Abstract
Alterations in the composition of the commensal microbiota (dysbiosis) seem to be a pathogenic component of functional gastrointestinal disorders, mainly irritable bowel syndrome (IBS), and might participate in the secretomotor and sensory alterations observed in these patients.We determined if a state antibiotics-induced intestinal dysbiosis is able to modify colonic pain-related and motor responses and characterized the neuro-immune mechanisms implicated in mice. A 2-week antibiotics treatment induced a colonic dysbiosis (increments in Bacteroides spp, Clostridium coccoides and Lactobacillus spp and reduction in Bifidobacterium spp). Bacterial adherence was not affected. Dysbiosis was associated with increased levels of secretory-IgA, up-regulation of the antimicrobial lectin RegIIIγ, and toll-like receptors (TLR) 4 and 7 and down-regulation of the antimicrobial-peptide Resistin-Like Molecule-β and TLR5. Dysbiotic mice showed less goblet cells, without changes in the thickness of the mucus layer. Neither macroscopical nor microscopical signs of inflammation were observed. In dysbiotic mice, expression of the cannabinoid receptor 2 was up-regulated, while the cannabinoid 1 and the mu-opioid receptors were down-regulated. In antibiotic-treated mice, visceral pain-related responses elicited by intraperitoneal acetic acid or intracolonic capsaicin were significantly attenuated. Colonic contractility was enhanced during dysbiosis. Intestinal dysbiosis induce changes in the innate intestinal immune system and modulate the expression of pain-related sensory systems, an effect associated with a reduction in visceral pain-related responses. Commensal microbiota modulates gut neuro-immune sensory systems, leading to functional changes, at least as it relates to viscerosensitivity. Similar mechanisms might explain the beneficial effects of antibiotics or certain probiotics in the treatment of IBS.
Abbreviations
| AMP | = | antimicrobial peptide |
| CB1/2 | = | cannabinoid receptor type 1 or 2 |
| FGD | = | functional gastrointestinal disorder |
| FISH | = | fluorescent in situ hybridization |
| GCM | = | gut commensal microbiota |
| GI | = | gastrointestinal |
| IBS | = | irritable bowel syndrome |
| iNOS | = | inducible nitric oxide synthase |
| MOR | = | mu-opioid receptor |
| NGF | = | nerve growth factor |
| PPR | = | pattern recognition receptor |
| Reg3γ | = | regenerating islet-derived protein 3 gamma |
| RELMβ | = | resistin-like molecule-β |
| RT-qPCR | = | reverse transcription quantitative polymerase chain reaction |
| SFB | = | segmented filamentous bacteria |
| sIgA | = | secretory IgA |
| TLR | = | toll-like receptor |
| TPH 1/2 | = | tryptophan hydroxylase isoforms 1 or 2 |
| TRPV1/3 | = | transient receptor potential vanilloid types 1 or 3 |
Introduction
Functional gastrointestinal disorders (FGDs) are highly prevalent alterations characterized by an altered gastrointestinal (GI) functionality in the absence of overt structural changes. Although FGDs might affect any segment of the GI tract, most of the patients present symptoms related to lower GI (colon) dysfunction, and are grouped as irritable bowel syndrome (IBS) patients. Main IBS symptoms include abdominal pain or discomfort, bloating, abdominal distension and altered bowel habits.Citation1 Although still partially unknown, IBS has a multifactorial pathogenesis involving psychosocial factors (such as stress), an intestinal immune activation (with a persistent low grade inflammation) and altered brain-gut-brain communication and host-microbial interactions.Citation1-4
Within the intestine, microbial community is established shortly from birth and acts as an entire organ.Citation5,6 Recent works have identified gut commensal microbiota (GCM) as a dynamic ecosystem that maintains a bidirectional relationship with the host and that is essential for physiological and pathophysiological states.Citation7-10 Within the GI tract, GCM has a distinct distribution, with the higher bacterial counts localized in the more distal areas (large intestine). Colonic microbiota is composed mainly by microorganisms from the Firmicutes and Bacteroidetes phyla (mainly Clostridium spp, Lactobacillus spp and Segmented Filamentous Bacteria), sharing the colonic niche with less abundant bacteria from the Actinobacteria and Proteobacteria phyla (mainly Bifidobacterium spp, Verrucobacteria and Enterobacteria).Citation11,12 Alterations in the normal composition of GCM, known as intestinal dysbiosis, have been linked to several diseases of the GI tract, including inflammatory conditions and IBS.Citation2,Citation13-18 For instance, in IBS patients, intestinal dysbiosis with altered host-microbial interactions seems to be important generating a local immune response that might lead to the sensorial and secretomotor alterations characteristic of the disease. The underlying mechanisms remain largely unknown, although some evidences support a local modulation of sensory-related systems leading to altered functional responses.Citation18-21 For instance, we have recently shown that specific alterations in the composition of the GCM modify the expression of the intestinal endocannabinoid system, affecting nociceptive responses in mice.Citation19
The intestinal immune system is in the front line of defense against bacteria; tolerating GCM, but, at the same time, maintaining appropriate immune responses to pathogens.Citation20,Citation22-25 In this context, the innate immune system represents a pivotal player in controlling host resistance and maintaining the mucosal immune balance. The innate immune system provides a primary host response to bacterial invasion by using pattern recognition receptors (PRRs), mainly Toll-like receptors (TLRs), to recognize microbial agents. TLRs-mediated host-bacterial interactions trigger the sequential activation of intracellular signaling pathways leading to the induction of a range of mediators that drive the primary host resistance to pathogens. Additional innate immune components include the mucous barrier and the secretion of IgA and antimicrobial peptides (AMPs), that modulate luminal microbiota avoiding bacterial attachment to the epithelium.Citation6,23,24,26
In the present study, to further understand the role of microbiota influencing gut secretomotor and sensory responses, we assessed changes in the local immune system and in the expression of sensory-related systems within the colon of mice after a 2-week antibiotic treatment-induced dysbiosis. Furthermore, we also assessed if these changes lead to functional alterations manifested as changes in colonic contractility and viscerosensitivity.
Results
Clinical and macroscopical assessment of the animals
During the 2-week antibiotic treatment, no clinical signs were observed, with all animals showing a similar rate of body weight gain (data not shown). Water intake was similar across experimental groups (data not shown). At necropsy, the only significant change observed was the enlargement of the cecum in antibiotic-treated animals (507.7 ± 18.43 mg, P < 0.0009 vs. vehicle group: 409.0 ± 20.24 mg; n = 19 for each). These differences were maintained when the cecal weight was expressed as relative to the total body weight (data not shown).
Histological evaluation
Microscopic analysis of colonic tissue samples showed a normal histological structure in all animals. Occasionally, a moderate multifocal-to-diffuse inflammatory infiltrate and/or desquamation of some epithelial cells were observed, but no treatment-related incidence could be established. No significant differences in the final histopathological scores (vehicle: 0.66 ± 0.25; antibiotic: 1.27 ± 0.25; n = 7–8 per group) were observed ().
Figure 1. Colonic histopathology in vehicle- and antibiotic-treated mice. (A) Histopathological scores. (B) Goblet cell counts from PAS/AB pH = 2.5 stained-sections. (C) length of colonic crypts. (D) Thickness of the mucus layer, assessed on PAS/AB pH = 2.5 stained-sections. Bars represent the mean ± SEM, symbols represent individual animals. n = 7–8 per group, *: P < 0.05 vs. vehicle.
