Abstract
Mass immunization of children has the potential to decrease infection rates and prevent the transmission of influenza. We evaluated the immunogenicity, safety, and tolerability of different formulations of cell-derived MF59-adjuvanted and nonadjuvanted A/H1N1 influenza vaccine in children and adolescents. This was a randomized, single-blind, multicenter study with a total of 666 healthy subjects aged 6 months–17 y in one of 3 vaccination groups, each receiving formulations containing different amounts of influenza A/H1N1 antigen with or without MF59. A booster trivalent seasonal MF59 vaccine was administered one year after primary vaccinations. Antibody titers were assessed by hemagglutination inhibition (HI) and microneutralization assays obtained on days 1, 22, 43, 366, and 387 (3 weeks post booster). Safety was monitored throughout the study. One vaccination with 3.75 μg of A/H1N1 antigen formulated with 50% MF59 (3.75_halfMF59) or 7.5 μg of A/H1N1 antigen formulated with 100% MF59 (7.5_fullMF59) induced an HI titer ≥1:40 in >70% of children in the 1–<3, 3–8, and 9–17 y cohorts; however, 2 vaccinations with nonadjuvanted 15 μg A/H1N1 antigen were needed to achieve this response in the 1–<3 and 3–8 y cohorts. Among children aged 6–11 months, 1 dose of 7.5_fullMF59 resulted in an HI titer ≥1:40 in >70% while 2 doses of 3.75_halfMF59 were required to achieve this result. All vaccines were well tolerated. Our findings support the immunogenicity and safety of the 3.75_halfMF59 (2 doses for children <12 months) and 7.5_fullMF59 vaccine formulations for use in children and adolescents aged 6 months to 17 y The use of the 3.75_halfMF59 could have the benefit of antigen and adjuvant sparing, increasing the available vaccine doses allowing vaccination of more people.
Introduction
Young people from birth to 18 y of age were at significantly higher risk from the 2009 A/H1N1 influenza pandemic than older adults,Citation1-3 with the most severe infections initially reported in children.Citation4 Estimates suggest that the cumulative rate of developing clinical illness was increased by 30- to 80-fold among people aged ≤24 years compared with individuals aged ≥65 years.Citation1 In addition to their heightened susceptibility to seasonal and pandemic influenza viruses, children are also important contributors to virus transmission.Citation5,6
Mass immunization of children is considered essential to achieve disease control that will decrease infection rates and the risk of viral transmission within families and communities.Citation6-9 Health authorities and vaccine manufacturers have focused on the development of vaccines based on the A/California/7/2009 viral strain, with various adjuvanted and nonadjuvanted formulations produced using both traditional and novel methods.Citation10 One important advance in the manufacture of influenza vaccines is the use of cell-culture to replace traditional egg-based production. This technology is not dependent on the availability of eggs, and facilitates enhanced manufacturing control and potentially reduction in production lead times, all of which could be pivotal in the event of another influenza pandemic. A number of controlled clinical trials confirm that oil-in-water adjuvants, such as MF59, meet the qualities required of a safe vaccine for diverse populations.Citation11-19 In addition, MF59 has been shown to heighten antibody response to the A/H1N1 pandemic virusCitation18,20–24 and has been endorsed by the World Health Organization (WHO) for this indication.Citation25 This study evaluated the immunogenicity, safety, and tolerability of different formulations of cell-derived MF59-adjuvanted and nonadjuvanted A/H1N1 vaccine in healthy children and adolescents.
Results
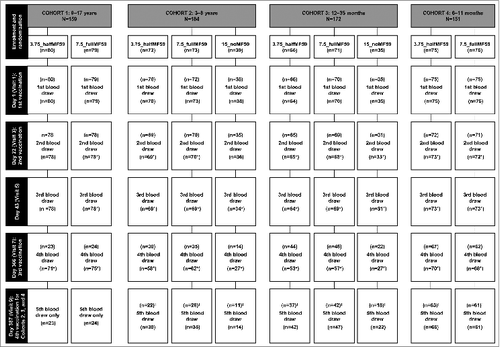
A total of 666 subjects were enrolled (cohort 1; n = 159; cohort 2; n = 184; cohort 3; n = 172; cohort 4; n = 151). Data from one site (n = 86) were excluded from the analysis as a result of noncompliance with protocol requirements for a different clinical study. Subject disposition and demographics are shown in and , respectively.
