DNA methylation, and childhood atopic dermatitis. Clin Exp Allergy 2013; 43:535-43; PMID:23600544; http://dx.doi.org/10.1111/cea.12108
- Wilhelm-Benartzi CS, Houseman EA, Maccani MA, Poage GM, Koestler DC, Langevin SM, et al. In utero exposures, infant growth, and DNA methylation of repetitive elements and developmentally related genes in human placenta. Environ Health Perspect 2012; 120:296-302; PMID:22005006; http://dx.doi.org/10.1289/ehp.1103927
- Suter M, Ma J, Harris A, Patterson L, Brown KA, Shope C, et al. Maternal tobacco use modestly alters correlated epigenome-wide placental DNA methylation and gene expression. Epigenetics 2011; 6:1284-94; PMID:21937876; http://dx.doi.org/10.4161/epi.6.11.17819
- Appleton AA, Armstrong DA, Lesseur C, Lee J, Padbury JF, Lester BM, et al. Patterning in placental 11-B hydroxysteroid dehydrogenase methylation according to prenatal socioeconomic adversity. PLoS One 2013; 8:e74691; PMID:24040322; http://dx.doi.org/10.1371/journal.pone.0074691
- Liu F, Killian JK, Yang M, Walker RL, Hong JA, Zhang M, et al. Epigenomic alterations and gene expression profiles in respiratory epithelia exposed to cigarette smoke condensate. Oncogene 2010; 29:3650-64; PMID:20440268; http://dx.doi.org/10.1038/onc.2010.129
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, et al. In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 2010; 59:1481-90; PMID:20462615; http://dx.doi.org/10.1016/j.metabol.2010.01.013
- Breton CV, Byun HM, Wenten M, Pan F, Yang A, Gilliland FD. Prenatal tobacco smoke exposure affects global and gene-specific DNA methylation. Am J Respir Crit Care Med 2009; 180:462-7; PMID:19498054; http://dx.doi.org/10.1164/rccm.200901-0135OC
- El Hajj N, Pliushch G, Schneider E, Dittrich M, Müller T, Korenkov M, et al. Metabolic Programming of MEST DNA Methylation by Intrauterine Exposure to Gestational Diabetes Mellitus. Diabetes 2013; 62:1320-8; PMID:23209187; http://dx.doi.org/10.2337/db12-0289
- Armstrong DA, Lesseur C, Conradt E, Lester BM, Marsit CJ. Global and gene-specific DNA methylation across multiple tissues in early infancy: implications for children's health research. FASEB J 2014; 28:2088-97; PMID:24478308; http://dx.doi.org/10.1096/fj.13-238402
- Lokk K, Modhukur V, Rajashekar B, Märtens K, Mägi R, Kolde R, et al. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol 2014; 15:R54.
- Vyhlidal CA, Riffel AK, Haley KJ, Sharma S, Dai H, Tantisira KG, et al. Cotinine in human placenta predicts induction of gene expression in fetal tissues. Drug Metab Dispos 2013; 41:305-11; PMID:23209192; http://dx.doi.org/10.1124/dmd.112.049999
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 2005; 102:15545-50; PMID:16199517; http://dx.doi.org/10.1073/pnas.0506580102
- Breton CV, Siegmund KD, Joubert BR, Wang X, Qui W, Carey V, et al. Prenatal tobacco smoke exposure is associated with childhood DNA CpG methylation. PLoS One 2014; 9:e99716; PMID:24964093; http://dx.doi.org/10.1371/journal.pone.0099716
- Breton CV, Salam MT, Gilliland FD. Heritability and role for the environment in DNA methylation in AXL receptor tyrosine kinase. Epigenetics 2011; 6:895-8; PMID:21555911; http://dx.doi.org/10.4161/epi.6.7.15768
- Toledo-Rodriguez M, Lotfipour S, Leonard G, Perron M, Richer L, Veillette S, et al. Maternal smoking during pregnancy is associated with epigenetic modifications of the brain-derived neurotrophic factor-6 exon in adolescent offspring. Am J Med Genet B Neuropsychiatr Genet 2010; 153B:1350-4; PMID:20583129; http://dx.doi.org/10.1002/ajmg.b.31109
- Stroud LR, Papandonatos GD, Rodriguez D, McCallum M, Salisbury AL, Phipps MG, et al. Maternal smoking during pregnancy and infant stress response: Test of a prenatal programming hypothesis. Psychoneuroendocrinology 2014; 48:29-40; PMID:24999830; http://dx.doi.org/10.1016/j.psyneuen.2014.05.017
