Abstract
MUC1 is a transmembrane mucin that is often overexpressed in metastatic cancers and often used as a diagnostic marker for metastatic progression. The extracellular domain of MUC1 can serve as a ligand for stromal and endothelial cell adhesion receptors, and the cytoplasmic domain engages in several interactions that can result in increased migration and invasion, as well as survival. In this review, we address the role of MUC1 in metastatic progression by assessing clinical studies reporting MUC1 levels at various disease stages, reviewing mouse models utilized to study the role of MUC1 in metastatic progression, discuss mechanisms of MUC1 upregulation, and detail MUC1 protein interactions and signaling events. We review interactions between MUC1 and the extracellular environment, with proteins colocalized in the plasma membrane and/or cytoplasmic proteins, and summarize the role of MUC1 in the nucleus as a transcriptional cofactor. Finally, we review recent publications describing current therapies targeting MUC1 in patients with advanced disease and the stage of these therapies in preclinical development or clinical trials.
Keywords: :
Introduction
MUC1, a transmembrane member of the mucin family, has long been associated with metastatic progression, both clinically and experimentally. Progression from a contained tumor to one that can metastasize to a distant organ requires a multitude of steps, including the gaining of invasive capacity, intra- and extra-vasation, and the ability to colonize and grow at a secondary site (reviewed in Steeg).Citation1 MUC1 is involved in metastatic progression through both its extracellular, O-glycosylated serine/threonine repeat region (the “mucin” domain, MUC1-ECD), as well as through activities of its intracellular domain (MUC1-CD). This role in metastatic progression is highlighted by the frequent observation of MUC1 overexpression in metastatic tissues and circulating tumor cells from patients with advanced adenocarcinoma, and the ability to use anti-MUC1 antibodies as diagnostics for metastatic disease. Mechanistically, MUC1 (both ECD and CD) engages in intercellular and intracellular interactions with other transmembrane proteins, such as ICAM-1 and the epidermal growth factor receptor (EGFR), which have prometastatic capacity themselves. In addition, MUC1 can engage cytoplasmic signaling proteins, such as Src and β-catenin, thereby driving changes in the cytoskeleton and adhesive capacity of the transformed cell. Finally, MUC1 can directly drive transcription of pro-invasive genes, through the proteolytic cleavage and nuclear translocation of MUC1-CD. In this review, we will summarize recent data regarding the expression profile of MUC1 in metastatic cancers and circulating tumor cells, review the direct role of MUC1 in pro-metastatic signal transduction and gene transcription, and discuss the current efforts to target metastatic disease by developing MUC1 targeted therapies. The reader is referred to other excellent reviews regarding the structure, oncogenic properties and clinical utility of MUC1 as a biomarker, including reviews by Baldus et al.,Citation2 Gendler,Citation3 Bafna et al.,Citation4 KufeCitation5 and Singh et al.Citation6
MUC1 Expression Correlates with Metastasis
In many tumor types, MUC1 expression correlates with aggressive, metastatic disease, poor response to therapy and poor survival. While MUC1 expression is limited to the apical surface of most ductal epithelium, in metastatic disease, MUC1 is overexpressed and becomes localized throughout the cell.Citation7 This has perhaps been most intensively studied in breast cancer, in which MUC1 expression has been evaluated clinically at the level of immunohistochemistry,Citation8,Citation9 RNA,Citation10 shed MUC1 in sera, expression on circulating tumor cells (discussed below) and biochemically,Citation11 and has correlated with poor disease-free and overall survival, as well as axillary node metastases.Citation9 MUC1 expression is seen in all subtypes of breast cancer, including luminal, HER2+ and basal, although in each of these cancer types, expression is highest in those tumors that have metastasized.Citation9,Citation12
In other hormonally responsive cancers, including ovarian and prostate, a similar overexpression of MUC1 is observed in advanced disease. In ovarian cancer, patients with metastatic, treatment-resistant disease display elevated levels of MUC1, with greater than 90% of these patients producing antibodies to MUC1.Citation13 Additionally, MUC1 expression is high in both primary epithelial ovarian cancers and in metastatic ovarian cancer (> 90%),Citation14 with MUC1 cytoplasmic expression correlating with poor overall survival and invasive capacity.Citation15 Likewise, in prostate cancer, less than 60% of primary lesions were found to express MUC1 in one study, whereas 90% of lymph node metastases expressed MUC1,Citation16 indicating that MUC1 is enriched in metastatic tumors.
In the gastrointestinal system, MUC1 is also strongly correlated with metastatic progression. In gastric cancer, MUC1 is not only expressed in metastatic disease, but also found to be highly expressed in virtually all isolated cancer cells invading throughout the stroma of the primary tumor, indicating it may be promoting initial spread.Citation17,Citation18 High MUC1 expression is also associated with invasive intraductal papillary neoplasms of the bile duct,Citation19 metastatic liver cancerCitation20 and pancreas,Citation21,Citation22 as well as lymph node metastasis and vascular invasion in oral squamous cell carcinoma.Citation23 As such, MUC1 was found as a useful biomarker to identify occult lymph node metastases in oral squamous cell carcinoma.Citation24 Similarly, MUC1 is associated with higher grade tumors and shorter metastasis-free survival in renal cell carcinoma, malignant thyroid cancer, leukemias and lymphomas.Citation25-Citation27 Overall, these studies reveal a strong link between MUC1 expression and metastatic progression, inversely correlating MUC1 and disease free survival due to metastatic spread.
MUC1 expression on circulating tumor cells and MUC1 serum markers
Circulating tumor cells (CTCs) are typically identified in approximately 50–80% of patients with defined metastatic breast, colon, or prostate cancer.Citation28,Citation29 Whether the remaining patients have CTCs that are either too rare to be captured, lack the surface markers for capture, or may not be present in the blood stream (e.g., the metastatic cells have remained solely in the tissue or lymphatics) is currently unknown. For those patients with detectable CTCs, the surface antigens currently used clinically to select these cells are epithelial cell adhesion molecule (EpCAM), cytokeratin-19Citation30,Citation31 and MUC1.Citation32,Citation33 The use of MUC1 as a capture antigen is based on the examples of MUC1 overexpression on CTCs. MUC1 is typically found to be expressed in greater than 60% of captured CTCs from metastatic breast, lung, pancreatic and colon cancer patients, among othersCitation33-Citation35.
The detection of MUC1 (which is also defined as serum antigens CA 15-3, KL-6 and BM7Citation36,Citation37) in patient sera is currently used clinically as a marker of response to therapy and as a prognostic indicator for survival.Citation38 In fact, the serum antigen CA 15-3 is currently one of the most widely used serum antigens in breast cancer, with high CA 15-3 levels correlating with higher grade tumors, lymph node involvement, and presence of distant metastases.Citation39 Cellular localization of MUC1 is also important, as studies in metastatic breast, colorectal, gall bladder, non-small cell lung and gastric cancer, using KL-6, discovered that MUC1 expression at the circumference of the plasma membrane and/or in the cytoplasm frequently correlated with deep invasion, lymph node or liver metastasis and decreased five-year survival.Citation40-Citation43 These studies demonstrate that MUC1 detection in patient circulation and localization of expression in the tumor are important prognostic factors for metastasis and disease progression.
Mouse models demonstrate MUC1 promotes metastatic progression
MUC1 expression is strongly correlated with metastatic progression in patient samples, both in tissue and circulated tumor cells. In order to further explore this relationship, mouse models of cancer have been employed which demonstrate a role for MUC1 in metastasis, a number of which provide direct evidence for MUC1 in driving metastatic progression. The first model demonstrating a potential role for MUC1 in driving metastatic progression was the MMTV-PyMT mouse model of breast cancer, which was crossed onto a Muc1 (note: in mice, MUC1 is annotated as Muc1) knockout background in a study by Spicer et al.Citation44 The MMTV-PyMT transgenic mouse develops multiple tumors in the mammary gland by approximately 10–12 weeks of age, with greater than 90% of these animals displaying lung metastases.Citation45 When crossed onto a Muc1 knockout, incidence of lung metastases was found to be lower, although the reduction did not reach statistical significance.Citation44 In a separate study, MMTV-Muc1 transgenic mice were created, resulting in late-onset mammary gland tumors that were metastatic to the lung, but only in animals overexpressing full-length Muc1 (Muc1 lacking the cytoplasmic domain was not tumorigenic).Citation46 In addition, our laboratory evaluated the role of Muc1 in epidermal growth factor receptor (EGFR)-driven breast cancer by crossing the WAP-TGFα transgenic model onto a Muc1 knockout background. The WAP-TGFα transgenic mouse develops mammary gland carcinoma in 100% of multiparous females, with fewer than 25% of mice presenting lung metastases.Citation47 Upon crossing the WAP-TGFα onto a Muc1−/− background, mammary gland carcinoma is reduced to less than 50%, and presentation of lung metastases drops to 0%.Citation48 Together, these studies implicate MUC1 as a promoter of metastatic progression in mouse models of breast cancer.
