Abstract
Diarrheal diseases remain a leading cause of global childhood mortality and morbidity. Several recent epidemiological studies highlight the rate of diarrheal diseases in different parts of the world and draw attention to the impact on childhood growth and survival. Despite the well-documented global burden of diarrheal diseases, currently there are no combination diarrheal vaccines, only licensed vaccines for rotavirus and cholera, and Salmonella typhi-based vaccines for typhoid fever. The recognition of the impact of diarrheal episodes on infant growth, as seen in resource-poor countries, has spurred action from governmental and non-governmental agencies to accelerate research toward affordable and effective vaccines against diarrheal diseases. Both travelers and children in endemic countries will benefit from a combination diarrheal vaccine, but it can be argued that the greater proportion of any positive impact will be on the public health status of the latter. The history of combination pediatric vaccines indicate that monovalent or single disease vaccines are typically licensed first prior to formulation in a combination vaccine, and that the combinations themselves undergo periodic revision in response to need for improvement in safety or potential for wider coverage of important pediatric pathogens. Nevertheless combination pediatric vaccines have proven to be an effective tool in limiting or eradicating communicable childhood diseases worldwide. The landscape of diarrheal vaccine candidates indicates that there now several in active development that offer options for potential testing of combinations to combat those bacterial and viral pathogens responsible for the heaviest disease burden—rotavirus, ETEC, Shigella, Campylobacter, V. cholera and Salmonella.
Keywords:
Introduction
Several recent large scale studies of global diarrheal disease burden and epidemiology, renewed recognition of multiple diarrhea episodes as a serious impediment to the health and development of children in resource-poor countries, an upsurge in the investment by charitable foundations and governmental entities in combatting global infectious diseases and the emergence of new concepts in vaccination strategies collectively point to opportunities to develop new vaccines against very old diseases. In this paper, we first review up-to-date information on diarrheal disease burden as a rationale for the pursuit of vaccine development. The history of the development and challenges of combination pediatric vaccines are presented as a model for combination diarrheal vaccines for children in endemic parts of the world as well as for travelers. There are very few existing licensed vaccines against diarrheal diseases, and none are combinations. However, a survey of the current vaccine development landscape indicates that there may be multiple options for a combination vaccine against the leading enteric pathogens. The historic success of combination pediatric vaccines indicates that combinations may be the best approach to address the multiple pathogens responsible for diarrheal diseases worldwide. Combination vaccines require special attention to manufacture and formulation issues, and specific combinations must take into consideration the target population and disease burden. We discuss the potential for a combination ETEC/Shigella vaccine as an example that addresses 2 of the most frequent causes of both endemic and traveler's diarrhea.
Global burden of diarrheal diseases
The Global Burden of Disease (GBD) study, 2010, estimated that although annual rates of childhood mortality due to diarrheal diseases have decreased from 2.5 million in 1990 to 1.4 million in 2010, the number of deaths in children <5 years of age due to diarrheal diseases remain significant.Citation1,2 The burden of childhood diarrhea in 2010 amounted to almost a billion episodes with ˜2% being severe episodes with an estimated >500,000 deaths in children 1–4 years of age.Citation3-7 Globally, diarrheal disease also remains one of the leading causes of disability-adjusted life years or DALYs.Citation8,9 Nearly 3-quarters of childhood diarrhea and pneumonia are concentrated in 15 countries, with the highest burden in Africa, South and South East (SE) Asia.Citation10 In the GBD 2010 study, rotavirus was identified to be the most important pathogen contributing to the global rates of disease followed by Cryptosporidia, the 2 together causing one-third of the diarrheal diseases in children <4 years of age.Citation1 The remaining share of the disease burden was attributed to several gram negative bacteria that include enterotoxigenic E. coli (ETEC), enteropathogenic E. coli (EPEC), Shigella, Campylobacter, V. cholera, Salmonella, with a high percentage of cases having no identified pathogensCitation1,8,11 ().
Table 1. Global etiology of diarrheal disease. The population evaluated for each list is given in parenthesis under each listed column
The recently published Global Enteric Multicenter Study (GEMS) is a large-scale survey of the incidence and causative agents of moderate-to-severe diarrheal disease in young children 0–59 months of age residing in low income parts of 7 countries in Africa and South Asia.Citation12-15 The GEMS study pointed out that just 4 pathogens contributed to the majority of moderate to severe diarrhea and these included rotavirus, ETEC, Shigella and Cryptosporidia. Other pathogens such as Aeromonas, V. cholera and Campylobacter were more region-specific. Among the youngest children 0–11 months of age, rotavirus and ETEC encoding heat stable toxin (ST) was often the leading agents of moderate-to-severe-diarrhea while in the older children ages 12–59 months, Shigella was either the lead agent or among the top 2 lead agents overall (). The GEMS study also followed a birth cohort till 2 years of age and provided additional evidence that children who have repeated episodes of moderate to severe diarrhea are comparatively underweight, stunted in physical growth, and have decreased cognitive functions as compared to age-controlled children with no disease.Citation16-19
Traveler's diarrhea (TD)
In the US and other developed countries the risk of diarrheal diseases is low, and when it occurs, is usually self-limiting and underreported and often treated with over-the-counter medications and antibiotics. The FoodNet program in the US that conducts population-based surveillance for laboratory-confirmed cases of diarrhea indicated that in 2011 there were 18,964 laboratory confirmed cases of diarrhea with 4398 hospitalizations and 82 deaths. Of these cases 41% and 36% were due to Salmonella and Campylobacter species, followed by Shigella (8.1%) and Cryptosporidia (8%).Citation20 Campylobacter infections are commonly associated with eating contaminated chicken, and 80% of these cases can be eliminated by proper poultry farming and cooking. Although ETEC has been recognized as an etiological agent of diarrhea since the 1960's, poor detection methods resulted in infrequent identification of ETEC as a cause of food-related gastroenteritis. Traditional methods of detection included culture, animal bioassays and later ELISA assays with antibodies to toxins.Citation21 More recent molecular based techniques have shown higher sensitivities for detection.Citation22
Diarrheal diseases are important causes of morbidity and mortality in Sub-Saharan Africa, North Africa and the Middle East, South and SE Asia, Central Asia, and several parts of Latin America.Citation8,23 Travelers including military populations who visit these endemic regions risk incurring diarrhea from multiple causes due to lack of pre-exisiting immunity.Citation24-28 The Centers for Disease Control, Atlanta, GA (CDC) notes that “Traveler's Diarrheal (TD) is the most predictable travel-related illness,” and that attack rates can be as high as 70%.Citation29 As seen in , diarrhea is caused by infection with a number of bacterial pathogens as well as several viruses and parasites.Citation30,31 In an age of globalization, millions of travelers move from one part of the world to another for business, pleasure or outreach. Some of these regions are recognized to be endemic for diarrheal disease. A case can therefore be made for a commercially viable TD vaccine that protect travelers against the discomfort, not to mention, wastage of time and expenses incurred due to treatment of episodes of diarrhea.
There are region-specific distribution patterns of diarrheal pathogens that need to be taken into account for vaccine development.Citation8 In one study ETEC was found to be the most common pathogen identified overall and shared the primary burden of TD disease (30–35%) in Africa, South Asia (India), and Latin America while enteroaggregative E. coli (EAEC) was the second most common pathogen identified in traveler's who had visited Latin America (24.7%) and South Asia (16%).Citation32 In SE Asia Campylobacter was more prevalent (32%) with Salmonella and V. cholera causing an equal share of disease episodes (˜9%). EPEC was also identified in 14.3%, 18% and 7.7% in traveler's returning from Latin America, SE Asia and Africa. Shigella species were most commonly found in TD in Africa and South Asia (8–9%).Citation33 Among the viruses, norovirus, rotavirus and adenovirus were the main etiologic agents causing diarrheal disease while the most prevalent parasites causing diarrhea were Cyclospora, Cryptosporidia, Entamoeba and Giardia.Citation29,34-36 In 40–50% of the cases no pathogen was identified indicating that better detection methods may be needed to obtain a more complete list of pathogens causing diarrhea. It is believed that 80% of all TD are caused by bacterial species that are transmitted by contaminated food and water. Currently the most common agents of TD are ETEC, followed by EAEC, C. jejuni, Shigella spp and Salmonella spp.Citation29,37-45 Prophylactic antibiotics may be prescribed, but increased resistance to commonly used drugs such as fluoroquinolones Citation29 has been observed in Campylobacter, Shigella and Salmonella. Although TD is generally an acute, short-term infection, in some cases it can trigger chronic conditions such as idiopathic inflammatory bowel disease.Citation25-27,46 Thus there is significant potential benefit to vaccination of travelers to high-risk parts of the world.
Based on prevalence, severity of disease caused by the pathogen and stage of current clinical development, provides an initial list of pathogens for which vaccines could be developed within the next 10 years. Development of a multi-cause childhood diarrheal disease vaccine, as well as a TD vaccine, that will require antigens from multiple pathogens can be developed pursuing a pathway that has been successfully employed for the formulation of several modern-day multivalent pediatric combination vaccines. A brief history of combination vaccine is also provided.
Table 2. Diarrheal disease-causing pathogens for combination vaccine development
History of combination vaccines
One of the earliest examples of a combination vaccine was a typhoid-paratyphoid A and B vaccine (TAB) that was administered by the intradermal route (ID) and was composed of S. typhi, S. paratyphi A and S. paratyphi B. The TAB vaccine contained 1–2 × 109 CFU/ml of the enteric organisms suspended in 0.5% phenol-saline.Citation47,48 Later, TAB was combined with a tetanus vaccine (TABT) and introduced into the British Army, Royal Navy and Air Force.Citation49 The ID route reportedly caused minimal reactogenicity and elicited higher agglutinating antibody titers.Citation50,51 TAB was also combined with heat-killed and phenol-preserved V. cholera strain Ogawa (TAB/Ch) and evaluated singly or combined and administered by the ID route.Citation48 The combined TAB/Ch vaccine contained 5 × 109 CFU of enteric organisms and 8 × 109 CFU of cholera organisms per ml suspended in 0.5% phenol saline. The combined vaccine in this case was obtained by adding equal quantities of double-strength TAB and cholera vaccines. Human volunteers were vaccinated ID with 2 0.1 ml volumes of TAB/Ch spaced 21 days apart.Citation48 The data indicated 2–8 fold higher agglutinin responses to the O-antigens of S. typhi and V. cholera and was validated by animal studies in mice. The conclusions drawn from this study was that a combined enteric and cholera vaccine could be given ID and was preferable over the administration of the 2 components separately.Citation48 Thus ID vaccination with a combined vaccine became a routine procedure in the British Services. This vaccine however, did not meet with the same success in the US because the immunogenicity patterns to the 3 different pathogens were not duplicated.Citation52,53
Modern era of combination vaccines
The Food and Drug Administration (FDA) defines “combination vaccine” as 2 or more vaccines that have been combined by the manufacturer or supplied as vaccine components that are formulated to be combined immediately before administration.Citation54 However, in practice, it is most likely that a combination vaccine is supplied as a pre-blended, single entity. The combination is intended to either prevent multiple diseases or a single disease caused by different strains or serotypes.Citation54 Since multiple species of bacteria, viruses and parasites can cause diarrheal disease, vaccine development must consider the population to be vaccinated (). In the case of diarrheal diseases in endemic parts of the world there would be special attention to the age range of the recipients as most of them would be very young children. Other factors to consider would be the etiological burden and attack rates in these populations, consideration of severity of disease, health care-seeking attitudes within the population, requirement for hospital care, complex treatment regimen, post-infectious sequelae, and prevalent species of each type of organism that is responsible for disease burden.Citation14,46,55-57 Combination vaccines against diarrheal diseases could be a mixture of 1) live or whole cell-killed vaccines that are reconstituted and mixed before oral ingestion 2) subunit vaccines against individual pathogens that are reconstituted and administered as a single injection or 3) whole cell and subunit vaccines, including DNA vaccines, given concurrentlyCitation57 Another category would be live vaccine organisms expressing heterologous antigens (vectored vaccines). Whole cell or subunit vaccines may each contain single or multiple antigens from a single pathogenic species or multiple antigens from several species that cause diarrheal disease. Some of these changes could also be engineered at the manufacturing step. Adjuvants may be added during reconstitution of the vaccine or manufactured along with the antigen. The stepwise development of several combination vaccines for pediatric use provides a case study as strategies for a combination vaccine against diarrheal diseases are contemplated.