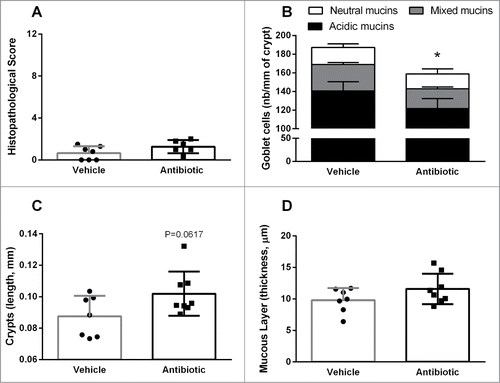
Evaluation of PAS/AB-stained sections showed a reduction in the density of goblet cells (per crypt length) in antibiotic-treated animals (158.8 ± 8.7 cells mm−1, n = 8) compared with the vehicle-treated group (187.2 ± 7.4 cells mm−1, n = 7, P < 0 .05; ). This was associated to a tendency for colonic crypts length to be increased in antibiotic–treated mice (vehicle: 87.52 ± 4.9 μm; antibiotic: 101.9 ± 4.97 μm; P = 0.061; ). When differentiating between acidic, mixed or neutral mucins, antibiotic-treated mice showed a relative increase in the number of goblet cells containing a mixture of acidic and neutral mucins (antibiotic: 21.1 ± 1.9 cells mm−1; vehicle: 28.5 ± 2.0 cells mm−1; P < 0.05; ). No differences were observed for the thickness of the mucus layer ().
A 2-week antibiotic treatment results in a dysbiosis of the colonic commensal microbiota
Total bacterial counts were increased in antibiotic-treated mice for a 2-week period when compared with the counts in vehicle-treated animals (5.33 ± 0.54 × 1013 cells/g of feces vs. Two.79 ± 0.38 × 1013 cells/g of feces; P = 0.004). The treatment with antibiotics increased Bacteroides spp. and Clostridium coccoides counts by 2- and 4-fold, respectively (both P < 0.05; ). Similarly, the Lactobacilli group was increased by 3-fold after the antibiotic treatment, although statistical significance was not reached, probably because of the relative large variability observed in control conditions (P = 0.07; ). On the other hand, the Bifidobacteria group showed a 10-fold reduction in antibiotic-treated animals (P = 0.006; ).
Figure 2. Characterization and quantification of luminal gut commensal microbiota. Data shows qPCR quantification of total bacteria and the main bacterial groups present in the colonic microbiota (see methods for details). Data are median (interquartile range) ± SD and are expressed as cells/g of feces; n = 7–8 for each group. *, **: P < 0.05 or 0.01 vs. vehicle group. The bottom-right graph shows the relative distribution of the ceco-colonic microbiota in vehicle- and antibiotic-treated mice. Data represent the relative abundance (percent) of the main bacterial groups present in the gut microbiota as quantified using qPCR. Relative percent composition was calculated taking as 100% the total counts of the different bacterial groups assessed (C. coccoides, Bacteroides spp., Bifidobacterium spp and Lactobacillus/Enterococcus spp).
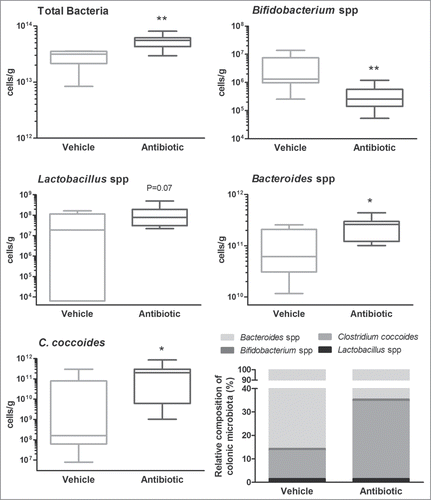
When assessing the relative composition of the microbiota (proportion of each bacterial group assessed within the total counts), the most abundant bacteria characterized, regardless the treatment, were Bacteroides spp and C. coccoides (cluster XIVa), representing 99% of the total bacterial counts. During antibiotic treatment the main change was an increase in the ratio of C. coccoides (cluster XIVa), from about 15% in control conditions to approximately 35% of the total counts. Regardless the treatment, the lactobacilli group was very scarce (<0.05% of the total bacterial counts) and the Bifidobacteria group was the less abundant (<0.01% of the total bacterial counts) ().
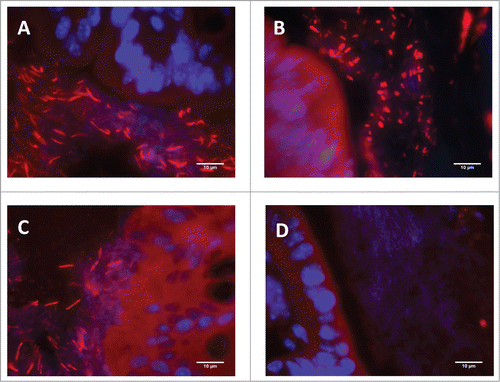
In control conditions, the main bacterial group adhered to the colonic epithelium was Clostridia (incidence of attachment: 100%), followed by Enterobacteria (incidence of attachment: 38%) and Segmented Filamentous Bacteria (Incidence of attachment: 25%). Overall, the 2-week period of antibiotic treatment did not affect the ratios of bacterial wall adherence (). Nevertheless, FISH images revealed that antibiotic-treated mice showed a higher proportion of coccoid-shaped Clostridia adhered to the epithelium than vehicle-treated animals, in which Clostridia hybridized mainly as a fusiform ballici ().
Table 1. Incidence of bacterial wall adherence.Citation1
Figure 3. Representative colonic tissue images showing Clostridium spp (identified by FISH using the EREC 482 probe) adherence to the colonic epithelium. (A) Vehicle-treated animal. (B) Antibiotic-treated animal. (C) Non-treated naïve animal maintained in the same conditions as the experimental groups; included here for comparative purposes. (D) Negative control (hybridized with the control non-specific fluorescent probe NON338). In all cases (A–C) abundant bacteria was observed attached to the colonic epithelium. Note, however, that bacillary-shaped bacteria were observed in vehicle-treated animals (A) (similarly to that observed in the non-treated naïve animal, (C) while in antibiotic-treated animals (B) a shift in morphology, with the appearance of abundant coccoidal forms, can be observed.
Antibiotics modulated the local innate immune system
S-IgAs were detected in all luminal contents analyzed. Levels of s-IgA were increased by 10-fold in antibiotic-treated animals (P < 0.05, ).
Figure 4. Changes in immune and host-bacterial interaction markers. (A) Changes in innate immune-related markers: luminal levels of secretory IgA (S-IgA) and gene expression levels of antimicrobial peptides. (B) Changes in expression levels of pro- (IL-12p40, IL-6 and TNFα) and anti-inflammatory (IL-10) cytokines and the inducible nitric oxide synthase (iNOS). (C) Changes in the expression levels of TLRs. Data are mean ± SEM, n = 7–8 group, *: P < 0 .05 vs. vehicle.
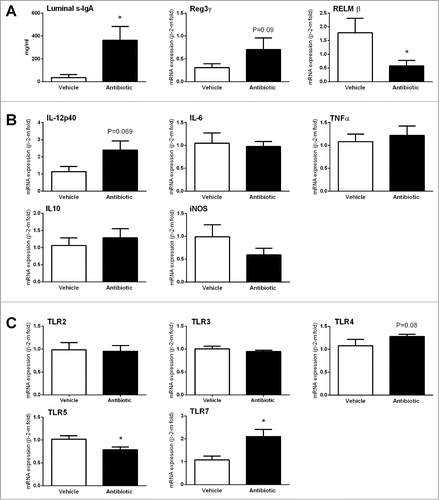
A relatively high variability was observed in the expression levels or AMPs. In control conditions, the relative expression levels of AMPs were: RELMβ > RegIIIγ > defensin-α24/6; with defensin-β4 being undetectable in all samples analyzed. In antibiotic-treated mice, RELMβ was downregulated (P < 0.05 vs. vehicle group) while RegIIIγ tended to be upregulated, although without reaching statistical significance ().
No differences in the expression of pro- (IL-6, IL-12p40 and TNFα) or anti-inflammatory (IL-10) cytokines was observed between vehicle- and antibiotic-treated animals. Only IL-12p40 tended to be up-regulated in antibiotic-treated mice (P = 0.069, ). During inflammation, activated immune cells are a source of NO through the expression of the inducible form of the NO synthase (iNOS).Citation27 In the present conditions, no differences in iNOS expression were observed between vehicle- and antibiotic-treated mice (P = 0.1982; ).