In addition to breast cancer, a role for MUC1 in driving pancreatic and lung cancer metastasis has also been examined. Evaluation of Muc1 during pancreatic cancer progression was performed in the KC or Cre-LSL-KRASG12D mouse model, a spontaneous mouse model of pancreatic ductal adenocarcinoma that relies on an activating KRAS mutation for tumor progression.Citation49 Crossing this mouse onto a Muc1 null background (KC-Muc1-null) resulted in reduced tumor burden and an increase in overall survival as well as a 50% reduction in distant metastasis compared with a KC mouse crossed with a Muc1 transgenic (KC-Muc1), which had Muc1 levels above wild-type. 61% of KC-Muc1 mice exhibited lung metastases, 33% had liver metastases and 23% had peritoneal metastases, whereas only 30% of KC mice with wild-type Muc1 levels had lung metastases, 20% had liver metastases and 10% had peritoneal metastases. The incidence of lung, liver and peritoneal metastases was further reduced in the KC-Muc1-null mice, only 10% of which developed metastases in any of the three organs examined, defining a role for MUC1 in pancreatic tumor growth and metastasis in this model.Citation49
The KC, or Cre-LSL-KRASG12D, mouse crossed with a Muc1 transgenic has also been used to look at the effect of MUC1 in lung cancer progression. In this model, Cre-LSL-KRASG12D mice developed lung tumors nine weeks after the administration of a Cre-adenovirus. The addition of transgenic Muc1 (KC-Muc1) induced the formation of twice as many premalignant and malignant masses, and Muc1 positive mouse tumors exhibited many more infiltrating cells.Citation50
Orthotopic models of cancer have also been evaluated for a role of MUC1 in driving metastatic progression. In a model for breast cancer metastasis to the brain, MUC1-expressing bone marrow cells (MA11 cells) were injected into the left ventricle heart chamber of athymic nude mice. The MA11 cell line was derived from a breast cancer which had metastasized to the bone. To isolate the cells, bone marrow was enriched for mucin-expressing cells which were then purified and maintained in culture for a period of time before injection. 87% of mice injected displayed brain metastases, with accompanying neurological symptoms. MUC1 was detected in the serum of 82% of mice showing histological evidence of brain metastasis, demonstrating that MUC1 is prevalent in this cell line and correlating expression with metastatic progression.Citation51 Additionally, an orthotopic lung cancer model was used to assess the significance of Muc1 expression. In an orthotopic model of H358 lung cancer cells, cells which stably express MUC1 siRNA or control siRNA were evaluated for their ability to induce metastatic disease. While 71% of MUC1-expressing tumors progressed to lung metastases, only 14% of mice with non-MUC1-expressing tumors displayed any metastases.Citation52 Similar results were found in an orthotopic pancreatic cancer model wherein S2-013 pancreatic cancer cells were stably transfected with MUC1 siRNA or control siRNA and implanted subcutaneously into mice. Tumors from these mice were excised and a normalized amount of the tumor was then implanted into ceca of recipient mice and metastasis was evaluated. 75% of S2-013-control-siRNA-injected mice displayed local invasion into lymph nodes vs. 29% of S2-013-MUC1-siRNA-injected mice. Furthermore, 64% of S2-013-control-siRNA-injected mice had lung metastases whereas only 32% of S2-013-MUC1-siRNA-injected mice displayed lung metastases.Citation53 Together, these studies demonstrate a role for MUC1 in driving metastatic progression in transgenic, knockout, knock-in and xenograft mouse models of cancer.
Mechanism of Overexpression
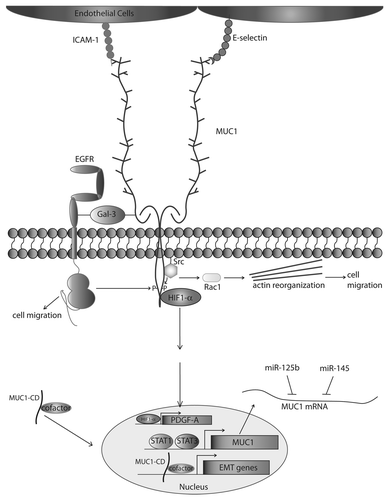
As we have described, MUC1 expression strongly increases during the clinical progression from normal tissue to metastatic disease. We will next summarize the observations regarding the mechanisms by which MUC1 expression is controlled and any correlations these have to metastatic progression. MUC1 expression is controlled by multiple factors, including alterations in transcription, amplification and post-translational modifications, and many of these regulatory pathways are strongly activated in the invasive, migratory or metastatic state ().
Figure 1. MUC1 drives metastatic progression. The protein core of underglycosylated MUC1 interacts with ICAM-1, E-selectin, and Galectin-3 using the extracellular domain. The cytoplasmic domain of MUC1 is phosphorylated by EGFR and Src, among other proteins, and upon Src phosphorylation can induce Rac activity and cytoskeletal change leading to an increase in cell motility. Phosphorylation by EGFR promotes cell motility, and interaction with HIF1-α drives PDGF-A transcription, positively affecting β-catenin transcriptional activity. The cytoplasmic domain of MUC1 interacts with cofactors, such as β-catenin, p120-catenin, and Estrogen Receptor β among other transcription factors, promoting nuclear translocation of these proteins and driving expression of Epithelial to Mesenchymal Transition (EMT) genes. MUC1 expression is upregulated by STAT1/STAT3 binding to the MUC1 promoter, and MUC1 mRNA is downregulated by binding of miR-125b/miR-145.
Transcriptional regulation
MUC1 is regulated at the transcriptional level by multiple factors, including hypoxia, STATs, hormones and growth factors. In a recent study of clear renal cell carcinoma, MUC1 was found to be a transcriptional target of hypoxia inducible factor 1 α (HIF1-α), which is upregulated in metastatic cancer, and promotes migration and invasion.Citation54 Hypoxia-enhanced MUC1 transcription was also observed in a human lung adenocarcinoma cell line,Citation55 demonstrating that control of MUC1 levels by hypoxia is not tissue-specific. Furthermore, MUC1 is transcriptionally upregulated by STATs in response to interferon gamma (IFNγ) and interleukin-6 (IL-6) signaling.Citation56 In addition, EGFR can also activate STAT1 and STAT3 in breast cancer tissuesCitation57,Citation58 and EGFR activation promotes MUC1 expression.Citation59 Intriguingly, MUC1 and EGFR can interact in cancer cells, resulting in a MUC1-dependent prolongation of EGFR activity.Citation60,Citation61 This may represent an EGFR-STAT-MUC1 positive feedback loop that is a source of MUC1 upregulation in breast cancer, although further studies need to be done in this area.
In prostate cancer cell lines, androgen receptor (AR) was found to regulate MUC1 expression through interaction with a consensus AR-element in the MUC1 promoter, although this result was cell line-specific.Citation62 In this study, AR was found to downregulate MUC1 expression in androgen-dependent, but not androgen-independent cell lines. Interestingly, in another study examining the phenotypic behavior of AR and MUC1, androgen treatment was found to increase expression of MUC1, and increased MUC1 expression correlated with loss of cell-cell adhesion.Citation63 These varied results indicate that there is more work necessary to fully sort out the role of androgen receptor in MUC1 transcriptional regulation.
Amplification and miRNA regulation of MUC1 expression
While transcriptional regulation of MUC1 is well-established as a mechanism for MUC1 regulation during cancer progression, amplification was recently also identified as a contributing factor for MUC1 overexpression. Examination of 83 patients for MUC1 gene amplification and protein expression found that 12% of benign lesions and 38% of primary invasive breast carcinoma samples displayed MUC1 genomic amplification.Citation64 Further-more, meta-analysis of 886 primary invasive breast carcinoma samples from 22 studies demonstrated that 44% had genomic gain of the MUC1 gene. MUC1 gene amplification also correlated significantly with protein expression, indicating that MUC1 gene amplification may be an untapped and important source for MUC1 protein in breast cancer samples, and that amplification of MUC1 may select cells for metastatic progression.Citation64
MUC1 expression and corresponding metastatic phenotypes are also regulated at the post-transcriptional level by microRNAs. Two miRs have been identified to regulate MUC1 translation, including miR-125b, which is upregulated by androgen receptor, and miR-145. The first study to identify miR regulation of MUC1 found that MUC1 translation is repressed by miR-125b, and mir-125b is downregulated in breast cancer cells.Citation65,Citation66 AR also promotes miR-125b expression, resulting in a suppression of MUC1 translation,Citation62 and together with AR directly downregulating MUC1 transcription,Citation62 this may indicate an androgen-dependent MUC1 downregulation program in certain circumstances. Finally, miR-145 was found to suppress MUC1 translation, and reduce MUC1-dependent cell invasion,Citation67 and miR-145 is frequently found to be lost during colorectal cancer progression.Citation68
Molecular Mechanisms of Metastasis
The many interaction domains of MUC1 allow for distinct mechanisms by which MUC1 promotes metastasis. These include interactions with proteins in the extracellular matrix, at the cell membrane, in the cytoplasm, and in the nucleus where MUC1 acts as a cofactor for gene transcription ().