Current pediatric combination vaccines
The inactivated polio vaccine (IPV) is composed of inactivated poliovirus types 1, 2, 3 vaccine strains and is an example of a combination vaccine that protects against multiple variants of a single disease.Citation58-60 The diphtheria, tetanus and pertussis (whooping cough) vaccine (DTP & DTaP) and the measles-mumps-rubella (MMR) vaccines are examples of combination vaccines that protect against multiple diseases and are comprised of previously licensed monovalent vaccines.Citation61-66 The MMR vaccine is a trivalent mixture of live attenuated viruses of 3 diseases administered via injection. Although individual licensed vaccines against all 3 diseases were available since the 1960s, the 3 vaccines were combined in 1971 to become the MMR vaccine.Citation66 DTP & DTaP are subunit-based vaccines composed of purified antigens from Corynebacterium diptheriae, Clostridium tetani and Bordetella pertussis.Citation67-69 Prior to the development of DTP, monovalent toxoid-based vaccines were available to protect against diphtheria, pertussis and tetanus. However, in 1948 DTP was licensed by the FDA. The DTP vaccine became the first version of a combined diphtheria, tetanus and pertussis vaccine that was routinely administered to children from the 1940's to the mid 1990s.Citation69,70 Since then, individual components in the combination vaccine have been replaced by other antigens to improve the safety profile. For example, due to reported long term neurological effects with the whole-cell pertussis vaccine component of the DTP vaccine (also referred to as DTwP), an acellular version, DTaP, using purified pertussis antigens was incorporated into a new formulation and approved in 1991 for use in the US.Citation68,69 Two versions of DTaP exist, one with 3 and the other with 5 pertussis antigens (DTaP3 and DTaP5).Citation71 Because DTaP uses fewer purified antigens than the whole-cell vaccines, it is less reactogenic and therefore considered safer, but it is also more expensive. Recent research suggests that DTwP is more effective than DTaP in conferring immunity due to DTaP's narrower antigen base.Citation72,73 DTaP vaccines contain alum as the adjuvant and the vaccines from different manufacturers differ mainly in the number, amount and detoxification of the pertussis components. Additionally, other vaccines have been added to DTaP, such as IPV, vaccines for hepatitis B (HepB) and Haemophilus influenza type B (Hib), to obtain licensed vaccines for DTaP-IPV, DTaP-IPV-Hib, DTaP-HepB-IPV and the latest combination that is DTaP-HepB-Hib/IPV hexavalent vaccine.Citation74-83 In each case aluminum hydroxide or aluminum phosphate is the adjuvant. At the same time various combinations of HepB, pneumococcal conjugate vaccine (PCV) and pneumococcal polysaccharide-based vaccines, Neisseria meningitis serogroup C and Y tetanus toxoid conjugate vaccines, that are either separately coadministered or combined in a single injection with routine pediatric vaccines are also being evaluated in clinical trials for safety and for demonstration of lack of immunological interference with other coadministered vaccine antigens.Citation84-91 The potential advantages of such combination vaccines accrue from giving fewer injections that protect against several diseases, accommodating the administration of the combination vaccines within the WHO-recommended Expanded Program of Immunization (EPI) schedule, lowering pain and anxiety to the infants and caregivers, reducing overhead costs for administration and vaccine storage and overall increasing vaccine coverage and compliance.
An important factor in the formulation of licensed combination vaccines is the availability of vaccine-induced immune mesasures that correlate with protection against a specific disease.Citation92 For diarrheal vaccines against individual pathogens, correlates or surrogates of protection will be required before vaccines can be combined successfully. When two or more vaccines are combined, evaluation of immunological interference is judged by determining the levels of antibodies that are known to confer protection against a specific pathogen.Citation92 Most currently used vaccines act through functional antibodies such as bactericidal or opsonophagocytic antibodies against encapsulated bacteria such as Hib, pneumococci and meningococci or anti-toxin antibodies to diphtheria, tetanus and pertussis toxins. For example, a bactericidal serum antibody level of 0.15 ug/ml and 0.18–0.35 ug/ml has been determined to be sufficient for protection against bacteremia caused by Hib and pneumococci respectively.Citation92 These correlates may vary with the population, exposure rates, serotype and clinical end-point.Citation92 Anti-toxin antibody levels of 0.1 ug/ml for tetanus and diphtheria and 5–10 units of anti-pertussis toxin antibody levels for pertussis correlate with protection. The most commonly reported example of immune interference during the formulation of a combination vaccine is the lower antibody titers to the Hib component of a DTaP-based combination vaccine.Citation93,94 The exact mechanism for this reduced response is not understood although interference of the Hib-conjugate with free unconjugated tetanus toxoid, interaction with the alum adjuvant and other factors have been advanced.Citation94 The polio vaccines (IPV, OPV) elicit antibodies that prevent viremia and neutralization titers of 1:8 or 1:4 are considered protective.Citation92
Assuming that there are licensed vaccines already in use and correlates of protection are known, a major question during development of a combination vaccine is when and how to combine 2 different vaccines. Further, as the safety and efficacy of initial combination vaccines become established, individual combinations may become combined with others to create a single entity with expanded coverage. Obviously vaccines cannot be mixed at will except when specifically approved by the FDA and packaged for that purpose. The Hib conjugate vaccine (ActHIB, Sanofi and Hiberix, GSK) was introduced in the US in 1987 for use in children 2 months–18 months and is composed of purified polyribosylribitol phosphate (PRP) capsular polysaccharide of Hib conjugated with tetanus toxoid (PRP-T). It is manufactured as a lyophilized powder and is reconstituted at the time of delivery with either saline to obtain the monovalent vaccine or combined with DTP or DTaP vaccine to obtain a combination DTP-Hib or DTaP-Hib vaccine.Citation78,82 The more recent development of a licensed liquid pentavalent vaccine DTaP5-IPV-Hib (Pediacel, Sanofi Pasteur, licensed 2008) for infants and toddlers is made up of 3 different combination vaccines protecting against 5 diseases, diphtheria, tetanus, pertussis, polio and Hib.Citation84 The licensing of this pentavalent vaccine was based on the observation that no clinically important differences in the safety or immunologic profiles were noted between infants receiving the pentavalent vaccine and those that received separately administered DTaP, IPV and Hib vaccines.Citation79 A fully liquid combination vaccine has the advantage of delivering an accurate dose with fewer injections for the infant. Such combination multivalent vaccines results in increased vaccine compliance and enables more infants to be safely immunized and to complete their immunization regimen against multiple childhood diseases more successfully.
Current licensed diarrheal vaccines
Currently there are licensed vaccines against only 2 strictly diarrheal pathogens, rotavirus and V. cholera. Both vaccines are orally administered. Two licensed vaccines against S. typhi, an oral live attenuated vaccine, Ty21a, and a subunit vaccine based on the Vi polysaccharide antigen are administered to protect against typhoid fever.Citation11,41,95-97 A number of live and subunit Salmonella vaccine candidates have been evaluated for non-typhoidal Salmonella but this area of effort will not be discussed further in this review.Citation98-100
Rotavirus is the most common cause of severe gastroenteritis in infants and young children worldwide and causes approximately half a million deaths each year among children aged <5 years, with >80% of deaths occurring in developing countries. Two live attenuated oral vaccines, RotaTeq® (RV5, Merck) and Rotarix® (RV1, GSK) have been licensed in over 100 countries and are recommended for administration concurrently with DTaP-IPV vaccines at 2, 4, 6 months or at 6, 10, and 14 weeks in developing countries.Citation101,102 Clinical trials have indicated that 2 doses of live attenuated rotavirus vaccines given orally to infants did not interfere with the immune responses to concurrently administered intramuscular injections of DTaP, Hib, HepB and PCV.Citation103 Combining 2 different routes of immunization for different vaccines is another example of maximizing the efficiency of infant immunization. The licensed rotavirus vaccines have reduced the rates of hospitalization in the US by ∼80%.Citation104,105 However, in endemic countries like India and Pakistan, the rotavirus vaccines have shown reduced efficacy indicating that higher doses and more doses may be required to immunize such an endemic population.Citation106 Similar observations have been previously noted with live polio and cholera vaccines. RotaTeq® (RV5) is a human-bovine reassortant which was licensed by the FDA in February 2006 and Rotarix® (RV1) is a human rotavirus strain attenuated by multiple passages through cultured cells that was licensed by the FDA in April 2008. The Biologics Licensure Application (BLA) for RV1 contained 6 phase II trials and 5 phase III trials and the BLA for RV5 contained 3 phase III trials (see WHO website on rotavirus vaccines).
Two licensed oral cholera vaccines exist to reduce the burden of disease in endemic regions and during outbreaks.Citation107 Dukoral, composed of whole cell killed V. cholera combined with a recombinant cholera toxin B subunit (WC-rBS) has shown very high short-term protection in different age-groups in Bangladesh and Peru and significant long-term efficacy in endemic populations.Citation108 Dukoral has also been used as a traveler's diarrheal vaccine against ETEC infections. The cholera toxin B subunit shares sequence homology with the B subunit of the heat labile toxin (LT) of ETEC and is believed to confer this cross protection. Shanchol, that was developed at the International Vaccine Institute in Seoul, Korea, and licensed in India in 2011, is a bivalent vaccine containing whole cell-killed bivalent cholera vaccine against O1 and O139 serotypes.Citation109 A live attenuated cholera vaccine CVD 103-HgR that has demonstrated efficacy in multiple populations has been licensed in several countries and is currently in the US licensure process.Citation110
Current landscape of vaccine development for enteric pathogens
It was generally believed that mucosal pathogens would require intestinal immunity and therefore the oral route would be the most immunogenic route of vaccine administration. Oral delivery would mimic the course of natural infection that is known to confer immunity against many diarrheal diseases. It would also be the easiest and cheapest for vaccine delivery. However, with the evaluation of non-oral routes of immunization such as intranasal, intradermal, and sublingual, in addition to the conventional intramuscular route, killed whole-cell and subunit vaccines are becoming more attractive for reasons of safety as well as the capability to deliver both a systemic and a mucosal immune response.
