Abstract
Brucella is the causing agent of a chronic zoonosis called brucellosis. The Brucella β-1,2 cyclic glucan (CβG) is a virulence factor, which has been described as a potent immune stimulator, albeit with no toxicity for cells and animals. We first used a genome-wide approach to characterize human myeloid dendritic cell (mDC) responses to CβG. Transcripts related to inflammation (IL-6, IL2RA, PTGS2), chemokine (CXCR7, CXCL2) and anti-inflammatory pathways (TNFAIP6, SOCS3) were highly expressed in CβG-treated mDC. In mouse GMCSF-derived DC, CβG triggered the expression of both activation (CXCL2, KC) and inhibition (SOCS3 and TNFAIP6) molecules. We then characterized the inflammatory infiltrates at the level of mouse ear when injected with CβG or LPS. CβG yielded a lower and transient recruitment of neutrophils compared to LPS. The consequence of these dual pro- and anti-inflammatory signals triggered by CβG corresponds to the induction of a controlled local inflammation.
Abbreviations
| CβG | = | Beta-1,2 cyclic glucan |
| LPS | = | lipopolysaccharide |
| DC | = | dendritic cells |
| E. coli | = | Escherichia coli |
Introduction
Brucellosis is a zoonosis affecting humans, farm animals and livestock. Brucella, the agent of brucellosis, is a pathogenic Gram-negative bacterium belonging to the α-2 proteobacteria group. The World Health Organization (WHO) has classified brucellosis among the top 7 “neglected zoonoses,” a group of diseases that are simultaneously a threat to human health and a cause of poverty.Citation1 It is now recognized that in countries such as Mongolia and northern China, brucellosis is becoming a threat for populations living in close contact with animals.
Pathogenic brucellae can efficiently replicate within the endoplasmic reticulum of infected macrophages and dendritic cells, a safe intracellular niche located at the crossroad of many vital host cell functions. The three main species leading to brucellosis in humans are B. melitensis, B. suis and B. abortus. B melitensis is the most potent among the Brucella species, since 1–5 bacteria are enough to cause the disease. In addition, the existing vaccine against B. melitensis (vaccine Rev 1) is virulent for humans, resistant to some antibiotics used to treat human brucellosis, and not stable (spontaneous change from smooth (S) full potency to a more attenuated rough (R) phenotype), which leads to reduced protection efficacy.
Over the last decade, most studies have focused on the understanding of the role of virulence factors expressed by pathogenic brucellae. However, their role in virulence and the characterization of the mechanisms involved, including the characterization of associated host cell counterparts, have only been described for a few of them and reviewed in refs.Citation2,3 In addition to their role in Brucella replication and intracellular trafficking, some virulence factors were shown to play an important role in dampening both innate and adaptive immunity. For example, BtpA and BtpB act as inhibitors of TLR signaling Citation4,5 and the wadB and wadC genes encoding mannosyl transferases bring mannosyl residues in the core region of Brucella LPS to avoid its recognition by TLR4.Citation6 Mutant strains lacking these proteins lead to enhanced recognition by innate receptors, increased inflammation and a strong adaptive immune response. wadC and wadB mutants can even induce protection in mice.Citation6
Recently, we described the β-1,2 cyclic glucan (CβG), a virulence factor synthesized by Brucella and concentrated in the periplasm of the bacterium. This polysaccharide is composed of a cyclic backbone of 17 to 25 glucose units in β-1,2 linkages and can harbor substitutions such as succinyl, mevalonyl and methyl groups. Brucella CβG was demonstrated to modulate lipid raft organization both at the plasma membrane of infected cells and intracellularly at the site of the Brucella-containing vacuole.Citation7 CβG is expressed in large amounts, representing 1–5% of the bacteria dry weight. When bacteria are killed by the host immune system, CβG is therefore released in the surrounding inflammatory environment in μM concentrations. This may have important consequences for the modulation of intracellular trafficking of the bacterium by shaping the lipid microdomain composition of the Brucella-containing vacuole and by modulating host immune responses. Brucella CβG seems to signal through TLR4 without the contribution of CD14.Citation8 In contrast to BtpA, BtpB and WadC virulence factors, which are involved in the inhibition of immune responses, Brucella CβG is a strong activator of both human and mouse DC, promoting pro-inflammatory cytokine expression, antigen cross-priming and cross-presentation to specific CD8+ T cells. However, unlike E. coli LPS and its derivatives, CβG does not show endotoxicity both in vitro and in vivo, and has recently been referred as a new class of adjuvants.
