Abstract
Hyperthermia is a known effect induced by psychomotor stimulants and pathological hyperthermia is a prominent symptom of acute intoxication with these drugs in humans. In this manuscript, I will review our recent work concerning the brain hyperthermic effects of several known and recently appeared psychostimulant drugs of abuse (cocaine, methamphetamine, MDMA, methylone, and MDPV). Specifically, I will consider the role of activity state and environmental conditions in modulating the brain temperature effects of these drugs and their acute toxicity. Although some of these drugs are structurally similar and interact with the same brain substrates, there are important differences in their temperature effects in quiet resting conditions and the type of modulation of these temperature effects under conditions that mimic basic aspects of human drug use (social interaction, moderately warm environments). These data could be important for understanding the potential dangers of each drug and ultimately preventing adverse health complications associated with acute drug-induced intoxication.
Abbreviations
| Iv | = | intravenous |
| MDMA | = | 3, 4-methylenedioxymethamphetamine (Ecstasy) |
| METH | = | methamphetamine |
| methylone | = | 3, 4-methylenedioxymethcathinone |
| MDPV | = | 3, 4-methylenedioxypyrovalerone |
| NAc | = | nucleus accumbens, sc, subcutaneous |
Introduction: Psychoactive Drugs of Abuse
In contrast to therapeutic psychoactive drugs, which are either taken voluntarily or given by a medical professional to alleviate specific pathological symptoms, modern people also take drugs recreationally to induce desired psychoactive effects. The variety and popularity of these drugs differ greatly, ranging from the very “safe” consumption of caffeine-containing drinks to the compulsive intravenous injections of heroin, cocaine or methamphetamine (METH). While the desire to experience new, unusual or pleasurable psychic effects is the initial drive for the recreational use of most drugs, experience (i.e., repeated drug intake) slowly adds another powerful contributor, a subjective or physical discomfort of being without the drug, that promotes further drug intakes.
Each psychoactive drug induces specific behavioral, physiological and psycho-emotional effects, which vary significantly depending upon the drug dose as well as the individual's activity state and the environmental conditions under which the drug is taken. While some physiological effects of the drugs at low doses mimic those induced by natural arousing stimuli, at higher doses these effects could exceed the physiological range, resulting in acute drug-induced intoxication and posing a significant risk for human health. The drug dose is an obvious critical parameter for inducing acute intoxication, but it also influenced by individual responsiveness to the drug, previous drug experience, simultaneous use of other drugs, silent pre-existing pathology, and the conditions under which the drug is taken.
In this review, I want to focus on the latter variable and consider both the literature and our recent data which suggest that both activity state and environmental conditions play a critical role in modulating the physiological and behavioral effects of several psychostimulant drugs of abuse. Some of these drugs (i.e., cocaine and methamphetamine or METH) have been known for decades and exhibited up-and-down trends of popularity, whereas other drugs like ecstasy (3,4-methylenedioxymethamphetamine or MDMA, “Molly”) and new synthetic cathinones (as 3,4-methylenedioxymethcathinone or methylone and 3,4-methylenedioxypyrovalerone or MDPV) became popular among young adults in the last few years. Hyperthermia appears to be a common physiological effect of all these drugs and extreme hyperthermia is a typical symptom of acute intoxication with amphetamine-like stimulants. As such, the focus will be on drug-induced perturbations in temperature homeostasis with a special emphasis on brain temperature as an important parameter that not only reflects metabolic aspects of brain activity, but also affects all forms of neural activity and neural functioning.Citation1
Brain Temperature
While it is traditionally believed that brain temperature in healthy homeothermic organisms is stable and close to 37°C, abundant data obtained in different animals suggest that relatively large fluctuations in brain temperature occur during different types of natural motivated behavior and following exposure to various environmental challenges.Citation2-9 By using miniature thermocouple sensors chronically implanted in different brain structures and peripheral locations, we showed that hypothalamic temperature in awake, freely moving male rats could fall to ∼35°C during deep sleepCitation10 and could phasically peak to ∼39.5°C at time of ejaculation during copulatory behavior.Citation11 These limits obviously define the range of “normal” brain temperature fluctuations (∼4°C) within the entire physiological continuum.
While the recording of brain temperature in rats is a relatively simple procedure, human data are limited and often restricted to neurological patientsCitation12-15 and indirect measurementsCitation16-18 that are still questionable with respect to their validity and accuracy. Therefore, it is not yet definitely proven that similar, relatively large brain temperature fluctuations could occur in healthy humans. However, several observations suggest that this could be the case. First, monkeys, which are much closer evolutionarily to humans show robust physiological changes in brain temperature similar to the range observed in rats.Citation3,5,6 Second, humans show pathological hyperthermia (>40-41°C) during acute intoxication by METH or MDMA,Citation19-22 also similar to the range seen in rats. Finally, direct measurements from healthy human volunteers suggest that brain temperatures (assessed by venous outflow) could reach 39.5-40.0°C during a 30-min bicycle exercise when they are wearing water-impermeable clothingCitation23-24 that impaired normal heat dissipation to the external environment. The physical and mental states of these volunteers at these high brain temperatures remained within the normal range.
Basic Mechanisms Underlying Brain Temperature Homeostasis
Brain temperature is determined by the balance of two opposing forces, which tend to either increase it by metabolism-related intra-brain heat production or decrease it through heat dissipation by cerebral blood outflow to the rest of the body and then to the external environment. The brain represents ∼2% of body weight in humans and accounts for ∼20% of organism's total energy consumption.Citation25,26 The heat continuously generated by brain tissue is removed by cerebral circulation due to a temperature differential between arterial blood inflowing to the brain and brain tissue.Citation6,27,28
While it seems mechanistic, the cooling of an internal combustion engine appears to be a good analogy to brain temperature exchange. Similar to circulating coolant that continuously removes heat from a working engine, cool, oxygenated arterial blood removes heat from the brain via heat exchange and the now warmed venous blood returns to the heart to be cooled and oxygenated again in the lungs. Such an arrangement determines the critical role of cerebral blood flow in brain temperature homeostasis and the essential inter-dependence of temperature in the brain and the rest of the body. While brain temperature tends to increase due to metabolism-related intra-brain heat production, it also rises when brain-generated heat cannot be properly dissipated to the body and then to the external environment. Similarly, a decrease in cerebral metabolism tends to lower brain temperature, but this effect could be strongly enhanced by peripheral vasodilatation that promotes heat loss to a cooler environment. Since most psychoactive drugs affect metabolism as well as the state of peripheral and cerebral blood vessels, drug-induced brain temperature responses should depend significantly upon the ongoing organism's state and the environmental conditions. A drug at a certain dose could induce minimal temperature effects under one set of environmental conditions, when adaptive mechanisms of heat loss are fully effective. However, the same drug at the same dose could induce pathological hyperthermia when used in different environmental conditions when heat dissipation mechanisms are significantly impaired. Since peripheral vasodilatation and perspiration are powerful means for heat loss in humans, drug-induced impairment of these adaptive mechanisms could be a very important determinant of drug-induced increases in brain and body temperatures.