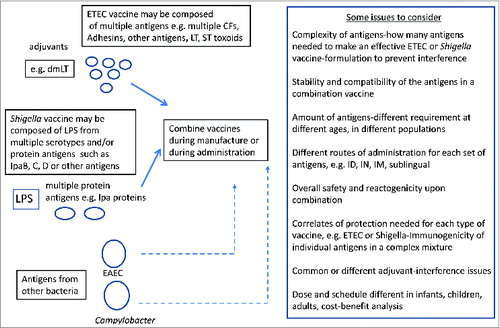
A key consideration for a combination diarrheal vaccine will be the list of antigens to be included for obtaining broad protection against multiple pathogens. For example, in Shigella, current data indicates that protection is serotype-specific and is based on the O-antigen type of the LPS molecule. Shigella has more than 50 serotypes suggesting that vaccines against all 50 serotypes may be needed for 100% protection. However, epidemiology studies show that 4–6 serotypes predominate and vaccine candidates against S. sonnei and S. flexneri serotypes 2a, 3a and 6 may suffice to protect 80% of shigellosis worldwide.Citation111 Therefore an effective O antigen-based vaccine against Shigella must contain the respective component from these 4 prevalent serotypes. There are efforts to develop a Shigella vaccine based on the highly conserved Type III secretion system antigens IpaB and IpaD Citation112 but proof of concept in humans remains to be addressed. ETEC strains are made up of almost 100 different O-antigenic types and express a heat-labile (LT) and a heat-stable enterotoxin (ST) along with more than 25 different colonization factors (CFs) or coli surface antigens (CS) that enable the bacteria to colonize the small intestine and induce diarrhea. While several CFs predominate, such as CFA/1, CS1-CS7, CS14, 17 and 21, 30–50% of ETEC strains do not express an identifiable CF on their surface. Approximately 30% of ETEC strains express either LT or ST while the remaining express both enterotoxin types. ETEC vaccine development is directed toward immune responses to LT, ST, the predominant CFs and their tip adhesins.Citation108,113 Campylobacter species express a capsule and lipooligosaccharide and using a traditional Penner serotyping scheme, 47 different Campylobacter serotypes have been described.Citation114,115 A capsule conjugate vaccine against Campylobacter that has shown promise in non-human primates will have to take into consideration the predominant capsule types circulating in the world. A combination diarrheal vaccine against any 2 or 3 of these enteric pathogens will therefore be composed of multiple antigens from each species.
There are 3 main issues in the field of diarrheal disease vaccine development 1) to determine whether live, whole-cell killed or subunit vaccines provides the best strategy for obtaining durable protection 2) to determine how many live attenuated or whole cell-killed strains or how many antigens will comprise an efficacious vaccine against an individual pathogen listed in ) what are the correlates of protection for each vaccine so that when monospecific vaccines are combined, immune responses can be quantitated and immunological interferences due to the combination can be evaluated. Currently there are no licensed vaccines against Campylobacter, non-typhoidal Salmonella, ETEC, EAEC, Cryptosporidia and Shigella. A limited number of live attenuated, killed whole-cell and subunit vaccine candidates have been previously evaluated in Phase 1, 2b and even Phase 3 clinical trials for some of these pathogens and results obtained have provided valuable information on safety and immunogenicity, although correlates of protection for any of the diarrheal diseases-causing pathogens are lacking.Citation108,116-124 A Phase 3 evaluation of S. sonnei and S. flexneri 2a O-specific polysaccharide conjugate was completed in children 1–4 years of age in Israel.Citation121 The S. sonnei conjugate showed 71% efficacy in 3–4 year old, an efficacy rate previously demonstrated with similar conjugates in Israeli adults. Efficacy was minimal in children less than 3 years of age.Citation121 A phase 3 study was also conducted with a skin-patch vaccine containing heat-labile toxin (LT) from ETEC. This was carried out in a population of travelers to Mexico and Guatemala.Citation124 The LT patch vaccine did not protect travelers against diarrhea caused by ETEC or other organisms. With increasing knowledge of bacterial pathogenesis and identification of novel and better characterized antigens, a number of promising candidates are currently in the development phase with some entering clinical trials. Based on current developmental stage, monovalent vaccines against ETEC, Shigella and Campylobacter may become a reality in the next decade, constituting the first step toward the development of a combination diarrhea vaccine.Citation113,114,118,125-127 Although correlates of protection for all 3 pathogens are unclear, mucosal responses along with systemic responses will be important for determining protection. Since ETEC and Shigella vaccine development have had a relatively earlier start, lists some of the promising strategies that are being implemented for these 2 pathogens.Citation122,125,128-142 For Campylobacter, a capsule conjugate vaccine is currently in clinical trials.Citation114,115
Table 3. Current ETEC vaccine landscape
Combination vaccines for diarrheal diseases
Traveler's, including members of the military and young children living in endemic parts of the world are the most in need of vaccines against diarrheal diseases.Citation4,26,28,143,144,145 A combination vaccine would greatly simplify immunization of these target groups, but the nature of the combination vaccine is likely very different for each group.
Based on recent data on infection rates, ETEC and Campylobacter are reasonable targets for an initial combination vaccine against TD. There are currently no vaccines licensed in the US for either pathogen. Dukoral, an oral cholera vaccine, is being used in some countries as a TD vaccine against ETEC on the basis of cross-reactivity of the B subunits of the respective toxins. In most cases, no prescription is needed for Dukoral and the vaccine can be self-administered. Vaccination requires 2 doses and the traveler is advised to take the last dose at least 1 week prior to travel. Ideally, similar to Dukoral, a combination TD vaccine could be self-administered by the oral route, which is the only practical route for this approach. A combination of subunit vaccines administered parenterally is also feasible, but this would require more close coordination between the traveler and the travel physician. Since travelers to endemic countries are sensitive to the high risk of diarrheal disease, there is likely a fairly strong market for TD vaccines in the more developed part of the world.Citation143 Considerations for a combination diarrhea vaccine for young children living in resource-poor endemic parts of the world are much more complex compared to the scenario for TD vaccines. Some key factors include geographical disease burden and coverage of the most relevant pathogens, route of vaccination, a defined schedule for immunization of the very young, who may already be on a vaccination regimen based on an EPI schedule, and options for manufacture of the vaccine. Based on the recent findings from the GBD 2010 study and the GEMS study, vaccines against ETEC and Shigella, along with the licensed rotavirus vaccine, would have the greatest impact on improving the health status of the most vulnerable – the children under 5 living in endemic parts of the world. However, both the GEMS and the GBD 2010 studies have indicated that a vaccine against Cryptosporidia has to be taken into consideration although clinical data for a vaccine against Cryptosporidia is lacking. In SE. Asia where Campylobacter is responsible for ˜30% of diarrheal disease, a combination vaccine against ETEC, Shigella, and Campylobacter will be protective against 70–80% of the diarrhea seen in that region. Overall, the path of development of a combination diarrheal vaccine would be similar to the steps used to license current pediatric vaccines.
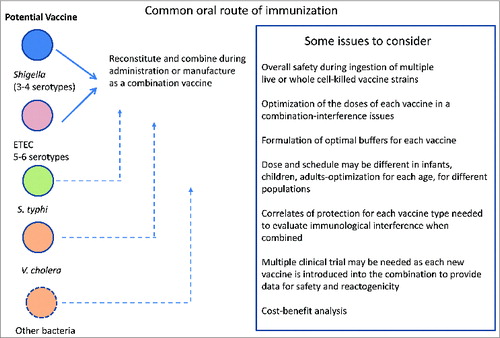
There are no vaccines currently available for either ETEC, Shigella or Campylobacter but there are multiple development efforts ongoing that may yield suitable candidates for either a stand-alone or a combination vaccine (). If the vaccine were to be delivered orally, consideration must be given to potential interference by the tropical enteropathy that is often observed in malnourished young children, and the general need for multiple oral doses to obtain protective immunity in these children.Citation146 Another challenge is the size or volume of the inoculum, which likely cannot exceed a few milliliters for the youngest children, and the palatability of the vaccine vehicle. Nonetheless, live or inactivated whole cells offer one possible option for a combination ETEC-Shigella vaccine. Parenteral vaccines are also being developed for ETEC, Shigella and Campylobacter and the challenge here may be how to optimize mucosal immunity by immunization through a non-mucosal route. A hypothetical scenario for an initial combination vaccine, either a live or whole-cell killed combination vaccine or a subunit vaccine against these 2 pathogens is schematized in . Such a licensed initial combination vaccine could be further expanded to include either existing vaccine candidates such as for cholera, rotavirus and typhoidal Salmonella or combined with newer vaccines as they are developed against other diarrheal pathogens (). Novel adjuvants such as dmLT may be able to stimulate mucosal as well as systemic immune responses even when the vaccine is given by injection rather than orally.Citation139,147 Some of the issues affecting each type of combination is also outlined ().
Ideally, combination diarrheal vaccines could be given in accordance with the in-country EPI schedule, which typically includes the first 6, 10 and 14 weeks of life (2, 4, and 6 months of age in the US). Vaccines against childhood illnesses such tuberculosis, diphtheria, pertussis, tetanus, polio and measles are usually given on the EPI schedule. Most of these are given by the parenteral route, although oral polio vaccine is still being used in many parts of the world. However, the licensed rotavirus vaccines are given orally and are shown to be efficaciious given concurrently with the EPI vaccines. If a combination diarrhea vaccine were to be incorporated, one critical step would be to demonstrate that the new vaccine does not interfere with immunogenicity or efficacy of the EPI vaccines already in place. An ETEC and Shigella combination is an obvious option, but others may be considered. Since rotavirus and ETEC are of greatest threat to younger children, a combination vaccine against these may be an attractive approach (). Existing oral rotavirus vaccines are already being taken up in parts of the developing world for early childhood immunization. Shigella becomes important in children after the first year of life, so vaccination at a later age may optimize efficacy. A Shigella vaccine could be given at 9 and 12 months, concurrent with the schedule for measles vaccine. For this scenario, a combination Shigella-typhoid vaccine could be considered, since both are invasive pathogens that tend to affect older children. Recent studies have indicated that as few as 4 serotypes of Shigella can protect against >80% of shigellosis worldwide.Citation148 There are oral and parenteral vaccines for typhoid already, but these are not currently used in children under 2 years of age. The injectable version and the oral version is only for those aged 6 years and above. Nonetheless, recent studies indicate that the oral typhoid vaccine may be safe and immunogenic in younger children ages 2–5.Citation96 For this potential Shigella/typhoid combination, there is much developmental work to be done.
