Abstract
For treating colonic diseases, conventional oral drug delivery systems are not effective, as they fail to reach the appropriate site of action. Thus, there is a need to develop effective and safe therapy for the treatment of colonic disorders. The aim of the present study was to design a colon-specific delivery system for an anti-inflammatory drug, mesalamine, with minimal degradation and optimum delivery of the drug with relatively higher local concentration, which may provide more effective therapy for inflammatory bowel disease including Crohn disease and ulcerative colitis. Factorial designs (four factors and two levels) for eudragit S-100 (pH-dependent polymer)-coated, pectin (natural polysaccharides)-based microspheres of mesalamine were constructed and conducted in a fully randomized manner to study all possible combinations. Based on the desirability function formulation, F14 was found to be the best formulation. The overall desirability coefficient of formulation F14 was found to be 0.825. The formulation F14 was subjected to in vitro release studies, and the results were evaluated kinetically and statistically. The microspheres started releasing the drug at the beginning of 7th hour, which corresponds to the arrival time at proximal colon. The cumulative percent drug release for formulation F14 at the end of 16 h was found to be 98%. The release kinetics showed that the release followed the Higuchi model, and the main mechanism of drug release was diffusion. The study presents a new approach for colon-specific drug delivery.
Introduction
Drug delivery to the colon is beneficial for many drug molecules, like proteins and peptide drugs, drugs used to treat ulcerative colitis, Crohn syndrome, diarrhea and colon cancer. Colon as a site offers various advantages on account of a far from neutral pH, a longer transit time, reduced digestive enzymatic activity and a much better responsiveness to absorption enhancers.Citation1 A colon-specific drug delivery system is expected to prevent drug release in the upper gastrointestinal tract and effect an rapid onset of drug release upon entry into the colon.Citation2
There are several approaches utilized in achieving colon targeting, including the use of prodrugs, coating with pH-sensitive polymers, design of time-release dosage forms, utilization of biodegradable polymers, such as azopolymers and polysaccharides (e.g., pectins and dextrans) that degrade exclusively by the colonic bacteria.Citation3,Citation4 Each system has advantages as well as disadvantages. The poor site-specificity of pH-dependent and time-release systems due to the large variations in the pH of the gastrointestinal tract and variations in gastric emptying times across the ileo-cecal junction, respectively, is well-documented.Citation5 Non-starch polysaccharides-based, microflora-activated systems are very promising, because they are degraded by the vast anaerobic microflora present in the colon. Furthermore, they are not digested in the human stomach and intestine.Citation6 This strategy exploiting the abrupt increase of the bacteria present in the colon and corresponding enzyme activities will also accomplish better site specificity. Moreover, the polysaccharides for colonic delivery are also naturally occurring, reasonably priced and available abundantly.Citation7,Citation8
Single-unit dosage forms for colonic delivery may suffer from the disadvantage of unwarrented disintegration of the formulation due to high inter- and intra-subject viability and poor reproducibility, which may lead to loss of local therapeutic action in the colon. Therefore little emphasis is being laid on the preparation of multi-particulate delivery systems in comparison to single-unit dosage forms due to their possible benefits, like better bioavailability, decreased risk of local irritation and predictable gastric emptying.Citation9
The microspheres are characteristically free-flowing powders composed of synthetic polymers, which are biodegradable in nature, and ideally, having a particle size less than 200 µm.Citation10 Microspheres may spread out on their arrival in the colon, with a sharp increase of surface area exposed to bacterial breakdown that produces a rapid drug release and thereby improves drug availability and gives site-specific action to the colon.
In order to treat inflammatory bowel diseases, oral administration of anti-inflammatory agents such as 5-amino salicylic acid, chemotherapy agents, antibiotics and corticosteroids to the colon is required. Mesalamine is a drug of choice to treat inflammatory bowel disease. It is unstable in acidic environments and is also absorbed by the small intestine and produces side effects. The aim of present study was to design a mesalamine-loaded, colon-specific delivery system to minimize its degradation and to achieve optimum delivery of the drug with relatively higher local concentration to provide more effective therapy for inflammatory bowel diseases, including Crohn disease and ulcerative colitis. A four-factor, two-level full factorial design was used in a fully randomized order to study all possible combinations of all the factors at all levels. Such an experiment allows studying the effect of each factor on the response variable as well as the effects of interactions between factors on the response variable. The response surface was visualized graphically, as the graphs are useful to determine the shape of a response surface; hills, valleys and ridge lines.