Table 1. Demographics and other baseline characteristics of subjects in cohorts 1, 2, 3 and 4 (enrolled population, excluding non-compliant site)
Figure 1. Subject disposition in each age cohort and vaccine group. *N values include blood draws outside the specified time window; †Fourth vaccination to be done only on the subset of naïve subjects <9 years of age. HI – hemagglutination inhibition.
Immunogenicity
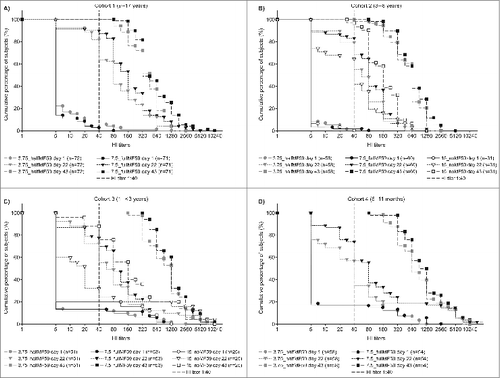
shows the cumulative percentage of subjects by hemagglutination inhibition (HI) titer following the initial injections (up to 43 days) in the 4 cohorts. In cohort 1 (9–17 years), there were 13- and 24-fold increases in geometric mean titer (GMT) in the 3.75_halfMF59 and 7.5_fullMF59 vaccine groups, respectively, 3 weeks following the first vaccination (). By day 43 (3 weeks following the second vaccination), GMT had increased 47-fold in the 3.75_halfMF59 group and 80-fold in the 7.5_fullMF59 group (). There were 6- to 14-fold increases in GMT across the 3 vaccine groups at day 22 for cohort 2 (3–8 years), with 28- to 115-fold increases across the 3 vaccine groups by day 43 (). Higher GMTs were evident for both adjuvanted groups compared with the nonadjuvanted group (). Three weeks following the first vaccination for cohort 3 (1–<3 years), there was a 6- to 7-fold increase in GMT from baseline in the adjuvanted vaccine groups compared with a 2-fold increase in the nonadjuvanted group (). By day 43, there was an 89- to 108-fold increase in GMT in the adjuvanted groups and an 11-fold increase in the nonadjuvanted group (). At day 22, there was an approximate 5- to 10-fold increase in GMT in the 3.75_halfMF59 and 7.5_fullMF59 groups, respectively, in cohort 4 (6–11 months), with a 92- to 129-fold increase by day 43 ().
Table 2. Geometric mean titers (GMT) and geometric mean ratios (GMR) – hemagglutination inhibition assay, per-protocol set*
Figure 2. Reverse cumulative distribution of hemagglutination inhibition (HI) titers in cohort 1 (A), cohort 2 (B), cohort 3 (C) and cohort 4 (D) on days 1, 22 and 43.
All three European Committee for Medicinal Products for Human Use (CHMP) criteria were met for both the nonadjuvanted and adjuvanted groups following the second vaccination (day 43) in all cohorts (). Following the first vaccination (day 22) for the adjuvanted formulations, the 3 CHMP criteria were met in cohorts 1, 2, and 3 and in the 7.5_fullMF59 group in cohort 4. The 3.75_halfMF59 group in cohort 4 did not meet the criterion of HI ≥1:40 at day 22 but met the other 2 CHMP criteria. At day 22, the 15 μg nonadjuvanted group met the CHMP geometric mean ratio (GMR) and seroconversion criteria but not the HI ≥1:40 criterion in cohort 2, and did not meet any CHMP criteria in cohort 3.