We therefore asked how the lack of endotoxicity and the strong immune response generated by CβG could be reconciled. Herein, we identify differential expression profiles between Escherichia coli (E.coli) LPS and CβG-stimulated mDC, highlighting specific anti-inflammatory networks. CβG leads to a transient neutrophil recruitment at the site of injection, thus leading to a reduced inflammation compared to E. coli LPS.
Results
Brucella CβG differentially modulates transcriptomic responses in human blood mDC
We previously described a number of immune pathways modulated by the Brucella CβG in human blood mDC.Citation8 Herein, we compared transcriptional profiles of mDC stimulated with CβG or E. coli LPS. We identified 34 genes that were expressed at a level at least 4.5-fold higher in CβG-stimulated mDC than in E. coli LPS-stimulated cells (). As previously described,Citation8 some of these genes were related to inflammation (IL-6, BATF, IL2RA, PTGS2), or chemokines pathways (CXCR7, CXCL2) while others such as TNFAIP6 and SOCS3 were related to anti-inflammatory pathways.Citation9
Table 1. Fold changes in gene expression in CβG- versus E. coli LPS-treated human blood mDC. lists 34 genes over-expressed at least 4.5-fold higher in CβG-stimulated cells compared to E. coli LPS-stimulated cells (at 6 h post-treatment). Data were normalized against cells treated for 6 h with culture media only
Here, we show that Brucella CβG triggers the transcription of regulatory genes, including those involved in the inhibition of pro-inflammatory cytokine secretion. An hypothesis would be to slow down the inflammatory process and perhaps to prevent further toxicity, a unique property of this new class of adjuvant ().Citation7,8 Several transcripts encoding chemokines (CXCL2, IL8, CCL5, and CCL20) were highly expressed in CβG-stimulated mDC ().Citation10 Interestingly, CβG as well as E. coli LPS induced the transcription of SOCS genes, which are known to encode regulatory elements involved in the control of inflammation ().Citation11 We focused on the expression levels of CXCL2, TNFAIP6, PTGS2, SOCS3, and IL8 () since these transcripts were highly expressed in both CβG-stimulated and E. coli LPS-stimulated mDC. CXCL2 and IL8 are chemokines that were previously described to promote neutrophil recruitment,Citation10 while SOCS3 negatively regulates pro-inflammatory cytokine secretion.Citation12,13 PTGS2 is involved in prostaglandins metabolism and TNFAIP6 has been shown to be an anti-inflammatory molecule.Citation14,15
Figure 1. Transcriptional profiling of human blood mDC from healthy donors stimulated with CβG (n = 5) or LPS (n = 4), respectively.(A) Heatmap representing the transcriptional expression levels of 15 regulatory genes differentially expressed between CβG and medium control (Welch T-test, P < 0.05). Data are normalized to the 6 h medium control for each donor. (B) Heatmap representing the transcriptional expression levels of 18 chemokines differentially expressed in mDc treated with CβG, LPS or cell culture medium. (C) Barcharts representing the raw expression value of CXCL2, IL8, PTGS2, SOCS3 and TNFAIP6 for the average of the 4 donors. Error bars represent the standard deviation. Ordinary one-way ANOVA with Tukey post-hoc test was conducted (***: P < 0.001; **: P < 0.01; *: P < 0.05; n.s: not significant).