MUC1 extracellular domain drives migration and invasion
MUC1 is co-translationally processed into two polypeptides that then associate to form the mature transmembrane MUC1 heterodimer. This heterodimer is composed of the extracellular MUC1 (MUC1-ECD), which is non-covalently associated with the transmembrane MUC1-CD via the extracellular stem region. The MUC1-ECD contains many O-glycosyl groups covalently attached to serine/threonine repeats throughout this domain.Citation5 Tumor-specific MUC1 is underglycosylated, enabling interactions between the MUC1 core protein and many transmembrane receptors and components of the extracellular matrix, such as ICAM-1, an adhesion receptor on the surface of endothelial and peritumoral stromal cells.Citation69,Citation70 E-selectin, a receptor present on the endothelial cell surface, also interacts with epithelial cells either via interactions with under-glycosylated MUC1 itself or through the binding of other E-selectin ligands present adjacent to MUC1 on the cell surface. In addition, interactions between MUC1 and E-selectin may promote MUC1 binding to ICAM-1 on the endothelial cell surface.Citation71 The MUC1-ICAM-1 interaction promotes the migratory capacity of tumor cells through the microenvironment, by facilitating interaction between epithelial and endothelial cells, enabling adhesion of circulating cancer cells to the inner lining of the blood vessel, slowing cell velocity and allowing escape from the blood vessel (reviewed in ref. Citation72).
In addition to promoting the ability of transformed cells to interact with vascular endothelium, MUC1-ICAM-1 interactions alter the metastatic phenotype of the cancer cell itself. Upon interacting with ICAM-1, Src interacts with the MUC1-CD, an interaction that promotes Src-mediated cytoskeletal rearrangements.Citation73,Citation74 The Src family of nonreceptor tyrosine kinases, through their ability to regulate integrin activation and cytoskeletal function, has long been regarded as key mediators of metastatic progression (reviewed in AleshinCitation75). Interactions between MUC1 and Src induce pro-migratory Rac1- and Cdc42-dependent actin reorganization at sites of contact with endothelial cells, thereby promoting an invasive phenotype in the tumor cell.Citation73 Lamellipodia and filopodia formation as a result of these interactions are induced via Src-CrkL complexes with MUC1-CD, with Src-dependent kinase activity driving cytoskeletal rearrangements.Citation73 Overall, these studies demonstrate that MUC1 can drive intercellular interactions that promote metastatic spread, as well as intracellular interactions that promote migratory behavior.
In addition to studies focused on interactions of the MUC1 extracellular domain with ICAM-1 and E-selectin, a truncated version of MUC1 spanning only the external stem region, the transmembrane domain, and the juxtamembrane domain was demonstrated to be sufficient to induce cellular EMT, although a precise mechanism was not described.Citation76 In this study, mouse mammary carcinoma cells expressing this truncated MUC1 showed mesenchymal morphology, decreased E-cadherin expression, increased vimentin expression, and increased invasion through a Matrigel matrix.
MUC1 expression promotes angiogenesis
One key aspect of metastatic progression is angiogenesis, which provides an escape route for migratory tumor cells. In addition to promoting the migratory capacity and invasive phenotype of tumor cells, MUC1 can also drive tumor angiogenesis. MUC1 overexpression in non-small cell lung cancer and breast cancer was found to upregulate vascular-endothelial growth factor (VEGF), thereby promoting endothelial migration and tube formation.Citation77 Though MUC1 is a transcriptional target of HIF1-α as discussed above, MUC1 also interacts with HIF1-α in the cytoplasm and promotes transcriptional upregulation of HIF1-α targets such as leptin, TGFβ3 and VEGF.Citation78-Citation80 Furthermore, MUC1 and VEGF expression correlate in human breast cancer cell lines, and MMTV-PyMT mice expressing human MUC1 display more angiogenesis than MMTV-PyMT mice on a Muc1 null background,Citation81 further supporting a role for MUC1 in the onset of angiogenesis.
Promotion of pro-metastatic activities of transmembrane receptors
In polarized epithelium, the MUC1 heterodimer is apically localized. Alternatively, in primary and metastatic tumors, MUC1 is found throughout the plasma membrane, in the cytoplasm and in the nucleus. Membrane-bound MUC1 is constitutively recycled via endocytosis and trafficking through the Golgi, resulting in re-glycosylation of the MUC1-ECD.Citation82 During cancer progression, when apical and basolateral proteins become co-localized due to a breakdown of tight junctions, MUC1 interacts with a number of previously sequestered transmembrane receptors, including the EGFR.Citation48 Interactions with EGFR result in the “hijacking” of normal EGFR trafficking, and EGFR becomes preferentially recycled instead of trafficked to the lysosome for degradation,Citation60 or to other compartments of the cell such as the nucleusCitation83 or mitochondria.Citation84 The result is prolonged EGFR signaling, which can drive pro-metastatic interaction and/or regulation of integrins, cadherins, phospholipase Cγ (PLCγ), phopho-inositide 3 kinase (PI3K) and matrix metalloproteinases (MMPs) which contribute to disruption of cell adhesion, induction of cell motility, and degradation of the extracellular matrix (ECM) (reviewed in Haley).Citation85 In addition, overexpression of MUC1 in primary canine malignant mammary tumors (CMMT) was accompanied by downregulation of galectin-3, which results in upregulation of endogenous MUC1, enabling an auto-feedback loop. Additionally, CMMT cells that have invaded into the vasculature express MUC1 and EGFR at focal adhesions as opposed to uniform cell membrane expression, further suggesting a role for MUC1 and EGFR in cell motility.Citation86
Recently, our laboratory published evidence that MUC1 and EGFR regulate the expression of c-Met, the hepatocyte growth factor/scatter factor receptor. c-Met is a receptor tyrosine kinase that is often upregulated in metastatic cancers and is involved in metastatic progression (reviewed in Peschard).Citation87 We found that treatment with a competitive MUC1 inhibitor downregulates c-Met in breast cancer cells, and that MUC1 promotes EGF- and c-Met-dependent cell motility, scattering and the formation of invasive protrusions.Citation88 In addition, MUC1 has recently been found to be upregulated in a subset of lung cancers which also have upregulated or constitutively active EGFR and c-Met.Citation89 In this study, lung cancer cell lines were analyzed for expression of genes associated with tumorigenesis, and MUC1, EGFR and c-Met expression were positively correlated. Cell lines with high expression of all three proteins also displayed high expression of Rho-family GTPases, and SNAIL transcription factor, in addition to other genes involved in EMT and cell motility,Citation89 suggesting that this subset of lung cancer is very motile and that MUC1, EGFR and c-Met expression may contribute to this motility.
Studies also show that MUC1 interacts with ErbB2, another member of the EGFR family of receptor tyrosine kinases, in breast cancer cells.Citation90 ErbB2 does not have a ligand-binding domain, but can heterodimerize and phosphorylate EGFR, and ErbB3 and ErbB4, the remaining members of the family. ErbB3 and ErbB4 bind several ligands to become activated, among them Heregulin (reviewed in Citri).Citation91 The interaction between MUC1 and ErbB2 was found to be driven by Heregulin-binding of ErbB3 or ErbB4 and heterodimerization of either of these proteins with ErbB2. Importantly, this interaction was then observed to promote the nuclear localization of a MUC1-γ-catenin complex.Citation90 γ-catenin, like β-catenin, is a transcription factor in the Wnt pathway, and can affect genes involved in motility and metastasis. γ-catenin suppresses cell motility and metastasis by downregulating fibronectin,Citation92 by organizing the actin cytoskeleton through modulation of Rho-family GTPases,Citation92 and by upregulating Nm23-H1, a known metastasis suppressor.Citation93 The authors of this study speculate that MUC1 could be sequestering γ-catenin in order to promote cell motility.Citation90
Pro-metastatic cytoplasmic interactions
MUC1 has no kinase domain itself, but protein-protein interactions in the cytoplasm allow it to activate signal transduction cascades, many of which have direct roles in driving metastatic progression (reviewed in Singh et al.).Citation6 Many of these interacting partners have been studied for their roles in affecting transformation directly, including inhibitory interactions with the pro-apoptotic protein BaxCitation94 and c-abl kinase.Citation95 MUC1-CD can also directly activate the JAK-1/STAT3 signaling pathway, promoting tumor growth and metastasis in an orthotopic model of lung cancer.Citation52 Finally, interactions with the tyrosine kinase Src or PKCδ can modulate the interactions between MUC1-CD, GSK3-β and β-catenin.Citation61,Citation96 Many of these interactions play a role not only in primary transformation, but in metastatic progression as well.