One year after the primary vaccinations, hemagglutinating antibodies were still present in all vaccine groups in subjects of all 4 cohorts, with a trend to higher HI titers in subjects primed with 7.5_fullMF59 (). In addition, greater persistence was achieved in cohorts 2 and 3 in response to both doses of the adjuvanted formulation vs. the nonadjuvanted vaccine (). Following the booster vaccination, all vaccine groups and cohorts achieved an HI titer ≥1:40.
Table 3. Number (percentage and 95% confidence interval) of subjects with hemagglutination inhibition titer ≥1:40 (per-protocol set)*
For the post-hoc analysis using an HI titer cutoff of ≥1:330, at day 43, 49–94% of subjects receiving the adjuvanted vaccines achieved this titer (), while only 10% and 24% of subjects achieved this titer following vaccination with the nonadjuvanted vaccines in cohorts 2 and 3, respectively. There was no apparent difference between the 2 adjuvanted formulations in the percentages of subjects achieving HI titers ≥1:330. Following the booster vaccination, 90–100% of subjects achieved an HI titer ≥1:330 with no difference between subjects primed with adjuvanted or non-adjuvanted formulations.
Table 4. Number (percentage and 95% confidence interval) of subjects who achieved seroconversion or significant increase in hemagglutination inhibition (per-protocol set)*
Table 5. Number (percentage and 95% confidence interval) of subjects with hemagglutination inhibition ≥1:330 (per-protocol set)
Analysis of results from the microneutralization (MN) titer assay revealed findings consistent with those for the HI assay, with the percentage of subjects with MN titer ≥1:40 substantially increased after the first and second vaccinations. The MN results for cohorts 2 and 3 were consistently lower for the nonadjuvanted groups. However, almost all subjects in the 3 vaccine groups in cohorts 2 and 3 demonstrated a 4-fold increase in MN titers following the second vaccination. A 4-fold increase in MN titers was evident 3 weeks after the first vaccination for approximately 50% of subjects in both vaccine groups in cohort 4, increasing to 100% at 3 weeks following the second vaccination ().
Table 6. Summary of immunogenicity data based on the microneutralization (MN) assay (per-protocol set)*
Table 7. Numbers (%) of subjects with any (and severe/>100 mm) local reaction within 7 days after each vaccination (safety population)
Table 8. Numbers (%) of subjects with any (and severe) systemic reaction within 7 d after each vaccination (safety population)
Safety
For all formulations of study vaccine, the majority of solicited local and systemic reactions were considered to be mild or moderate and most subjects recovered within 7 d of onset without sequelae (). The rate of local and systemic reactions was lower after the second vaccination for all groups and cohorts. Within each cohort, the occurrence of local and systemic reactions was higher in the 7.5_fullMF59 groups than the 3.75_halfMF59 groups. Systemic reactions were reported by more subjects in the adjuvanted groups than in the nonadjuvanted group in cohort 3, but there was no clear difference in the occurrence of systemic reactions between adjuvanted and non-adjuvanted groups in cohort 2. There was lower reactogenicity in all cohorts following the administration of the seasonal MF59 vaccine after one year.
Unsolicited adverse events (AEs) through day 43 were reported by 32–34% of subjects in cohort 1 and 35–36% of cohort 2, with higher rates for cohort 3 (37–61%) and for cohort 4 (63–64%). Nasopharyngitis, diarrhea, vomiting, eating disorder, and pyrexia were the most frequently reported unsolicited AEs for cohorts 3 and 4 that were considered possibly related to vaccine. The rate of unsolicited AEs following booster vaccinations was low, ranging from 5% in cohort 1, 7% cohort 2, 10% cohort 3, and 24% of subjects in cohort 4.