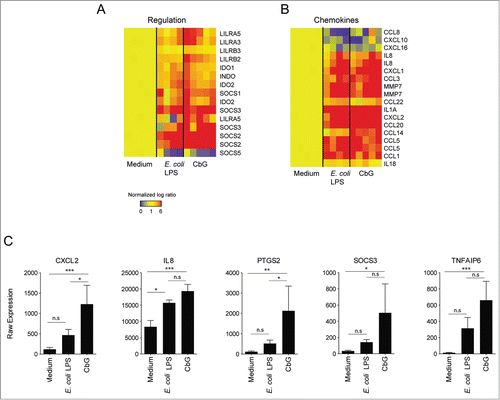
Corroborating previous results, Brucella CβG triggered pro-inflammatory cytokine expression Citation8 but also induced the transcription of anti-inflammatory genes such as socs, tnfaip6, lilrb and ido2 ().Citation16-18
Brucella CβG induces both inflammatory and anti-inflammatory responses in mouse DC
The expression of CXCL2, KC, PTGS2, SOCS3 and TNFAIP6 mRNAs in Brucella CβG and E. coli LPS-stimulated murine BMDC was analyzed by RT-PCR (). We observed a strong induction of all these genes at 8 h post-stimulation, which declined at 24 h post-stimulation. CβG- and E. coli LPS-treated BMDC expressed similar levels of CXCL2 and TNFAIP6 transcripts at 8 h post-stimulation ().
Figure 2 (See previous page). Induction of gene expression in murine DC stimulated with Brucella CβG or E. coli LPS. (A) Murine BMDC were stimulated for 8 h and 24 h with E. coli LPS (black bars) or Brucella CβG (gray bars). mRNA was extracted from stimulated cells and qPCR performed to determine transcript expression levels of CXCL2, KC and PTGS2. Fold-increases were estimated by comparing with cells that had been stimulated with PBS as a negative control. Basal expression levels for each gene are indicated (dash line). HPRT was used as a housekeeping gene to normalize the data. An induction level of more than 2 is considered as significant. Mean ± standard deviation of 3 independent experiments is represented here. Mann-Whitney test, one-tailed (analysis on GraphPad Prism) has been done and P < 0.05 is denoted by “*."(B) Expression levels of SOCS3 and TNFAIP6 mRNA were assessed. Experiments were processed as described above. Three independent experiments were carried out. An induction level of more than 2 is considered as significant. Mean ± standard deviation of 3 independent experiments is represented here. (C) BMDC stimulated for 8 h or 24 h with PBS (control), E. coli LPS or Brucella CβG were lysed and protein purified. The expression of tsg-6 protein was assessed by western blot using 10 μg of recombinant tsg-6 as a positive control. β-actin expression was used as control. At least 3 independent experiments were carried out and one representative is shown here.
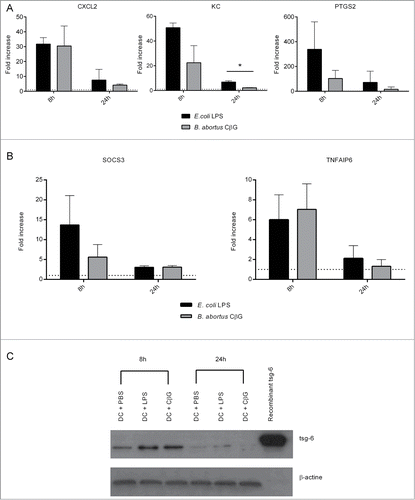
We then validated BMDC transcriptional profiles at the protein level. We measured the expression level of the tsg-6 protein, the product of the tnfaip6 gene (). tsg-6 was highly expressed in murine BMDC at 8 h stimulation and, as observed at the transcriptional level, its expression decreased at 24 h post-injection in all conditions ().
In BMDC, E. coli LPS and Brucella CβG both act on inflammatory and on anti-inflammatory pathways, through the control of expression of chemokines on one hand and on SOCS and TNFAIP6, respectively. The inflammatory and anti-inflammatory induction corresponded to an early response (8 h post-stimulation).
Injection of Brucella CβG into mouse ear induces the recruitment of innate immune cells in vivo
Since we observed a high expression of chemokines transcripts in both murine and human DC stimulated with E. coli LPS and Brucella CβG, we measured the levels of inflammation triggered at the site of injection in mouse ears after intradermal immunization. At 48 h post-injection, ear tissue was recovered and hematoxylin/eosin stained.
Injection with CβG or E. coli LPS led to the formation of an edema with cell infiltrates, which was not observed in PBS-injected control mice (). However, in CβG-injected ears, the edema was significantly smaller than in E. coli LPS-injected ears, suggesting a lower level of inflammation in response to CβG.