In control conditions, relative colonic expression of TLRs was: TLR4 > TLR3 > TLR2 > TLR5 > TLR7. In antibiotic-treated animals specific changes in expression were detected; with a significant upregulation of TLR7 (P = 0.008) and a tendency for TLR4 (P = 0.08); while TLR5 showed a slight (less than 1-fold) downregulation (P = 0.02) (). Expression of other TLRs was not affected.
Antibiotics modulated the local expression of sensory-related markers
With the exception of tryptophan hydroxylase 2 (which was in general undetectable) all markers assessed were expressed at detectable levels in all samples analyzed. In antibiotic-treated animals, only a selective down-regulation of CB1, MOR and NGF was detected (). CB2 expression showed a tendency to be upregulated; however, statistical significance was not reached probably because of the relatively high variability observed in antibiotic-treated animals. Other secretomotor and sensory markers assessed were not affected by the antibiotic treatment.
Figure 5. Changes in sensory-related markers. (A) Changes in colonic gene expression of cannabinoid receptors 1 and 2 (CB1/2), mu-opioid receptors (MOR) and nerve growth factor (NGF). Data are mean ± SEM, n = 5–8 animals per group. *, **, ***: P < 0.05, 0.01 or 0.001 vs. vehicle. (B) Quantification of immunorreactive ganglionic cells within the myenteric plexus in vehicle- and antibiotic-treated animals. Data are mean ± SEM, of 5–8 animals per group; see methods for details of the quantification procedures.
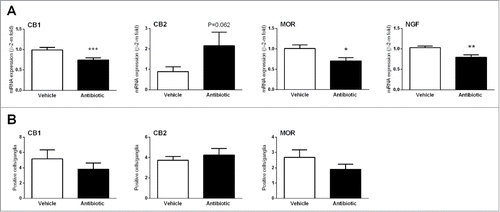
Evaluation of CB1, CB2 and MOR content in the colonic myenteric plexus using immunohistochemistry correlated with the gene expression data, although statistical significances were not reached. The number of CB1- or MOR-positive ganglionic cells within the myenteric plexus was reduced by 25% and 30%, respectively, in antibiotics-treated mice. On the other hand, the number of CB2-positive ganglionic cells was increased by 13% in antibiotic-treated animals (). The mean area of the myenteric ganglia was similar in vehicle- and antibiotic-treated mice (vehicle: 57.1 ± 10.1 μm2, antibiotic: 71.0 ± 19.0 μm2, P > 0.05).
Bacterial counts correlated with host-bacterial interaction and nociceptive markers
Significant correlations were found between bacterial counts and the expression changes in host-bacterial interaction and nociceptive markers. Total bacterial counts correlated negatively with the colonic expression of CB1 (P = 0.01; r2 = 0.39) and TLR-5 (P = 0.02; r2 = 0.36) and positively with CB2 (P = 0.03; r2 = 0.31) and TLR-7 (P = 0.02; r2 = 0.32) ().
Figure 6. (A) Correlations between total luminal bacterial counts and sensory-related (CB1 and CB2) markers or TLRs. (B) Correlations between expression levels of TLR7 and sensory-related markers. Each point represents an individual animal. Broken lines represent the 95 % confidence interval.
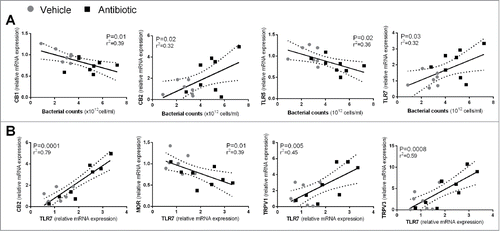
Moreover, regardless the treatment applied, positive correlations between TLR7 and the nociceptive markers CB2 (P = 0.0001; r2 = 0.79), TRPV1 (P = 0.005; r2 = 0.45) and TRPV3 (P = 0.0008; r2 = 0.59) were found, while negatively correlating with MOR expression (P = 0.01; r2 = 0.39) (). In addition, expression levels of IL-12p40 (P = 0.0008; r2 = 0.59) and IL-10 (P = 0.003; r2 = 0.49) correlated positively with TLR7 expression.
Visceral pain-related responses were altered in antibiotic-treated mice
Intraperitoneal acetic acid produced repeated characteristic stretching contractions (abdominal contractions) during the 30 min period after administration, with a maximal response observed at 10 min post-administration. Time-course responses to IP acetic acid were similar in vehicle- and antibiotic-treated mice, but the overall response was attenuated by 33% in antibiotics-treated mice (40.9 ± 7.6 abdominal contractions/30 min, n = 6) when compared to vehicle treated animals (61.4 ± 4.0 abdominal contractions/30 min, n = 6; P < 0 .001; ). Abdominal contractions were absent in animals injected IP with vehicle.
Figure 7. Effects of antibiotic treatment on visceral pain-related responses. A: Intraperitoneal acetic acid- (AA, 0.6%) induced abdominal contractions. The left graph shows the total number of abdominal contractions during the observation time (30 min) in the different experimental groups. Each point represents an individual animal; the horizontal lines with errors correspond to the mean ± SEM. ***: P < 0 .001 vs. respective non-AA-treated control group. #: P < 0.05 vs. vehicle-AA group. The graph to the right shows the time-course (in 5-min intervals) for the pain-related responses in the same animals. B: Intracolonic capsaicin- (Caps) evoked visceral pain-related behaviors. The left graph shows the total number of behaviors during the observation time (30 min) in the different experimental groups. Each point represents an individual animal; the horizontal lines with errors correspond to the mean ± SEM. ***: P < 0.001 vs. respective non-capsaicin-treated control group. #: P < 0.05 vs. vehicle-Caps group. The graph to the right shows the time-course (in 5-min intervals) for the observation of pain-related behaviors in the same animals.
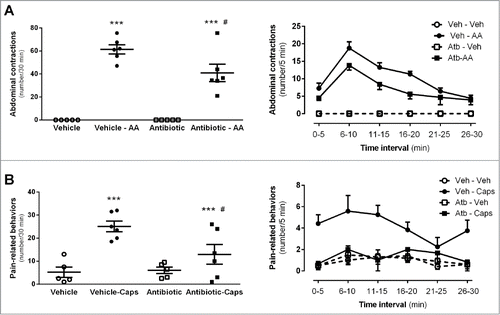
Intracolonic administration of capsaicin induced pain-related behaviors in all mice during the 30 min observation period, with a maximal response observed at 10 min post-administration. Capsaicin-induced pain-related behaviors were reduced by 48% in antibiotic-treated mice (12.9 ± 4.2 behaviors/30 min, n = 6) when compared to vehicle-treated mice (25.1 ± 2.3 behaviors/30 min, n = 6; P < 0.05; ). The predominant behavior after intracolonic capsaicin was the licking of the abdominal area, observed in all animals. Stretching of the abdomen and squashing of the lower abdomen against the floor behaviors, considered to reflex the highest levels of pain, were seen only in vehicle-treated mice.
Colonic contractility was altered in antibiotic-treated mice
Spontaneous colonic contractility, as assessed in vitro, was increased in the antibiotic-treated group (AUC/10 min: 2.73 ± 0.46 g, n = 6) when compared to vehicle-treated animals (AUC/10 min: 1.60 ± 0.14 g, n = 5; P < 0.05; ). Regardless the experimental group considered, carbachol produced a concentration-dependent contractile response. In antibiotic-treated mice the EC50 for carbachol was 2.4-fold lower than that determined in control conditions (vehicle: 1.24 ± 0.30 10−6 M; antibiotic: 5.19 ± 1.59 10−7 M; n = 5–6; P = 0.056; ).
Figure 8. Effects of antibiotic treatment on colonic contractility assessed in vitro: basal contractility; contractile responses to NO-synthase inhibition with LNNA; Concentration-response curves to cholinergic stimulation with carbachol (CCh) and corresponding EC50s. Data are mean ± SEM, n = 5–6 per group, each point represents an individual animal (except for the concentration-response curves, where only mean ± SEM is shown). *: P < 0.05 vs. vehicle.