From day 1–546, 48 subjects experienced 65 serious AEs (SAEs), with higher rates evident for cohorts 3 and 4. A SAE considered possibly related to vaccine was reported for 3 subjects: multiple seizure episodes at day 98 affecting one subject in cohort 1 (7.5_fullMF59); vomiting in one subject in cohort 3, 26 d after the third vaccination (7.5_fullMF59); and febrile convulsion at day 10 in one subject in cohort 4 (3.75_halfMF59). Eight subjects, 2 in each of the 4 cohorts (3.75_halfMF59 n = 4; 7.5_fullMF59 n = 2; 15_noMF59 n = 2), discontinued study participation from day 1–546 due to at least one AE, with all withdrawals occurring before administration of the booster. No deaths occurred during the study.
Discussion
Effective, well-tolerated A/H1N1 vaccines are needed for children, particularly those aged 6–12 months, who are at high risk of contracting the virus due to lack of protection and priming of the immune system by exposure to the influenza virus. Children also contribute to virus transmission to family members and the community at large.Citation5,6 Our results demonstrate that nonadjuvanted A/H1N1 vaccines are less immunogenic than adjuvanted formulations for children. Low immunogenicity of nonadjuvanted vaccines in children has also been observed in other studies; for example, HI titers ≥1:40 were observed in 45% and 69% of subjects aged 6–35 months and 3–9 years, respectively, following a single dose of nonadjuvanted A/California/07/2009 vaccine, compared with rates of 95% and 94% in subjects aged 18–64 y and ≥65 years, respectively.Citation26
Our study showed that one vaccination with 3.75_halfMF59 or 7.5_fullMF59 induced an HI titer of ≥1:40 in >70 % of children and adolescents in the 1–<3 year, 3–8 year, and 9–17 y cohorts; however, 2 vaccinations with nonadjuvanted 15 μg A/H1N1 antigen were needed to achieve a similar response in the 1–<3 and 3–8 y cohorts. Among children aged 6–11 months, 1 dose of the fully adjuvanted vaccine was associated with an HI titer of ≥1:40 in >70% of children while 2 doses of the half-adjuvant formulation were required to achieve the same result. On a practical level, and considering cost effectiveness, administering 1 vaccine dose rather than 2 doses would be preferable. However, this approach would require commercial availability of the 2 presentations (3.75_halfMF59 and 7.5_fullMF59) which, during the very hectic times of a pandemic, may not be easy to achieve. A study conducted in children and adolescents aged 6 months to 18 y found that a single 15 μg dose of A/H1N1 monovalent vaccine resulted in HI titers ≥1:40 in >70% of those aged 9 to <18 years of age; however a single 7.5 or 15 μg dose did not achieve the same result in subjects 6 months to 8 y of age.Citation27 Together, these results suggest that the number and formulation of adjuvant vaccine doses should be tailored to children's age in order to achieve HI titers ≥1:40.
Our study established that the 3 CHMP criteria for immunogenicity were met 3 weeks following administration of the booster adjuvanted vaccine in the 4 cohorts across all vaccine groups. This was the case regardless of the priming formulation and irrespective of HI titers at day 366. However, one concern with the CHMP criteria is that they are based on studies conducted in adults and the applicability of these criteria for studies in children is not clear. While we were conducting this study, a separate study in 777 vaccine-naïve children aged 6–72 months 50 d following administration of 2 doses of MF59-adjuvanted seasonal vaccine was published.Citation28 Findings from this study suggested that the conventional adult HI titer of ≥1:40 is only associated with 22% protection in children aged 6–72 months.Citation28 Notably, titers of 1:110, 1:215, 1:330, and 1:629 were associated with protection rates of 50%, 70%, 80%, and 90%, respectively. The investigators concluded that a titer of 1:330 would have more favorable public health implications. To address this, we conducted a post-hoc analysis to establish the percentages of subjects achieving an HI titer ≥1:330. We observed that at day 43, 49–94% of subjects receiving the adjuvanted vaccines achieved this titer, compared with only 10% and 24% of subjects in cohorts 2 and 3 following vaccination with the nonadjuvanted vaccines. Following the booster, ≥90 % of subjects achieved an HI titer ≥1:330, with no difference between those subjects primed with adjuvanted or non-adjuvanted vaccine.