State-Dependent Effects of Cocaine on Brain Temperature
Cocaine is a widely used drug of abuse that acts on multiple neural substrates localized both in the central and peripheral nervous systems. Cocaine is a non-selective inhibitor of monoamine reuptake and cocaine's action on monoamine transporters, particularly the dopamine transporter, is usually viewed as the critical action in mediating its reinforcing properties.Citation29,30 Cocaine's action on uptake suggests that its effects should depend on the ongoing activity of monoamine neurons and release of monoamines, both of which are affected by an organism's functional state. This modulatory, activity-dependent pattern of cocaine's action on monoamine systems as well as other multiple actions of this drug in the brain and periphery (i.e., its interaction with ionic channels of different subtypes) should be considered as a whole to understand the highly variable physiological and behavioral effects of this drug. Moreover, these effects also depend upon the drug dose and route of administration. Since cocaine is interesting primarily as a drug of abuse, the effects induced by this drug in experimental animals should always be considered with respect to the doses and routes of administration used by humans. In contrast to animal research, when the effects of cocaine are typically assessed under quiet resting conditions, humans always self-administer this drug during high activity levels when various physiological parameters are altered as a part of motivated drug-seeking behavior. This psycho-physiological activation appears to be an essential force of cocaine-taking behavior and it could dramatically alter the pharmacological effects of this drug as seen in drug-naive, quietly resting animals.
In our studies of cocaine, all measurements were conducted in freely moving rats with thermocouple microsensors chronically implanted in different brain structures and several peripheral locations. Cocaine was delivered as an intravenous (iv) injection at 1 mg/kg, a dose optimal for drug-self-administration in rats.Citation31 First, we examined how brain temperature is affected by cocaine administered to quietly resting animals.Citation32,33 Second, we examined the pattern of temperature fluctuations during cocaine self-administration in drug-experienced, trained rats.Citation34 Finally, to explore how the effects of cocaine are modulated by ongoing behavior, we conducted a yoked control experiment, in which cocaine was delivered passively at the dose/pattern that mimicked its self-administration.Citation35
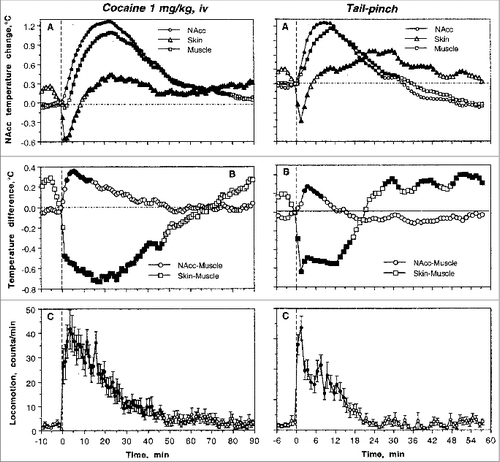
As shown in , cocaine delivered to quietly resting rats at 22-23°C (standard ambient temperature) induced moderate temperature increases in nucleus accumbens (NAc) and temporal muscle, a biphasic, down-up fluctuation in skin temperature (A), and a strong locomotor activation (C). The temperature increase in the NAc was always more rapid and stronger than in temporal muscle that received the same arterial supply as the brain (B). This difference (brain-muscle differential) was maintained for ∼20 min post-injection, suggesting metabolic neural activation and intra-brain heat production as a contributor to cocaine-induced brain temperature elevation. Skin temperature decrease is consistent with peripheral vasoconstriction, a known rapid effect of cocaine following its iv administration. Since skin temperature is also affected by the temperature of arterial blood, exclusion of this factor (i.e. skin-muscle temperature differential) revealed a powerful vasoconstrictive effect of cocaine that was maintained for about an hour after iv drug administration (B). Importantly, the pattern of temperature response induced by iv cocaine under quiet resting conditions was qualitatively similar to that induced by natural arousing stimuli such as a 3-min tail-pinch; see ). Tail-pinch, which also induced locomotor activation, increased brain and muscle temperatures and resulted in a rapid down-up fluctuation in skin temperature (D and F). Similar to cocaine, tail-pinch induced transient increases in NAc-Muscle differential and a biphasic, down-up change in skin-muscle differential. These changes, however, were less robust and more transient than those seen with cocaine.
Figure 1. Changes in brain (NAc), temporal muscle and skin temperatures induced by iv injection of cocaine (1 mg/kg) and 3-min tail-pinch in freely moving rats under quiet resting conditions. Top graphs (A) show relative changes in temperatures, middle graphs (B) show changes in NAc-Muscle and Skin-Muscle temperature differentials and bottom graphs (C) show changes in locomotor activity. Filled symbols mark values significantly different from baseline.
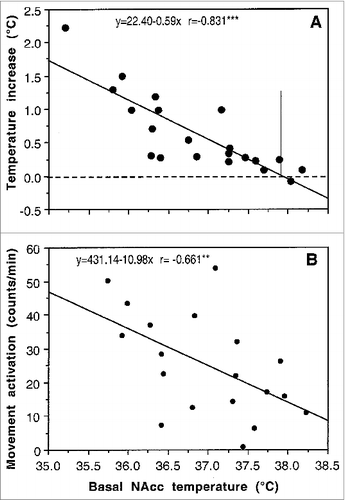
Similar to natural arousing stimuli, the hyperthermic effects of cocaine were strongly dependent upon baseline temperatures (). When basal NAc temperatures were low (35.5-37.0°C), cocaine increased them strongly. The effect, however, became progressively weaker when basal temperatures were higher [r = −0.831, p < 0.001]. As shown by the regression analysis, the effect disappeared at ∼38°C suggesting that cocaine delivered at brain temperatures higher than 38°C might induce an opposite response – a decrease in brain temperature. Although the correlation was weaker, cocaine-induced locomotor activation was also dependent on basal brain temperature (), being stronger at lower NAc temperatures and weaker at higher temperatures [r = −0.661, p < 0.01]. In contrast to the inversion of brain temperature response at high brain temperatures, cocaine increased locomotor activity at high brain temperatures but the response became progressively weaker. These relationships are also typical to various arousing stimuli (i.e., tail-pinch, social interaction) and they could reflect the known “law of initial values” which postulates that the magnitude and even direction of the autonomic response to the stimulus depends on the pre-stimulus basal values.Citation36
Figure 2. Relationships between basal brain (NAc) temperature and its changes induced by cocaine (1 mg/kg, iv) in freely moving rats (A). As can be seen, cocaine-induced NAc temperature increase (assessed as a peak value) was stronger when basal NAc temperatures were lower and weaker when basal temperatures were higher (r = −0.831; p < 0.001). Cocaine-induced locomotor activation was also dependent upon basal NAc temperature (r = −0.661; p < 0.01) but correlation was weaker in this case. Vertical line in (A) shows intersection of the regression line with the line of no effect, suggesting that the hyperthermic effect of cocaine disappears when basal NAc temperature is close to 38°C.