β-catenin, serving as both an activator of oncogenic transcription and as a suppressor of invasion, has both a protumorigenic and anti-metastatic role to play. As such, its interactions with MUC1 have been shown to promote transformation, primarily through MUC1-β-catenin protein complexes driving transcription of such genes as cyclin D1.Citation97 In addition, a study by Yuan et al. demonstrated that the downregulation of MUC1 promoted E-cadherin/β-catenin complex formation, reduced nuclear β-catenin, upregulated both E-cadherin and β-catenin expression, and decreased invasive potential in PANC1 pancreatic cancer cells and MCF-7 breast cancer cells.Citation98 MUC1 can also interact with the SH3 domain of Src via the RXPPXR domain in the MUC1-CD,Citation99 and the SH2 domain of Grb2 through a phosphorylated tyrosine at the YTNP motif of the MUC1 cytoplasmic domain.Citation100 These interactions result in an increase in MUC1-dependent metastatic cell behavior including cytoskeletal rearrangement leading to invasive protusion.Citation73
In addition to the interactions described above, MUC1 was also found to induce the expression of platelet-derived growth factor (PDGF-A), thereby promoting cell migration.Citation101 In a mouse model expressing mutant KRASG12D in the pancreas to drive pancreatic cancer, MUC1 influences the expression and secretion of PDGF-A through interaction with HIF1-α, a known effector of PDGF-A expression.Citation101 In this system, MUC1-overexpressing cells are highly dependent on PDGF-A for growth and migration, and PDGF-A and MUC1 are necessary for nuclear translocation of β-catenin. MUC1 is phosphorylated by activated PDGFR-β at two tyrosine residues in the cytoplasmic tail, and PDGF-A expression increases nuclear colocalization of β-catenin and MUC1-CD. Although these interactions do not affect cell proliferation, PDGF-A expression does increase invasion in vitro and tumor growth and metastasis in vivo.Citation102
Pro-metastatic roles in the nucleus
In addition to the numerous roles for MUC1 in altering pro-metastatic signaling cascades described above, MUC1 can also directly affect gene transcription by acting as a transcriptional cofactor. γ-secretase-dependent MUC1 cleavage at the juxtamembrane domain can result in soluble MUC1-CD that is capable of interacting with a variety of proteins. MUC1 has a non-canonical nuclear import domain that interacts with importin β1, a nuclear importer.Citation103 MUC1-CD can directly act as a transcription factor, altering the transcription of a number of genes that directly affect transformation, including p53 and EGFR.Citation104-Citation106 Induction of MUC1 intracellular cleavage has been shown to be promoted by EGFR activation, as cells pulsed with EGF show an increase in MUC1-CD relative to full-length MUC1.Citation107
Once cleaved from the transmembrane domain, MUC1-CD can interact with proteins such as β-catenin, p120catenin, Src, estrogen receptor β (ERβ) and EGFR. Furthermore, interaction between MUC1-CD and these proteins promotes their nuclear translocation (reviewed in Singh).Citation6 Nuclear localization of MUC1 correlates with poor prognosis, tumor-node-metastasis staging and lower survival and MUC1 has been found co-localized with β-catenin at the invasion front in colorectal carcinoma.Citation108 Importantly, while all of the gene targets have yet to be mapped, these transcriptional cofactor interactions have been shown to drive an EMT phenotypic-switch. This includes the activation of Slug and Snail transcription factors and the resulting induction of EMT genes, such as vimentin, and N-cadherin.Citation109 In pancreatic cancer, ChIP-ChIP promoter analysis and microarray demonstrated that MUC1-CD is a cofactor for transcription of genes related to invasion, angiogenesis, and metastasis.Citation110 Additionally, MUC1-CD promotes expression of connective tissue growth factor (CTGF), a mediator of ECM remodeling and angiogenesis.Citation110
MUC1 Targeted Therapies
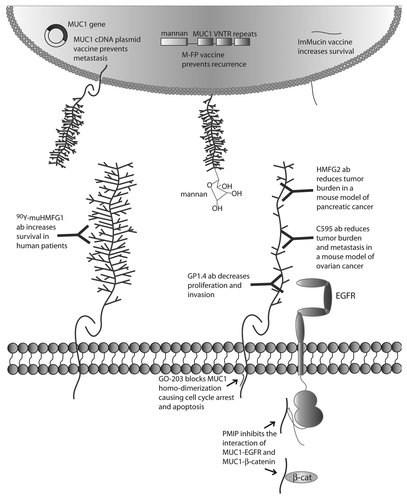
The role of MUC1 in both transformation and metastatic progression has led to extensive focus on this protein for the development of targeted therapies to treat metastatic disease (). A number of groups have developed vaccine-like therapies to target MUC1, largely focusing on the primary tumor, and reviewed in Singh et al.Citation6 Below, we summarize a number of therapeutic interventions with an emphasis on metastatic disease ().
Table 1. MUC1-dependent metastasis inhibitors
Figure 2. Targeted therapies directed against MUC1. MUC1 cDNA vaccine, M-FP vaccine and ImMucin vaccine induce immune response to MUC1 tumor antigen. 90Y-muHMGF1 antibody binds glycosylated extracellular MUC1 and increases survival in human patients. HMFG2 and C595 antibodies bind the protein core of underglycosylated MUC1 and reduce tumor burden in mouse models of cancer. GP1.4 binds to MUC1 protein and decreases proliferation and invasion. GO-203 peptide binds to the juxtamembrane domain of MUC1 and blocks MUC1 homodimerization, preventing MUC1 activity and causing cell cycle arrest and apoptosis. PMIP decoy peptide inhibits MUC1-EGFR interaction and MUC1-β-catenin interaction, decreasing EGFR activity and inhibiting proliferation and invasion and inhibiting tumor growth and metastasis in mouse models of cancer.
Peptide-based therapies
Upon the identification of protein-protein interactions that drive metastatic progression, we and others began the development of peptide-based therapeutics to block these interactions. In our laboratory, we developed a peptide therapeutic that mimicked the domain of MUC1 that interacts with β-catenin/Src/EGFR. The peptide, PTD-4 MUC1 inhibitory peptide (PMIP), was conjugated to the cell-penetrating peptide sequence PTD-4 to allow for cellular uptake.Citation111,Citation112 PMIP blocks interactions by serving as a decoy to endogenous MUC1 binding partners, resulting in an inhibition of both proliferation and invasion in vitro. Importantly, PMIP treatment resulted in a significant decrease in metastatic progression in orthotopic models of breast cancer, demonstrating that inhibition of MUC1 activities can directly inhibit metastasis.Citation113 Follow-up studies with PMIP further demonstrated the utility of PMIP as a therapeutic intervention for lung cancer, although neither of these studies focused on metastatic disease.Citation114,Citation115
In the Kufe laboratory, the ability of MUC1-CD to form dimers was targeted with a peptide-based therapy termed GO-203.Citation116 GO-203 is a decoy peptide conjugated to the cell penetrating peptide and targeted to the juxtamembrane region of MUC1, which serves as both the non-canonical nuclear localization signal and the dimerization domain.Citation116 Treatment of chronic myelogenous leukemia, and non-small cell lung cancer cell lines demonstrated that blocking MUC1 dimerization resulted in cell cycle arrest, an increase in reactive oxygen species and apoptosis.Citation117 GO-203 treatment of non-small cell lung cancer (NSCLC) cell lines in xenograft tumors caused tumor regression with no evidence of metastasis in treated animals,Citation116 demonstrating efficacy of this dimerization-blocking drug against MUC1-driven cancer.