All study vaccines were safe and well tolerated, with the majority of solicited reactions mild and self-limiting. The safety profile of the adjuvanted vaccine was generally similar to that of the nonadjuvanted vaccine, including in subjects who received repeat doses of the adjuvanted formulation; in line with previous studies, local reactogenicity was higher with the adjuvanted formulation, especially in older age groups.Citation29 Furthermore, the safety profile of the cell-derived vaccine was similar to the safety profile previously reported for an equivalent egg-derived vaccine.Citation30 Together, these findings support the acceptable safety profile of the cell-derived halfMF59 and fullMF59 formulations when administered with 3.75 μg or 7.5 μg of A/H1N1 antigen to children aged 6 months to 17 y
Our findings support the immunogenicity and acceptable safety of the cell-derived MF59-adjuvanted A/H1N1 influenza vaccine in children aged 6 months to 17 y.
Methods
Study design and objectives
This randomized, single-blind, dose-ranging study was conducted between 2009 and 2011 at 8 centers in Germany, 2 in Belgium, 2 in the Dominican Republic, and one in the Netherlands. The protocol was approved by the Institutional Review Board or Ethics Committee at each center and the study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from each subject or their legal guardians before enrollment. The study was registered with the National Institutes of Health database, ClinicalTrials.gov (NCT00971100).
The primary study objective was to identify the preferred vaccine formulation (with or without MF59), dosage of antigen and adjuvant, and schedule (1 or 2 administrations) of the cell-derived A/H1N1 monovalent vaccine in healthy children and adolescents. Secondary objectives included evaluation of long-term persistence of antibodies and vaccine safety throughout the study.
Subjects
Eligible subjects were healthy males and females aged 6 months to 17 y on the day of enrollment without documented influenza in the 3 months preceding enrollment. Influenza was considered documented by positive serology test, viral culture, or rapid antigen tests. Exclusion criteria are detailed in the supplementary section.
Four cohorts were enrolled: children aged 9–17 y (cohort 1), 3–8 y (cohort 2), 1–<3 years (cohort 3), and 6–11 months (cohort 4).
Vaccines
Each subject received 2 doses of one of 3 vaccine formulations (3.75_halfMF59, 7.5_fullMF59 and 15_noMF59 containing 3.75, 7.5 and 15 μg A/H1N1 and 0.125, 0.25 and 0 mL MF59 adjuvant, respectively), (Supplementary Table 1) administered approximately 3 weeks apart. One year after the first vaccination, subjects received a booster with the trivalent seasonal MF59 vaccine (Fluad®) recommended for the 2010/11 influenza season (15 μg antigen from each influenza strain [including A/H1N1] + 0.35 mL MF59). A second dose of trivalent seasonal vaccine was administered 3 weeks after the booster to naïve subjects <9 years of age, to complete the primary immunization against influenza according to WHO recommendations. Vaccinations were administered by intramuscular injection in the deltoid muscle of the non-dominant arm and the anterolateral thigh for children ≥24 months and <24 months, respectively.
Immunogenicity analysis
Blood samples were taken on day 1 prior to vaccination, on days 22, 43, and 366 following the first vaccination, and on day 387 (3 weeks after the booster). Immunogenicity was evaluated by homologous (A/California/7/2009) H1N1 strain-specific HI and MN assays. Immunogenicity measures were: 1) GMT for HI and MN for the primary vaccine and for the booster; 2) GMR of HI and MN; 3) percentage of subjects achieving seroconversion (HI ≥1:40 for subjects negative at baseline [HI <1:10]), or a significant increase in HI (minimum 4-fold increase in HI titer for subjects positive at baseline); 4) percentage of subjects with a MN titer ≥1:40, 1:80, and 1:160; 5) percentage of subjects with an HI titer ≥1:40; and 6) percentage of subjects achieving a minimum 4-fold increase in MN titer. Following publication of HI antibody titers necessary for protection for inactivated influenza vaccines in children, a further analysis was conducted to evaluate the percentage of subjects with an HI titer ≥1:330.Citation28
Safety analysis
Safety reactions were collected on diary cards and reviewed at each follow-up visit. The safety analysis included the number of subjects exposed to study vaccine with solicited local and systemic reactions and unsolicited AEs reported per group. Solicited reactions occurring within 7 d post each vaccination were considered indicators of reactogenicity that might possibly be related to study vaccine. These included local and systemic reactions collected for 7 d following each vaccination.