A strong dependence of cocaine's effects on brain temperature upon the behavioral context of drug administration was evident during self-administration behavior.Citation34,35 In this case, the first cocaine self-injection of a session induced a strong increase in NAc temperature (). This increase, moreover, was preceded by an equally strong temperature increase that occurred after the trained rat was exposed to a cocaine-related sensory cue that triggered drug-seeking behavior. This pre-injection increase was fully absent before the first passive cocaine injection in equally drug-experienced rats (yoked control), suggesting its association with behavior, but in this case NAc temperatures similarly increased after drug injection. In sharp contrast to the initial self-injection of a session, clear biphasic fluctuations of much smaller magnitude (∼0.1°C) were typical of subsequent self-injections of the session when NAc temperature stabilized at higher levels (A and C). In these cases, NAc temperature gradually increased before the lever-press for cocaine and transiently decreased after cocaine infusion. Despite their relatively small magnitude, these biphasic fluctuations were highly significant and occurred at higher and relatively stable NAc temperatures (∼38.5°C or ∼1.0-1.5°C above initial baseline; see A). As shown above with regression analysis (), cocaine loses its ability to increase brain temperatures at these levels. These biphasic fluctuations also occurred during repeated passive cocaine injections, but they were half as large in magnitude (C). Therefore, these biphasic changes are determined not only by behavior, but also reflect the superimposition of repeated drug effects.
Figure 3. Changes in brain (NAc) and temporal muscle temperatures during cocaine self-administration behavior in trained rats. (A) shows mean (±SEM) values of absolute temperatures at each event of cocaine self-administration session. L+S, presentation of a light-sound cue. (B, C and D) show rapid time-course dynamics of NAc temperatures associated with critical events of self-administration behavior (initial self-administration of a session; “regular” self-injections, the last self-injection of a session). Close circles show data obtained from self-administering animals and open circles represent data obtained from yoked-control rats. For clarity, standard errors are not shown in these graphs. The word “regular” refer to multiple self-injections within a session and these mean data were obtained for all fifth, tenth, and fifteens injections. Original data of this study were reported in.Citation34,35
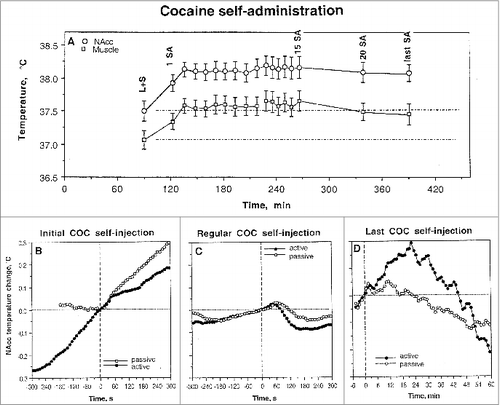
Interestingly, the post-injection decrease in NAc temperatures began from 40-60 s after the start of both active and passive cocaine injections, obviously reflecting the time necessary for the drug to travel to the brain, cross the blood-brain barrier (BBB), and diffuse to centrally located neural substrates. Although NAc temperature rises for ∼20 min after a single passive injection (), trained rats self-administer this drug (at 1 mg/kg dose) with a mean inter-injection interval of 7-8 min,Citation37 i.e., each subsequent injection occurs within the time of previous drug effect. Finally, NAc temperature dynamics changed dramatically after the last cocaine self-injection of a session when the lever was blocked. In this case, NAc temperature increased again after a transient decrease, correlating with rat's attempt to activate the blocked lever (). This effect was seen only in behaving animals and was absent in rats that received cocaine passively. Therefore, this increase in brain temperature is primarily dependent on a drug-seeking behavior.
Therefore, the effects of iv cocaine at a low, self-administering dose on brain temperature are clearly state-dependent, showing increases when the drug is passively administered during quiet resting conditions at low basal temperatures and biphasic responses (decreases followed by increases) when the drug is self-administered at high pre-injection “basal” temperatures. This state-dependency determines the appearance of biphasic, down-up temperature fluctuations around relatively stable tonically elevated temperature baselines. However, this behavior-associated pattern appeared after initial self-injections of a session that shifted temperature baseline to higher levels. Although these differences in the effects of cocaine depend upon the behavioral context of drug administration, our yoked control data suggest that basic aspects of these differences are pharmacologically determined and related to the super-imposition of repeated drug effects. Finally, this study clearly demonstrated that the effects of cocaine that is self-administered differ substantially from the effects of the same drug administered passively in quiet resting conditions.
Brain Hyperthermia Induced by Psychostimulant Drugs: State-Dependency and Environmental Modulation
METH and MDMA are widely used psychomotor stimulant drugs of a similar chemical structure. Both of these drugs increase metabolism and induce hyperthermia,Citation38-44 which is believed to be an important contributor to pathological changes associated with both acute intoxication with these drugs and their chronic abuse.Citation45-49 Both METH and MDMA are often considered “club drugs” that are used by young adults under conditions of physical and emotional activation typically in a warm and humid environment. While the effects of any neuroactive drug are modulated by environmental conditions and specific activity states, these factors may be especially important for METH and MDMA because, in addition to metabolic activation, these drugs induce peripheral vasoconstriction,Citation40,50 thus diminishing heat dissipation from body surfaces and enhancing heat accumulation in the brain.
Our initial thermorecording studies were focused on METH.Citation51 To assess the thermogenic effects of METH, we examined temperature changes in the NAcc, hippocampus, and temporal muscle induced in male rats by METH at different doses (1-9 mg/kg, sc) under quiet resting conditions at normal laboratory temperatures (23°C). To examine how the thermogenic effects of METH are modulated by activity state and environmental conditions that mimic some basic aspects of human drug use, similar parameters were monitored when METH at a higher dose (9 mg/kg) was administered during social interaction with a female (23°C) and at moderately warm ambient temperatures (29°C). While most drug experiments in rats are conducted at standard laboratory temperatures (22-23°C), 29°C used in our experiments is not a true hot temperature and is within the range of normothermy or a temperature comfort in rats,Citation52 where basal metabolism is maintained at its lowest levels.