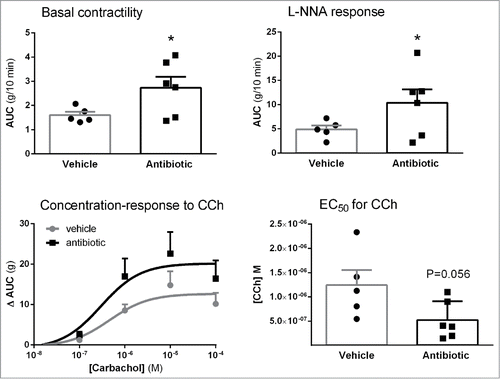
Spontaneous colonic contractility during L-NNA addition to the organ bath, to block NO synthesis, had a tendency to be higher in antibiotic-treated mice (AUC/10 min: 10.37 ± 2.77 g, n = 6) compared with vehicle controls (AUC: 4.88 ± 0.82 g, n = 5; P = 0.0578; ).
Discussion
In the present study, we show that a 2-week treatment with antibiotics generates a moderate dysbiotic state, with increments in total bacterial counts, in mice. These changes imply a modulation of both host-bacterial interaction systems and local neuro-immune systems, leading to functional alterations revealed as changes in colonic sensitivity and contractility.
The 2-week antibiotic treatment caused a specific dysbiosis accompanied with an enlargement of the cecum, although no signs of inflammation were observed, as previously described.Citation19,28,29 Overall, this dysbiotic state is similar to that described in previous studies in mice and indicates antibiotic-induced temporal and spatial changes in the GCM composition.Citation19,28,30 In the present conditions, total bacterial load was increased in antibiotic-treated animals; thus suggesting that the treatment favored the expansion of antibiotic-resistant bacterial groups. This contrasts with other studies indicating that antibiotics alter bacterial community richness decreasing overall bacterial density.Citation26,28,29 In the same way, similar antibiotic treatment might cause different microbial changes depending upon the doses administered, the basal microbial composition, the strain and commercial breeder of the animals used or the environmental conditions. Specifically, short- and long-term antibiotic treatments seem to generate different states of dysbiosisCitation28,30 and be, in consequence, related to different responses of the host.
In our conditions, luminal ceco-colonic dysbiosis was characterized by increments in Bacteroides spp., C. coccoides (cluster XIVa) and Lactobacillus-Enterococcus spp. and reductions in Bifidobacterium spp. counts. All these bacteria have been implicated in both GI physiology and pathology.Citation18,21,26,Citation31-33 Recent evidences suggest a key role of some bacterial groups in immune functions (mainly segmented filamentous bacteria, SFB, and Clostridia from the clusters XIVa and VI) which are, in normal conditions, in direct contact to the host and, therefore, directly influencing host immune responses.Citation19,Citation34-36 In agreement with this, we found Clostridia, SFB, and Enterobacteria adhered, in large percentages, to the colonic epithelium. However, despite the luminal dysbiosis observed, antibiotics did not modify the rate of attachment, in agreement to that previously reported during long-term antibiotic treatment in mice.Citation29 Nevertheless, these results contrast with data obtained during short-term antibiotic treatment, which suggested a facilitation in bacterial attachment.Citation19 These differences further enfatize the importance of the duration of the antibitotic exposure in the microbial and functional responses elicited. Interestingly, a change in bacterial morphology for epithelium-attached Clostridia was observed in antibiotic-treated animals. While in control conditions Clostridia showed a predominant bacillary shape after antibiotics treatment a higher proportion of coccoidal Clostridia was observed in close contact to the colonic epithelium. This agrees with the key role given to C. coccoides in immune activation within the gut.Citation34 Together with the increase in the ratio of luminal Clostridium spp. observed in antibiotic-treated animals, these observations further suggest that antibiotics favored the proliferation of some bacterial groups, particularly C. coccoides.
In antibiotic-treated mice, changes in markers related to host-bacterial interactions were detected. TLRs are primary sensors of luminal bacteria and key components in host-bacterial interactions. In our conditions, there was a type-specific modulation of TLRs expression, with an up-regulation of TLR4 and 7 and a downregulation of TLR5. Changes in TLR5 and TLR7 might be difficult to correlate with specific microbial changes because they recognize components of a wide variety of ligands from both Gram positive and negative bacteria.Citation37 On the other hand, TLR4 is mainly activated by lipopolysaccharides (LPS) from Gram negative bacteria.Citation37 Therefore, in our conditions, the slight TLR4 upregulation observed might represent and adaptive response to the proliferation of Bacteroides spp. In fact, similar up-regulation of TLR4 was observed in estates of colitis characterized by increases in the counts of gram-negative bacteria (Bacteroides spp.)Citation38 and, on the contrary, a TLR4 down-regulation was detected when Bacteroides spp. counts were reduced.Citation39
Other markers assessed indicate more extended changes in the local innate immune system. These included changes in the mucosal barrier, the secretion of sIgA and the upregulation of AMPs and pro-inflammatory cytokines. Overall, these changes indicate an activation of the innate immune system, which might be related to the dysbiotic state. Antibiotic-treated mice showed similar morphological (cecal enlargement, changes in goblet cells) and innate immune-related responses as those observed in other models of antibiotic-induced dysbiosis, in germ free animals or in different models of intestinal inflammation.Citation28,40,41 These changes might represent a general adaptive pattern of the host in response to alterations in the composition of the microbiota and, therefore, in host-bacterial interactions. Interestingly, this innate immune activation was not associated to structural changes (macroscopical or microscopical) consistent with a state of overt inflammation within the colon. A similar situation is described in IBS patients, considered to have a persistent intestinal immune activation (low grade inflammation) in the absence of structural alterations.Citation2-4
In addition, markers involved in colonic sensitivity and secretomotor responses were also affected during the antibiotic treatment, thus indicating that local adaptive processes to microbial modifications might take place at multiple levels and affect various regulatory systems. From the markers assessed, CB1 and MOR showed a downregulation while CB2 had a tendency to be up-regulated. Similarly to that observed here, during spontaneous adaptive changes of the microbiota or during the administration of probiotics, both the endocannabinoid and opioid systems were modulated.Citation19,21,42,43 However, the changes observed contrast with those after short (1-week) treatment with the same antibiotic regime, in which CB2 was upregulated, without changes in CB1 expression.Citation19 These differences are likely to relate to the different duration of the antibiotic treatment, reflecting time-related variations (1-week vs. 2-week) in the adaptive process of sensory mechanisms. In any case, these observations indicate that modulation of cannabinoid and opioid pathways might be important in host-bacterial interactions and might mediate neural-related functional changes associated to alterations of the GCM. Moreover, gene expression changes translated at the protein level, since CB1, CB2 and MOR immunoreactivity in the enteric nervous system was also modified in the same direction as the gene expression. Nevertheless, these data result from a semi-quantitative analysis that should be further confirmed. Furthermore, these changes seem to have functional significance since visceral pain-related responses were also affected in antibiotic-treated animals. Indeed, visceral pain-related responses elicited by intraperitoneal or direct intracolonic chemical stimulation were attenuated by 40% in antibiotic-treated mice, thus suggesting that treatment with antibiotics can generate an analgesic-like state within the gut. Cannabinoid receptors and MOR are directly involved in visceral pain, eliciting analgesic responses.Citation42,44 Therefore, the down-regulation of MOR and CB1 contrasts with the analgesic-like state observed, an effect likely compensated by the moderate up-regulation of CB2, which has been implicated in pain modulation in states of inflammation and immune activation.Citation45 Additionally, NGF has been implicated in the sensitization of visceral afferents, leading to the development of hypersensitivity.Citation46 Therefore, NGF downregulation might also contribute to the analgesic-like responses observed here.