Figure 3. Brucella CβG induces the recruitment of CD11b+, LyG6+, and Gr1+ cells at the site on injection. (A) Mouse ears intradermally injected with PBS, E. coli LPS or Brucella CβG were recovered at 48 h post-injection and stained for hematoxylin and eosin. (B) Mouse ears injected with PBS, E. coli LPS or Brucella CβG were recovered at 48 h post-injection, embedded in tissueteck OCT compound and frozen in isopentan. 15 μm thick cryosections were then stained with CD11b (blue), Gr1 (red), Ly6G (green) and DAPI (gray) before observation under a Zeiss LSM 510. Bar: 0.25 mm.
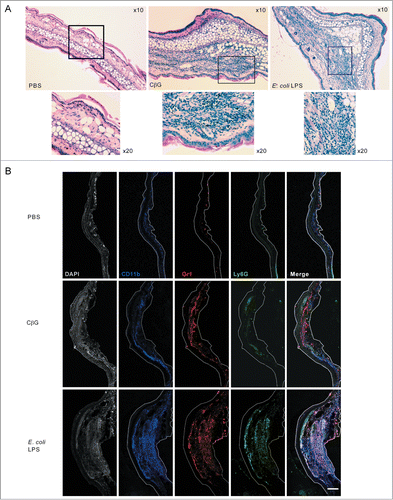
To determine which cells were recruited at the inflammatory site, we immuno-stained ear sections to detect monocytes (CD11b+, Gr1+) and neutrophils (CD11b+, Gr1+ and Ly6G+) by confocal microscopy (). Although these 3 markers were localized in both E. coli LPS- and CβG-treated ears, LPS-injected ears showed a higher recruitment of neutrophils (white labeling indicating the presence of neutrophils positive for CD11b+, Gr1+ Ly6G+) ().
Brucella CβG induces a transient recruitment of neutrophils
We then characterized the kinetics of neutrophil recruitment at the site of injection. To this end, we injected PBS, E. coli LPS or CβG and harvested ears at 2 h, 6 h, 12 h, 24 h or 48 h post-injection. Cells were stained for F4/80 and Ly6G and analyzed by flow cytometry ().
Figure 4. Brucella CβG induces a transient neutrophil recruitment at 12 h post-injection. (A) Cells were extracted from mouse ears at 2 h, 6 h, 12 h, 24 h and 48 h following injection with PBS (white bars), E. coli LPS (black bars) or Brucella CβG (gray bars) (see ) and neutrophils were quantified by flow cytometry. Mean ± SD of 3 independent experiments is represented here, Mann-Whitney test, one-tailed (analysis on GraphPad Prism) has been done and P < 0.05 is denoted by “*."(B) Dot-plots of neutrophil recruitment to the ear at 12 h post-injection with PBS, E. coli LPS or CβG. Cells were gated on CD45+, MHC II−, CD11b+, Ly6C+. Neutrophils represented in blue population are negative for F4/80 and positive for Ly6G. (C) Dot-plots of neutrophil recruitment to the ear at 24 h post-injection with PBS, E. coli LPS or CβG. Cells were gated on CD45+, MHC II−, CD11b+ and Ly6C+. Neutrophils (in blue) are negative for F4/80 and positive for Ly6G. Three independent experiments were carried out, and one representative is shown here.
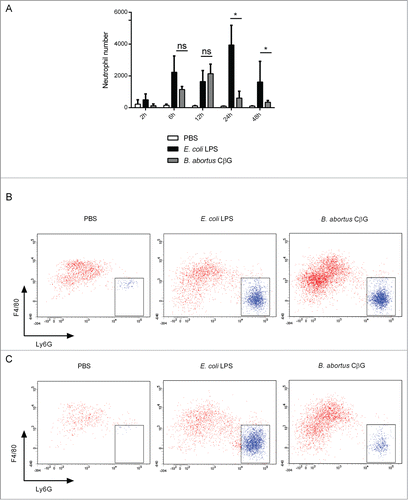
In E. coli LPS-injected ears, neutrophils (F4/80−, Ly6G+) were recruited as soon as 2 h post-injection and increased in number until 24 h when almost 4,000 neutrophils were recruited to finally reach about 2,000 cells at 48 h post-injection (). In contrast to E. coli LPS, in CβG-treated ears, neutrophil recruitment was first detected at 6 h post-injection, reached a maximum at 12 h post-injection with more than 2,000 neutrophils recruited (), and then strongly decreased from 24 h onwards (), thereby corroborating histology experiments ().