All unsolicited AEs were collected for 3 weeks following each vaccination. All AEs resulting in study discontinuation, SAEs, onset of chronic health conditions, and associated medications were collected for the whole study (i.e., days 1–546).
Statistical analysis
Approximately 720 subjects (80 per age group receiving adjuvanted vaccines; 40 per age group in cohorts 2 and 3 receiving non-adjuvanted vaccines) were planned for study enrollment to provide adequate power for evaluation of the primary endpoint. Sample size was defined as sufficient to provide adequate estimates for the endpoints specified in the CHMP criteria, and was calculated to demonstrate non-inferiority with a power of 90%, if the real GMT ratio is 0.93.The full analysis set included all subjects who received a study vaccination and provided at least one evaluable serum sample before and after baseline. The per-protocol set (PPS) comprised all subjects who received the correct doses of vaccine, provided evaluable serum samples at the appropriate time points, and had no major protocol violations. The PPS was the primary analysis set for the immunogenicity analyses.
Immunogenicity criteria comprised those of the CHMP for adults,Citation31 including: 1) the percentage of subjects with seroconversion or significant increase in HI antibody is >40%; 2) the percentage of subjects achieving an HI titer ≥1:40 is >70%; and 3) GMR is ≥2.5. All three criteria assessed at day 43 and at least one of the 3 criteria assessed 3 weeks post-booster dose had to be met by each cohort to fulfill regulatory requirements. Results from day 22 following the first vaccination were also evaluated against these criteria. Vaccine group differences were assessed using 2-way analysis of variance with factors for vaccine group and center.
The safety population included all subjects who received study vaccination and provided post-baseline safety data. Descriptive statistics were calculated for all safety data and expressed as the proportion and number of subjects with solicited local or systemic reactions, unsolicited AEs, SAEs, and AEs resulting in study discontinuation. All statistical analyses were performed by Novartis Vaccines using SAS, version 9.1 or higher.
Disclosure of Potential Conflicts of Interest
MK received honoraria and compensation of travel expenses from Novartis, Astra Zeneca and GSK for presentations and advisory activities. GL-R received honoraria and compensation of travel expenses from Novartis, GSK and Immune Targeting Systems for presentations and advisory activities. H.R. and L.R, have no conflicts to report. PP, AKA, ML and GDC are full time employees of Novartis Vaccines companies. DK received honoraria and compensation of travel expenses from Novartis, Wyeth/Pfizer and GSK for presentations and advisory activities.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
Author Contributions
MK, GL-R, HR, LR and DK participated in the conduct of the study, data acquisition and interpretation, and contributed to the development of the initial draft of the manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted. PP participated in the conception, design, statistical analyses, implementation of the study, interpretation of analyzed data, contributed to the development of the initial draft of the manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted. AKA, ML and GDC participated in the conception, design and implementation of the study, interpretation of analyzed data, and contributed to the development of the initial draft of the manuscript, reviewed and revised the manuscript, and approved the final manuscript as submitted.
987014_Supplementary_Material.docx
Download MS Word (13 KB)Acknowledgments
We would like to thank the study teams, the children and their parents. Medical writing assistance for this manuscript was provided by Dr Helen Swainston of Bioscript Medical (London, UK) and funded by Novartis Vaccines. Editorial support was provided by Drs Yvonna Fisher-Jeffes and Shanthi Voorn.
Funding
This study was supported by funds provided by Novartis Vaccines.