A combination diarrheal vaccine against multiple pathogens will require development of efficacious vaccines against individual bacterial pathogens. The combination itself will depend upon whether it serves as a pediatric vaccine or a TD vaccine. A combination pediatric vaccine could be given in conjunction with other childhood immunizations. Interestingly, studies on the infant's immune system has indicated that neonates are capable of mounting a protective immune response to vaccines within hours of birth and that they are capable of generating both a humoral and cellular immune responses to pathogens.Citation149,150 The neonates immune system has the capacity to respond to extremely large numbers of antigens and one study estimated that each infant has the theoretical capacity to respond to at least 10,000 vaccines, with each vaccine composed of 100 antigens.Citation150 Response of B cells in infants to T-independent antigens such as polysaccharides are considerably less than adults till they reach 2 years of age.Citation150 Thus vaccines against the most common diarrheal disease-causing pathogens can be accommodated within the schedule of childhood immunizations, whether given as live oral vaccines such as the rotaviral vaccine, or whole-cell killed and subunit vaccines. Whether administered concurrently or sequentially, the vaccination should not negatively affect the safety and immunogenicity of the routine pediatric vaccines. For example, the MMRV vaccine, a combined measles, mumps, rubella and varicella vaccine, has been proposed as a replacement for the MMR vaccine to simplify administration of the vaccines.Citation151,152 However, preliminary data indicating a rate of fever-induced seizure of 9 per 10,000 vaccinations with MMRV, as opposed to 4 per 10,000 for separate MMR and varicella injections has convinced US health officials not to recommend use of MMRV vaccine over separate injections.Citation153
As greater understanding of pathogenesis and other vaccines come into effect for Salmonella, EAEC and Cryptosporidia, these can be added stepwise to the mixture analogous to the manner in which current pediatric vaccines are formulated.Citation154-156 For the infant group the combination vaccine, be it live or subunit, could be given as part of the normal pediatric vaccination schedule while a traveler's diarrheal vaccine, given as 2 or 3 doses, could be scheduled to be taken ahead of the planned travel dates. In this context it may be worth mentioning that a norovirus vaccine, based on virus-like particles, has shown promising safety and efficacy in limited Phase 1 and 2 trials. Such a vaccine could be used for high-risk populations or situations such as the elderly, in day care, on cruise ships, nursing homes or in the military.Citation157
Manufacturing issues for development of a combination diarrheal vaccine
Under manufacturing issues, there are several key considerations relating to formulation (). Of primary importance is the compatibility of the individual components:
Table 4. Current Shigella vaccine landscape
Table 5. Manufacturing issues for combination vaccinesFootnote
The potential for immunological interference should be evaluated in an animal model to detect effects of the combination on potency and immunogenicity. For diarrhea vaccines, mucosal immunity would be a key parameter for evaluation when determining compatibility. Here again, some knowledge can be gained from evaluating the formulation of pediatric combination vaccines. As described above, the most commonly reported example of immune interference in DTaP-based vaccines is the reduction in antibody titers to the Hib component of the vaccine PRP antigens. Consistent with the clinical data this interference has also been seen in animal models.Citation94,158 Several explanations have been provided including interference of tetanus toxoid and the FHA pertussis antigen with Hib and incompatibility with the alum adjuvant in DTaP vaccines. As a result, the DTaP-Hib vaccines have been licensed in Europe but not in the US.
Depending on the nature of the components, formulation of the final combination must be assessed by a battery of physicochemical, biochemical and biological assays. For example, in the case of live attenuated bacterial strains combined in a single formulation, assays would be needed to determine the viability and potency of each strain. If the combination is one of subunit proteins, there may be challenges in terms of identifying preservatives, excipients and delivery vehicles that effectively maintain the stability of each component and yet do not interfere with required assays. If the combination vaccine includes an adjuvant, additional areas of assessment would be required. In addition to lack of any negative effect on safety or immunogenicity of any component, the selected adjuvant should not interfere with assays for each component in the final product. Besides alum, 2 other adjuvants AS03 and AS04 have been licensed in the US. AS03 is a oil-in-water emulsion of D,L-alphatocopherol (vitamin E) and squalene and an emulsifier polysorbate 80 and AS04 is aluminum hydroxide and monophosphoryl lipid A. New mucosal adjuvants such as dmLT or liposomes that may be particularly useful for diarrhea vaccines are also being investigated.Citation159,160 If a subunit enteric combination vaccine is formulated, non-alum based adjuvants such as the oil-in-water emulsion MF59 and polylactide coglycolide (PLG) microparticles, could also be evaluated.Citation161 New approaches are needed to evaluate formulation of non-alum-based adjuvants as part of a combination diarrhea vaccine.
Demonstration of potency, which may be a reflection of vaccine stability, is likely the most important and challenging part of product testing. Potency is often demonstrated by immunogenicity in animal models but development of improved in vitro assays to measure batch-to-batch consistency of vaccine production would be useful to reduce reliance on animal testing. For example, antibody-based ELISA assays for quantifying diphtheria toxoid antigen in DTP-based combination vaccines have several advantages over other biochemical and biophysical methods due to their sensitivity and to the fact that they can be used on the final combined product even in the presence of antigen adsorbed to the adjuvant alum. Whether supplied as a pre-blended formulation or separate components for co-administration at the time of vaccination, the FDA guideline states that in general the potency requirement of each component in the combination should comply with the potency requirement for each component as a stand-alone product. For vaccines already licensed, potency standards would have been previously established. However, since there are so few vaccines licensed for diarrheal diseases, the combination vaccine may be one of new candidates for which potency will have to be established for each as part of the vaccine development plan.
The FDA guidance on clinical evaluation of safety and immunogenicity requires that the immunogenicity of combination vaccines should not be decreased when compared to those of individual components delivered simultaneously. In the case of licensed components such as those for childhood diseases, in some cases they are given simultaneously (oral rotavirus vaccine given along with intramuscularly administered DTP-based vaccines), and in other cases, such as DTP-based combination vaccines, they are formulated together for a single delivery. For example, Kinrix is a combination pediatric vaccine of DTaP-IPV (GSK, licensed 2008) that is injected into the muscle in a 4-dose EPI schedule. The diphtheria and tetanus toxins are extracted from the respective cultures, detoxified with formaldehyde, and individually adsorbed to aluminum phosphate.Citation162 Pertussis toxin (PT), filamentous haemagglutinin antigen (FHA) and pertactin (PRN) are isolated separately from the supernatant of B. pertussis cultures.Citation162 Fimbrial antigens (FIM) are copurified from bacterial cells. The pertussis antigens are purified by sequential filtration, salt precipitation, ultrafiltrations and chromatography. Glutaraldehyde and formaldehyde are used to detoxify PT and FHA respectively. The individual antigens are adsorbed separately onto aluminum phosphate (AlPO4). Polioviruses types 1, 2, 3 are each grown in seperate cultures of human fetal lung cells (MRC-5). The viral suspensions are inactivated by formaldehyde after concentration by ultrafiltration and liquid chromatography. The monovalent IPVs are combined to produce the trivalent poliovirus concentrate.Citation162 The adsorbed DTP antigens are combined with aluminum phosphate as an adjuvant and water for injection. The trivalent polio concentrate is then added and the DTaP-IPV component is diluted to its final concentration.Citation162 Again, because of the very limited number of licensed diarrhea vaccines, there may be very little pre-existing data to support a combination vaccine. On the other hand, most diarrheal vaccines will be administered either orally or through an alternate mucosal route, so the simultaneous administration of the individual components as a mixture may be nearly the same as the administration of a pre-blended combination vaccine formulation. Additional information on manufacture, testing and clinical trials of combination vaccines is available in the FDA website.Citation162
Conclusion
Despite the well-documented global burden of diarrheal diseases, currently there are no combination diarrheal vaccines to protect against the diversity of enteric pathogens which include bacteria, viruses and parasites. The recent success with rotavirus and cholera vaccines has stimulated developmental efforts to advance vaccine candidates against other diarrheal pathogens such as ETEC, Shigella, Salmonella, Campylobacter and Norovirus. In the future, research will also be directed toward combining monovalent vaccines against individual pathogens to provide broader coverage against multiple causes of diarrheal disease. The American College of Immunization practices (ACIP) recommends that combination vaccines be used whenever possible. As new vaccines that target previously unaddressed diseases are added to the vaccination calendar, the use and improvement of currently available combination vaccines will be paramount if high vaccine coverage is to be maintained. Areas for future research would be to increase compatibility of antigens, testing more potent adjuvants and developing better methods to monitor vaccine production and potency.
In an article summarizing the issues concerning combination vaccines for less developed countries, the question was raised as to “how can vaccines of special epidemiological importance for the developing world be developed”. Successful development of an affordable combination vaccine against diarrheal diseases for pediatric public health will rely on subsidy by international organizations, potential private sector markets among developed world travelers and the middle to upper income class of developing countries, and collaboration between the commercial manufacturer (who likely hold the patents and IP rights) and a local producer.Citation163 Ultimately governments and health-policy decision makers in individual countries will determine if such combination diarrheal vaccines benefit their population and add value to the well-being of their children.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclaimer
The opinions or assertions contained herein are the private views of the author, and are not to be construed as official, or as reflecting true views of the Department of the Army or the Department of Defense or PATH.
Acknowledgments
We thank Allison Clifford and Richard Walker of PATH-EVI and Stephen Savarino of the Naval Medical Research Institute for their review of this paper and thoughtful comments. We also thank Thomas L Hale for reading the mauscript.
References
- Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, Naghavi M, Salomon JA, Shibuya K, Vos T, et al. GBD 2010: a multi-investigator collaboration for global comparative descriptive epidemiology. Lancet 2012; 380:2055-8; PMID:23245598; http://dx.doi.org/10.1016/S0140-6736(12)62134-5
- Walker CL, Aryee MJ, Boschi-Pinto C, Black RE. Estimating diarrhea mortality among young children in low and middle income countries. PloS one 2012; 7:e29151; PMID:22235266; http://dx.doi.org/10.1371/journal.pone.0029151
- UNICEF-WHO. Diarrhea: why children are still dying and what can be done? NY, United Nations Children Fund. 2009.
- Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E. Diarrhoea: why children are still dying and what can be done. Lancet 2010; 375:870-2; PMID:19833382; http://dx.doi.org/10.1016/S0140-6736(09)61798-0
- WHO. Years of life lost due to premature mortality-trends and causes. World Health Statistics 2014 2014:45-9.
- Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE, Child Health Epidemiology Reference Group of the World Health Organization and UNICEF. Global causes of diarrheal disease mortality in children. PloS one 2013; 8:e72788; PMID:24023773; http://dx.doi.org/10.1371/journal.pone.0072788
- Fischer Walker CL, Munos MK, Black RE. Quantifying the indirect effects of key child survival interventions for pneumonia, diarrhoea, and measles. Epidemiol Infect 2013; 141:115-31; PMID:22793874; http://dx.doi.org/10.1017/S0950268812001525
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2095-128; PMID:23245604; http://dx.doi.org/10.1016/S0140-6736(12)61728-0
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2197-223; PMID:23245608; http://dx.doi.org/10.1016/S0140-6736(12)61689-4
- Fischer Walker CL, Sack D, Black RE. Etiology of diarrhea in older children, adolescents and adults: a systematic review. PloS Negl Trop Dis 2010; 4:e768; PMID:20689809; http://dx.doi.org/10.1371/journal.pntd.0000768
- Haeusler GM, Curtis N. Non-typhoidal Salmonella in children: microbiology, epidemiology and treatment. Adv Exp Med Biol 2013; 764:13-26; PMID:23654054; http://dx.doi.org/10.1007/978-1-4614-4726-9_2
- Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, Adegbola RA, Alonso PL, Breiman RF, Faruque AS, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis 2012; 55 Suppl 4:S232-45; PMID:23169936; http://dx.doi.org/10.1093/cid/cis753
- Levine MM, Kotloff KL, Nataro JP, Muhsen K. The Global Enteric Multicenter Study (GEMS): impetus, rationale, and genesis. Clin Infect Dis 2012; 55 Suppl 4:S215-24; PMID:23169934; http://dx.doi.org/10.1093/cid/cis761
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209-22; PMID:23680352; http://dx.doi.org/10.1016/S0140-6736(13)60844-2
- Lindsay B, Ochieng JB, Ikumapayi UN, Toure A, Ahmed D, Li S, Panchalingam S, Levine MM, Kotloff K, Rasko DA, et al. Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol 2013; 51:1740-6; PMID:23536399; http://dx.doi.org/10.1128/JCM.02713-12
- Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, Mølbak K, Valentiner-Branth P, Lanata CF, Black RE, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. IntJ Epidemiol 2008; 37:816-30; PMID:18567626; http://dx.doi.org/10.1093/ije/dyn099
- Richard SA, Black RE, Gilman RH, Guerrant RL, Kang G, Lanata CF, Mølbak K, Rasmussen ZA, Sack RB, Valentiner-Branth P, et al. Diarrhea in early childhood: short-term association with weight and long-term association with length. AmJ Epidemiol 2013; 178:1129-38; PMID:23966558; http://dx.doi.org/10.1093/aje/kwt094
- Fischer Walker CL, Lamberti L, Adair L, Guerrant RL, Lescano AG, Martorell R, Pinkerton RC, Black RE. Does childhood diarrhea influence cognition beyond the diarrhea-stunting pathway? PloS One 2012; 7:e47908; PMID:23118906
- Lamberti LM, Fischer Walker CL, Black RE. Systematic review of diarrhea duration and severity in children and adults in low- and middle-income countries. BMC Public Health 2012; 12:276; PMID:22480268; http://dx.doi.org/10.1186/1471-2458-12-276
- CDC. Foodborne diseases active surveillance network (FoodNet): foodnet surveillance report for 2011. 2012. http://www.cdc.gov/foodnet/PDFs/2011_annual_report_508c.pdf
- Caeiro JP, Estrada-Garcia MT, Jiang ZD, Mathewson JJ, Adachi JA, Steffen R, DuPont HL. Improved detection of enterotoxigenic Escherichia coli among patients with travelers' diarrhea, by use of the polymerase chain reaction technique. The Journal of infectious diseases 1999; 180:2053-5; PMID:10558969; http://dx.doi.org/10.1086/315121
- Lothigius A, Janzon A, Begum Y, Sjoling A, Qadri F, Svennerholm AM, Bölin I. Enterotoxigenic Escherichia coli is detectable in water samples from an endemic area by real-time PCR. Journal of applied microbiology 2008; 104:1128-36; PMID:17976169; http://dx.doi.org/10.1111/j.1365-2672.2007.03628.x
- Harvey K, Esposito DH, Han P, Kozarsky P, Freedman DO, Plier DA, Sotir MJ; Centers for Disease Control and Prevention (CDC). Surveillance for travel-related disease–GeoSentinel Surveillance System, United States, 1997–2011. Morbidity and mortality weekly report Surveillance summaries 2013; 62:1-23; PMID:23863769
- Putnam SD, Sanders JW, Frenck RW, Monteville M, Riddle MS, Rockabrand DM, Sharp TW, Frankart C, Tribble DR. Self-reported description of diarrhea among military populations in operations Iraqi Freedom and Enduring Freedom. Journal of travel medicine 2006; 13:92-9; PMID:16553595; http://dx.doi.org/10.1111/j.1708-8305.2006.00020.x
- Porter CK, Choi D, Cash B, Pimentel M, Murray J, May L, Riddle MS. Pathogen-specific risk of chronic gastrointestinal disorders following bacterial causes of foodborne illness. BMC gastroenterology 2013; 13:46; PMID:23510245; http://dx.doi.org/10.1186/1471-230X-13-46
- Porter CK, Gloor K, Cash BD, Riddle MS. Risk of functional gastrointestinal disorders in U.S. military following self-reported diarrhea and vomiting during deployment. Digestive diseases and sciences 2011; 56:3262-9; PMID:21643738; http://dx.doi.org/10.1007/s10620-011-1762-3
- Porter CK, Thura N, Riddle MS. Quantifying the incidence and burden of postinfectious enteric sequelae. Military medicine 2013; 178:452-69; PMID:23707833; http://dx.doi.org/10.7205/MILMED-D-12-00510
- Connor P, Porter CK, Swierczewski B, Riddle MS. Diarrhoea during military deployment: current concepts and future directions. Current opinion in infectious diseases 2012; 25:546-54; PMID:22907281; http://dx.doi.org/10.1097/QCO.0b013e3283582ebc
- Connor BA. Traveler's Diarrhea. CDC Health Information for International Travel 2014, ed GW Brunette, Oxford University Press, New York, NY 10016 2014; Chapter 2, under Self Treatable Conditions.
- Greenwood Z, Black J, Weld L, O'Brien D, Leder K, Von Sonnenburg F, Pandey P, Schwartz E, Connor BA, Brown G, et al. Gastrointestinal infection among international travelers globally. Journal of travel medicine 2008; 15:221-8; PMID:18666921; http://dx.doi.org/10.1111/j.1708-8305.2008.00203.x
- Swaminathan A, Torresi J, Schlagenhauf P, Thursky K, Wilder-Smith A, Connor BA, Schwartz E, Vonsonnenberg F, Keystone J, O'Brien DP, et al. A global study of pathogens and host risk factors associated with infectious gastrointestinal disease in returned international travellers. The Journal of infection 2009; 59:19-27; PMID:19552961; http://dx.doi.org/10.1016/j.jinf.2009.05.008
- Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers' diarrhea: systematic review from 1973 to the present. The American journal of tropical medicine and hygiene 2009; 80:609-14; PMID:19346386
- Rathaur VK, Pathania M, Jayara A, Yadav N. Clinical study of acute childhood diarrhoea caused by bacterial enteropathogens. Journal of clinical and diagnostic research : JCDR 2014; 8:PC01-5; PMID:24995223
- Stark D, Roberts T, Ellis JT, Marriott D, Harkness J. Evaluation of the EasyScreen enteric parasite detection kit for the detection of Blastocystis spp., Cryptosporidium spp., Dientamoeba fragilis, Entamoeba complex, and Giardia intestinalis from clinical stool samples. Diagnostic microbiology and infectious disease 2014; 78:149-52; PMID:24286625; http://dx.doi.org/10.1016/j.diagmicrobio.2013.10.013
- Sanchez-Vega JT, Cabrera-Fuentes HA, Romero-Olmedo AJ, Ortiz-Frias JL, Sokolina F, Barreto G. Cyclospora cayetanensis: this emerging protozoan pathogen in Mexico. The American journal of tropical medicine and hygiene 2014; 90:351-3; PMID:24379243; http://dx.doi.org/10.4269/ajtmh.12-0782
- Ahmed SM, Hall AJ, Robinson AE, Verhoef L, Premkumar P, Parashar UD, Koopmans M, Lopman BA. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. The Lancet infectious diseases 2014; 14:725-30; PMID:24981041; http://dx.doi.org/10.1016/S1473-3099(14)70767-4
- Adachi JA, Jiang ZD, Mathewson JJ, Verenkar MP, Thompson S, Martinez-Sandoval F, Steffen R, Ericsson CD, DuPont HL. Enteroaggregative Escherichia coli as a major etiologic agent in traveler's diarrhea in 3 regions of the world. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2001; 32:1706-9; PMID:11360211; http://dx.doi.org/10.1086/320756
- Ruiz J, Marco F, Oliveira I, Vila J, Gascon J. Trends in antimicrobial resistance in Campylobacter spp. causing traveler's diarrhea. APMIS : acta pathologica, microbiologica, et immunologica Scandinavica 2007; 115:218-24; PMID:17367467; http://dx.doi.org/10.1111/j.1600-0463.2007.apm_567.x
- Navia MM, Ruiz J, Vila J. Molecular characterization of the integrons in Shigella strains isolated from patients with traveler's diarrhea. Diagnostic microbiology and infectious disease 2004; 48:175-9; PMID:15023426; http://dx.doi.org/10.1016/j.diagmicrobio.2003.10.007
- Vila J, Vargas M, Henderson IR, Gascon J, Nataro JP. Enteroaggregative Escherichia coli virulence factors in traveler's diarrhea strains. The Journal of infectious diseases 2000; 182:1780-3; PMID:11069254; http://dx.doi.org/10.1086/317617
- Sanchez-Vargas FM, Abu-El-Haija MA, Gomez-Duarte OG. Salmonella infections: an update on epidemiology, management, and prevention. Travel medicine and infectious disease 2011; 9:263-77; PMID:22118951; http://dx.doi.org/10.1016/j.tmaid.2011.11.001
- Cabrera R, Marco F, Vila J, Ruiz J, Gascon J. Class 1 Integrons in Salmonella Strains causing traveler's diarrhea. Antimicrobial agents and chemotherapy 2006; 50:1612-3; PMID:16569899; http://dx.doi.org/10.1128/AAC.50.4.1612-1613.2006
- Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infection and immunity 2007; 75:3961-8; PMID:17548483; http://dx.doi.org/10.1128/IAI.00459-07
- Qadri F, Svennerholm AM, Faruque AS, Sack RB. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clinical microbiology reviews 2005; 18:465-83; PMID:16020685; http://dx.doi.org/10.1128/CMR.18.3.465-483.2005
- Das SK, Ahmed S, Ferdous F, Farzana FD, Chisti MJ, Leung DT, Malek MA, Talukder KA, Bardhan PK, Salam MA, et al. Changing emergence of Shigella sero-groups in Bangladesh: observation from four different diarrheal disease hospitals. PloS one 2013; 8:e62029; PMID:23658619; http://dx.doi.org/10.1371/journal.pone.0062029
- Connor BA. Sequelae of traveler's diarrhea: focus on postinfectious irritable bowel syndrome. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2005; 41 Suppl 8:S577-86; PMID:16267722; http://dx.doi.org/10.1086/432956
- Bardhan PN, Dutta HN, Krishnaswami P. Intradermal Tab Immunization against Enteric Infections. The Journal of hygiene 1963; 61:365-72; PMID:14066593; http://dx.doi.org/10.1017/S0022172400039656
- Noble JE, Fielding P. Combined enteric and cholera vaccination by the intradermal route. The Journal of hygiene 1965; 63:345-55; PMID:5212837; http://dx.doi.org/10.1017/S002217240004523X
- Barr M, Sayers, MHP, Stamm, WP. Intradermal TABT vaccine for immunization against enteric. Lancet 1959; 1:816-17; PMID:13642909
- Kamp M. Mass typhoid fever immunization: Intradermal method. J Ind med Ass 1943; 36:584.
- Felix A, Bensted HJ. Proposed standard agglutinating sera for typhoid and paratyphoid A and B fevers. Bulletin of the World Health Organization 1954; 10:919-26; PMID:13199655
- Vella W. On vaccines and vaccination: typhoid-paratyphoid fevers. Postgraduate medical journal 1972; 48:98-106; PMID:4622606; http://dx.doi.org/10.1136/pgmj.48.556.98
- Hornick RB. Typhoid immunization recommendation of the Immunization Practices Advisory Committee. MMWR-CDC 1990; 39:1-5; PMID:2165557
- FDA. http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm175909.pdf. 2014.