Discussion
Brucella is considered as a stealthy pathogen aiming at: keeping the host immune response under control and avoiding strong inflammatory processes detrimental to the survival of the bacterium. Up to now, most virulence factors expressed by pathogenic Brucella were found to be involved in dampening immune responses whereas others were shown to be activators. For instance, activation of macrophages by PrpA leads to the proliferation of B cells.Citation19,20 In contrast, Brucella BtpA and BtpB are effector proteins that limit inflammation in infected DC.Citation5,8 WadC is a mannosyltransferase, which has been shown to protect Brucella LPS from detection and recognition by TLR4.Citation6 Interestingly, CβG has been described as a virulence factor in macrophages but not in dendritic cells and shown to be a strong activator of the immune systemCitation4,7,8 since DC infected with cgs− synthase null-mutants do not show any sign of DC activation.Citation8
Whereas most virulence factors act as inhibitors of host cell function, CβG may bring infected DC to an intermediate level of maturation, which might turn T lymphocyte responses toward a Th2 profile, thus favoring the pathogen. Moreover, CβG does not show any toxicity, a characteristic that fits very well with Brucella and its goal to induce a chronic disease where the pathogen can survive and persist.Citation7 In this context, Brucella may have selected upon evolution the CβG molecule to allow a transient activation of host immune pathways in the absence of toxicity. As we demonstrate herein, CβG induces a combination of inflammatory and anti-inflammatory pathways that directs a transient inflammation to better facilitate the control of neutrophil recruitment, corroborating previous studies on neutrophil-Brucella interactions.Citation21 Indeed, injection of CβG into mouse ear led to a local inflammation characterized by an edema and a fast and transient neutrophil recruitment. In contrast, in E. coli LPS-injected ears, edema was larger and neutrophil stayed longer in the tissue suggesting a higher and prolonged inflammation. This difference could be due to a faster resolution of inflammation in CβG-injected ears or to a less important inflammation triggered at the onset of injection. A recent in vivo infection study showed that neutrophils could play different roles, protective or deleterious for the host, depending on the stage of the disease.Citation21 At early time point (5 d post-infection) recruitment of neutrophils induced a decrease of bacterial load.Citation21 Then, the presence of neutrophils seemed to be less important to control the chronic phase of the disease and bacteria could multiply.Citation21 The killing of bacteria at early time points may correspond to the release of large quantities of CβG in the tissue participating to a transient neutrophil recruitment. This action would be associated to a dual pro-inflammatory and anti-inflammatory response mediated by DC.
In vivo, Brucella enhances the recruitment and activation of DC in infected organs such as the spleen.Citation22 These cells also participate to granuloma formation. Interestingly, among the cells surrounding granulomas, Ly6G+ CD11b+ granulocytes were found.Citation22 These cells may correspond to Ly6G+ CD11b+ neutrophils we detected in CβG-treated ears.
CβG also represents a potential new adjuvant that could be used in vaccine design. Different Brucella components such as outer membrane proteins have been studied for their role in inducing protective immunity in cattle, which are of the main reservoir for the bacterium.Citation23 Studying CβG properties in relevant animals such as cows or sheep would lead to an enhancement of vaccine efficacy by using appropriate adjuvants such as CβG. Moreover, the need of new adjuvants without toxicity enhancing CD8+ memory responses is a priority in order to find new ways of inducing protective Th1 and Th2 immunity.
Materials and Methods
Cell culture
BMDC were prepared from 6–10 week-old female C57BL/6 mice as previously described.Citation4 Briefly, tibias and femur were removed from mice; bone marrow was harvested by flushing with RPMI-1640 (Gibco, Life Technologies). Red blood cells are then lysed, and after 3 washes cells were seeded onto 6 well plates at 0.6 × 106 cells/ml. Cells were grown in RPMI-1640, 5% FCS, 50 μM β-mercaptoethanol. Human monocyte-derived DC were purified from blood using Ficoll (Ge Healthcare), cells were cultivated in serum-free Cellgro DC culture media supplemented with 100 ng/ml GM-CSF and 20 ng/ml IL-4.