Results and Discussion
A four-factor, two-level full factorial designs was conducted and constructed to study all possible combinations of all the factors at all levels in fully randomized order. In order to estimate the relationship between the independent variables and the response variables, a statistical model was used.
Before application of the design, a number of preliminary trials were conducted to establish the control factors and their levels. The statistical evaluation of the results was performed by analysis of variance (ANOVA) using Microsoft Excel Version 2000. The significant parameters in the equations for the calculation of regression analysis can be selected using a step-wise forward and backward elimination. In this study, the full model with a non-significant p value (p > 0.05) was used in obtaining dependent variables, and thus, the results of the study were neglected.
Interaction plots were obtained for the deliberate response on the basis of the model using the software (Design-Expert 8) to further explain the relationship between the independent variables and the response.
Effect of drug-polymer ratio
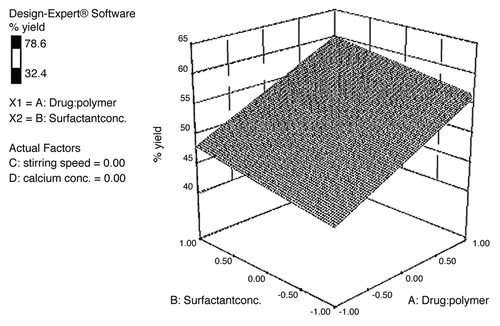
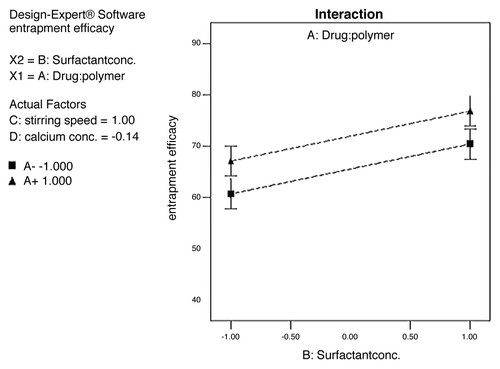
The surface appearance and inner structure of the microspheres were examined using scanning electron microscopy (SEM), which is shown in . The microspheres were found to be uniform without any drug crystal of free or unentraped drug on the surface. shows that the drug-to-polymer ratio has considerable effect on the size of microspheres. It was noted that, as the ratio of drug-to-polymer was increased, the particle size was also increased. As depicted in , in case of formulation F4, with low drug-polymer ratio (1:3), the smaller microspheres (130.2 ± 1.62 µm) were obtained, while in formulation F12, with high drug-polymer ratio (1:6), larger microspheres (185.5 ± 2.31 µm) were observed. This might be due to the fact that higher concentrations of polymer produced more viscous dispersion, which formed larger droplets and, consequently, larger microspheres. It was also observed that as the ratio of drug-to-polymer was increased, entrapment efficiency was increased. This might be due to the fact that a high drug-to-polymer ratio produces large microspheres, which, in turn, shows higher drug loading efficiency. Production yield, entrapment efficiency and mean particle size of formulations F1–F16 are given in . The entrapment efficiency and production yield of the formulations F1–F16 were found to be between 32–78% and 45–86%, respectively. Statistical analysis using software (Design-Expert®) for production yield of microspheres was done. The model F-value of 3.41 implies that the model is significant. A value of “Prob > F” less than 0.05 indicates that model terms are significant. In this case, significant model terms are A (drug:polymer ratio), B (surfactant concentration), C (stirring speed) and D (calcium chloride concentration) (). Statistical analysis for entrapment efficiency was also done. The Model F-value of 24.97 implies the model is significant. A value of “Prob > F” less than 0.05 indicates that model terms are significant. In this case, significant model terms are A (drug:polymer ratio), B (surfactant concentration), C (stirring speed) and D (calcium-ion concentration) ().