MUC1 antibodies/conjugates
Anti-MUC1 antibodies have been utilized to directly target MUC1-positive tumors, with several showing potential in a variety of cancer types. GP1.4 antibody promotes the internalization of MUC1, resulting in decreased signaling of EGFR in pancreatic cancer cells, inhibiting ERK phosphorylation and decreasing both proliferation and migration of these cells.Citation118 Pancreatic cancer cells coated in MUC1-specific HMFG2 antibody conjugated to CpG effectively activate natural killer cells, and intratumoral injection of conjugated MUC1 antibody reduced tumor burden in metastatic mouse models of pancreatic cancer.Citation119 In addition, in a xenograft mouse model of ovarian cancer, a MUC1 antibody C595 reduced tumor growth, whereas the C595 in combination with docetaxel inhibited tumor growth and metastasis while increasing survival.Citation120
Additionally, in a study of 447 patients with epithelial ovarian cancer at a variety of stages and who had already undergone chemotherapy, patients were given either standard treatment or one injection of an yttrium90-labeled anti-MUC1 antibody (murine HMFG1 antibody), referred to as 90Y-muHMFG1. This antibody was designed to bind MUC1 epitopes and kill cancer cells with the radioactive yttrium moiety. Patients who were given the 90Y-muHMFG1 treatment had higher circulating anti-MUC1 antibodies in response to the treatment, and their CA 15-3 serum assessments were lower, indicating that the levels of circulating MUC1 were being reduced in these patients. Patients with the highest levels of circulating anti-MUC1 antibodies did have higher overall survival (80th percentile of 90Y-muHMGF1 treated group), though there was no significant difference in survival between the 90Y-muHMGF1 treated group as a whole and the group who received the standard treatment.Citation121
Vaccination against MUC1
In a separate study, 31 post-menopausal women with stage 2 breast cancer were treated with tamoxifen and either seven injections of oxidized mannan conjugated to a MUC1-GST fusion protein (M-FP), or seven injections of placebo after mastectomy.Citation122 While none of the patients treated with M-FP experienced metastases, 20% of the patients treated with placebo presented with metastases. Moreover, after the injection series, patients treated with M-FP elicited immunity when subjected to an additional injection of a different MUC1 fusion protein, indicating that the M-FP treatment acted as a vaccine against MUC1.Citation122
Several vaccines have recently shown promise against MUC1-driven cancers.Citation121-Citation124 Evaluation of a MUC1 cDNA as a vaccine has shown promise as a metastasis suppressor. In this study, wild-type C57/Bl6 mice were given weekly intradermal injections of a full-length MUC1 cDNA plasmid and assessed against mice injected with empty vector. B16-F10 melanoma cells stably overexpressing MUC1 were then intravenously introduced to the mice and animals were evaluated for the formation of lung metastases. Mice vaccinated with the MUC1 cDNA had significantly fewer lung metastases than mice injected with empty vector, providing evidence that vaccination with MUC1 cDNA suppresses MUC1-dependent lung metastasis development.Citation123
One MUC1 vaccine just finished Phase 2 clinical trials to determine its efficacy against MUC1-driven cancers. The MUC1 vaccine, ImMucin, is composed of a short 21-mer peptide sequence at the N-terminal signal peptide region of MUC1, and was shown in preliminary studies to bind to both human MHC class 1 and 2 immune cell proteins derived from cancer patient tissues. Pre-clinically, mice injected with MUC1-overexpressing DA3 metastatic mouse mammary carcinoma cells experienced longer survival after vaccination with ImMucin than mice injected with the carcinoma cells and treated with vehicle.Citation125 Phase 1 and Phase 2 human clinical trials in which multiple myeloma patients were treated with the vaccine have shown promise but have not been formally reported.Citation126
Summary
Numerous factors affect the metastatic cascade, including cell motility and invasion, degradation of ECM, neo-vascularization, intra- and extravasation and dormancy and survival at a secondary site.Citation127 Of these, MUC1 has been shown to affect tumor invasion and neo-vascularization, interactions with the vasculature and survival and growth at a secondary site. Clinically, MUC1 expression is highly correlated with advanced disease, poor survival and tumor dissemination. Mechanistically, MUC1 can drive metastatic progression by altering the interaction between tumor cells and their environment, altering the composition of the tumor microenvironment and altering the genetic makeup of the tumor cell itself to produce a pro-metastatic phenotype. Due to the prevalence of MUC1 expression in metastatic disease, and the role of metastasis in patient mortality, directly targeting MUC1 would appear to be of paramount importance. While still in the pre-clinical and early clinical stages, MUC1 targeting has now begun in earnest, and appears to hold significant promise for a number of tumor types, including lung, pancreatic and breast.
| Abbreviations: | ||
| MUC1 | = | mucin 1 (also called CA 15-3, KL-6 and BM7) |
| ECD | = | extracellular domain |
| CD | = | cytoplasmic domain |
| EGFR | = | epidermal growth factor receptor |
| CTC | = | circulating tumor cell |
| HIF-1α | = | hypoxia inducible factor 1α |
| IFNγ | = | interferon γ |
| IL-6 | = | interleukin 6 |
| AR | = | androgen receptor |
| VEGF | = | vascular endothelial growth factor |
| PLCγ | = | phospholipase Cγ |
| PI3K | = | phosphoinositide 3 kinase |
| MMP | = | matrix metalloproteinase |
| ECM | = | extracellular matrix |
| EMT | = | epithelial to mesenchymal transition |
| PDGF-A | = | platelet-derived growth factor A |
| PDGFRβ | = | platelet-derived growth factor receptor β |
| ERβ | = | estrogen receptor β |
| CTGF | = | connective tissue growth factor |
| PMIP | = | PTD4 (protein transduction domain 4) MUC1 inhibitory peptide |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med 2006; 12:895 - 904; http://dx.doi.org/10.1038/nm1469; PMID: 16892035
- Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: a family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci 2004; 41:189 - 231; http://dx.doi.org/10.1080/10408360490452040; PMID: 15270554
- Gendler SJ. MUC1, the renaissance molecule. J Mammary Gland Biol Neoplasia 2001; 6:339 - 53; http://dx.doi.org/10.1023/A:1011379725811; PMID: 11547902
- Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010; 29:2893 - 904; http://dx.doi.org/10.1038/onc.2010.87; PMID: 20348949
- Kufe DW. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene 2012; In press http://dx.doi.org/10.1038/onc.2012.158; PMID: 22580612
- Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol 2006; 16:467 - 76; http://dx.doi.org/10.1016/j.tcb.2006.07.006; PMID: 16904320
- Rahn JJ, Dabbagh L, Pasdar M, Hugh JC. The importance of MUC1 cellular localization in patients with breast carcinoma: an immunohistologic study of 71 patients and review of the literature. Cancer 2001; 91:1973 - 82; http://dx.doi.org/10.1002/1097-0142(20010601)91:11<1973::AID-CNCR1222>3.0.CO;2-A; PMID: 11391575
- Lavrsen K, Madsen CB, Rasch MG, Woetmann A, Odum N, Mandel U, et al. Aberrantly glycosylated MUC1 is expressed on the surface of breast cancer cells and a target for antibody-dependent cell-mediated cytotoxicity. Glycoconj J 2012; In press http://dx.doi.org/10.1007/s10719-012-9437-7; PMID: 22878593
- McGuckin MA, Walsh MD, Hohn BG, Ward BG, Wright RG. Prognostic significance of MUC1 epithelial mucin expression in breast cancer. Hum Pathol 1995; 26:432 - 9; http://dx.doi.org/10.1016/0046-8177(95)90146-9; PMID: 7705823