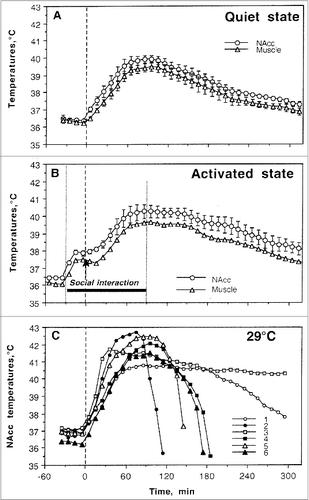
As shown in , METH (9 mg/kg, sc) administered in quiet resting conditions strongly increased brain and muscle temperatures (A). In contrast to moderate temperature increases (∼1°C) elicited by natural stimuli or cocaine at low, self-administering dose (see above), METH-induced hyperthermia was much stronger (3-4°C) and longer in its duration, exceeding 4-5 hours. Similar to that with natural arousing stimuli and cocaine, the increase was more rapid and stronger in the NAc than that in temporal muscle, pointing to metabolic brain activation (and intra-brain heat production) as the primary cause of brain hyperthermia and a factor behind a more delayed and weaker body hyperthermia. This change, however, was stronger and much more prolonged than that induced by arousing stimuli and cocaine. When METH was injected during social interaction with another animal, it induced significantly stronger and more prolonged increases in NAc and muscle temperatures (B). While thermogenic effects of social interaction and METH were not additive, under activated conditions NAc temperature was maintained above 40°C for about 90 min, while it only touched this value under quiet resting conditions. However, the temperature effects of METH were greatly potentiated by a slight increase in ambient temperature (C). When administered at 29°C, METH induced robust increases in NAc and muscle temperatures in all animals, rising to clearly pathological values (>41-42°C) and resulting in death of 5/6 animals within six hours post-injection. A similar potentiation of hyperthermic effects by social interaction and moderately warm environment was also found with MDMA in our early study.Citation53
Figure 4. Changes in brain (NAcc) and temporal muscle temperatures induced by methamphetamine (9 mg/kg, sc) under quiet resting conditions at standard ambient temperatures (A), during social interaction with female (B, activated conditions) and at moderately warm ambient temperatures (29°C). (A, B) show mean (±SEM) changes in absolute temperatures and (C) shows individual temperature responses in all tested rats. As can be seen, 4 of 6 rats tested died during the experimental session and one more rat (No 1) died overnight.
Although 9 mg/kg of METH used in our studies exceeds the typical dose range used by humans, this dose corresponds to only 1/6 of the LD50 in rats (49 mg/kg for ip injection, respectivelyCitation54,55) and does not induce lethality under standard environmental conditions. Therefore, the large LD50 determined in healthy rats in quiet resting conditions in a standard laboratory settings could be misleading and much smaller drug doses used under specific environmental conditions could induce life-threatening health complications, including lethality.
During recent years, there has been a rapid increase in the abuse of synthetic cathinone analogs that are sold with innocuous names such as “bath salts” or “plant food.”Citation56,57 Such products were designed to circumvent the regulations controlling the sale and use of psychoactive substances. Two very popular synthetic cathinones are 3,4-methylenedioxymethcathinone (methylone) and 3,4-methylenedioxypyrovalerone (MDPV).Citation58 While low recreational doses of synthetic cathinones enhance mood and increase energy, high doses or chronic use can cause serious medical complications, including agitation, psychosis, tachycardia, hyperthermia, and even death.Citation59,60 Due to these risks, methylone and MDPV have been classified as Schedule I controlled substances in the United States.
Methylone and MDPV are structurally similar to MDMA. Like MDMA, methylone and MDPV exert their major effects by interacting with monoamine transporter proteins in the central and peripheral nervous systems.Citation56 Methylone is a non-selective transporter substrate that evokes the release of dopamine, norepinephrine and serotonin, analogous to the effects of MDMA.Citation61-63 By contrast, MDPV is a potent transporter blocker that inhibits the uptake of dopamine and norepinephrine, with minimal effects on serotonin uptake.Citation64,65
Although robust increases in body temperature have been reported during acute intoxication with both methyloneCitation66 and MDPV,Citation67-69 the reported temperature effects of methylone and MDPV in laboratory animals are limited and controversial, varying according to species, dose, and experimental conditions.Citation61,Citation70-72 MDPV is reported to cause hyperthermia in mice (3-30 mg/kg, i.p.) but only under elevated ambient temperature.Citation71 In rats, acute MDPV (1.0-5.6 mg/kg s.c.) has no effect on core body temperature.Citation70
Since these drugs are structurally similar to MDMA, it was of interest to compare the thermogenic effects of MDPV and methylone with those induced by MDMA and assess whether they are equally affected by social interaction and moderately warm environment. Therefore, we first examined the effects of these three drugs at different doses on temperatures in the NAc, temporal muscle, and facial skin in quiet resting conditions at standard ambient temperatures (22°C). Then we examined the effect of methylone, MDPV and MDMA on these measures during social interaction (exposure to a conspecific male at 22-23°C) and at increased ambient temperatures (29°C). By using this three-point recording procedure we were able to assess how these drugs affect intra-brain heat production (NAc-muscle differential) and skin vascular tone (i.e., vasoconstriction/vasodilation; Skin-muscle differential), two factors determining brain and body hyperthermia.Citation73,74
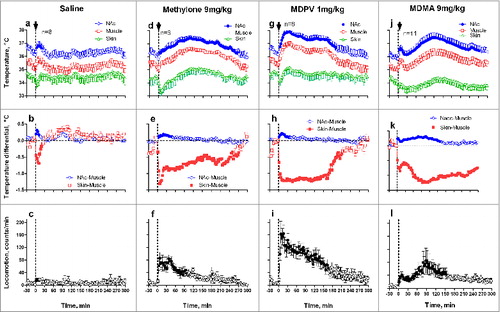
shows that MDMA, MDPV and methylone (9, 1 and 9 mg/kg, sc) each increase brain and muscle temperatures, rapidly decrease skin temperature, and induce locomotor activation. All these changes differed from a transient hyperthermic effect of control saline injection that did not result in evident motor response (a-c). While brain temperature increases induced by all three drugs were approximately equal in its magnitude (1.5-1.8°C), their time-course and duration differed in each case. Methylone and MDMA induce prolonged temperature increases, but the effect of MDPV was shorter and more rapid. Injections of each drug induced rapid increases in NAc-Muscle differentials, but this effect, suggesting metabolic brain activation and enhanced intra-brain heat production, was clearly larger vs. saline injection only with MDMA (k). Each drug also rapidly decreased Skin-Muscle differential, suggesting cutaneous vasoconstriction; this effect was about the same for each drug. Finally, all three drugs induced locomotor activation; this effect was clearly the greatest for MDPV.