Figure 1. SEM photomicrographs of (A) uncoated microspheres (B) uncoated microspheres in groups (C) enteric-coated microspheres (D) T.S. of enteric-coated microspheres (E) enteric-coated micerospheres after dissolution.
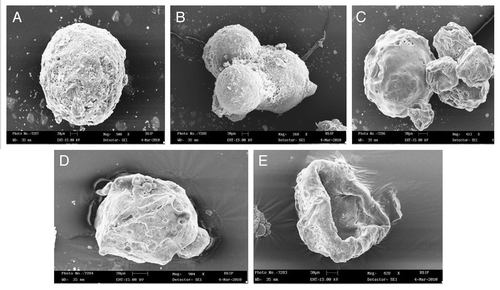
Table 2. Design of experiment
Table 3. Characterization of Microspheres
Effect of surfactant concentration on microspheres
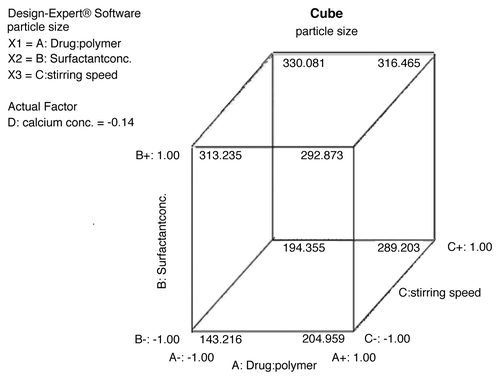
An increased amount of surfactant resulted in increased particle size. As shown in , in formulation F4 with a low concentration of surfactant (0.75% w/v), smaller microspheres (130.2 ± 1.62 µm) were obtained, while in formulation F15 at higher concentrations of surfactant (1.5% w/v), larger microspheres (280.5 ± 2.45 µm, respectively) were obtained. This could be due to the increased viscosity, where larger emulsion droplets formed in larger microspheres.
Effect of stirring rate on morphology of microspheres
The mean diameter of microspheres decreased on increasing agitation speed from 1,000 rpm to 2,000 rpm. As shown in , in case of formulation F4 at low stirring speed (1,000 rpm), larger microspheres (130.2 ± 1.62 µm) were obtained, while in formulation F5 at high stirring speed (2,000 rpm), smaller microspheres (112.3 ± 0.89 µm) were obtained. This result was expected, because high stirring rates provide the necessary shearing force required to separate the oil phase into smaller globules.
The model F-value of 4.81 implies the model is significant. A value of “Prob > F” less than 0.05 that indicated that model terms are significant. In this case, significant model terms are B (surfactant concentration) and D (calcium-ion concentration). The effect of independent variables on particle size (responcse variables) is shown in .
In vitro release studies
Desirability function was used to find out the best formulation; among all formulations, F14 showed the highest overall desirability of 0.825. Therefore, this formulation was considered to be the best formulation, and the values of independent variables of this formulation were considered to be optimum values for the preparation of enteric-coated microspheres.Citation16
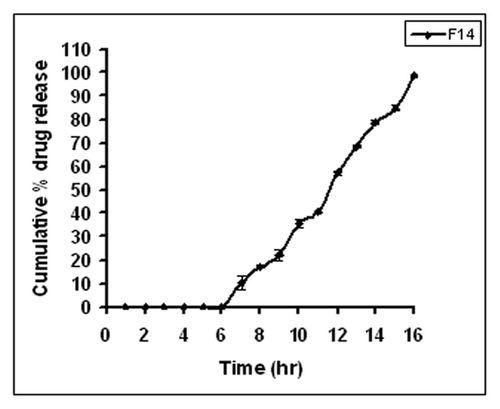
In vitro drug release studies of the developed microspheres were performed by the Souder and Ellenbogen extractionCitation15 technique using USP XXIII a type II, i.e., paddle type, dissolution apparatus with a stirring rate of 100 rpm at 37 ± 0.5°C. The release plot obtained for the formulation F14 is shown in . No drug release was observed during the first six hours. After a lag time of 6 h, the drug started releasing at the 7th hour due to the dissolution of Eudragit S-100 in the presence of the pectinex Ultra-SPL. The overall cumulative percent release for formulation F14 at the end of 16 h was found to be 98%.