- Haworth KE, Farrell WE, Emes RD, Ismail KM, Carroll WD, Borthwick HA, et al. Combined influence of gene-specific cord blood methylation and maternal smoking habit on birth weight. Epigenomics 2013; 5:37-49; PMID:23414319; http://dx.doi.org/10.2217/epi.12.72
- Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics 2009; 4:526-31; PMID:19923908; http://dx.doi.org/10.4161/epi.4.8.10265
- Blakey JD, Sayers I, Ring SM, Strachan DP, Hall IP. Positionally cloned asthma susceptibility gene polymorphisms and disease risk in the British 1958 Birth Cohort. Thorax 2009; 64:381-7; PMID:19237393; http://dx.doi.org/10.1136/thx.2008.102053
- Torgerson DG, Capurso D, Mathias RA, Graves PE, Hernandez RD, Beaty TH, et al. Resequencing candidate genes implicates rare variants in asthma susceptibility. Am J Hum Genet 2012; 90:273-81; PMID:22325360; http://dx.doi.org/10.1016/j.ajhg.2012.01.008
- Wu H, Romieu I, Shi M, Hancock DB, Li H, Sienra-Monge JJ, et al. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J Allergy Clin Immunol 2010; 125:321-7.e13; PMID:19910030; http://dx.doi.org/10.1016/j.jaci.2009.09.007
- Liang P, Song F, Ghosh S, Morien E, Qin M, Mahmood S, et al. Genome-wide survey reveals dynamic widespread tissue-specific changes in DNA methylation during development. BMC Genomics 2011; 12:231; PMID:21569359; http://dx.doi.org/10.1186/1471-2164-12-231
- Hogg K, Blair JD, von Dadelszen P, Robinson WP. Hypomethylation of the LEP gene in placenta and elevated maternal leptin concentration in early onset pre-eclampsia. Mol Cell Endocrinol 2013; 367:64-73; PMID:23274423; http://dx.doi.org/10.1016/j.mce.2012.12.018
- Jia RZ, Zhang X, Hu P, Liu XM, Hua XD, Wang X, et al. Screening for differential methylation status in human placenta in preeclampsia using a CpG island plus promoter microarray. Int J Mol Med 2012; 30:133-41; PMID:22552323
- Nelissen EC, Dumoulin JC, Daunay A, Evers JL, Tost J, van Montfoort AP. Placentas from pregnancies conceived by IVF/ICSI have a reduced DNA methylation level at the H19 and MEST differentially methylated regions. Hum Reprod 2013; 28:1117-26; PMID:23343754; http://dx.doi.org/10.1093/humrep/des459
- Wan ES, Qiu W, Baccarelli A, Carey VJ, Bacherman H, Rennard SI, et al. Cigarette smoking behaviors and time since quitting are associated with differential DNA methylation across the human genome. Hum Mol Genet 2012; 21:3073-82; PMID:22492999; http://dx.doi.org/10.1093/hmg/dds135
- Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet 2011; 88:450-7; PMID:21457905; http://dx.doi.org/10.1016/j.ajhg.2011.03.003
- Monick MM, Beach SR, Plume J, Sears R, Gerrard M, Brody GH, et al. Coordinated changes in AHRR methylation in lymphoblasts and pulmonary macrophages from smokers. Am J Med Genet B Neuropsychiatr Genet 2012; 159B:141-51; PMID:22232023; http://dx.doi.org/10.1002/ajmg.b.32021
- Flom JD, Ferris JS, Liao Y, Tehranifar P, Richards CB, Cho YH, et al. Prenatal smoke exposure and genomic DNA methylation in a multiethnic birth cohort. Cancer Epidemiol Biomarkers Prev 2011; 20:2518-23; PMID:21994404; http://dx.doi.org/10.1158/1055-9965.EPI-11-0553
- Marsit CJ, Maccani MA, Padbury JF, Lester BM. Placental 11-β hydroxysteroid dehydrogenase methylation is associated with newborn growth and a measure of neurobehavioral outcome. PLoS One 2012; 7:e33794; PMID:22432047; http://dx.doi.org/10.1371/journal.pone.0033794
- Filiberto AC, Maccani MA, Koestler D, Wilhelm-Benartzi C, Avissar-Whiting M, Banister CE, et al. Birthweight is associated with DNA promoter methylation of the glucocorticoid receptor in human placenta. Epigenetics 2011; 6:566-72; PMID:21521940; http://dx.doi.org/10.4161/epi.6.5.15236
- UniProt 2013. http://www.uniprot.org/uniprot/Q6P1K8. Accessed Dec12, 2013.