Figure 5. Mean changes in brain (NAc), temporal muscle, and skin temperatures and locomotor responses induced by sc injections of methylone (9 mg/kg), MDPV (1 mg/kg), MDMA (9 mg/kg), and saline in freely moving rats under quiet resting conditions. Top graphs show mean (±SEM) values of absolute temperature changes; middle graphs show changes in NAc-Muscle and Skin-Muscle differentials; and bottom graphs show mean (±SEM) changes in locomotor activity. Filled symbols mark values significantly different from pre-injection baselines. Bold arrows mark the moment of injection. Original data shown in this graph were reported in Citation73,74
To examine how the temperature effects of drugs are affected by associated physiological activation, we examined temperature dynamics during social interaction of two rats. Consistent with our previous work with METH and MDMA,Citation51,53 the introduction of a novel rat into the chamber occupied by the experimental rat undergoing recording induced locomotor activation, a rapid, strong rise in NAc and muscle temperatures (∼1.5°C), and a brief decrease in skin temperature that was rapidly transformed into a more tonic, rebound-like increase (). While the changes in NAc and muscle temperatures were generally parallel, the rise was stronger and more rapid in the brain. This resulted in a significant increase in NAc-Muscle differentials that indicated metabolic brain activation. Social interaction was also accompanied by a strong decrease in Skin-Muscle temperature differentials, indicating stimulus-induced cutaneous vasoconstriction. While NAc-Muscle differentials rapidly returned to baseline after the end of the social interaction period, Skin-Muscle differentials increased above baseline, suggesting a rebound vasodilation. All these physiological parameters and locomotion showed consistent changes at the start and end of the social interaction. A saline injection during social interaction (+10 min after its onset; black arrow in ) had no effect on the parameters above.
Figure 6. Mean changes in brain (NAc), temporal muscle, and skin temperatures and locomotor responses induced by sc injections of methylone (9 mg/kg), MDPV (1 mg/kg), MDMA (9 mg/kg), and saline during social interaction. Top graphs show mean (±SEM) values of absolute temperature changes; middle graphs show changes in NAc-Muscle and Skin-Muscle differentials; and bottom graphs show mean (±SEM) changes in locomotor activity. Filled symbols mark values significantly different from the pre-injection baseline. The first and third vertical hatched lines in each graph show onset and offset of social interaction (60 min) and black arrows at the second hatched lines mark the moment of drug administration. Original data shown in this graph were reported in. Citation73,74
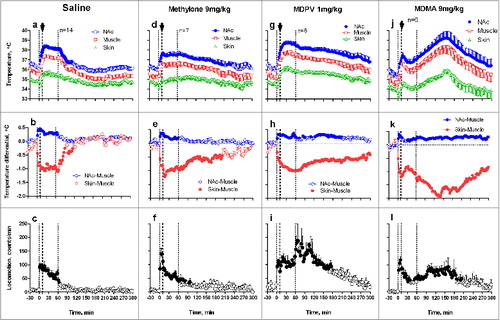
When instead of saline rats received methylone, temperature differences were minimal, but the decreases in NAcc and muscle temperatures and Skin-Muscle differentials after the end of social interaction were more prolonged (). Interestingly, the combination of two hyperthermic effects (drug + social interaction) did not result in their summation or potentiation. Mean values of all parameters in the Methylone group did not differ statistically from those seen with saline and with this drug used under quiet resting conditions. For example, mean increase in NAc temperature calculated for the entire 5-hour post-drug interval as the area under the curve was maximal with methylone used under quiet resting conditions (1.12±0.23°C) and even slightly lower when methylone was used during social interaction (0.88±0.18°C, no significant change).
Similar to methylone, injection of MDPV delayed brain and muscle temperature decreases after social interaction (). However, in contrast to methylone, changes were more robust and mean NAc temperature increase during social interaction (1.91±0.18°C) was significantly larger than those seen with this drug during quiet rest (0.79±0.25°C; p<0.01). The “pure” effect of MDPV (difference vs. saline control for each condition) was also clearly amplified when drug was administered during social interaction (1.38°C vs. 0.74°C). In contrast to methylone, MDPV also potentiated increases in NAc-Muscle differentials and decreases in skin-muscle differentials, suggesting a relatively weak potentiation of metabolic and vasoconstrictive effects of this drug used during physiological activation.
The most robust changes in hyperthermic effects were found with MDMA used during social interaction (). In this case, mean NAc temperatures at their peak exceed 39°C and response was most prolonged, exceeding the 5-hr observation interval. These changes were highly variable in individual rats, with some rats showing values exceeding 40°C. One rat that showed very strong hyperthermic effect even died overnight. The mean temperature elevation was maximal in this case (2.39±0.27°C), significantly exceeding the effect of this drug in quiet resting conditions (0.95±0.25°C; p<0.01). The pure effects of MDMA on brain temperature were also significantly stronger during social interaction than in quiet resting conditions (1.86°C vs. 0.89°C), suggesting super-additive interaction. Strong potentiation of MDMA effects during social interaction was also evident in other parameters. First, the increase in NAc-Muscle differential was very prolonged (> 5 hrs) although its amplitude did not differ from that seen with the drug used in quiet resting conditions. Second, when used during social interaction, MDMA robustly decreased Skin-Muscle differentials (-1.31±0.29°C) and this effect was larger and more prolonged than with other drugs (-0.56±0.13 and -0.66±0.27°C for Methylone and MDPV, respectively; p<0.05 for both cases). This drug effect was also clearly larger than that seen in quiet resting conditions (-0.95±0.24°C).
To examine how the hyperhermic effects of methylone, MDPV and MDMA are modulated under conditions of diminished heat dissipation, these drugs were administered under quiet resting conditions at 29°C, a temperature within rats’ thermoneutrality zone.Citation52 Consistent with previous studies,Citation51,53 rats exposed to a warm ambient temperature maintained stable but slightly higher internal temperatures (mean: 36.7±0.2, 36.2±0.2 and 35.2±0.4°C for NAc, muscle, and skin, respectively) than rats housed under standard laboratory conditions at 22-23°C (mean: 35.9±0.2, 35.2±0.3 and 34.0±0.26°C, respectively). The difference was minimal for the brain (Δ = 0.76°C) and maximal for skin (Δ = 1.17°C), suggesting weak tonic vasodilation as a way to promote dissipation of metabolic heat into the warmer environment. Saline injections under these conditions induced weak, transient injection-related temperature responses similar to that seen with saline injection at standard room temperature ().
Figure 7. Mean changes in brain (NAc), temporal muscle, and skin temperatures and locomotor responses induced by sc injections of methylone (9 mg/kg), MDPV (1 mg/kg), MDMA (9 mg/kg), and saline in freely moving rats at warm ambient temperatures (29°C). Top graphs show mean (±SEM) values of absolute temperature changes; middle graphs show changes in NAc-Muscle and Skin-Muscle differentials; and bottom graphs show mean (±SEM) changes in locomotor activity. Filled symbols mark values significantly different from the pre-injection baseline. Black arrows at the hatched lines mark the moment of drug administration. Since all rats exposed to MDMA died within 6 hrs post-injection, MDMA data are shown as individual changes (j) and mean values of NAc-Muscle, Skin-Muscle differentials and locomotion (k and l) for the first 80 min post-injection when all rats were still alive. Original data shown in this graph were reported in. Citation73,74
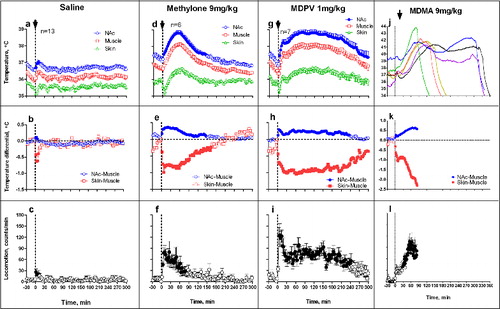
Although methylone (9 mg/kg) injected at 29°C increased NAc temperature (), this change was more transient than at 23°C and its mean value assessed as the area under the curve (0.47 ± 0.15°C) was significantly lower than in quiet resting conditions (1.12 ± 0.23°C, p < 0.01). Under these conditions, drug-induced increases in NAc-Muscle differentials were larger than those in quiet rest, but decreases in Skin-Muscle differentials were shorter in duration and significantly weaker than in control (−0.30 ± 0.05°C vs. −0.72 ± 0.15°C; p<0.05).