Reagents and antibodies
Purified cyclic glucan was obtained from Brucella abortus 2308 as previously described. Citation8 E. coli LPS (055:B5) was purchased from Sigma Aldrich. Cells were stimulated with 10 μg/ml of CβG or 100 ng/ml or LPS corresponding to the same molarity (0.25 μM). Flow cytometry antibodies were: anti Ly6G-V450 (1A8), anti CD45.2-PerCP-Cy5.5 (104), anti Ly6C-PE-CF594 (AL-21), anti CD64-AF647 (X54–5/7.1) from BD Biosciences; anti CD24-AF488 (M1/69), anti F4/80-PE (CI :A3-1), anti CD11b-APC/Cy7 (M1/70), anti CD150-PECy7 (TC15-12F12.2) from BioLegend, anti I-A/I-E-A700 (M5/114–15.2) from eBiosciences. Antibodies used for confocal microscopy were: anti Gr1-PE (RB6–8C5), anti CD11b-AF647 (M1/70) from BioLegend; anti-Ly6G (1A8) from BD Biosciences; anti-rat AF555 (A21434) from Invitrogen. Human/Mouse TSG-6 MAb (RD systems) was used to detect the tsg-6 protein in western blots using anti-mouse HRP (Invitrogen) as a secondary antibody. Western blots were revealed using Amersham ECL Detection system (GE Healthcare).
Mouse immunization
6–10 weeks old C57BL/6 females were intradermally injected into the internal face of the ear with 200 μg of CβG, 10 μg of E. coli LPS (Sigma Aldrich) or PBS as a negative control. At 48 h post-injection mice were sacrificed and ears recovered for further analyses.
mRNA extraction and hybridization for transcriptomic analysis
RNA was extracted and purified from purified blood human mDC. RNA hybridization performed using Illumina HT12 v4 Beadchip arrays was performed as previously described.Citation8
Bioinformatics analysis of microarrays was performed as previously described.Citation8 Briefly, pathway analysis was carried out using the Ingenuity Pathway Analysis (IPA) software. A non-parametric test was applied to the different samples from 4 donors with a false discovery rate of 0.01. Only significant transcripts were considered, transcripts presented were normalized to their control, the transcripts showed here have an absolute fold changes equal or superior to 2. Genespring 7.3 was used for analysis and generating heatmaps and GraphPad Prism 5 was used for barcharts.
mRNA extraction and RT
Total RNAs were extracted from infected BMDC using RNeasy Mini Kit (Qiagen) following the manufacturer's instructions. cDNA were generated by using Quantitech Reverse Transcription Kit (Qiagen) following the manufacturer's instructions and using 300 ng of RNA as matrix.
qPCR
Recovered cDNA (2 μl) was used as a template for qPCR following the manufacturer's instructions using a 7500 Fast Real-time PCR (Applied Biosystem). Primers used are listed in . HPRT was used as a housekeeping gene to determine ΔCt. Fold increases were determined by comparing control (non-treated) and treated cells. mRNA expressed more than 2 fold more were considered as significantly upregulated.
Table 2. Sequence of the qPCR primers used
Protein extraction
BMDC were harvested, washed once in PBS, and cell pellets frozen at – 80° C. For protein purification, cell pellets were resuspended in lysis buffer (PBS containing 0.5% of NP-40 and proteases inhibitor (Roche Diagnosis)) and the resulting cell lysate was centrifuged at 10,000 g for 10 min at 4°C. The supernatants were recovered and analyzed by SDS-PAGE and protein gel blot.
Immunohistochemistry
Ears were recovered and fixed with 10% formalin for 48 h and embedded in paraffin. A Leica RM2245 microtome was used to prepare 5 μm slides, which were then stained with hematoxylin and eosin. Images were subsequently acquired using a Nikon Eclispe Ci.
Confocal microscopy
Ear tissues were embedded in tissue-tec OCT (Sakura Finetek). 15 μm cryosections were first incubated in PBS containing 2% BSA for 30 min, then overnight with primary antibodies, and thereafter for 1 h with secondary antibodies. Slides were mounted with ProLong Gold containing DAPI (Invitrogen). Images were acquired using a LSM 510 confocal microscope (Carl Zeiss, Inc.), before analyzed and assembled using ImageJ software (ImageJ).