- Nasrin D, Wu Y, Blackwelder WC, Farag TH, Saha D, Sow SO, Alonso PL, Breiman RF, Sur D, Faruque AS, et al. Health care seeking for childhood diarrhea in developing countries: evidence from seven sites in Africa and Asia. The American journal of tropical medicine and hygiene 2013; 89:3-12; PMID:23629939; http://dx.doi.org/10.4269/ajtmh.12-0749
- Manna B, Nasrin D, Kanungo S, Roy S, Ramamurthy T, Kotloff KL, Levine MM, Sur D. Determinants of health care seeking for diarrheal illness in young children in urban slums of Kolkata, India. The American journal of tropical medicine and hygiene 2013; 89:56-61; PMID:23629936; http://dx.doi.org/10.4269/ajtmh.12-0756
- Bohles N, Busch K, Hensel M. Vaccines against human diarrheal pathogens: Current status and perspectives. Human vaccines & immunotherapeutics 2014; 10; PMID:24861668; http://dx.doi.org/10.4161/hv.29241
- Modlin JF. Inactivated polio vaccine and global polio eradication. The Lancet infectious diseases 2012; 12:93-4; PMID:22071250; http://dx.doi.org/10.1016/S1473-3099(11)70312-7
- Thompson KM, Duintjer Tebbens RJ. National choices related to inactivated poliovirus vaccine, innovation and the endgame of global polio eradication. Expert review of vaccines 2014; 13:221-34; PMID:24308581; http://dx.doi.org/10.1586/14760584.2014.864563
- Thomassen YE, van 't Oever AG, van Oijen MG, Wijffels RH, van der Pol LA, Bakker WA. Next generation inactivated polio vaccine manufacturing to support post polio-eradication biosafety goals. PloS one 2013; 8:e83374; PMID:24349497; http://dx.doi.org/10.1371/journal.pone.0083374
- Jensen H, Benn CS, Aaby P. DTP in low income countries: improved child survival or survival bias? Bmj 2005; 330:845-6; PMID:15817558; http://dx.doi.org/10.1136/bmj.330.7495.845-a
- Robbins JB, Schneerson R, Keith JM, Shiloach J, Miller M, Trollors B. The rise in pertussis cases urges replacement of chemically-inactivated with genetically-inactivated toxoid for DTP. Vaccine 2007; 25:2811-6; PMID:17291636; http://dx.doi.org/10.1016/j.vaccine.2006.12.013
- Corbel MJ, Das RG, Lei D, Xing DK, Horiuchi Y, Dobbelaer R, WHO Working Group. WHO Working Group on revision of the Manual of Laboratory Methods for Testing DTP Vaccines-Report of two meetings held on 20-21 July 2006 and 28-30 March 2007, Geneva, Switzerland. Vaccine 2008; 26:1913-21; PMID:18336960; http://dx.doi.org/10.1016/j.vaccine.2008.02.013
- He H, Chen E, Chen H, Wang Z, Li Q, Yan R, Guo J, Zhou Y, Pan J, Xie S. Similar immunogenicity of measles-mumps-rubella (MMR) vaccine administrated at 8 months versus 12 months age in children. Vaccine 2014; 32:4001-5; PMID:24837773; http://dx.doi.org/10.1016/j.vaccine.2014.04.044
- Le Menach A, Boxall N, Amirthalingam G, Maddock L, Balasegaram S, Mindlin M. Increased measles-mumps-rubella (MMR) vaccine uptake in the context of a targeted immunisation campaign during a measles outbreak in a vaccine-reluctant community in England. Vaccine 2014; 32:1147-52; PMID:24440207; http://dx.doi.org/10.1016/j.vaccine.2014.01.002
- Stokes J, Jr., Weibel RE, Villarejos VM, Arguedas JA, Buynak EB, Hilleman MR. Trivalent combined measles-mumps-rubella vaccine. Findings in clinical-laboratory studies. JAMA : the journal of the American Medical Association 1971; 218:57-61; http://dx.doi.org/10.1001/jama.1971.03190140033006
- Howe BJ. Diphtheria-tetanus-acellular pertussis (DTaP) vaccine. JAMA : the journal of the American Medical Association 1996; 276:1803-4; PMID:8946898; http://dx.doi.org/10.1001/jama.1996.03540220027023
- MMWR. Food and Drug Administration approval of use of diptheria and tetanus toxoids and acellualr pertussis vaccine 1991; 40:881-2.
- Fine A. Diptheria, tetanus and acellular pertussis vaccine (DTaP): a case study. Institute of Medicine, 500 5th st, NW, Washington DC. 2003.
- Miller JJ, Jr., Ryan ML. Immunization with combined diptheria and tetanus toxoids (aluminum hydroxide adsorbed) containing H. pertussis vaccine. The duration of serologic immunity. Pediatrics 1948; 1:8-22; PMID:18905779
- Vesikari T, Silfverdal SA, Boisnard F, Thomas S, Mwawasi G, Reynolds D. Randomized, controlled, multicenter study of the immunogenicity and safety of a fully liquid combination diphtheria-tetanus toxoid-five-component acellular pertussis (DTaP5), inactivated poliovirus (IPV), and Haemophilus influenzae type b (Hib) vaccine compared with a DTaP3-IPV/Hib vaccine administered at 3, 5, and 12 months of age. Clin Vaccin Immunol 2013; 20:1647-53; PMID:23966556; http://dx.doi.org/10.1128/CVI.00414-13
- Sheridan SL, Ware RS, Grimwood K, Lambert SB. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a North American outbreak. ClinIinfect Dis 2012; 55:1434-5; author reply 5-6; PMID:22871826; http://dx.doi.org/10.1093/cid/cis672
- Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA : the journal of the American Medical Association 2012; 308:454-6; PMID:22851107
- Capeding MR, Cadorna-Carlos J, Book-Montellano M, Ortiz E. Immunogenicity and safety of a DTaP-IPV//PRP approximately T combination vaccine given with hepatitis B vaccine: a randomized open-label trial. Bull World Health Org 2008; 86:443-51; PMID:18568273
- Aristegui J, Dal-Re R, Diez-Delgado J, Mares J, Casanovas JM, Garcia-Corbeira P, De Frutos E, Van Esso D, Verdaguer J, De la Flor J, et al. Comparison of the reactogenicity and immunogenicity of a combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio (DTPa-HBV-IPV) vaccine, mixed with the Haemophilus influenzae type b (Hib) conjugate vaccine and administered as a single injection, with the DTPa-IPV/Hib and hepatitis B vaccines administered in two simultaneous injections to infants at 2, 4 and 6 months of age. Vaccine 2003; 21:3593-600; PMID:12922087; http://dx.doi.org/10.1016/S0264-410X(03)00420-1
- Kanra G, Silier T, Yurdakok K, Yavuz T, Baskan S, Ulukol B, Ceyhan M, Ozmert E, Türkay F, Pehlivan T. Immunogenicity study of a combined diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis vaccine used to reconstitute a freeze-dried Haemophilus influenzae type b vaccine (DTaP-IPV//PRP-T) administered simultaneously with a hepatitis B vaccine at two, three and four months of life. Vaccine 1999; 18:947-54; PMID:10580209; http://dx.doi.org/10.1016/S0264-410X(99)00331-X
- Carlsson RM, Claesson BA, Selstam U, Fagerlund E, Granstrom M, Blondeau C, Hoffenbach A. Safety and immunogenicity of a combined diphtheria-tetanus-acellular pertussis-inactivated polio vaccine-Haemophilus influenzae type b vaccine administered at 2-4-6-13 or 3-5-12 months of age. Pediatr Infect Dis J 1998; 17:1026-33; PMID:9849987; http://dx.doi.org/10.1097/00006454-199811000-00013
- Eskola J, Ward J, Dagan R, Goldblatt D, Zepp F, Siegrist CA. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 1999; 354:2063-8; PMID:10636384; http://dx.doi.org/10.1016/S0140-6736(99)04377-9
- Guerra FA, Blatter MM, Greenberg DP, Pichichero M, Noriega FR, Pentacel Study G. Safety and immunogenicity of a pentavalent vaccine compared with separate administration of licensed equivalent vaccines in US infants and toddlers and persistence of antibodies before a preschool booster dose: a randomized, clinical trial. Pediatrics 2009; 123:301-12; PMID:19117896; http://dx.doi.org/10.1542/peds.2007-3317
- Southern J, Crowley-Luke A, Borrow R, Andrews N, Miller E. Immunogenicity of one, two or three doses of a meningococcal C conjugate vaccine conjugated to tetanus toxoid, given as a three-dose primary vaccination course in UK infants at 2, 3 and 4 months of age with acellular pertussis-containing DTP/Hib vaccine. Vaccine 2006; 24:215-9; PMID:16112255; http://dx.doi.org/10.1016/j.vaccine.2005.07.060
- Rennels MB, Englund JA, Bernstein DI, Losonsky GA, Anderson EL, Pichichero ME, et al. Diminution of the anti-polyribosylribitol phosphate response to a combined diphtheria-tetanus-acellular pertussis/Haemophilus influenzae type b vaccine by concurrent inactivated poliovirus vaccination. Pediatr Infecti Dis J 2000; 19:417-23; PMID:10819337; http://dx.doi.org/10.1097/00006454-200005000-00006
- Punjabi NH, Richie EL, Simanjuntak CH, Harjanto SJ, Wangsasaputra F, Arjoso S, Munoz FM, Wolff MC. Immunogenicity and safety of four different doses of Haemophilus influenzae type b-tetanus toxoid conjugated vaccine, combined with diphtheria-tetanus-pertussis vaccine (DTP-Hib), in Indonesian infants. Vaccine 2006; 24:1776-85; PMID:16303216; http://dx.doi.org/10.1016/j.vaccine.2005.10.023
- Dhillon S. DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa): A Review of its Use as Primary and Booster Vaccination. Drugs 2010; 70:1021-58; PMID:20481658; http://dx.doi.org/10.2165/11204830-000000000-00000
- Johns TL, Hutter GE. New combination vaccines: DTaP-IPV (Kinrix) and DTaP-IPV/Hib (Pentacel). Ann Pharmacother 2010; 44:515-23; PMID:20197476; http://dx.doi.org/10.1345/aph.1M468
- Bar-On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane database of Syst Rev 2012; 4:CD005530; PMID:22513932
- Marshall GS, Marchant CD, Blatter M, Friedland LR, Aris E, Miller JM. Co-administration of a novel Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y-tetanus toxoid conjugate vaccine does not interfere with the immune response to antigens contained in infant vaccines routinely used in the United States. Human Vaccin 2011; 7:258-64; PMID:21307655; http://dx.doi.org/10.4161/hv.7.2.14170
- Tejedor JC, Moro M, Ruiz-Contreras J, Castro J, Gomez-Campdera JA, Navarro ML, Merino JM, Martín-Ancel A, Roca J, García-del-Río M, et al. Immunogenicity and reactogenicity of primary immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type B vaccine coadministered with two doses of a meningococcal C-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J 2006; 25:713-20; PMID:16874171; http://dx.doi.org/10.1097/01.inf.0000227725.61495.c4