In contrast, MDPV used under warm ambient temperatures induced a stronger and more prolonged temperature elevation (1.51 ± 0.16°C), which was significantly larger that that induced at 23°C (0.79 ± 0.25°C; p < 0.05). This potentiation of hyperthermic effects was coupled with stronger and more prolonged increases in NAc-Muscle differentials and stronger decreases in Skin-Muscle differentials, but these changes were not significant vs. 23°C. Therefore, in contrast to the lack of habituation and unusual weakening of hyperthermic effects of methylone occurred during social interaction and at 29°C, hyperthermic effects of MDPV are moderately enhanced by both social interaction and warm external temperatures.
In contrast to two cathinones, MDMA administered at 29°C induced robust increases in NAc temperatures which reached fatal values (>41°C), inducing lethality in all 6 tested rats within 6 hours post-injection (). Peak values of NAc temperatures after MDMA administration ranged from 41.4°C to 43.8°C, and their means were more than 4°C larger than in the control condition (42.2 ± 0.4°C vs. 37.7 ± 0.4°C; p < 0.01). When calculated as the pre-lethality temperature peak, the mean increase in NAc temperature was 2.29 ± 0.22°C, significantly larger than mean MDMA-induced Nac temperature elevation in 23°C environment (0.95 ± 0.25°C; p < 0.01). By this parameter, the potentiation effect for MDMA was two-fold larger than for MDPV (1.34°C vs. 0.72°C).
The potentiation effect was also evident with other measures. While significant increases in NAc-Muscle differentials were evident with MDMA at quiet rest and standard ambient temperatures (mean = 0.17 ± 0.07°C), this effect more than doubled at 29°C (0.36 ± 0.04°C; p<0.05). When used at warm temperatures, MDMA also induced much stronger and more prolonged vasoconstriction as evidenced by Skin-Muscle differentials, which decreased more on average than 2°C below pre-injection baseline ().
Conclusions and Functional Implications
Data presented in this review demonstrate that brain hyperthermic effects of several popular psychostimulant drugs of abuse differ significantly depending upon the environmental conditions and the activity state associated with their administration. While each of these four drugs considered in this review increased brain temperature when administered under standard laboratory conditions (quiet rest, 22-23°C), this increase in each case differed in magnitude and duration. Each drug also showed a specific pattern of temperature responses when used under conditions of physiological activation (social interaction), when brain temperature was naturally increased, and at moderately warm environments that impair the adaptive mechanisms of heat loss. While cocaine decreased its ability to elevate brain temperature when used during physiological activation, the effects of methylone, MDPV and MDMA show different types of modulation (). When tested at standard laboratory conditions, each of three drugs induced a moderate, approximately equal brain hyperthermic effect. However, this effect for methylone (blue bars) did not change and even slightly decreased when the drug was used during social interaction and at 29°C, when basal brain temperature increased and heat dissipation was impaired. This pattern of interaction was especially evident when we calculated the net effects of methylone (drug - saline; ) in each condition. While atypical for METHCitation51 and MDMA,Citation53,73 this pattern of state dependency suggests that methylone and social interaction share common effector mechanisms (e.g., sympathetic activation) to induce brain hyperthermia. When these mechanisms are naturally activated during social interaction (i.e., when brain metabolic activity is increased and/or cutaneous vessels are physiologically constricted), the effects of the drug per se become weaker, but the overall hyperthermic response does not change.
Figure 8. Mean values of brain (NAc) temperature increases (area under curve for 5-hrs post-injection) induced by sc injections of methylone (9 mg/kg), MDPV (1 mg/kg), MDMA (9 mg/kg), and saline in rats under quiet resting conditions, during social interaction, and at warm ambient temperatures. Top graphs (A) show mean (±SEM) temperature changes and bottom graphs (B) show “net” or “pure” drug effects (drug - saline). Horizontal hatched lines in (B) show values induced by each drug under quiet resting conditions.
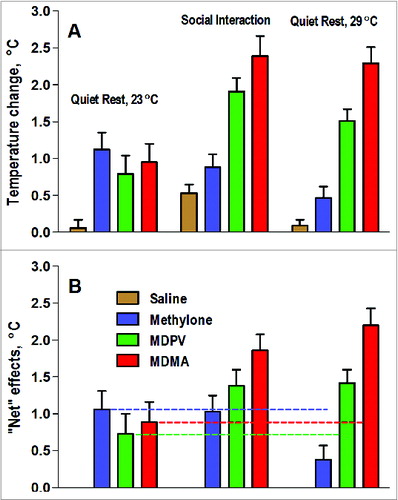
However, a different pattern of interaction occurred with MDPV (, green bars). Brain temperature increase induced by this drug during social interaction and at 29°C becomes significantly stronger (A) and net effect of MDPV was larger (B), suggesting an additive interaction and the involvement of different mechanisms in mediating the enhanced brain temperature response to MDPV. Finally, potentiation was supra-additive with MDMA (, red bars), which induced pathological brain temperature increases when administered during social interaction and lethality when used in moderately warm environments. This pattern of modulation was clearly evident in mean values of temperature elevation (A) and the “net” effect of drug in each condition (B).
A powerful enhancement of the hyperthermic effects of MDMA by environmental conditions seen in rats may help to explain the exceptionally strong, sometimes fatal, responses of some individuals induced by this drug under rave party conditions. However, some caution should be taken in extrapolating these findings to human conditions because humans have much more sophisticated mechanisms of heat loss from the body than do rats,Citation75 thus making them more resistant to high environmental temperatures and thermogenic effects of psychomotor stimulants. In contrast to rats, humans have a well-developed ability to sweat and have a very high dynamic range of flow rates in the skin, thus allowing them to lose more metabolic heat (1 kW) than could be maximally produced in the body.Citation76 These differences in the effector mechanisms of heat loss could explain weaker MDMA-induced body temperature increases and their lesser dependence on ambient temperatures found in monkeysCitation44,77,78 and humans.Citation39,79 Despite their high efficiency, the compensatory mechanisms of heat loss in humans could be greatly impaired under specific conditions, resulting in progressive heat accumulation in the organism. A simple bicycle exercise that produces ∼1°C brain temperature elevation under normal conditions produced strong hyperthermia (39.0-39.5°C) when the exercise is conducted in a special water-impermeable cloth that prevents heat dissipation to the external environment.Citation23 Therefore, pathological brain hyperthermia induced by overdose of psychomotor stimulants under rave conditions results not only from excessive heat production due to drug-induced and psycho-physiological activation, but also from the impaired ability to dissipate metabolic heat due to warm, humid environment and powerful drug-induced peripheral vasoconstriction. Since partygoers are often actively engaged with other individuals (dancing, sexual activity, etc.), drug effects are additionally potentiated by psycho-physiological activation typical to these conditions.