- Greenberg R, Barnea Y, Schneebaum S, Kashtan H, Kaplan O, Skornik Y. Detection of hepatocyte growth factor/scatter factor receptor (c-Met) and MUC1 from the axillary fluid drainage in patients after breast cancer surgery. Isr Med Assoc J 2003; 5:649 - 52; PMID: 14509156
- Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene 2003; 22:1324 - 32; http://dx.doi.org/10.1038/sj.onc.1206291; PMID: 12618757
- Park S, Ahn HK, Park LC, Hwang DW, Ji JH, Maeng CH, et al. Implications of different CA 15-3 levels according to breast cancer subtype at initial diagnosis of recurrent or metastatic breast cancer. Oncology 2012; 82:180 - 7; http://dx.doi.org/10.1159/000336081; PMID: 22433564
- Budiu RA, Mantia-Smaldone G, Elishaev E, Chu T, Thaller J, McCabe K, et al. Soluble MUC1 and serum MUC1-specific antibodies are potential prognostic biomarkers for platinum-resistant ovarian cancer. Cancer Immunol Immunother 2011; 60:975 - 84; http://dx.doi.org/10.1007/s00262-011-1010-x; PMID: 21461842
- Wang L, Ma J, Liu F, Yu Q, Chu G, Perkins AC, et al. Expression of MUC1 in primary and metastatic human epithelial ovarian cancer and its therapeutic significance. Gynecol Oncol 2007; 105:695 - 702; http://dx.doi.org/10.1016/j.ygyno.2007.02.004; PMID: 17368732
- Dong Y, Walsh MD, Cummings MC, Wright RG, Khoo SK, Parsons PG, et al. Expression of MUC1 and MUC2 mucins in epithelial ovarian tumours. J Pathol 1997; 183:311 - 7; http://dx.doi.org/10.1002/(SICI)1096-9896(199711)183:3<311::AID-PATH917>3.0.CO;2-2; PMID: 9422987
- Cozzi PJ, Wang J, Delprado W, Perkins AC, Allen BJ, Russell PJ, et al. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis 2005; 22:565 - 73; http://dx.doi.org/10.1007/s10585-005-5376-z; PMID: 16475027
- Retterspitz MF, Mönig SP, Schreckenberg S, Schneider PM, Hölscher AH, Dienes HP, et al. Expression of beta-catenin, MUC1 and c-met in diffuse-type gastric carcinomas: correlations with tumour progression and prognosis. Anticancer Res 2010; 30:4635 - 41; PMID: 21115917
- Yonezawa S, Kitajima S, Higashi M, Osako M, Horinouchi M, Yokoyama S, et al. A novel anti-MUC1 antibody against the MUC1 cytoplasmic tail domain: use in sensitive identification of poorly differentiated cells in adenocarcinoma of the stomach. Gastric Cancer 2012; 15:370 - 81; http://dx.doi.org/10.1007/s10120-011-0125-2; PMID: 22237656
- Ohtsuka M, Kimura F, Shimizu H, Yoshidome H, Kato A, Yoshitomi H, et al. Similarities and differences between intraductal papillary tumors of the bile duct with and without macroscopically visible mucin secretion. Am J Surg Pathol 2011; 35:512 - 21; http://dx.doi.org/10.1097/PAS.0b013e3182103f36; PMID: 21412069
- Zhang K, Tang W, Qu X, Guo Q, Inagaki Y, Seyama Y, et al. KL-6 mucin in metastatic liver cancer tissues from primary colorectal carcinoma. Hepatogastroenterology 2009; 56:960 - 3; PMID: 19760920
- Mizumoto M, Honjo G, Kobashi Y, Awane M, Matsusue S. Molecular profile of apomucin and p53 protein as predictors of malignancy in intraductal papillary mucinous neoplasms of the pancreas. Hepatogastroenterology 2011; 58:1791 - 5; PMID: 22086702
- Xu H, Inagaki Y, Seyama Y, Du G, Wang F, Kokudo N, et al. Expression of KL-6/MUC1 in pancreatic cancer tissues and its potential involvement in tumor metastasis. Oncol Rep 2011; 26:371 - 6; PMID: 21617869
- Hamada T, Nomura M, Kamikawa Y, Yamada N, Batra SK, Yonezawa S, et al. DF3 epitope expression on MUC1 mucin is associated with tumor aggressiveness, subsequent lymph node metastasis, and poor prognosis in patients with oral squamous cell carcinoma. Cancer 2012; 118:5251 - 64; http://dx.doi.org/10.1002/cncr.27542; PMID: 22434549
- Liu XY, Chen G, Wang Z, Liu FY. Clinical significance of detecting mucin 1 mRNA in diagnosing occult lymph node micrometastasis in esophageal cancer patients. Ai Zheng 2007; 26:194 - 9; PMID: 17298752
- Leroy X, Zerimech F, Zini L, Copin MC, Buisine MP, Gosselin B, et al. MUC1 expression is correlated with nuclear grade and tumor progression in pT1 renal clear cell carcinoma. Am J Clin Pathol 2002; 118:47 - 51; http://dx.doi.org/10.1309/1F99-BPDY-7DHH-9G97; PMID: 12109855
- Hasegawa H, Komoda M, Yamada Y, Yonezawa S, Tsutsumida H, Nagai K, et al. Aberrant overexpression of membrane-associated mucin contributes to tumor progression in adult T-cell leukemia/lymphoma cells. Leuk Lymphoma 2011; 52:1108 - 17; http://dx.doi.org/10.3109/10428194.2011.559671; PMID: 21599593
- Kaira K, Murakami H, Serizawa M, Koh Y, Abe M, Ohde Y, et al. MUC1 expression in thymic epithelial tumors: MUC1 may be useful marker as differential diagnosis between type B3 thymoma and thymic carcinoma. Virchows Arch 2011; 458:615 - 20; http://dx.doi.org/10.1007/s00428-011-1041-x; PMID: 21253760
- Marrinucci D, Bethel K, Kolatkar A, Luttgen MS, Malchiodi M, Baehring F, et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys Biol 2012; 9:016003; http://dx.doi.org/10.1088/1478-3975/9/1/016003; PMID: 22306768
- Sanislo L, Vertakova-Krakovska B, Kuliffay P, Brtko J, Galbava A, Galbavy S. Detection of circulating tumor cells in metastatic breast cancer patients. Endocr Regul 2011; 45:113 - 24; http://dx.doi.org/10.4149/endo_2011_03_113; PMID: 21793623
- Deng G, Herrler M, Burgess D, Manna E, Krag D, Burke JF. Enrichment with anti-cytokeratin alone or combined with anti-EpCAM antibodies significantly increases the sensitivity for circulating tumor cell detection in metastatic breast cancer patients. Breast Cancer Res 2008; 10:R69; http://dx.doi.org/10.1186/bcr2131; PMID: 18687126
- Sha MY, Xu H, Natan MJ, Cromer R. Surface-enhanced Raman scattering tags for rapid and homogeneous detection of circulating tumor cells in the presence of human whole blood. J Am Chem Soc 2008; 130:17214 - 5; http://dx.doi.org/10.1021/ja804494m; PMID: 19053187
- de Albuquerque A, Kaul S, Breier G, Krabisch P, Fersis N. Multimarker Analysis of Circulating Tumor Cells in Peripheral Blood of Metastatic Breast Cancer Patients: A Step Forward in Personalized Medicine. Breast Care (Basel) 2012; 7:7 - 12; http://dx.doi.org/10.1159/000336548; PMID: 22553466
- de Albuquerque A, Kubisch I, Breier G, Stamminger G, Fersis N, Eichler A, et al. Multimarker gene analysis of circulating tumor cells in pancreatic cancer patients: a feasibility study. Oncology 2012; 82:3 - 10; http://dx.doi.org/10.1159/000335479; PMID: 22270149
- de Albuquerque A, Kubisch I, Ernst D, Breier G, Stamminger G, Fersis N, et al. Development of a molecular multimarker assay for the analysis of circulating tumor cells in adenocarcinoma patients. Clin Lab 2012; 58:373 - 84; PMID: 22783565
- Aktas B, Müller V, Tewes M, Zeitz J, Kasimir-Bauer S, Loehberg CR, et al. Comparison of estrogen and progesterone receptor status of circulating tumor cells and the primary tumor in metastatic breast cancer patients. Gynecol Oncol 2011; 122:356 - 60; http://dx.doi.org/10.1016/j.ygyno.2011.04.039; PMID: 21605893
- Thie H, Toleikis L, Li J, von Wasielewski R, Bastert G, Schirrmann T, et al. Rise and fall of an anti-MUC1 specific antibody. PLoS One 2011; 6:e15921; http://dx.doi.org/10.1371/journal.pone.0015921; PMID: 21264246
- Kruit A, Gerritsen WB, Pot N, Grutters JC, van den Bosch JM, Ruven HJ. CA 15-3 as an alternative marker for KL-6 in fibrotic lung diseases. Sarcoidosis Vasc Diffuse Lung Dis 2010; 27:138 - 46; PMID: 21319596
- Duffy MJ, Shering S, Sherry F, McDermott E, O’Higgins N. CA 15-3: a prognostic marker in breast cancer. Int J Biol Markers 2000; 15:330 - 3; PMID: 11192829
- González-Sistal A, Arias JI, Ruibal A. CA 15-3 serum levels in patients with ductal breast carcinoma: relationship with clinicopathological parameters and tumor markers. Int J Biol Markers 2012; 27:47 - 52; http://dx.doi.org/10.5301/JBM.2011.8591; PMID: 21928245