References
- Health Protection Agency. Pandemic (H1N1) 2009 in England: an overview of initial epidemiological findings and implications for the second wave (v4 2 December 2009). Available from: http://webarchive.nationalarchives.gov.uk/20140714084352/http://www.hpa.org.uk/web/HPAwebFile/HPAweb_C/1258560552857
- Miller E, Hoschler K, Hardelid P, Stanford E, Andrews N, Zambon M. Incidence of 2009 pandemic influenza A H1N1 infection in England: a cross-sectional serological study. Lancet 2010; 375:1100-8; PMID:20096450; http://dx.doi.org/10.1016/S0140-6736(09)62126-7
- Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: an observational population-based study. Lancet 2010; 376:1846-52; PMID:21030071; http://dx.doi.org/10.1016/S0140-6736(10)61195-6
- World Health Organization. New influenza A (H1N1) virus: global epidemiological situation, June 2009. Wkly Epidemiol Rec 2009; 84:249-60; PMID:19537358
- Glezen WP, Couch RB. Interpandemic influenza in the Houston area, 1974-76. N Engl J Med 1978; 298:587-92; PMID:628375; http://dx.doi.org/10.1056/NEJM197803162981103
- Basta NE, Chao DL, Halloran ME, Matrajt L, Longini IM, Jr. Strategies for pandemic and seasonal influenza vaccination of schoolchildren in the United States. Am J Epidemiol 2009; 170:679-86; PMID:19679750; http://dx.doi.org/10.1093/aje/kwp237
- Medlock J, Galvani AP. Optimizing influenza vaccine distribution. Science 2009; 325:1705-8; PMID:19696313; http://dx.doi.org/10.1126/science.1175570
- Yang Y, Sugimoto JD, Halloran ME, Basta NE, Chao DL, Matrajt L, Potter G, Kenah E, Longini IM Jr.. The transmissibility and control of pandemic influenza A (H1N1) virus. Science 2009; 326:729-33; PMID:19745114; http://dx.doi.org/10.1126/science.1177373
- Jordan R, Connock M, Albon E, Fry-Smith A, Olowokure B, Hawker J, Burls A. Universal vaccination of children against influenza: are there indirect benefits to the community? A systematic review of the evidence. Vaccine 2006; 24:1047-62; PMID:16298026; http://dx.doi.org/10.1016/j.vaccine.2005.09.017
- Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med 2009; 361:2424-35; PMID:19745215; http://dx.doi.org/10.1056/NEJMoa0907650
- Alghisi F, Palma P, Montemitro E, Bernardi S, Pontrelli G, Rossi P, Lucidi V. Immunogenicity and safety profile of the monovalent A/H1N1 MF59-adjuvanted vaccine in patients affected by cystic fibrosis. Thorax 2011; 66:259-60; PMID:21228426; http://dx.doi.org/10.1136/thx.2010.156018
- Banzhoff A, Haertel S, Praus M. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Hum Vaccin 2011; 7:539-48; PMID:21422814; http://dx.doi.org/10.4161/hv.7.5.14821
- Esposito S, D'Angelo E, Daleno C, Peia F, Scala A, Serra D, Mirra N, Galeone C, Principi N. Immunogenicity, safety and tolerability of monovalent 2009 pandemic influenza A/H1N1 MF59-adjuvanted vaccine in patients with beta-thalassemia major. Vaccine 2010; 28:7825-8; PMID:20888873; http://dx.doi.org/10.1016/j.vaccine.2010.09.058
- Esposito S, Meregalli E, Daleno C, Peia F, Scala A, Serra D, Mirra N, Galeone C, Principi N. An open-label, randomized clinical trial assessing immunogenicity, safety and tolerability of pandemic influenza A/H1N1 MF59-adjuvanted vaccine administered sequentially or simultaneously with seasonal virosomal-adjuvanted influenza vaccine to paediatric kidney transplant recipients. Nephrol Dial Transplant 2011; 26:2018-24; PMID:20974645; http://dx.doi.org/10.1093/ndt/gfq657