The analysis of variance for percent drug release (partial sum of squares type III) was determined using factorial design. The model F-value of 4.15 implies the model is significant. The release data was analyzed using various mathematical models (). The correlation coefficient and release rate constant values for zero, first, Higuchi, Matrix, Peppas and Hixson-Crowell models were computed. The correlation coefficient values of F14 formulation for different models were found: 0.9683 for zero order, 0.9522 for first order, 0.9983 for Higuchi matrix, 0.9741 for Peppas and 0.9808 for Hixson-Crowell respectively. The R values were much closer to 1 for the Higuchi matrix kinetics. From the correlation coefficient values, it was concluded that drug release from the F14 microspheres formulation followed the Higuchi model. The Higuchi model explained the matrix diffusion mechanism of drug release for F14 microspheres formulation. Based on highest regression value, the best fit was observed for Higuchi matrix. The n value for the Peppas model was found to be between 0.5–1, indicating non-Fickian diffusion.
Table 4. Kinetic modeling of drug release of F14 formulation
Materials and Methods
Materials
Mesalamine was purchased from Sigma Aldrich. Pectin Classic with low methoxylated grade (AU701) and Pectin Amid, Amidated with low methoxylated grade (CF020) were a generous gift from Herbstreith and Fox. Eudragit S-100 was obtained as a gift sample from Evonik India Pvt Ltd. Pectinase ultra was obtained from HiMedia Laboratories Ltd. Methanol and potassium di-hydrogen phosphate were procured from Qualigens Fine Chemicals, Mumbai. Sodium hydroxide pellets, n-octanol, hydrochloric acid and calcium chloride were procured from Central Drug House (P) Ltd.
General method of preparation of enteric coated microspheres
Factorial design and desirability function
A four-factor, two-level full factorial design was constructed and conducted in a fully randomized order to study all possible combinations of all the factors at all levels (). The dependent variables included particle size, yield of microspheres, entrapment efficiency and percent drug release. The concentration of drug and polymer (A), the concentration of surfactant (B), stirring speed (C) and concentration of calcium chloride (D) were set at two different levels i.e., high and low (), which was coded as +1 and -1, respectively. The range of a factor was chosen so as to adequately measure its effects on the response variables. This design was selected, as it provides sufficient degrees of freedom to resolve the main effects as well as the factor interactions. Step-wise regression analysis was used to find out the control factors that significantly affect response variables.Citation11 By using full factorial design, 16 formulations were prepared.
Table1. Independent variables and levels (16)
Preparation of enteric coated microspheres
Mesalamine microspheres were prepared by emulsion dehydration technique. Both pectins (AU701, 2% w/v and CF020, 8% w/v) were homogeneously dispersed in 20 ml of distilled water using a high speed homogenizer (Remi RQ 1217-D). 200 mg of mesalamine was added to this under stirring.Citation12 The drug polymer dispersion was added in 50 ml isooctane containing span 80 and stirred at variable speed continuously to obtain stable w/o emulsion. The emulsion was rapidly cooled to 15°C, and then 50 ml of acetone was added in order to dehydrate the pectin droplets. The system was maintained under mechanical agitation for 30 min at 1,000 rpm at 25°C to effect complete evaporation of the solvent. Microspheres were filtered and dispersed in calcium chloride solution for cross-linking for 20 min, separated and washed with distilled water and consequently suspended in hardening agent, glutaraldehyde solution. The microspheres were filtered, rewashed and dried.
Characterization of microspheres
Scanning electron microscopy
The morphology, surface appearance and inner structure of the microspheres were examined using scanning electron microscopy (LEO 430 SEM analyzer). The samples were fixed on a brass stub using double-sided tape and coated with gold-palladium alloy under vacuum. The photographs were then taken using an excitation voltage of 20 kV.