Ear mouse skin cell isolation
Ear skin tissues were split in 2 sheets (dorsal and ventral) and incubated overnight in PBS containing 2.5 mg/ml dispase II (Roche) at 4°C to separate the dermal and epidermal sheets. The separated epidermal and dermal sheets were then cut in to pieces and incubated for 90 min at 37°C with RPMI containing 1 mg/ml DNase (Sigma Aldrich) and 1 mg/ml collagenase type IV (Worthington Biochemical) to obtain an homogeneous cell suspension.
Flow cytometry
Skin cells were incubated for 10 min with the 2.4G2 antibody to block non-specific signals before staining for 20 min at 4°C with the corresponding fluorescent antibodies. Cells were then washed once in 2% FCS in PBS and once in PBS before fixing in 3% PFA for 20 min at room temperature (RT). At least 400,000 events were collected by flow cytometry using a FACSCantoII (Becton Dickinson) or FACSLSRII UV. Analyses were performed using FlowJo software (TreeStar) and FACS DIVA (BD).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Sean Hanniffy for critical reading and suggestions. We also thank the CIML histology core platform and especially Lionel Chasson.
Funding
CD and AG held fellowships from Aix Marseille Université. This work was supported by the Center National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, Aix-Marseille Université, and the Baylor Institute for Immunology Research.
References
- Ian Maudlin SW-M. The Control of Neglected Zoonotic Diseases – A Route to Poverty Alleviation. Geneva: WHO, 2006.
- de Jong MF, Tsolis RM. Brucellosis and type IV secretion. Future Microbiol 2012; 7:47-58; PMID:22191446; http://dx.doi.org/10.2217/fmb.11.136
- Lacerda TL, Salcedo SP, Gorvel JP. Brucella T4SS: the VIP pass inside host cells. Curr Opin Microbiol 2013; 16:45-51; PMID:23318140; http://dx.doi.org/10.1016/j.mib.2012.11.005
- Salcedo SP, Marchesini MI, Lelouard H, Fugier E, Jolly G, Balor S, Muller A, Lapaque N, Demaria O, Alexopoulou L, et al. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein Btp1. PLoS Pathogens 2008; 4:e21; PMID:18266466; http://dx.doi.org/10.1371/journal.ppat.0040021
- Salcedo SP, Marchesini MI, Degos C, Terwagne M, Von Bargen K, Lepidi H, Herrmann CK, Santos Lacerda TL, Imbert PR, Pierre P, et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front Cell Infect Microbiol 2013; 3:28; PMID:23847770; http://dx.doi.org/10.3389/fcimb.2013.00028
- Conde-Alvarez R, Arce-Gorvel V, Iriarte M, Mancek-Keber M, Barquero-Calvo E, Palacios-Chaves L, Chacon-Diaz C, Chaves-Olarte E, Martirosyan A, von Bargen K, et al. The lipopolysaccharide core of Brucella abortus acts as a shield against innate immunity recognition. PLoS Pathog 2012; 8:e1002675; PMID:22589715; http://dx.doi.org/10.1371/journal.ppat.1002675
- Arellano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, Moreno E, Moriyon I, Gorvel JP. Cyclic beta-1,2-glucan is a Brucella virulence factor required for intracellular survival. Nat Immunol 2005; 6:618-25; PMID:15880113; http://dx.doi.org/10.1038/ni1202
- Martirosyan A, Perez-Gutierrez C, Banchereau R, Dutartre H, Lecine P, Dullaers M, Mello M, Salcedo SP, Muller A, Leserman L, et al. Brucella beta 1,2 cyclic glucan is an activator of human and mouse dendritic cells. PLoS Pathog 2012; 8:e1002983; PMID:23166489; http://dx.doi.org/10.1371/journal.ppat.1002983