- Guo Q, Tang W, Inagaki Y, Midorikawa Y, Kokudo N, Sugawara Y, et al. Clinical significance of subcellular localization of KL-6 mucin in primary colorectal adenocarcinoma and metastatic tissues. World J Gastroenterol 2006; 12:54 - 9; PMID: 16440417
- Kaira K, Nakagawa K, Ohde Y, Okumura T, Takahashi T, Murakami H, et al. Depolarized MUC1 expression is closely associated with hypoxic markers and poor outcome in resected non-small cell lung cancer. Int J Surg Pathol 2012; 20:223 - 32; http://dx.doi.org/10.1177/1066896911429296; PMID: 22108499
- Rakha EA, Boyce RW, Abd El-Rehim D, Kurien T, Green AR, Paish EC, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol 2005; 18:1295 - 304; http://dx.doi.org/10.1038/modpathol.3800445; PMID: 15976813
- Ghosh M, Kamma H, Kawamoto T, Koike N, Miwa M, Kapoor VK, et al. MUC 1 core protein as a marker of gallbladder malignancy. Eur J Surg Oncol 2005; 31:891 - 6; http://dx.doi.org/10.1016/j.ejso.2005.03.008; PMID: 15922536
- Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem 1995; 270:30093 - 101; http://dx.doi.org/10.1074/jbc.270.50.30093; PMID: 8530414
- Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 1992; 12:954 - 61; PMID: 1312220
- Schroeder JA, Masri AA, Adriance MC, Tessier JC, Kotlarczyk KL, Thompson MC, et al. MUC1 overexpression results in mammary gland tumorigenesis and prolonged alveolar differentiation. Oncogene 2004; 23:5739 - 47; http://dx.doi.org/10.1038/sj.onc.1207713; PMID: 15221004
- Sandgren EP, Schroeder JA, Qui TH, Palmiter RD, Brinster RL, Lee DC. Inhibition of mammary gland involution is associated with transforming growth factor alpha but not c-myc-induced tumorigenesis in transgenic mice. Cancer Res 1995; 55:3915 - 27; PMID: 7641211
- Pochampalli MR, Bitler BG, Schroeder JA. Transforming growth factor alpha dependent cancer progression is modulated by Muc1. Cancer Res 2007; 67:6591 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-4518; PMID: 17638868
- Besmer DM, Curry JM, Roy LD, Tinder TL, Sahraei M, Schettini J, et al. Pancreatic ductal adenocarcinoma mice lacking mucin 1 have a profound defect in tumor growth and metastasis. Cancer Res 2011; 71:4432 - 42; http://dx.doi.org/10.1158/0008-5472.CAN-10-4439; PMID: 21558393
- Finn OJ, Gantt KR, Lepisto AJ, Pejawar-Gaddy S, Xue J, Beatty PL. Importance of MUC1 and spontaneous mouse tumor models for understanding the immunobiology of human adenocarcinomas. Immunol Res 2011; 50:261 - 8; http://dx.doi.org/10.1007/s12026-011-8214-1; PMID: 21717081
- Rye PD, Norum L, Olsen DR, Garman-Vik S, Kaul S, Fodstad O. Brain metastasis model in athymic nude mice using a novel MUC1-secreting human breast-cancer cell line, MA11. Int J Cancer 1996; 68:682 - 7; http://dx.doi.org/10.1002/(SICI)1097-0215(19961127)68:5<682::AID-IJC20>3.0.CO;2-2; PMID: 8938153
- Gao J, McConnell MJ, Yu B, Li J, Balko JM, Black EP, et al. MUC1 is a downstream target of STAT3 and regulates lung cancer cell survival and invasion. Int J Oncol 2009; 35:337 - 45; PMID: 19578748
- Tsutsumida H, Swanson BJ, Singh PK, Caffrey TC, Kitajima S, Goto M, et al. RNA interference suppression of MUC1 reduces the growth rate and metastatic phenotype of human pancreatic cancer cells. Clin Cancer Res 2006; 12:2976 - 87; http://dx.doi.org/10.1158/1078-0432.CCR-05-1197; PMID: 16707592
- Aubert S, Fauquette V, Hémon B, Lepoivre R, Briez N, Bernard D, et al. MUC1, a new hypoxia inducible factor target gene, is an actor in clear renal cell carcinoma tumor progression. Cancer Res 2009; 69:5707 - 15; http://dx.doi.org/10.1158/0008-5472.CAN-08-4905; PMID: 19549898
- Mikami Y, Hisatsune A, Tashiro T, Isohama Y, Katsuki H. Hypoxia enhances MUC1 expression in a lung adenocarcinoma cell line. Biochem Biophys Res Commun 2009; 379:1060 - 5; http://dx.doi.org/10.1016/j.bbrc.2009.01.002; PMID: 19141292
- Gaemers IC, Vos HL, Volders HH, van der Valk SW, Hilkens J. A stat-responsive element in the promoter of the episialin/MUC1 gene is involved in its overexpression in carcinoma cells. J Biol Chem 2001; 276:6191 - 9; http://dx.doi.org/10.1074/jbc.M009449200; PMID: 11084045
- Berclaz G, Altermatt HJ, Rohrbach V, Siragusa A, Dreher E, Smith PD. EGFR dependent expression of STAT3 (but not STAT1) in breast cancer. Int J Oncol 2001; 19:1155 - 60; PMID: 11713584
- Xia L, Wang L, Chung AS, Ivanov SS, Ling MY, Dragoi AM, et al. Identification of both positive and negative domains within the epidermal growth factor receptor COOH-terminal region for signal transducer and activator of transcription (STAT) activation. J Biol Chem 2002; 277:30716 - 23; http://dx.doi.org/10.1074/jbc.M202823200; PMID: 12070153
- Khodarev N, Ahmad R, Rajabi H, Pitroda S, Kufe T, McClary C, et al. Cooperativity of the MUC1 oncoprotein and STAT1 pathway in poor prognosis human breast cancer. Oncogene 2010; 29:920 - 9; http://dx.doi.org/10.1038/onc.2009.391; PMID: 19915608
- Pochampalli MR, el Bejjani RM, Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene 2007; 26:1693 - 701; http://dx.doi.org/10.1038/sj.onc.1209976; PMID: 16983337
- Li Y, Kuwahara H, Ren J, Wen G, Kufe D. The c-Src tyrosine kinase regulates signaling of the human DF3/MUC1 carcinoma-associated antigen with GSK3 beta and beta-catenin. J Biol Chem 2001; 276:6061 - 4; http://dx.doi.org/10.1074/jbc.C000754200; PMID: 11152665
- Rajabi H, Joshi MD, Jin C, Ahmad R, Kufe D. Androgen receptor regulates expression of the MUC1-C oncoprotein in human prostate cancer cells. Prostate 2011; 71:1299 - 308; PMID: 21308711
- Mitchell S, Abel P, Madaan S, Jeffs J, Chaudhary K, Stamp G, et al. Androgen-dependent regulation of human MUC1 mucin expression. Neoplasia 2002; 4:9 - 18; http://dx.doi.org/10.1038/sj.neo.7900194; PMID: 11922395
- Lacunza E, Baudis M, Colussi AG, Segal-Eiras A, Croce MV, Abba MC. MUC1 oncogene amplification correlates with protein overexpression in invasive breast carcinoma cells. Cancer Genet Cytogenet 2010; 201:102 - 10; http://dx.doi.org/10.1016/j.cancergencyto.2010.05.015; PMID: 20682394
- Rajabi H, Jin C, Ahmad R, McClary C, Joshi MD, Kufe D. MUCIN 1 ONCOPROTEIN EXPRESSION IS SUPPRESSED BY THE miR-125b ONCOMIR. Genes Cancer 2010; 1:62 - 8; http://dx.doi.org/10.1177/1947601909357933; PMID: 20729973
- O’Day E, Lal A. MicroRNAs and their target gene networks in breast cancer. Breast Cancer Res 2010; 12:201; http://dx.doi.org/10.1186/bcr2484; PMID: 20346098
- Sachdeva M, Mo YY. miR-145-mediated suppression of cell growth, invasion and metastasis. Am J Transl Res 2010; 2:170 - 80; PMID: 20407606
- Luo X, Burwinkel B, Tao S, Brenner H. MicroRNA signatures: novel biomarker for colorectal cancer?. Cancer Epidemiol Biomarkers Prev 2011; 20:1272 - 86; http://dx.doi.org/10.1158/1055-9965.EPI-11-0035; PMID: 21551242
- Regimbald LH, Pilarski LM, Longenecker BM, Reddish MA, Zimmermann G, Hugh JC. The breast mucin MUCI as a novel adhesion ligand for endothelial intercellular adhesion molecule 1 in breast cancer. Cancer Res 1996; 56:4244 - 9; PMID: 8797599
- Ciborowski P, Finn OJ. Non-glycosylated tandem repeats of MUC1 facilitate attachment of breast tumor cells to normal human lung tissue and immobilized extracellular matrix proteins (ECM) in vitro: potential role in metastasis. Clin Exp Metastasis 2002; 19:339 - 45; http://dx.doi.org/10.1023/A:1015590515957; PMID: 12090474
- Geng Y, Yeh K, Takatani T, King MR. Three to Tango: MUC1 as a Ligand for Both E-Selectin and ICAM-1 in the Breast Cancer Metastatic Cascade. Front Oncol 2012; 2:76; http://dx.doi.org/10.3389/fonc.2012.00076; PMID: 22866263
- Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol 2000; 190:310 - 29; http://dx.doi.org/10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P; PMID: 10685065