Recent evidences have linked activation of TLRs, particularly TLR-4, with changes in nociception.Citation47,48 In the present study, although changes in TLRs expression were relatively minor, we observed correlations between TLR expression (in particular TLR-7) and nociceptive markers (CB2, MOR and TRPV1/3). Although these correlations have to be interpreted with caution, this further supports the possibility that TLRs act as transducers of microbial-generated signals generating local changes in neuro-immune systems and leading to a modulation of viscerosensitivity. Moreover, several studies linking the gut microbiota with visceral sensitivity, have shown that increments in the Lactobacilli family (during probiotic treatment or during states of dysbiosis) are associated to visceral analgesic-like states.Citation18,19,21,33 In our conditions, we can speculate that the moderate increment in Lactobacillus spp. counts observed in antibiotic-treated animals might be important in the observed visceral pain-related responses. Overall, these observations might have relevance in IBS patients in which dysbiosis coexists with alterations in visceral sensitivity (visceral hyperalgesia) and this state ameliorates during antibiotic treatment and with administration of certain probiotics.Citation15,Citation49-52 Although further studies are needed, we can speculate that a similar modulation of sensory-related systems to that described here might operate during antibiotic/probiotic treatments in IBS, leading to an improvement in visceral sensitivity.
The microbiota and microbial-derived products are factors that also affect gastrointestinal motility.Citation53,54 In the present conditions, basal colonic contractility was increased in dysbiotic mice. This, together with the observed increased responses to carbachol and NO-synthase (neural and endothelial) inhibition suggests that during dysbiosis enhanced colonic contractility represents an imbalance between excitatory (mainly cholinergic) and inhibitory (mainly NO-dependent) systems. Several bacterial metabolic products, such as hydrogen sulphide production or short chain fatty acids, might act as mediators modulating colonic motility. Similarly, it has been suggested that direct TLR4-dependent host-bacterial interactions enhance motility through a neutrally-mediated effect.Citation53 Overall, these observations suggest that altered gut microbiota might be responsible, at least in part, for the colonic motor disturbances observed in IBS patients and might help to explain the beneficial effects observed after antibiotic or probiotic treatments.Citation52,55,56
In summary, the results presented here indicate that during states of dysbiosis there is a local neuro-immune adaptive response, likely associated to changes in host-bacterial interactions, which leads to functional alterations manifested as changes in viscerosensitivity and motor activity within the colon. According to previous observations, we can speculate that proliferation of C. coccoides, Lactobacillus–Enterococcus spp and Bacteroides spp. and reduction in Bifidobacterium spp counts might be significant for the molecular and functional changes observed. Similar microbial-dependent modulatory actions might explain the beneficial effects associated to the use of antibiotics or probiotic bacteria in IBS patients.
Materials and Methods
Animals
Female CD1 mice, 10–12 week-old (Charles River Laboratories) were used. All animals were maintained in conventional conditions in an environmentally controlled room (20–22°C, 12 h light:dark cycle), with food and water ad libitum. All procedures were approved by the Ethical Committee of the Universitat Autònoma de Barcelona (protocols 1099 and 1396) and the Generalitat de Catalunya (protocols 5646 and 7193).
Antibiotic treatment
Animals received a mixture of non-absorbable, broad spectrum antibiotics containing Bacitracin A (31626 - Vetranal™; Sigma-Aldrich) and Neomycin (N1876 - Neomycin trisulfate salt hydrate; Sigma-Aldrich). Amphotericin B (A9528; Sigma-Aldrich) was added to prevent yeast overgrowth. During a 2-week period, animals were dosed daily, by oral gavage, with 0.3 mL of the mixture (prepared fresh on a daily basis). This procedure ensured a minimum dose of 0.4 mg for bacitracin and neomycin and 0.1 mg for amphotericin B (per mouse and day). In addition, the same mixture was added to the drinking water during the same period of time. Vehicle-treated animals were dosed with deionized water by oral gavage (0.3 mL). Water consumption and body weight was assessed on a daily basis during the treatment period. Similar treatment protocols have been followed previously in comparable studies in mice and rats, demonstrating the induction of significant changes of the GCM.Citation18,19,57
Experimental protocols
Mice were treated with the antibiotic mixture (n = 28) or vehicle (n = 28) during 14 consecutive days. Different subgroups of vehicle- and antibiotics-treated animals were used to assess visceral pain responses (behavioral pain responses to IP acetic acid, n = 22 and IC capsaicin, n = 22) and in vitro colonic contractility (n = 12). On day 15, 24 h after the last antibiotic/vehicle administration and after all other procedures (pain tests), animals were euthanized, the weight of body organs assessed and samples of colonic tissue and fecal content obtained. Samples obtained from animals included as controls in the visceral pain tests were used for morphological/molecular studies. Each animal was used only for one procedure.
Samples collection
Mice were deeply anesthetized with isoflurane (Isoflo) and euthanatized by exsanguination through intracardiac puncture followed by cervical dislocation. Thereafter, a medial laparotomy was performed, the ceco-colonic region localized and the cecum and colon dissected. Afterward, ceco-colonic fecal contents and a tissue sample from the proximal colon were collected and frozen immediately in liquid nitrogen. Frozen samples were stored at –80°C until analysis. At the same time, tissue samples of the proximal and middle colon (about 1.5 cm each) were collected and fixed overnight in Meta-Carnoy fixative (methanol:chloroform:glacial acetic acid, 6:3:1, v:v:v) or in 4% paraformaldehyde. After an overnight fixing, tissues were paraffin embedded and 5 μm-tick sections obtained. During the necropsy, the adrenal glands, the thymus, the liver and the spleen were dissected and weighed.
Quantification of bacteria using real-time quantitative PCR (qPCR)
Total DNA was isolated from frozen fecal ceco-colonic content using QIAamp® DNA Stool Mini Kit (Qiagen), following the manufacturer's instructions. Thereafter, DNA was quantified using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies), diluted to equal concentrations with sterile deionized water and stored at −20°C until analysis.
The relative abundance of bacteria was measured using 16S rRNA gene-targeting hydrolysis probes (Custom TaqMan assays; Applied Biosystems) as previously described.Citation58-60 Probes used are detailed in . All samples and the negative controls were assayed in triplicates. The vehicle group served as the calibrator. For each assay, a positive (quantified) sample was used to generate a standard curve and to quantify the number of bacteria. For Clostridia cluster XIVa and total bacterial counts, C. coccoides (DSM 935; Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures) was used. In all cases, standard curves were derived from the serial dilutions in a customary way [EUB (r2 = 0.99): y = −0.2808x + 16.023; EREC (r2 = 0.99): y = −0.2964x + 16.749 ; LAB (r2 = 0.98): y = −0.3342x + 18.513; BIF (r2 = 0.99): y = −0.2777x + 12.908; and BAC (r2 = 0.99): y = −0.2906x + 13.935]. Relative concentrations were expressed in arbitrary units. Logarithms (base 10) of concentrations were plotted against crossing points and least square fit was used as the standard curve to obtain the bacterial number (expressed as cells/g of feces).
Table 2. Probes and primers used for bacterial identification (FISH and qPCR)
Identification of bacterial adherence by fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH) procedures for colonic tissue samples were followed as previously reported.Citation17,35 Oligonucleotide probes consisted in a single strain DNA covalently linked with a Cy3 (carbocyanine) reactive fluorescent dye at the 5′ end (Biomers and Isogen Lifescience). Probes used are detailed in .
Hybridized slides were viewed under oil immersion, using a Carl Zeiss Axioskop 40 FL epifluorescence microscope (Carl Zeiss, Jena, Germany) equipped with a digital camera (Zeiss AxioCam MRm) for obtaining digital images (Zeiss AxioVision Release 4.8.1). To provide a semi-quantitative assessment of bacterial attachment, analysis of the images was performed manually by 2 independent researchers that observed the pictures and localized hybridized bacteria within the mucus layer or attached to the epithelial surface. A coincidence between the 2 observers in bacterial location in at least 15% of the pictures observed (at least 3 out of 20) was required to decide that there was bacterial attachment to the epithelium. All procedures were performed on coded slides, to avoid bias.