Particle size analysis
The microspheres were examined under photomicroscope RXLr-3T (Radical Instrument, Ambala). Dried powder of microspheres was homogenously mixed in glycerol and was examined to measure the size of microspheres. Around 250 microspheres for each formulation were measured.
Determination of microspheres water content
The water content of the microspheres was determined by weighing the microspheres beforeCitation13 and after drying at 50°C for 24 h. The mean water loss (WL) was calculated according to the following equation:
where Wo is the initial weight of the microspheres before drying, and WD is the weight after drying.
Production yield of microspheres
The weight of the microspheres after drying was divided by the total dried weight of polymers and drug used to obtain the production yield.Citation14
Entrapment efficiency
The weighed amount of drug-loaded microspheres containing mesalamine (100 mg) was kept in phosphate buffer pH 6.8 (100 ml) for 12 h with continuous stirring. The samples were filtered using a membrane filter (0.45 micron) and analyzed at 230 nm using UV spectrophotometer model UV 1700E. The entrapment efficiency was calculated using the following formula:Citation7 Entrapment efficiency (%) = Ma/Mt x 100, where Ma is the actual drug content in microspheres and Mt is the amount of drug added to the microspheres.
Coating of microspheres
The enteric coating solution (12% w/v) was prepared by dissolving Eudragit S100 in acetone.Citation13 Coating was affected by immersion of microspheres in the coating solution followed by drying in a rotary evaporator. The process was repeated until the desired amount of coating was achieved. Microspheres were coated at different levels (weight gain ranging from 5 to 100% w/w). Coated microspheres were dried, weighed, and the mean coating weight was calculated. The mean coating thickness was determined by the difference between the mean diameter of microspheres before and after the coating.
In vitro drug release
Coated pectin microspheres were evaluated for the in vitro drug release in the media solution of different pH values using USP XXIII type II dissolution apparatus (Electrolab TDT-08L).Citation14,Citation15 Microspheres containing 200 mg drug with a magnetic bead were kept in a dialysis bag (Sigma Aldrich; molecular weight cut off, 12,000 Da). The bag was tied with a nylon thread to avoid the escape of microspheres. The dissolution test was performed at 100 rpm in 0.1 N hydrochloric acid for first hour, phthalate buffer pH 4.5 for second and third hour, phosphate buffer pH 6.8 for fourth and fifth hour, phosphate buffer pH 7.4 for sixth hour and, afterwards, in a mixture of phosphate buffer pH 6.8 and pectinex Ultra-SPL (1% v/v) in order to simulate the enzymatic action of the colonic bacteria.
At specified intervals, 5 ml aliquots were withdrawn, filtered, diluted appropriately with the same medium and assayed at 230 nm using a UV double-beam spectrophotometer (Shimadzu UV-1700). Samples withdrawn were replaced with equal volume of the dissolution medium. All the experiments mentioned above were conducted in triplicate.