- Esposito S, Tagliaferri L, Daleno C, Valzano A, Picciolli I, Tel F, Prunotto G, Serra D, Galeone C, Plebani A, et al. Pandemic influenza A/H1N1 vaccine administered sequentially or simultaneously with seasonal influenza vaccine to HIV-infected children and adolescents. Vaccine 2011; 29:1677-82; PMID:21199699; http://dx.doi.org/10.1016/j.vaccine.2010.12.047
- Kajaste-Rudnitski A, Galli L, Nozza S, Tambussi G, Di Pietro A, Pellicciotta G, Monti A, Mascagni P, Moro M, Vicenzi E. Induction of protective antibody response by MF59-adjuvanted 2009 pandemic A/H1N1v influenza vaccine in HIV-1-infected individuals. AIDS 2011; 25:177-83; PMID:21150561; http://dx.doi.org/10.1097/QAD.0b013e328341afa8
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959-65; PMID:19751689; http://dx.doi.org/10.1016/j.vaccine.2009.08.101
- Schultze V, D'Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine 2008; 26:3209-22; PMID:18462843; http://dx.doi.org/10.1016/j.vaccine.2008.03.093
- Tsai TF, Crucitti A, Nacci P, Nicolay U, Della Cioppa G, Ferguson J, Clemens R. Explorations of clinical trials and pharmacovigilance databases of MF59((R))-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand J Infect Dis 2011; 43:702-6; PMID:21534891
- Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respir Viruses 2008; 2:243-9; PMID:19453401; http://dx.doi.org/10.1111/j.1750-2659.2008.00059.x
- Durando P, Icardi G, Ansaldi F. MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin Biol Ther 2010; 10:639-51; PMID:20218923; http://dx.doi.org/10.1517/14712591003724662
- El Sahly H. MF59™ as a vaccine adjuvant: a review of safety and immunogenicity. Expert Rev Vaccines 2010; 9:1135-41; PMID:20923265; http://dx.doi.org/10.1586/erv.10.111
- O'Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines 2007; 6:699-710; PMID:17931151; http://dx.doi.org/10.1586/14760584.6.5.699
- Tsai T, Kyaw MH, Novicki D, Nacci P, Rai S, Clemens R. Exposure to MF59-adjuvanted influenza vaccines during pregnancy–a retrospective analysis. Vaccine 2010; 28:1877-80; PMID:19969117; http://dx.doi.org/10.1016/j.vaccine.2009.11.077
- Global alert and response: WHO recommendations on pandemic (H1N1) 2009 vaccines. Geneva. World Health Organization, 2009. Available from: http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/index.html
- Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet 2010; 375:41-8; PMID:20018365; http://dx.doi.org/10.1016/S0140-6736(09)62026-2
- Oh CE, Lee J, Kang JH, Hong YJ, Kim YK, Cheong HJ, Ahn YJ, Kim SH, Lee HJ. Safety and immunogenicity of an inactivated split-virus influenza A/H1N1 vaccine in healthy children from 6 months to. Vaccine 2010; 28:5857-63; PMID:20600483; http://dx.doi.org/10.1016/j.vaccine.2010.06.060
- Black S, Nicolay U, Vesikari T, Knuf M, Del Giudice G, Della Cioppa G, Tsai T, Clemens R, Rappuoli R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J 2011; 30:1081-5; PMID:21983214; http://dx.doi.org/10.1097/INF.0b013e3182367662
- Beyer WE, Nauta JJ, Palache AM, Giezeman KM, Osterhaus AD. Immunogenicity and safety of inactivated influenza vaccines in primed populations: a systematic literature review and meta-analysis. Vaccine 2011; 29:5785-92; PMID:21624411; http://dx.doi.org/10.1016/j.vaccine.2011.05.040
- Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O'Hagan DT, Podda A. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J 2009; 28:563-71; PMID:19561422; http://dx.doi.org/10.1097/INF.0b013e31819d6394
- European Medicines Agency. CPMP/BWP/214/96 Note for guidance on harmonization of requirements for influenza vaccines. Available from: http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500003945