Quantification of secretory immunoglobulin A
Luminal s-IgA was measured in fresh homogenates of cecal contents (equal diluted in PBS 1x) using a commercial double-antibody sandwich ELISA, following manufacturers’ instructions (MBS564073; MyBiosource).
mRNA analysis
Total RNA was extracted from frozen tissue samples using TRI reagent with Ribopure Kit (Ambion/Applied biosystems) using the FastPrep-12 instrument (MP Biomedicals, France). Thereafter, a 2-step quantitative real time PCR (RT-qPCR) was performed. cDNA was obtained using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The PCR reaction mixture was incubated on the ABI 7500Fast (Applied Biosystems). All samples, as well as the negative controls, were assayed in triplicate. The cycle thresholds for each sample were obtained and data were analyzed using the comparative Ct method (2−ΔΔCt) with the vehicle group serving as the calibrator.Citation61
TaqMan ® gene expression assays (hydrolysis probes) for cannabinoid receptor 1 (CB1) (Mm01212171_s1) and 2 (CB2) (Mm00438286_m1), mu-opioid receptor (MOR) (Mm01188089_m1), tryptophan hydroxylase 1 (TPH1) (Mm00493794_m1) and 2 (TPH2) (Mm00557715_m1), serotonin Transporter (SERT) (Mm00439391_m1), transient receptor potential vanilloid 1 (TRPV1) (Mm01246302_m1) and 3 (TRPV3) (Mm00455003_m1), nerve growth factor (NGF) (Mm00443039_m1), inducible nitric oxide synthase (iNOS) (Mm04440502_m1), interleukin 6 (IL-6) (Mm00446190_m1), tumor necrosis factor α (TNFα) (m00443258_m1), interleukin 12 (IL-12p40) (Mm00434174_m1), interleukin 10 (IL-10) (Mm00439614_m1), toll-like receptor (TLR) 2 (Mm0044-2346_m1), 3 (Mm01207404_m1), 4 (Mm00445273_m1), 5 (Mm00546288_s1) and 7 (Mm00446590_m1), defensin-α6/24 (Mm04205950_gH), defensing-β4 (Mm00731768_m1), the lectin regenerating islet-derived protein 3 gamma (RegIIIγ) (Mm00441127_m1) and the resistin-like molecule-β (RELMβ) (Mm00445845_m1) were used (Applied Biosystems). β-2-microglobulin (Mm00437762_m1) was used as endogenous reference gene.
Histology
For histological examination, hematoxylin-eosin-stained sections from the colon were obtained following standard procedures. A histopathological score (ranging from 0, normal, to 12, maximal alterations) was assigned to each animal. Specifically, parameters scored included: epithelial structure (0: normal; 1: mild alterations of the villi; 2: local villi destruction and/or fusion; 3: generalized villi destruction and/or fusion), structure of the crypts (0: normal; 1: mild alterations of the crypts; 2: local destruction of the crypts; 3: generalized destruction of the crypts), presence of edema (0: normal; 1: mild local edema in submucosa and/or lamina propria; 2: moderate diffuse edema in submucosa and/or lamina propria; 3: severe generalized edema in submucosa and/or lamina propria), presence of inflammatory infiltrate (0: normal; 1: mild localized infiltrate; 2: mild generalized infiltrate; 3: severe generalized infiltrate). Scoring was performed on coded slides by 2 independent researchers.
The mucous layer was assessed in Meta-Carnoy-fixed samples of colonic tissue. Thickness of the mucous layer was measured in 10 different fields, in representative regions covering, at least, 20% of the epithelial surface. All measurements were performed on coded slides by 2 independent investigators using the Zeiss AxioVision Release 4.8.1 software. Moreover, tissue sections were also stained with Alcian Blue pH 2.5/Periodic Acid Schiff (AB 2.5/PAS kit; Bio-Optica) in order to specifically stain neutral (pink), mixed (purple) or acidic (blue) mucins. Thereafter, colonic goblet cells were counted in 20 longitudinally-oriented villus-crypt units. Length of the villus-crypt unit was also determined to obtain goblet cells density (number of cells mm−1).
Immunohistochemistry and quantification of immune-positive signals in the myenteric plexus
Paraffin-embedded tissue sections (5 μm in thickness) were deparaffinized and rehydrated with a battery gradient of alcohols. Immunohistochemistry protocols for each antibody were followed by a customary way and as previously described.Citation58 Antigen retrieval for CB1 receptor and MOR was achieved by processing the slides in a microwave with 10 mM of citrate solution. Epitope retrieval for CB2 receptor was performed using a pressure cooker (at full pressure, for 3 min) in Tris–EDTA solution buffer. Primary antibodies included a rabbit polyclonal anti-CB1 (1:100; rabbit polyclonal to cannabinoid receptor 1, ab23703; Abcam), a rabbit polyclonal anti-CB2 [1:100; rabbit polyclonal to cannabinoid receptor 2 (H-60), sc-25494; Santa Cruz Biotechnology], and a rabbit polyclonal anti-MOR (1:2,500; rabbit polyclonal to mu opioid receptor AB1580; Chemicon/Millipore). The secondary antibody used was a biotinylated polyclonal swine anti-rabbit IgG (E 0353; DakoCytomation). Antigen–antibody complexes were reveled with 3–3′-diaminobenzidine (SK-4100 DAB; Vector Laboratories), with the same time exposure per antibody, and sections were counterstained with hematoxylin. Specificity of staining was confirmed by omission of the primary antibody.
For each marker assessed (CB1, CB2 and MOR), immunopositive cells were counted in 30, randomly selected, myenteric ganglia for each tissue sample, to provide a semi-quantitative estimation. Cells were considered to be immunopositive if they expressed more labeling than the background levels seen in the negative controls. Myenteric ganglia of the same animals were photographed (Eclipse 90i, Nikon), and the area quantified (in μm2) using the software ImageJ (National Institutes of Health). All procedures were performed on coded slides to avoid any bias.
Organ bath contractility of the colon
Full thickness preparations from the mid portion of the colon were cut 0.2 cm wide and hung for organ bath study oriented to record circular muscle activity. Strips were mounted under 1–2 g tension in a 10 mL organ bath containing carbogenated (95% O2–5% CO2) Krebs solution with glucose and maintained at 37 ± 1°C. One strip edge was tied to the bottom of the organ bath using 2/0 silk suture and the other one to an isometric force transducer (Harvard VF-1; Harvard Apparatus Inc.). Output from the transducer was fed to a PC through an amplifier. Data were digitalized (25 Hz) using Data 2001 software (Panlab). Strips were allowed to equilibrate for about 90 min; thereafter, to determine the spontaneous contractile activity, the tone was measured for 10 min. After this, responses to carbachol (CCh; 10−7 to 10−4 M; Sigma-Aldrich), added to the bath in a cumulative manner at 5-min intervals, were assessed. Thereafter, the bath solution was replaced, tissues were allowed to reequilibrate (20 min), and spontaneous contractile activity and responses to the NO-synthase inhibitor NG-nitro-L-arginine (L-NNA; 10−3 M; Sigma-Aldrich) were assessed. The amplitude of contractions from the baseline (maximal response) and the area under curve (AUC) during 10 min (spontaneous activity and effects of L-NNA) or 5 min (effects of CCh) were used to evaluate the contractile activity.
Behavioral pain responses to intraperitoneal acetic acid
0.6% glacial acetic acid (Sigma-Aldrich) in distilled water (10 ml/kg) or vehicle (distilled water) was administered IP and pain related responses were determined following previous protocols.Citation62 The pain response was scored by counting the number of abdominal contractions during the 30 min period after the IP treatment (in blocks of 5 min) by 2 independent researchers.
Behavioral responses to intracolonic capsaicin-evoked visceral pain
Spontaneous visceral pain-related behaviors induced by intracolonic capsaicin (Sigma-Aldrich) were assessed following previously described protocols, with minor modifications.Citation19 Mice were anesthetized with isoflurane (Isoflo) and capsaicin (0.05 ml/mice, 0.1% in ethanol:Tween 80:saline; 1:1:8, v:v:v; Sigma-Aldrich) or vehicle (ethanol:Tween 80:saline; 1:1:8, v:v:v) were administered intracolonically. Petroleum jelly was applied to the perianal area to avoid the stimulation of somatic areas due to any leakage on the capsaicin solution. After recovering consciousness, visceral pain-related behaviors (licking of the abdomen, stretching the abdomen, squashing of the lower abdomen against the floor or abdominal retractions) were assessed during a 30 min period (in blocks of 5 min). Pain behaviors were visually assessed by 2 independent researchers.