- Posselt G, Schwarz H, Duschl A, Horejs-Hoeck J. Suppressor of cytokine signaling 2 is a feedback inhibitor of TLR-induced activation in human monocyte-derived dendritic cells. J Immunol 2011; 187:2875-84; PMID:21844389; http://dx.doi.org/10.4049/jimmunol.1003348
- McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol 1999; 163:2829-35; PMID:10453028
- Krebs DL, Hilton DJ. SOCS: physiological suppressors of cytokine signaling. J Cell Sci 2000; 113(Pt 16):2813-9; PMID:10910765
- Carow B, Rottenberg ME. SOCS3, a major regulator of infection and inflammation. Front Immunol 2014; 5:58; PMID:24600449; http://dx.doi.org/10.3389/fimmu.2014.00058
- Babon JJ, Nicola NA. The biology and mechanism of action of suppressor of cytokine signaling 3. Growth Factors 2012; 30:207-19; PMID:22574771; http://dx.doi.org/10.3109/08977194.2012.687375
- Danchuk S, Ylostalo JH, Hossain F, Sorge R, Ramsey A, Bonvillain RW, Lasky JA, Bunnell BA, Welsh DA, Prockop DJ, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther 2011; 2:27; PMID:21569482; http://dx.doi.org/10.1186/scrt68
- Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood 2011; 118:330-8; PMID:21551236; http://dx.doi.org/10.1182/blood-2010-12-327353
- Anderson KJ, Allen RL. Regulation of T-cell immunity by leucocyte immunoglobulin-like receptors: innate immune receptors for self on antigen-presenting cells. Immunology 2009; 127:8-17; PMID:19368561; http://dx.doi.org/10.1111/j.1365-2567.2009.03097.x
- Katz HR. Inhibition of inflammatory responses by leukocyte Ig-like receptors. Adv Immunol 2006; 91:251-72; PMID:16938543; http://dx.doi.org/10.1016/S0065-2776(06)91007-4
- Trabanelli S, Ocadlikova D, Ciciarello M, Salvestrini V, Lecciso M, Jandus C, Metz R, Evangelisti C, Laury-Kleintop L, Romero P, et al. The SOCS3-independent expression of IDO2 supports the homeostatic generation of T regulatory cells by human dendritic cells. J Immunol 2014; 192:1231-40; PMID:24391212; http://dx.doi.org/10.4049/jimmunol.1300720
- Spera JM, Herrmann CK, Roset MS, Comerci DJ, Ugalde JE. A Brucella virulence factor targets macrophages to trigger B-cell proliferation. J Biol Chem 2013; 288:20208-16; PMID:23720774; http://dx.doi.org/10.1074/jbc.M113.453282
- Spera JM, Ugalde JE, Mucci J, Comerci DJ, Ugalde RA. A B lymphocyte mitogen is a Brucella abortus virulence factor required for persistent infection. Proc Nat Acad Sci U S A 2006; 103:16514-9; PMID:17053080; http://dx.doi.org/10.1073/pnas.0603362103
- Barquero-Calvo E, Martirosyan A, Ordonez-Rueda D, Arce-Gorvel V, Alfaro-Alarcon A, Lepidi H, Malissen B, Malissen M, Gorvel JP, Moreno E. Neutrophils exert a suppressive effect on Th1 responses to intracellular pathogen Brucella abortus. PLoS Pathog 2013; 9:e1003167; PMID:23458832; http://dx.doi.org/10.1371/journal.ppat.1003167
- Copin R, Vitry MA, Hanot Mambres D, Machelart A, De Trez C, Vanderwinden JM, Magez S, Akira S, Ryffel B, Carlier Y, et al. In situ microscopy analysis reveals local innate immune response developed around Brucella infected cells in resistant and susceptible mice. PLoS Pathog 2012; 8:e1002575; PMID:22479178; http://dx.doi.org/10.1371/journal.ppat.1002575
- Tabynov K, Kydyrbayev Z, Ryskeldinova S, Yespembetov B, Zinina N, Assanzhanova N, Kozhamkulov Y, Inkarbekov D, Gotskina T, Sansyzbay A. Novel influenza virus vectors expressing Brucella L7/L12 or Omp16 proteins in cattle induced a strong T-cell immune response, as well as high protectiveness against B. abortus infection. Vaccine 2014; 32:2034-41; PMID:24598723; http://dx.doi.org/10.1016/j.vaccine.2014.02.058