- Tejedor JC, Omenaca F, Garcia-Sicilia J, Esporrin C, Molina V, Mares J, Muro M, Sanjuan P, Méndez M, Teixidor R, et al. Antibody persistence after primary vaccination with a hexavalent DTPa-HBV-IPV/HiB vaccine coadministered with a meningococcal C-CRM197 vaccine and response to a DTPa-IPV/HiB booster at 18 months of age. Pediatr Infect Dis J 2006; 25:943-5; PMID:17006294; http://dx.doi.org/10.1097/01.inf.0000237917.60734.05
- Knuf M, Habermehl P, Cimino C, Petersen G, Schmitt HJ. Immunogenicity, reactogenicity and safety of a 7-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a DTPa-HBV-IPV/Hib combination vaccine in healthy infants. Vaccine 2006; 24:4727-36; PMID:16616973; http://dx.doi.org/10.1016/j.vaccine.2006.03.032
- Chevallier B, Vesikari T, Brzostek J, Knuf M, Bermal N, Aristegui J, Borys D, Cleerbout J, Lommel P, Schuerman L. Safety and reactogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with routine childhood vaccines. The Pediatr Infect Dis J 2009; 28:S109-18; PMID:19325447; http://dx.doi.org/10.1097/INF.0b013e318199f62d
- Gimenez-Sanchez F, Kieninger DM, Kueper K, Martinon-Torres F, Bernaola E, Diez-Domingo J, Steul K, Juergens C, Gurtman A, Giardina P, et al. Immunogenicity of a combination vaccine containing diphtheria toxoid, tetanus toxoid, three-component acellular pertussis, hepatitis B, inactivated polio virus, and Haemophilus influenzae type b when given concomitantly with 13-valent pneumococcal conjugate vaccine. Vaccine 2011; 29:6042-8; PMID:21704105; http://dx.doi.org/10.1016/j.vaccine.2011.06.026
- Plotkin SA. Correlates of protection induced by vaccination. Clin Vaccin Immunol 2010; 17:1055-65; PMID:20463105; http://dx.doi.org/10.1128/CVI.00131-10
- White C, Halperin SA, Scheifele DW. Pediatric combined formulation DTaP-IPV/Hib vaccine. Expert Rev Vaccines 2009; 8:831-40; PMID:19538110; http://dx.doi.org/10.1586/erv.09.59
- Skibinski DA, Baudner BC, Singh M, O'Hagan DT. Combination vaccines. J Global Infect Dis 2011; 3:63-72; PMID:21572611; http://dx.doi.org/10.4103/0974-777X.77298
- Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res 2012; 135:161-9; PMID:22446857
- Bhuiyan TR, Choudhury FK, Khanam F, Saha A, Sayeed MA, Salma U, Lundgren A, Sack DA, Svennerholm AM, Qadri F. Evaluation of immune responses to an oral typhoid vaccine, Ty21a, in children from 2 to 5 years of age in Bangladesh. Vaccine 2014; 32:1055-60; PMID:24440210; http://dx.doi.org/10.1016/j.vaccine.2014.01.001
- Bhutta ZA, Capeding MR, Bavdekar A, Marchetti E, Ariff S, Soofi SB, Anemona A, Habib MA, Alberto E, Juvekar S, et al. Immunogenicity and safety of the Vi-CRM197 conjugate vaccine against typhoid fever in adults, children, and infants in south and southeast Asia: results from two randomised, observer-blind, age de-escalation, phase 2 trials. Lancet Infect Dis 2014; 14:119-29; PMID:24290843; http://dx.doi.org/10.1016/S1473-3099(13)70241-X
- Simon R, Tennant SM, Galen JE, Levine MM. Mouse models to assess the efficacy of non-typhoidal Salmonella vaccines: revisiting the role of host innate susceptibility and routes of challenge. Vaccine 2011; 29:5094-106; PMID:21616112; http://dx.doi.org/10.1016/j.vaccine.2011.05.022
- Strugnell RA, Scott TA, Wang N, Yang C, Peres N, Bedoui S, Kupz A. Salmonella vaccines: lessons from the mouse model or bad teaching? Curr Opin Microbiol 2014; 17:99-105; PMID:24440968; http://dx.doi.org/10.1016/j.mib.2013.12.004
- Wahid R, Zafar SJ, McArthur MA, Pasetti MF, Levine MM, Sztein MB. Live oral Salmonella enterica serovar Typhi vaccines Ty21a and CVD 909 induce opsonophagocytic functional antibodies in humans that cross-react with S. Paratyphi A and S. Paratyphi B. Clin Vaccine Immunol 2014; 21:427-34; PMID:24429069; http://dx.doi.org/10.1128/CVI.00786-13
- Glass RI, Parashar UD. Rotavirus vaccines–balancing intussusception risks and health benefits. N Engl J Med 2014; 370:568-70; PMID:24422677; http://dx.doi.org/10.1056/NEJMe1315836
- Soares-Weiser K, Maclehose H, Bergman H, Ben-Aharon I, Nagpal S, Goldberg E, Pitan F, Cunliffe N. Vaccines for preventing rotavirus diarrhoea: vaccines in use. Cochrane Database Systemat Rev 2012; 11:CD008521; PMID:23152260
- Dennehy PH, Bertrand HR, Silas PE, Damaso S, Friedland LR, Abu-Elyazeed R. Coadministration of RIX4414 oral human rotavirus vaccine does not impact the immune response to antigens contained in routine infant vaccines in the United States. Pediatrics 2008; 122:e1062-6; PMID:18977955; http://dx.doi.org/10.1542/peds.2008-1059
- Leshem E, Moritz RE, Curns AT, Zhou F, Tate JE, Lopman BA, Parashar UD. Rotavirus vaccines and health care utilization for diarrhea in the United States (2007-2011). Pediatrics 2014; 134:15-23; PMID:24913793;http://dx.doi.org/10.1542/peds.2013-3849
- Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, Parashar UD. Effectiveness and impact of rotavirus vaccines in the United States - 2006–2012. Expert Rev Vaccines 2014; 13:365-76; PMID:24392657; http://dx.doi.org/10.1586/14760584.2014.877846
- Yen C, Tate JE, Hyde TB, Cortese MM, Lopman BA, Jiang B, Glass RI, Parashar UD. Rotavirus vaccines: Current status and future considerations. Hum Vaccines Immunother 2014; 10:1436-48; PMID:24755452
- Ryan ET, Calderwood SB, Qadri F. Live attenuated oral cholera vaccines. Expert Rev Vaccines 2006; 5:483-94; PMID:16989629; http://dx.doi.org/10.1586/14760584.5.4.483
- Svennerholm AM. From cholera to enterotoxigenic Escherichia coli (ETEC) vaccine development. Indian J Med Res 2011; 133:188-96; PMID:21415493
- Saha A, Chowdhury MI, Khanam F, Bhuiyan MS, Chowdhury F, Khan AI, Khan IA, Clemens J, Ali M, Cravioto A, et al. Safety and immunogenicity study of a killed bivalent (O1 and O139) whole-cell oral cholera vaccine Shanchol, in Bangladeshi adults and children as young as 1 year of age. Vaccine 2011; 29:8285-92; PMID:21907255; http://dx.doi.org/10.1016/j.vaccine.2011.08.108
- Chen WH, Greenberg RN, Pasetti MF, Livio S, Lock M, Gurwith M, Levine MM. Safety and immunogenicity of single-dose live oral cholera vaccine strain CVD 103-HgR, prepared from new master and working cell banks. Clin Vaccine Immunol 2014; 21:66-73; PMID:24173028; http://dx.doi.org/10.1128/CVI.00601-13
- Livio S, Strockbine NA, Panchalingam S, Tennant SM, Barry EM, Marohn ME, Antonio M, Hossain A, Mandomando I, Ochieng JB, et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 2014; 59:933-41; PMID:24958238; http://dx.doi.org/10.1093/cid/ciu468
- Martinez-Becerra FJ, Scobey M, Harrison K, Choudhari SP, Quick AM, Joshi SB, Middaugh CR, Picking WL. Parenteral immunization with IpaB/IpaD protects mice against lethal pulmonary infection by Shigella. Vaccine 2013; 31:2667-72; PMID:23602665; http://dx.doi.org/10.1016/j.vaccine.2013.04.012
- Savarino SJ, O'Dowd A, Poole S, Maciel M, Rollenhagen J, McVeigh A, et al. Adavancement of an adhesin-based ETEC vaccine into clinical evaluation. 6th International Conferenceon Vaccines for Enteric Diseases (VED) 2011 2011; Cannes, france:Sep 14-6.
- Monteiro MA, Baqar S, Hall ER, Chen YH, Porter CK, Bentzel DE, Applebee L, Guerry P. Capsule polysaccharide conjugate vaccine against diarrheal disease caused by Campylobacter jejuni. Infect Immun 2009; 77:1128-36; PMID:19114545; http://dx.doi.org/10.1128/IAI.01056-08
- Maue AC, Mohawk KL, Giles DK, Poly F, Ewing CP, Jiao Y, Lee G, Ma Z, Monteiro MA, Hill CL, et al. The polysaccharide capsule of Campylobacter jejuni modulates the host immune response. Infect Immun 2013; 81:665-72; PMID:23250948; http://dx.doi.org/10.1128/IAI.01008-12
- Scott DA. Vaccines against Campylobacter jejuni. J Infect Dis 1997; 176 Suppl 2:S183-8; PMID:9396708; http://dx.doi.org/10.1086/513791
- Kaminski RW, Wu M, Turbyfill KR, Clarkson K, Tai B, Bourgeois AL, Van De Verg LL, Walker RI, Oaks EV. Development and preclinical evaluation of a trivalent, formalin-inactivated Shigella whole-cell vaccine. Clin Vaccine Immunol 2014; 21:366-82; PMID:24403527; http://dx.doi.org/10.1128/CVI.00683-13
- Venkatesan MM, Ranallo RT. Live-attenuated Shigella vaccines. Expert Rev Vaccines 2006; 5:669-86; PMID:17181440; http://dx.doi.org/10.1586/14760584.5.5.669
- Riddle MS, Kaminski RW, Williams C, Porter C, Baqar S, Kordis A, Gilliland T, Lapa J, Coughlin M, Soltis C, et al. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine 2011; 29:7009-19; PMID:21787825; http://dx.doi.org/10.1016/j.vaccine.2011.07.033
- Harro C, Sack D, Bourgeois AL, Walker R, DeNearing B, Feller A, Chakraborty S, Buchwaldt C, Darsley MJ. A combination vaccine consisting of three live attenuated enterotoxigenic Escherichia coli strains expressing a range of colonization factors and heat-labile toxin subunit B is well tolerated and immunogenic in a placebo-controlled double-blind phase I trial in healthy adults. Clin Vaccine Immunol 2011; 18:2118-27; PMID:21994354; http://dx.doi.org/10.1128/CVI.05342-11
- Passwell JH, Ashkenzi S, Banet-Levi Y, Ramon-Saraf R, Farzam N, Lerner-Geva L, Even-Nir H, Yerushalmi B, Chu C, Shiloach J, et al. Age-related efficacy of Shigella O-specific polysaccharide conjugates in 1-4-year-old Israeli children. Vaccine 2010; 28:2231-5; PMID:20056180; http://dx.doi.org/10.1016/j.vaccine.2009.12.050
- Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol 2012; 19:1921-31; PMID:23035175; http://dx.doi.org/10.1128/CVI.00364-12
- Riddle MS, Savarino SJ. Moving beyond a heat-labile enterotoxin-based vaccine against enterotoxigenic Escherichia coli. Lancet Infecti Dis 2014; 14:174-5; PMID:24291167; http://dx.doi.org/10.1016/S1473-3099(13)70355-4