- Ren P, Rosas IO, Macdonald SD, Wu HP, Billings EM, Gochuico BR. Impairment of alveolar macrophage transcription in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007; 175:1151-7; PMID:17332483; http://dx.doi.org/10.1164/rccm.200607-958OC
- UniProt 2013. http://www.uniprot.org/uniprot/O60674. Accessed Dec 12, 2013.
- James C, Ugo V, Le Couédic JP, Staerk J, Delhommeau F, Lacout C, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434:1144-8; PMID:15793561; http://dx.doi.org/10.1038/nature03546
- Karkucak M, Yakut T, Ozkocaman V, Ozkalemkas F, Ali R, Bayram M, et al. Evaluation of the JAK2-V617F gene mutation in Turkish patients with essential thrombocythemia and polycythemia vera. Mol Biol Rep 2012; 39:8663-7; PMID:22722988; http://dx.doi.org/10.1007/s11033-012-1721-x
- Zhao R, Xing S, Li Z, Fu X, Li Q, Krantz SB, et al. Identification of an acquired JAK2 mutation in polycythemia vera. J Biol Chem 2005; 280:22788-92; PMID:15863514; http://dx.doi.org/10.1074/jbc.C500138200
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365:1054-61; PMID:15781101; http://dx.doi.org/10.1016/S0140-6736(05)71142-9
- Quintás-Cardama A. The role of Janus kinase 2 (JAK2) in myeloproliferative neoplasms: therapeutic implications. Leuk Res 2013; 37:465-72; http://dx.doi.org/10.1016/j.leukres.2012.12.006
- Weinberg I, Borohovitz A, Krichevsky S, Perlman R, Ben-Yehuda A, Ben-Yehuda D. Janus Kinase V617F mutation in cigarette smokers. Am J Hematol 2012; 87:5-8; PMID:21953826; http://dx.doi.org/10.1002/ajh.22180
- Wilhelm-Benartzi CS, Christensen BC, Koestler DC, Andres Houseman E, Schned AR, Karagas MR, et al. Association of secondhand smoke exposures with DNA methylation in bladder carcinomas. Cancer Causes Control 2011; 22:1205-13; PMID:21660454; http://dx.doi.org/10.1007/s10552-011-9788-6
- Barfield RT, Almli LM, Kilaru V, Smith AK, Mercer KB, Duncan R, et al. Accounting for population stratification in DNA methylation studies. Genet Epidemiol 2014; 38:231-41; PMID:24478250; http://dx.doi.org/10.1002/gepi.21789
- Bibikova M, Lin Z, Zhou L, Chudin E, Garcia EW, Wu B, et al. High-throughput DNA methylation profiling using universal bead arrays. Genome Res 2006; 16:383-93; PMID:16449502; http://dx.doi.org/10.1101/gr.4410706
- Dedeurwaerder S, Defrance M, Calonne E, Denis H, Sotiriou C, Fuks F. Evaluation of the Infinium Methylation 450K technology. Epigenomics 2011; 3:771-84; PMID:22126295; http://dx.doi.org/10.2217/epi.11.105
- Sean Davis PD, Sven Bilke, Tim Triche, Jr. and Moiz Bootwalla. methylumi: Handle Illumina methylation data. R package version 2.6.1., 2013.
- Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012; 28:882-3; PMID:22257669; http://dx.doi.org/10.1093/bioinformatics/bts034
- Smyth GK. Limma: linear models for microarray data. In: 'Bioinformatics and Computational Biology Solutions using R and Bioconductor'. R. Gentleman, V. Carey, S. Dudoit, R. Irizarry, W. Huber (eds), New York: Springer; pages 397-420.
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res 2000; 28:27-30; PMID:10592173; http://dx.doi.org/10.1093/nar/28.1.27
- Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014; 30:1363-9; PMID:24478339; http://dx.doi.org/10.1093/bioinformatics/btu049