About the Author
Eugene A. Kiyatkin () received his MD from Moscow Sechenov Medical Academy (1978) and PhD from the USSR Academy of Medical Sciences (1983, Neurophysiology). His primary interests are in applying physiological, pharmacological, and neurochemical techniques for understanding central mechanisms underlying motivated behavior and actions of psychoactive drugs. Recent studies are focused on electrophysiological and neurochemical correlates of learning, the role of peripheral actions of cocaine and nicotine in mediating its central effects, glutamate and glucose electrochemistry, and alterations in permeability of blood-brain barrier in adverse health effects of psychomotor stimulant drugs of abuse.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest are disclosed.
Acknowledgments
I like to thank Leon P. Brown and Albert H. Kim, who contributed to experimental work described in this review and Suelynn Ren for the language editing of this text.
Funding
This work was supported by the National Institute on Drug Abuse, Intramural Research Program (DA000445-14).
REFERENCES
- Kiyatkin EA. Brain temperature homeostasis: physiological fluctuations and pathological shifts. Front Biosci 2010; 15:73-92.
- Abrams R, Hammel HT. Hypothalamic temperature in unanesthetized albino rats during feeding and sleeping. Am J Physiol 1964; 206:641-6.
- Baker MA, Frye FM, Millet VE. Origin of temperature changes evoked in the brain by sensory stimulation. Exp Neurol 1973; 38:502-19.
- Delgado JMR, Hanai T. Intracerebral temperatures in free-moving cats. Am J Physiol 1966; 211:755-69.
- Fuller CA, Baker MA. Selective regulation of brain and body temperatures in the squirrel monkey. Am J Physiol 1983; 245:R293-7.
- Hayward JN, Baker MA. Role of cerebral blood flow in the regulation of brain temperature In the monkey. Am J Physiol 1968; 215:389-403.
- Kovalson VM. Brain temperature variations during natural sleep and arousal in white rats. Physiol Behav 1972; 10:667-70.
- McElligott JC, Melzack R. Localized thermal changes evoked in the brain by visual and auditory stimulation. Exp Neurol 1967; 17:293-312.
- Serota HM, Gerard RM. Localized thermal changes in cat's brain. J Neurophysiol 1938; 1:115-24.
- Smirnov MS, Kiyatkin EA. Fluctuations in central and peripheral temperatures associated with feeding behavior in rats. Am J Physiol 2008; 295:R1414-24.
- Kiyatkin EA, Mitchum R. Fluctuations in brain temperatures during sexual behavior in male rats: An approach for evaluating neural activity underlying motivated behavior. Neuroscience 2003; 119:1169-83.
- Rumana CS, Gopinath SP, Uzura M, Valadka AB, Robertson CS. Brain temperatures exceed systemic temperatures in head-injured patients. Clin Care Med 1998; 26:562-7.
- Mariak Z, Jadeszko M, Lewko J, Lebkowski W, Lyson T. No specific brain protection against thermal stress in fever. Acta Neurochir (Wien) 1998; 140:585-90.
- Mariak Z, Lebkowski W, Lyson T, Lewko J, Piekarski P. Brain temperature during craniotomy in general anesthesia. Neurol Neurochir Pol 1999; 33:1325-7.
- Mariak Z, Lyso, T, Peikarski P, Lewko J, Jadeszko M, Szydlik P. Brain temperature in patients with central nervous system lesions. Neurol Neurosurg Pol 2000; 34:509-22.
- Rango M, Arighi A, Bonifati C, Bresolin N. Increased brain temperature in Parkinson's disease. NeuroReport 2012; 23:129-33.
- Rango M, Arighi A, Bonifati C, Del Bo R, Comi G, Bresolin N. The brain is hypothermic in patients with mitochondrial diseases. J Cereb Blood Flow Metab 2014; 34:915-920.
- Sukstanskii AL, Yablonskiy DA. Theoretical model of temperature regulation in the brain during changes in functional activity. Proc Natl Acad Sci USA 2006; 103:12144-9.
- Armenian P, Mamantov TM, Tsutaoka BT, Gerona RR, Silman EF, Wu AH, Olson KR. Multiple MDMA (Ecstasy) overdoses at a rave event: a case series. J Intensive Care Med 2013; 28:252-8.
- Dafters RI. Hyperthermia following MDMA administration in rats: effects of ambient temperature, water consumption, and chronic dosing. Physiol Behav 1995; 58:877-82.
- Kalant H. The pharmacology and toxicology of “ecstasy” (MDMA) and related drugs. Can Med Ass J 2001; 165:917-28.
- Nimmo SM, Kennedy BW, Tullett WM, Blyth AS. Dougall JR. Drug-induced hyperthermia. Anesthesia 1993; 48:892-5.
- Nybo L, Secher NH, Nielson B. Inadequate heat release from the human brain during prolonged exercise with hyperthermia. J Physiol 2002; 545:697-704.
- Nybo L. Hyperthermia and fatigue. J Appl Physiol 2008; 104:871-7.
- Schmidt-Nielsen K. Animal Physiology. Adaptation and Environment. 5th Edition. Cambridge: Cambridge University Press, 1997.
- Siesjo B. Brain Energy Metabolism. New York: Wiley, 1978.
- Feitelberg S, Lampl H. Warmetonung der Grosshirnrinde bei Erregung und Ruhe. Functionshemmung. Arch Exp Path Pharmak 1935; 177:726-36 (in German).
- Kiyatkin EA, Brown PL, Wise RA. 2002. Brain temperature fluctuation: a reflection of functional neural activation. Eur J Neurosci 2002; 16:164-68.
- Kuhar MJ, Ritz MC, Boja JM. The dopamine hypothesis of the reinforcing properties of cocaine. Trends Neurosci 1991; 14:299-302.
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev 1987; 94:469-92.
- Pickens R, Thompson T. Cocaine-reinforced behavior in rats: effects of reinforcement magnitude and fixed–ratio size. J Pharmacol Exp Ther 1968; 16:122-9.
- Brown PL, Kiyatkin EA. Dopamine-dependent and dopamine-independent actions of cocaine as revealed by brain thermorecording in freely moving rats. Eur J Neurosci 2005; 22:930-8.
- Kiyatkin EA. Brain temperature responses to salient stimuli persis during dopamine receptor blockade despite a blockade of locomotor responses. Pharmacol Biochem Behav 2008; 91:233-42.