Conclusion
The purpose of this work was to a design mesalamine-loaded, colon-specific delivery system. To achieve site-specific drug delivery to the colon, eudragit S100-coated low methoxylated amidated pectin (CF020) and low methoxylated pectin (AU701) microspheres were prepared. The combination of both pectins provided microspheres with greater stability due to the formation of stiffer gel as compared with low methoxylated pectin alone. The microspheres protected the drug from being released in the stomach and small intestine and provided more uniform distribution of the drug in the colon. It was concluded that eudragit S100 microspheres prepared using the combination of low methoxylated amidated pectin (CF020) and low methoxylated pectin (AU701) can successfully be deliver the drug to the proximal colon.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Jain V, Singh R. Development and characterization of eudragit RS 100 loaded microsponges and its colonic delivery using natural polysaccharides. Acta Pol Pharm 2010; 67:407 - 15; PMID: 20635537
- Varshosaz J, Jaffarian Dehkordi A, Golafshan S. Colon-specific delivery of mesalazine chitosan microspheres. J Microencapsul 2006; 23:329 - 39; http://dx.doi.org/10.1080/02652040600612405; PMID: 16801244
- Sriamornsak P, Nunthanid J, Wanchana S, Luangtana-Anan M. Composite film-coated tablets intended for colon-specific delivery of 5-aminosalicylic acid: using deesterified pectin. Pharm Dev Technol 2003; 8:311 - 8; http://dx.doi.org/10.1081/PDT-120022159; PMID: 12901696
- Watts PJ, Illum L. Colonic Drug Delivery. Drug Dev Ind Pharm 1997; 23:893 - 913; http://dx.doi.org/10.3109/03639049709148695
- Krishnaiah YSR, Satyanarayana S, Rama Prasad YV, Narasimha Rao S. Gamma scintigraphic studies on guar gum matrix tablets for colonic drug delivery in healthy human volunteers. J Control Release 1998; 55:245 - 52; http://dx.doi.org/10.1016/S0168-3659(98)00057-1; PMID: 9795074
- Lee CM, Kim DW, Lee HC, Lee KY. Pectin microspheres for oral colon delivery: Preparation using spray drying method and in vitro release of indomethacin. Biotechnology and Bioprocess Engineering 2004; 9:191 - 5; http://dx.doi.org/10.1007/BF02942291
- Orlu M, Cevher E, Araman A. Design and evaluation of colon specific drug delivery system containing flurbiprofen microsponges. Int J Pharm 2006; 318:103 - 17; http://dx.doi.org/10.1016/j.ijpharm.2006.03.025; PMID: 16687222
- Vandamme TH, Lenourry A, Charroeau C, Chaumeil JC. The use of polysaccharides to target drugs to the colon. Carbohydr Polym 2002; 48:219 - 31; http://dx.doi.org/10.1016/S0144-8617(01)00263-6
- Asghar LFA, Chandran S. Multiparticulate formulation approach to colon specific drug delivery: current perspectives. J Pharm Pharm Sci 2006; 9:327 - 38; PMID: 17207416
- Vyas SP, Khar RK. Novel carrier system. In: Vyas SP, Ed (s). Targeted and controlled drug delivery: Novel carrier system. New Delhi: CBS Publishers and distributors 2008; 41-3.
- Ansel HC. Dosage form design: Pharmaceutical and formulation consideration. In: Allen LU, Popovich JNG, Ansel HC, Ed(s). Ansel’s Phrmaceutical dosage forms and drug delivery systems. Philadelphia, New York: Lippincott William & Wilkins 2007; 92-141.
- Paharia A, Yadav AK, Rai G, Jain SK, Pancholi SS, Agrawal GP. Eudragit coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 2007; 8:1 - 7; http://dx.doi.org/10.1208/pt0801012; PMID: 17408209
- Maestrelli F, Cirri M, Corti G, Mennini N, Mura P. Development of enteric-coated calcium pectinate microspheres intended for colonic drug delivery. Eur J Pharm Biopharm 2008; 69:508 - 18; http://dx.doi.org/10.1016/j.ejpb.2007.12.004; PMID: 18194852
- Anande NM, Jain SK, Jain NK. Con-A conjugated mucoadhesive microspheres for the colonic delivery of diloxanide furoate. Int J Pharm 2008; 359:182 - 9; http://dx.doi.org/10.1016/j.ijpharm.2008.04.009; PMID: 18486369
- Chourasia MK, Jain SK. Design and development of multiparticulate system for targeted drug delivery to colon. Drug Deliv 2004; 11:201 - 7; http://dx.doi.org/10.1080/10717540490445955; PMID: 15204639
- Mashru RC, Sutariya VB, Sankalia MG, Parikh PP. Development and evaluation of fast-dissolving film of salbutamol sulphate. Drug Dev Ind Pharm 2005; 31:25 - 34; PMID: 15704855
- Ibrahim HK, El-Setouhy DA. Valsartan orodispersible tablets: formulation, in vitro/in vivo characterization. AAPS PharmSciTech 2010; 11:189 - 96; http://dx.doi.org/10.1208/s12249-009-9354-7; PMID: 20112137