Statistical analysis
Data are expressed as mean ± SEM, except for bacterial quantification by qPCR which is given as median (interquartile range) ± SD. A robust analysis (one iteration) was used to obtain mean ± SEM for RT-qPCR data. Data were analyzed by a parametric unpaired t-test or by a non-parametric Mann-Whitney test as appropriate. A Chi square test was used to analyze bacterial adherence. Data were considered statistically significant when P < 0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
E. Martínez and A. Acosta are thanked for their technical assistance. Dr. I. Badiola is thanked for their help in the obtention of some of the reference bacterial strains for PCR quantification. Dr. J. Grootjans is thanked for kindly sharing the metha-carnoy protocol.
Funding
This study was supported by grants BFU2009–08229 and BES-2010–037699 (FPI program; M. A. personal support) from the Ministerio de Ciencia e Innovación (Spain) and 2009SGR708 from the Generalitat de Catalunya.
References
- Sood R, Law GR, Ford AC. Diagnosis of IBS: symptoms, symptom-based criteria, biomarkers or “psychomarkers”? Nat Rev Gastroenterol Hepatol2014; 11(11):683-91; http://dx.doi.org/10.1038/nrgastro.2014.127
- Simrén M. IBS with intestinal microbial dysbiosis: a new and clinically relevant subgroup? Gut 2014; 63:1685-6; PMID:24569059; http://dx.doi.org/10.1136/gutjnl-2013-306434
- Lee YJ, Park KS. Irritable bowel syndrome: emerging paradigm in pathophysiology. World J Gastroenterol 2014; 20:2456-69; PMID:24627583; http://dx.doi.org/10.3748/wjg.v20.i10.2456
- Ishihara S, Tada Y, Fukuba N, Oka A, Kusunoki R, Mishima Y, Oshima N, Moriyama I, Yuki T, Kawashima K, et al. Pathogenesis of irritable bowel syndrome-review regarding associated infection and immune activation. Digestion 2013; 87:204-11; PMID:23712295; http://dx.doi.org/10.1159/000350054
- O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7:688-93; PMID:16819463;http://dx.doi.org/10.1038/sj.embor.7400731
- Cerf-Bensussan N, Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 2010; 10:735-44; PMID:20865020; http://dx.doi.org/10.1038/nri2850
- Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol 2008; 8:411-20; PMID:18469830; http://dx.doi.org/10.1038/nri2316
- Collins SM, Bercik P. The relationship between intestinal microbiota and the central nervous system in normal gastrointestinal function and disease. Gastroenterology 2009; 136:2003-14; PMID:19457424; http://dx.doi.org/10.1053/j.gastro.2009.01.075
- De Palma G, Collins SM, Bercik P, Verdu EF. The microbiota-gut-brain axis in gastrointestinal disorders: stressed bugs, stressed brain or both? J Physiol 2014; 592:2989-97; PMID:24756641; http://dx.doi.org/10.1113/jphysiol.2014.273995
- Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med 2014; 68:122-33; PMID:24275541; http://dx.doi.org/10.1016/j.freeradbiomed.2013.11.008
- Duerkop BA, Vaishnava S, Hooper L V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 2009; 31:368-76; PMID:19766080; http://dx.doi.org/10.1016/j.immuni.2009.08.009
- Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 2013; 14:685-90; PMID:23778796; http://dx.doi.org/10.1038/ni.2608
- Foxx-Orenstein AE, Chey WD. Manipulation of the Gut Microbiota as a Novel Treatment Strategy for Gastrointestinal Disorders. Am J Gastroenterol Suppl 2012; 1:41-6.
- Jalanka-Tuovinen J, Salojärvi J, Salonen A, Immonen O, Garsed K, Kelly FM, Zaitoun A, Palva A, Spiller RC, de Vos WM. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2013; 63:1737-45; PMID:24310267; http://dx.doi.org/10.1136/gutjnl-2013-305994
- Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG. Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 2013; 62:159-76; PMID:22730468; http://dx.doi.org/10.1136/gutjnl-2012-302167
- Söderholm JD, Yang P-C, Ceponis P, Vohra A, Riddell R, Sherman PM, Perdue MH. Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology 2002; 123:1099-108; PMID:12360472; http://dx.doi.org/10.1053/gast.2002.36019
- Verdu EF, Collins SM. Irritable bowel syndrome and probiotics: from rationale to clinical use. Curr Opin Gastroenterol 2005; 21:697-701; PMID:16220048; http://dx.doi.org/10.1097/01.mog.0000182861.11669.4d
- Verdú EF, Bercik P, Verma-Gandhu M, Huang X-X, Blennerhassett P, Jackson W, Mao Y, Wang L, Rochat F, Collins SM. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut 2006; 55:182-90; PMID:16105890; http://dx.doi.org/10.1136/gut.2005.066100
- Aguilera M, Vergara P, Martínez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol Motil 2013; 25:e515-29; PMID:23711047; http://dx.doi.org/10.1111/nmo.12154
- Barbara G, Stanghellini V, Brandi G, Cremon C, Di Nardo G, De Giorgio R, Corinaldesi R. Interactions between commensal bacteria and gut sensorimotor function in health and disease. Am J Gastroenterol 2005; 100:2560-8; PMID:16279914; http://dx.doi.org/10.1111/j.1572-0241.2005.00230.x
- Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, Dubuquoy C, Merour E, Geboes K, Chamaillard M, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med 2007; 13:35-7; PMID:17159985; http://dx.doi.org/10.1038/nm1521
- Collins S, Verdu E, Denou E, Bercik P. The role of pathogenic microbes and commensal bacteria in irritable bowel syndrome. Dig Dis 2009; 27(Suppl 1):85-9; PMID:20203502; http://dx.doi.org/10.1159/000268126
- Goto Y, Ivanov II. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol Cell Biol 2013; 91:204-14; PMID:23318659; http://dx.doi.org/10.1038/icb.2012.80
- Salzman NH, Bevins CL. Dysbiosis—A consequence of Paneth cell dysfunction. Semin Immunol 2013; 25:334-41; PMID:24239045; http://dx.doi.org/10.1016/j.smim.2013.09.006
- Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ, Versalovic J, Verdu EF, Dinan TG, Hecht G, et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 2013; 4:17-27; PMID:23202796; http://dx.doi.org/10.4161/gmic.22973
- Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun 2011; 79:1536-45; PMID:21321077; http://dx.doi.org/10.1128/IAI.01104-10
- Domínguez PM, Ardavín C. Differentiation and function of mouse monocyte-derived dendritic cells in steady state and inflammation. Immunol Rev 2010; 234:90-104; PMID:20193014; http://dx.doi.org/10.1111/j.0105-2896.2009.00876.x
- Hill DA, Hoffmann C, Abt MC, Du Y, Kobuley D, Kirn TJ, Bushman FD, Artis D. Metagenomic analyses reveal antibiotic-induced temporal and spatial changes in intestinal microbiota with associated alterations in immune cell homeostasis. Mucosal Immunol 2010; 3:148-58; PMID:19940845; http://dx.doi.org/10.1038/mi.2009.132
- Puhl NJ, Uwiera RRE, Yanke LJ, Selinger LB, Inglis GD. Antibiotics conspicuously affect community profiles and richness, but not the density of bacterial cells associated with mucosa in the large and small intestines of mice. Anaerobe 2012; 18:67-75; PMID:22185696; http://dx.doi.org/10.1016/j.anaerobe.2011.12.007
- Croswell A, Amir E, Teggatz P, Barman M, Salzman NH. Prolonged impact of antibiotics on intestinal microbial ecology and susceptibility to enteric Salmonella infection. Infect Immun 2009; 77:2741-53; PMID:19380465; http://dx.doi.org/10.1128/IAI.00006-09
- Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 2012; 37:1369-78.; PMID:22483040; http://dx.doi.org/10.1016/j.psyneuen.2012.03.007
- Barbara G, De Giorgio R, Stanghellini V, Cremon C, Corinaldesi R. A role for inflammation in irritable bowel syndrome? Gut 2002; 51(Suppl 1):i41-4; PMID:12077063; http://dx.doi.org/10.1136/gut.51.suppl_1.i41
- Xu D, Gao J, Gillilland M, Wu X, Song I, Kao JY, Owyang C. Rifaximin alters intestinal bacteria and prevents stress-induced gut inflammation and visceral hyperalgesia in rats. Gastroenterology 2014; 146:484-96. e4; PMID:24161699; http://dx.doi.org/10.1053/j.gastro.2013.10.026
- Barnes MJ, Powrie F. Immunology. the gut's clostridium cocktail. Science 2011; 331:289-90; PMID:21252334; http://dx.doi.org/10.1126/science.1201291
- Koboziev I, Reinoso Webb C, Furr KL, Grisham MB. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med 2013; 68C:122-33.