- Behrens RH, Cramer JP, Jelinek T, Shaw H, von Sonnenburg F, Wilbraham D, Weinke T, Bell DJ, Asturias E, Pauwells HL, et al. Efficacy and safety of a patch vaccine containing heat-labile toxin from Escherichia coli against travellers' diarrhoea: a phase 3, randomised, double-blind, placebo-controlled field trial in travellers from Europe to Mexico and Guatemala. Lancet Infect Dis 2014; 14:197-204; PMID:24291168; http://dx.doi.org/10.1016/S1473-3099(13)70297-4
- Kaminski RW, Oaks EV. Inactivated and subunit vaccines to prevent shigellosis. Expert Rev Vaccines 2009; 8:1693-704; PMID:19943764; http://dx.doi.org/10.1586/erv.09.127
- Turbyfill KR, Kaminski RW, Oaks EV. Immunogenicity and efficacy of highly purified invasin complex vaccine from Shigella flexneri 2a. Vaccine 2008; 26:1353-64; PMID:18276045; http://dx.doi.org/10.1016/j.vaccine.2007.12.040
- Ruan X, Robertson DC, Nataro JP, Clements JD, Zhang W, Group STTVC. Characterization of heat-stable (STa) toxoids of enterotoxigenic Escherichia coli fused to double mutant heat-labile toxin peptide in inducing neutralizing Anti-STa antibodies. Infect Immun 2014; 82:1823-32; PMID:24549325; http://dx.doi.org/10.1128/IAI.01394-13
- Roy K, Hamilton DJ, Fleckenstein JM. Cooperative role of antibodies against heat-labile toxin and the EtpA Adhesin in preventing toxin delivery and intestinal colonization by enterotoxigenic Escherichia coli. Clinical and vaccine immunology : CVI 2012; 19:1603-8; PMID:22875600
- Tacket CO. Plant-based oral vaccines: results of human trials. Curr Topics Microbiol Immunol 2009; 332:103-17; PMID:19401823
- Dharmasena MN, Hanisch BW, Wai TT, Kopecko DJ. Stable expression of Shigella sonnei form I O-polysaccharide genes recombineered into the chromosome of live Salmonella oral vaccine vector Ty21a. Int J Med Microbiol 2013; 303:105-13; PMID:23474241; http://dx.doi.org/10.1016/j.ijmm.2013.01.001
- Ahmed T, Bhuiyan TR, Zaman K, Sinclair D, Qadri F. Vaccines for preventing enterotoxigenic Escherichia coli (ETEC) diarrhoea. Cochrane Database Systemat Rev 2013; 7:CD009029; PMID:23828581
- Ihssen J, Kowarik M, Dilettoso S, Tanner C, Wacker M, Thony-Meyer L. Production of glycoprotein vaccines in Escherichia coli. Microbial Cell Factories 2010; 9:61; PMID:20701771; http://dx.doi.org/10.1186/1475-2859-9-61
- Phalipon A, Tanguy M, Grandjean C, Guerreiro C, Belot F, Cohen D, Sansonetti PJ, Mulard LA. A synthetic carbohydrate-protein conjugate vaccine candidate against Shigella flexneri 2a infection. J Immunol 2009; 182:2241-7; PMID:19201878; http://dx.doi.org/10.4049/jimmunol.0803141
- Ranallo RT, Fonseka S, Boren TL, Bedford LA, Kaminski RW, Thakkar S, Venkatesan MM. Two live attenuated Shigella flexneri 2a strains WRSf2G12 and WRSf2G15: a new combination of gene deletions for 2nd generation live attenuated vaccine candidates. Vaccine 2012; 30:5159-71; PMID:22658966; http://dx.doi.org/10.1016/j.vaccine.2012.05.003
- Zhang W, Sack DA. Progress and hurdles in the development of vaccines against enterotoxigenic Escherichia coli in humans. Expert Rrev Vaccines 2012; 11:677-94; PMID:22873126; http://dx.doi.org/10.1586/erv.12.37
- Tokuhara D, Yuki Y, Nochi T, Kodama T, Mejima M, Kurokawa S, Takahashi Y, Nanno M, Nakanishi U, Takaiwa F, et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc Natl Acad Sci U S A 2010; 107:8794-9; PMID:20421480; http://dx.doi.org/10.1073/pnas.0914121107
- Camacho AI, Souza-Reboucas J, Irache JM, Gamazo C. Towards a non-living vaccine against Shigella flexneri: from the inactivation procedure to protection studies. Methods 2013; 60:264-8; PMID:23046911; http://dx.doi.org/10.1016/j.ymeth.2012.09.008
- Barnoy S, Baqar S, Kaminski RW, Collins T, Nemelka K, Hale TL, Ranallo RT, Venkatesan MM. Shigella sonnei vaccine candidates WRSs2 and WRSs3 are as immunogenic as WRSS1, a clinically tested vaccine candidate, in a primate model of infection. Vaccine 2011; 29:6371-8; PMID:21596086; http://dx.doi.org/10.1016/j.vaccine.2011.04.115
- Martinez-Becerra FJ, Chen X, Dickenson NE, Choudhari SP, Harrison K, Clements JD, Picking WD, Van De Verg LL, Walker RI, Picking WL. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect Immun 2013; 81:4470-7; PMID:24060976; http://dx.doi.org/10.1128/IAI.00859-13
- Barry EM, Pasetti MF, Sztein MB, Fasano A, Kotloff KL, Levine MM. Progress and pitfalls in Shigella vaccine research. Nat Rev Gastroenterol Hepatol 2013; 10:245-55; PMID:23419287; http://dx.doi.org/10.1038/nrgastro.2013.12
- Lundgren A, Leach S, Tobias J, Carlin N, Gustafsson B, Jertborn M, Bourgeois L, Walker R, Holmgren J, Svennerholm AM. Clinical trial to evaluate safety and immunogenicity of an oral inactivated enterotoxigenic Escherichia coli prototype vaccine containing CFA/I overexpressing bacteria and recombinantly produced LTB/CTB hybrid protein. Vaccine 2013; 31:1163-70; PMID:23306362; http://dx.doi.org/10.1016/j.vaccine.2012.12.063
- Ashkenazi S, Cohen D. An update on vaccines against Shigella. Therap Adv Vaccines 2013; 1:113-23; PMID:24757519; http://dx.doi.org/10.1177/2051013613500428
- de la Cabada Bauche J, Dupont HL. New Developments in Traveler's Diarrhea. Gastroenterology & hepatology 2011; 7:88-95; PMID:21475415
- Riddle MS, Rockabrand DM, Schlett C, Monteville MR, Frenck RW, Romine M, Ahmed SF, Sanders JW. A prospective study of acute diarrhea in a cohort of United States military personnel on deployment to the Multinational Force and Observers, Sinai, Egypt. Am J Trop Med Hyg 2011; 84:59-64; PMID:21212203; http://dx.doi.org/10.4269/ajtmh.2011.10-0093
- Link W. Diarrhoeal Diseases (Updated February 2009) page. World Health Organization website. Available at: www.who.int/topics/diarrhoea/en/
- Farthing MJ. Tropical Malabsorption Semin Gastrointest Dis 2002; 13:221-31; PMID:12465593
- Holmgren J, Bourgeois L, Carlin N, Clements J, Gustafsson B, Lundgren A, Nygren E, Tobias J, Walker R, Svennerholm AM. Development and preclinical evaluation of safety and immunogenicity of an oral ETEC vaccine containing inactivated E. coli bacteria overexpressing colonization factors CFA/I, CS3, CS5 and CS6 combined with a hybrid LT/CT B subunit antigen, administered alone and together with dmLT adjuvant. Vaccine 2013; 31:2457-64; PMID:23541621; http://dx.doi.org/10.1016/j.vaccine.2013.03.027
- Van de Verg L, Venkatesan, M. A Shigella vaccine against prevalent serotypes-editorial commentary. Clin Infec Dis 2014; 59:942-3.
- Siegrist CA. Neonatal and early life vaccinology. Vaccine 2001; 19:3331-46; PMID:11348697; http://dx.doi.org/10.1016/S0264-410X(01)00028-7
- Offit PA, Quarles J, Gerber MA, Hackett CJ, Marcuse EK, Kollman TR, Gellin BG, Landry S. Addressing parents' concerns: do multiple vaccines overwhelm or weaken the infant's immune system? Pediatrics 2002; 109:124-9; PMID:11773551; http://dx.doi.org/10.1542/peds.109.1.124
- Dhillon S, Curran MP. Live attenuated measles, mumps, rubella, and varicella zoster virus vaccine (Priorix-Tetra). Paediatric drugs 2008; 10:337-47; PMID:18754700; http://dx.doi.org/10.2165/00148581-200810050-00007
- Cha SH, Shin SH, Lee TJ, Kim CH, Povey M, Kim HM, Nicholson O. Immunogenicity and safety of a tetravalent measles-mumps-rubella-varicella vaccine: an open-labeled, randomized trial in healthy Korean children. Clin Exp Vaccine Res 2014; 3:91-9; PMID:24427766; http://dx.doi.org/10.7774/cevr.2014.3.1.91
- O'Leary ST, Suh CA, Marin M, Vaccine Policy Collaborative I. Febrile seizures and measles-mumps-rubella-varicella (MMRV) vaccine: what do primary care physicians think? Vaccine 2012; 30:6731-3; PMID:22975026; http://dx.doi.org/10.1016/j.vaccine.2012.08.075
- Valigurova A, Jirku M, Koudela B, Gelnar M, Modry D, Slapeta J. Cryptosporidia: epicellular parasites embraced by the host cell membrane. Inter J Parasitol 2008; 38:913-22; PMID:18158154; http://dx.doi.org/10.1016/j.ijpara.2007.11.003
- Maclennan CA, Martin LB, Micoli F. Vaccines against invasive Salmonella disease: Current status and future directions. Hum Vaccin Immunother 2014; 10; PMID:24804797; http://dx.doi.org/10.4161/hv.29054
- Boisen N, Scheutz F, Rasko DA, Redman JC, Persson S, Simon J, Kotloff KL, Levine MM, Sow S, Tamboura B, et al. Genomic characterization of enteroaggregative Escherichia coli from children in Mali. J Infect Dis 2012; 205:431-44; PMID:22184729; http://dx.doi.org/10.1093/infdis/jir757
- Treanor JJ, Atmar RL, Frey SE, Gormley R, Chen WH, Ferreira J, et al. A Novel Intramuscular Bivalent Norovirus Virus-Like Particle Vaccine Candidate-Reactogenicity, Safety, and Immunogenicity in a Phase 1 Trial in Healthy Adults. J Infectious Dis. 2014; 210:1763-71.
- Mawas F, Dickinson R, Douglas-Bardsley A, Xing DK, Sesardic D, Corbel MJ. Immune interaction between components of acellular pertussis-diphtheria-tetanus (DTaP) vaccine and Haemophilus influenzae b (Hib) conjugate vaccine in a rat model. Vaccine 2006;24:3505-12; PMID:16524648; http://dx.doi.org/10.1016/j.vaccine.2006.02.021
- Brito LA1 MP, O'Hagan DT. Vaccine adjuvant formulations: a pharmaceutical perspective. Semin Immunol 2013; 25:130-45; PMID:23850011; http://dx.doi.org/10.1016/j.smim.2013.05.007
- Baldwin SL, Bertholet S, Reese VA, Ching LK, Reed SG, Coler RN. The importance of adjuvant formulation in the development of a tuberculosis vaccine. J Immunol 2012; 188:2189-97; PMID:22291184; http://dx.doi.org/10.4049/jimmunol.1102696
- O'Hagan DT, Ott G, Nest GV, Rappuoli R, Giudice G. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines 2013; 12:13-30; PMID:23256736; http://dx.doi.org/10.1586/erv.12.140
- FDA. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm172495.htm. 2014.
- Di Fabio JL, de Quarles C. Considerations for combination vaccine development and use in the developing world. Clin Infect Dis 2001; 33 Suppl 4:S340-5; PMID:11709770; http://dx.doi.org/10.1086/322571