- Kiyatkin EA, Brown PL. Fluctuations in neural activity during cocaine self-administration: Clues provided by brain thermorecording. Neuroscience 2003; 116:525-38.
- Kiyatkin EA, Brown PL. Brain temperature fluctuations during repeated passive vs. active cocaine administration: Clues for understanding the pharmacological determination of drug-taking behavior. Brain Res 2004; 1005:101-16.
- Wilder J. The law of initial values in neurology and psychiatry; facts and problems. J Nerv Ment Dis 1957; 125:73-86.
- Kiyatkin EA, Stein EA. Fluctuations in nucleus accumbens dopamine during cocaine self-administration behavior: an electrochemical study. Neuroscience 1995; 64:599-617.
- Alberts DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther 1995; 275:1104-14.
- Freedman RR. Johanson C-E. Tancer ME. Thermoregulatory effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology 2005; 183:248-56.
- Gordon CJ, Watkinson WP, O’Callaghan PP, Miller DB. Effects of 3,4-methylenedioxymetamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav 1991; 38:339-44.
- Green AR, Mechan AO, Elliott JM, O’Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-Methylenedioxymethamphetamine (MDMA, “Ecstasy”). Pharmacol Rev 2003; 55:463-508.
- Mechan AO, Esteban B, O’Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenediomethamphetamine (MDMA, “ecstasy”) to rats. Br J Pharmacol 2002; 135:170-80.
- Sandoval V, Hanson GR, Fleckenstein AE. Methamphetamine decreases mouse striatal dopamine transport activity: roles of hyperthermia and dopamine. Eur J Pharmacol 2000; 409:265-71.
- Von Huben RD, Lay CC, Crean RD, Davis SA, Katner SN, Taffe MA. Impact of ambient temperature on hyperthermia induced by (+/-)3,4- methylenediomethamphetamine in rhesus macaques. Neuropsychopharmacology 2007; 32:673-81.
- Ali SF, Newport GD, Holson RR, Slikker W, Bowyer JF. Low environmental temperatures or pharmacological agents that produce hypothermia decrease methamphetamine neurotoxicity in mice. Brain Res 1994; 658:33-8.
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res Rev 2001; 36:1-22.
- Kuhn DM, Geddes TJ. Molecular footprints of neurotoxic amphetamine action. Ann NY Acad Sci 2008; 914:92-103.
- Schmued LC. Demonstration and localization of neuronal degeneration in the rat forebrain following a single exposure to MDMA. Brain Res 2003; 974:127-33.
- Seiden LS, Sabol KE. Methamphetamine and methylenedioxymethamphetamine neurotoxicity: possible mechanisms of cell destruction. NIDA Res Monogr 1996; 163:251-76.
- Pederson NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) on conscious rabbits. J Neurosci 2001; 21:8648-54.
- Brown PL, Wise RA, Kiyatkin E.A. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J Neurosci 2003; 23:3924-9.
- Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 2002; 92:2667-79.
- Brown PL, Kiyatkin EA. Brain hyperthermia induced by MDMA (“ecstasy”): modulation by environmental conditions. Eur J Neurosci 2004; 20:51-8.
- Davis WM, Hatoum HT, Walters IW. Toxicity of MDA (2.4-methylenedioxyamphetamine) considered for relevance to hazards of MDMA (Ecstasy) abuse. Alcohol Drug Res 1987; 7:123-34.
- Yamamoto BK, Zhu W. The effect of methamphetamine on the production of free radicals and oxidative stress. J Pharmacol Exp Ther 1998; 287:107-14.
- Baumann MH, Partilla JS, Lehner KR. Psychoactive “bath salts”: not so soothing. Eur J Pharmacol 2013; 698:1-5.
- German et al., 2014 German CL, Fleckenstein AE, Hanson GR. Bath salts and synthetic cathinones: an emerging designer drug phenomenon. Life Sci 2014; 97:2-8.
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 2011; 49:499-505.
- Prosser JM, Nelson LS. The toxicology of bath salts: a review of synthetic cathinones. J Med Toxicol 2012; 8:33-42.
- Ross EA, Reisfield GM, Watson MC, Chronister CW, Goldberger BA. Psychoactive “bath salts” intoxication with methylenedioxypyrovalerone. Am J Med 2012; 125:854-8.
- Baumann MH, Ayestas MA, Jr., Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012; 37:1192-203.
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol 2013; 85:1803-15.
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol 2013; 168:458-70.
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology 2013; 38:552-62.
- Cameron KN, Kolanos R, Solis E, Jr., Glennon RA, De Felice LJ. Bath salts components mephedrone and methylenedioxypyrovalerone (MDPV) act synergistically at the human dopamine transporter. Br J Pharmacol 2013; 168:1750-57.
- Pearson JM, Hargraves TL, Hair LS, Massucci CJ, Frazee CC, 3rd, Garg U, et al. Three fatal intoxications due to methylone. J Anal Toxicol 2012; 36:444-451.
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of “bath salts” containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med 2012; 60:103-5.
- Kesha K, Boggs CL, Ripple MG, Allan CH, Levine B, Jufer-Phipps R, et al. Methylenedioxypyrovalerone (“bath salts”), related death: case report and review of the literature. J Forensic Sci 2013; 58: 1654-9.
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV). J Med Toxicol 2012; 8:69-75.
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology 2013; 71: 130-40.
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology 2013; 38:563-73.
- Merluzzi AP, Hurwitz ZE, Briscione MA, Cobuzzi JL, Wetzell B, Rice KC, et al. Age-dependent MDPV-induced taste aversions and thermoregulation in adolescent and adult rats. Dev Psychobiol 2013; 56:943-54.
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Critical role of peripheral vasoconstriction in fatal brain hypewrthermia induced by MDMA (Ecstasy) under conditions that mimic human drug use. J Neurosci 2014; 34:7754-62.
- Kiyatkin EA, Kim AH, Wakabayashi KT, Baumann MH, Shaham Y. Effects of social interaction and warm ambient temperature on brain hyperthermia induced by the designer drugs methylone and MDPV. Neuropsychopharmacology 2014; in press
- Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav 1990; 47:963-91.
- Rowell LB. Cardiovascular aspects of human thermoregulation. Circ Res 1983; 52:367-79.
- Banks ML, Sprague JE, Kisor DF, Czoty PW, Nichols DE, Nader MA. Ambient temperature effects on 3,4-methylenedioxymethamphetamine-induced theremodysregulation and pharmacokinetics in male monkeys. Drug Metab Dispos 2007; 35:1840-5.
- Taffe MA, Lay CC, Von Huben SN, Davis SA, Crean RD, Katner SN. Hyperthermia induced by 3,4-methylenedioxymethamphetamine in unrestrained rhesus monkeys, Drug Alcohol Depend 2006; 20:276-81.
- Parrott AC. The potential dangers of using MDMA for psychotherapy. J Psychoactive Drugs 2014; 46:37-43.