- Swidsinski A, Loening-Baucke V, Lochs H, Hale L-P. Spatial organization of bacterial flora in normal and inflamed intestine: a fluorescence in situ hybridization study in mice. World J Gastroenterol 2005; 11:1131-40; PMID:15754393; http://dx.doi.org/10.3748/wjg.v11.i8.1131
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 10:131-44; PMID:20098461; http://dx.doi.org/10.1038/nri2707
- Terán-Ventura E, Aguilera M, Vergara P, Martínez V. Specific changes of gut commensal microbiota and TLRs during indomethacin-induced acute intestinal inflammation in rats. J Crohns Colitis 2014; 8:1043-54; PMID:24566169; http://dx.doi.org/10.1016/j.crohns.2014.02.001
- Terán-Ventura E, Roca M, Martin MT, Abarca ML, Martinez V, Vergara P. Characterization of housing-related spontaneous variations of gut microbiota and expression of toll-like receptors 2 and 4 in rats. Microb Ecol 2010; 60:691-702; PMID:20717659; http://dx.doi.org/10.1007/s00248-010-9737-z
- Reikvam DH, Erofeev A, Sandvik A, Grcic V, Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA, Johansen F-E. Depletion of murine intestinal microbiota: effects on gut mucosa and epithelial gene expression. PLoS One 2011; 6:e17996; PMID:21445311
- Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr 2001; 73:1131S-41S; PMID:11393191
- Boué J, Basso L, Cenac N, Blanpied C, Rolli-Derkinderen M, Neunlist M, Vergnolle N, Dietrich G. Endogenous regulation of visceral pain via production of opioids by colitogenic CD4(+) T cells in mice. Gastroenterology 2014; 146:166-75; PMID:24055279; http://dx.doi.org/10.1053/j.gastro.2013.09.020
- Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PLoS One 2013; 8:e63893; PMID:23691109
- Brusberg M, Arvidsson S, Kang D, Larsson H, Lindström E, Martinez V. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents. J Neurosci 2009; 29:1554-64; PMID:19193902; http://dx.doi.org/10.1523/JNEUROSCI.5166-08.2009
- Wright KL, Duncan M, Sharkey KA. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br J Pharmacol 2008; 153:263-70; PMID:17906675; http://dx.doi.org/10.1038/sj.bjp.0707486
- Jardí F, Fernández-Blanco JA, Martínez V, Vergara P. Plasticity of dorsal root ganglion neurons in a rat model of post-infectious gut dysfunction: potential implication of nerve growth factor. Scand J Gastroenterol 2014; 49:1296-303; PMID:25259719; http://dx.doi.org/10.3109/00365521.2014.958524
- Meseguer V, Alpizar YA, Luis E, Tajada S, Denlinger B, Fajardo O, Manenschijn J-A, Fernández-Peña C, Talavera A, Kichko T, et al. TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat Commun 2014; 5:3125; PMID:24445575; http://dx.doi.org/10.1038/ncomms4125
- Tramullas M, Finger BC, Moloney RD, Golubeva A V, Moloney G, Dinan TG, Cryan JF. Toll-like receptor 4 regulates chronic stress-induced visceral pain in mice. Biol Psychiatry 2014; 76:340-8; PMID:24331544; http://dx.doi.org/10.1016/j.biopsych.2013.11.004
- Cash BD. Emerging role of probiotics and antimicrobials in the management of irritable bowel syndrome. Curr Med Res Opin 2014; 30:1405-15; PMID:24666019; http://dx.doi.org/10.1185/03007995.2014.908278
- Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011; 33:1123-32; PMID:21418261; http://dx.doi.org/10.1111/j.1365-2036.2011.04633.x
- Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol 2010; 26:17-25; PMID:19881343; http://dx.doi.org/10.1097/MOG.0b013e328333dc8d
- Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol 2000; 95:3503-6; PMID:11151884; http://dx.doi.org/10.1111/j.1572-0241.2000.03368.x
- Anitha M, Vijay-Kumar M, Sitaraman S V, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology 2012; 143:1006-16. e4; PMID:22732731; http://dx.doi.org/10.1053/j.gastro.2012.06.034
- Bär F, Von Koschitzky H, Roblick U, Bruch HP, Schulze L, Sonnenborn U, Böttner M, Wedel T. Cell-free supernatants of Escherichia coli Nissle 1917 modulate human colonic motility: evidence from an in vitro organ bath study. Neurogastroenterol Motil 2009; 21:559-66, e16–7; PMID:19220758; http://dx.doi.org/10.1111/j.1365-2982.2008.01258.x
- Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier C-H, Matuchansky C. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther 2007; 26:475-86; PMID:17635382; http://dx.doi.org/10.1111/j.1365-2036.2007.03362.x
- Quigley EMM. Bacteria: a new player in gastrointestinal motility disorders–infections, bacterial overgrowth, and probiotics. Gastroenterol Clin North Am 2007; 36:735-48, xi; PMID:17950446; http://dx.doi.org/10.1016/j.gtc.2007.07.012
- Van der Waaij D, Berghuis-de Vries JM, Korthals Altes C. Oral dose and faecal concentration of antibiotics during antibiotic decontamination in mice and in a patient. J Hyg (Lond) 1974; 73:197-203; PMID:4528886
- Aguilera M, Vergara P, Martínez V. Environment-related adaptive changes of gut commensal microbiota do not alter colonic toll-like receptors but modulate the local expression of sensory-related systems in rats. Microb Ecol 2013; 66:232-43; PMID:23666270; http://dx.doi.org/10.1007/s00248-013-0241-0
- Furet J-P, Firmesse O, Gourmelon M, Bridonneau C, Tap J, Mondot S, Doré J, Corthier G. Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS Microbiol Ecol 2009; 68:351-62; PMID:19302550; http://dx.doi.org/10.1111/j.1574-6941.2009.00671.x
- Zwielehner J, Lassl C, Hippe B, Pointner A, Switzeny OJ, Remely M, Kitzweger E, Ruckser R, Haslberger AG. Changes in human fecal microbiota due to chemotherapy analyzed by TaqMan-PCR, 454 sequencing and PCR-DGGE fingerprinting. PLoS One 2011; 6:e28654; PMID:22194876
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8.
- Martínez V, Thakur S, Mogil JS, Taché Y, Mayer EA. Differential effects of chemical and mechanical colonic irritation on behavioral pain response to intraperitoneal acetic acid in mice. Pain 1999; 81:179-86; PMID:10353506; http://dx.doi.org/10.1016/S0304-3959(99)00008-1
- Harmsen HJM, Raangs GC, He T, Degener JE, Welling GW. Extensive set of 16S rRNA-based probes for detection of bacteria in human feces. Appl Environ Microbiol 2002; 68:2982-90; PMID:12039758; http://dx.doi.org/10.1128/AEM.68.6.2982-2990.2002
- Haarman M, Knol J. Quantitative real-time PCR analysis of fecal Lactobacillus species in infants receiving a prebiotic infant formula. Appl Environ Microbiol 2006; 72:2359-65; PMID:16597930; http://dx.doi.org/10.1128/AEM.72.4.2359-2365.2006
- Salzman NH, de Jong H, Paterson Y, Harmsen HJM, Welling GW, Bos NA. Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology 2002; 148:3651-60; PMID:12427955
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjöberg J, Amir E, Teggatz P, Barman M, Hayward M, Eastwood D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010; 11:76-83; PMID:19855381; http://dx.doi.org/10.1038/ni.1825