- Shen Q, Rahn JJ, Zhang J, Gunasekera N, Sun X, Shaw AR, et al. MUC1 initiates Src-CrkL-Rac1/Cdc42-mediated actin cytoskeletal protrusive motility after ligating intercellular adhesion molecule-1. Mol Cancer Res 2008; 6:555 - 67; http://dx.doi.org/10.1158/1541-7786.MCR-07-2033; PMID: 18403635
- Rahn JJ, Chow JW, Horne GJ, Mah BK, Emerman JT, Hoffman P, et al. MUC1 mediates transendothelial migration in vitro by ligating endothelial cell ICAM-1. Clin Exp Metastasis 2005; 22:475 - 83; http://dx.doi.org/10.1007/s10585-005-3098-x; PMID: 16320110
- Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia 2010; 12:599 - 607; PMID: 20689754
- Horn G, Gaziel A, Wreschner DH, Smorodinsky NI, Ehrlich M. ERK and PI3K regulate different aspects of the epithelial to mesenchymal transition of mammary tumor cells induced by truncated MUC1. Exp Cell Res 2009; 315:1490 - 504; http://dx.doi.org/10.1016/j.yexcr.2009.02.011; PMID: 19245809
- Yao M, Zhang W, Zhang Q, Xing L, Xu A, Liu Q, et al. Overexpression of MUC1 enhances proangiogenic activity of non-small-cell lung cancer cells through activation of Akt and extracellular signal-regulated kinase pathways. Lung 2011; 189:453 - 60; http://dx.doi.org/10.1007/s00408-011-9327-y; PMID: 21959954
- Chaika NV, Gebregiworgis T, Lewallen ME, Purohit V, Radhakrishnan P, Liu X, et al. MUC1 mucin stabilizes and activates hypoxia-inducible factor 1 alpha to regulate metabolism in pancreatic cancer. Proc Natl Acad Sci U S A 2012; 109:13787 - 92; http://dx.doi.org/10.1073/pnas.1203339109; PMID: 22869720
- Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem 2002; 277:42953 - 7; http://dx.doi.org/10.1074/jbc.M206775200; PMID: 12215445
- Scheid A, Wenger RH, Schäffer L, Camenisch I, Distler O, Ferenc A, et al. Physiologically low oxygen concentrations in fetal skin regulate hypoxia-inducible factor 1 and transforming growth factor-beta3. FASEB J 2002; 16:411 - 3; PMID: 11790723
- Woo JK, Choi Y, Oh SH, Jeong JH, Choi DH, Seo HS, et al. Mucin 1 enhances the tumor angiogenic response by activation of the AKT signaling pathway. Oncogene 2012; 31:2187 - 98; http://dx.doi.org/10.1038/onc.2011.410; PMID: 21927028
- Litvinov SV, Hilkens J. The epithelial sialomucin, episialin, is sialylated during recycling. J Biol Chem 1993; 268:21364 - 71; PMID: 8407976
- Marti U, Burwen SJ, Wells A, Barker ME, Huling S, Feren AM, et al. Localization of epidermal growth factor receptor in hepatocyte nuclei. Hepatology 1991; 13:15 - 20; http://dx.doi.org/10.1002/hep.1840130104; PMID: 1988335
- Boerner JL, Demory ML, Silva C, Parsons SJ. Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol Cell Biol 2004; 24:7059 - 71; http://dx.doi.org/10.1128/MCB.24.16.7059-7071.2004; PMID: 15282306
- Haley JD, Gullick WJ. EGFR Signaling Networks in Cancer Therapy / edited by John D. Haley, William John Gullick. New York, NY: Humana Press, 2008.
- de Oliveira JT, de Matos AJ, Santos AL, Pinto R, Gomes J, Hespanhol V, et al. Sialylation regulates galectin-3/ligand interplay during mammary tumour progression--a case of targeted uncloaking. Int J Dev Biol 2011; 55:823 - 34; http://dx.doi.org/10.1387/ijdb.113359jt; PMID: 22161838
- Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 2007; 26:1276 - 85; http://dx.doi.org/10.1038/sj.onc.1210201; PMID: 17322912
- Horm TMBB, Bitler BG, Broka DM, Louderbough JM, Schroeder JA. MUC1 Drives c-Met-Dependent Migration and Scattering. Mol Cancer Res 2012; In press http://dx.doi.org/10.1158/1541-7786.MCR-12-0296; PMID: 23193156
- Matsubara D, Ishikawa S, Sachiko O, Aburatani H, Fukayama M, Niki T. Co-activation of epidermal growth factor receptor and c-MET defines a distinct subset of lung adenocarcinomas. Am J Pathol 2010; 177:2191 - 204; http://dx.doi.org/10.2353/ajpath.2010.100217; PMID: 20934974
- Li YQ, Yu WH, Ren J, Chen W, Huang L, Kharbanda S, et al. Heregulin targets gamma-catenin to the nucleolus by a mechanism dependent on the DF3/MUC1 oncoprotein. Mol Cancer Res 2003; 1:765 - 75; PMID: 12939402
- Citri A, Yarden Y. EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 2006; 7:505 - 16; http://dx.doi.org/10.1038/nrm1962; PMID: 16829981
- Todorović V, Desai BV, Patterson MJS, Amargo EV, Dubash AD, Yin TF, et al. Plakoglobin regulates cell motility through Rho- and fibronectin-dependent Src signaling. J Cell Sci 2010; 123:3576 - 86; http://dx.doi.org/10.1242/jcs.070391; PMID: 20876660
- Aktary Z, Chapman K, Lam L, Lo A, Ji C, Graham K, et al. Plakoglobin interacts with and increases the protein levels of metastasis suppressor Nm23-H2 and regulates the expression of Nm23-H1. Oncogene 2010; 29:2118 - 29; http://dx.doi.org/10.1038/onc.2009.495; PMID: 20101217
- Ahmad R, Alam M, Rajabi H, Kufe D. The MUC1-C oncoprotein binds to the BH3 domain of the pro-apoptotic BAX protein and blocks BAX function. J Biol Chem 2012; 287:20866 - 75; http://dx.doi.org/10.1074/jbc.M112.357293; PMID: 22544745
- Raina D, Ahmad R, Kumar S, Ren J, Yoshida K, Kharbanda S, et al. MUC1 oncoprotein blocks nuclear targeting of c-Abl in the apoptotic response to DNA damage. EMBO J 2006; 25:3774 - 83; http://dx.doi.org/10.1038/sj.emboj.7601263; PMID: 16888623
- Ren J, Li Y, Kufe D. Protein kinase C delta regulates function of the DF3/MUC1 carcinoma antigen in beta-catenin signaling. J Biol Chem 2002; 277:17616 - 22; http://dx.doi.org/10.1074/jbc.M200436200; PMID: 11877440
- Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi MD, et al. MUC1-C oncoprotein induces TCF7L2 transcription factor activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem 2012; 287:10703 - 13; http://dx.doi.org/10.1074/jbc.M111.323311; PMID: 22318732
- Yuan Z, Wong S, Borrelli A, Chung MA. Down-regulation of MUC1 in cancer cells inhibits cell migration by promoting E-cadherin/catenin complex formation. Biochem Biophys Res Commun 2007; 362:740 - 6; http://dx.doi.org/10.1016/j.bbrc.2007.08.074; PMID: 17764657
- Bernier AJ, Zhang J, Lillehoj E, Shaw AR, Gunasekara N, Hugh JC. Non-cysteine linked MUC1 cytoplasmic dimers are required for Src recruitment and ICAM-1 binding induced cell invasion. Mol Cancer 2011; 10:93; http://dx.doi.org/10.1186/1476-4598-10-93; PMID: 21798038
- Pandey P, Kharbanda S, Kufe D. Association of the DF3/MUC1 breast cancer antigen with Grb2 and the Sos/Ras exchange protein. Cancer Res 1995; 55:4000 - 3; PMID: 7664271
- Sahraei M, Roy LD, Curry JM, Teresa TL, Nath S, Besmer D, et al. MUC1 regulates PDGFA expression during pancreatic cancer progression. Oncogene 2012; 31:4935 - 45; http://dx.doi.org/10.1038/onc.2011.651; PMID: 22266848
- Singh PK, Wen Y, Swanson BJ, Shanmugam K, Kazlauskas A, Cerny RL, et al. Platelet-derived growth factor receptor beta-mediated phosphorylation of MUC1 enhances invasiveness in pancreatic adenocarcinoma cells. Cancer Res 2007; 67:5201 - 10; http://dx.doi.org/10.1158/0008-5472.CAN-06-4647; PMID: 17545600
- Julian J, Dharmaraj N, Carson DD. MUC1 is a substrate for gamma-secretase. J Cell Biochem 2009; 108:802 - 15; http://dx.doi.org/10.1002/jcb.22292; PMID: 19711367
- Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, et al. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem 2007; 282:19321 - 30; http://dx.doi.org/10.1074/jbc.M703222200; PMID: 17500061
- Wei X, Xu H, Kufe D. Human mucin 1 oncoprotein represses transcription of the p53 tumor suppressor gene. Cancer Res 2007; 67:1853 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-06-3063; PMID: 17308127
- Bitler BG, Goverdhan A, Schroeder JA. MUC1 regulates nuclear localization and function of the epidermal growth factor. J Cell Sci 2010; 123:1716 - 23; http://dx.doi.org/10.1242/jcs.062661; PMID: 20406885
- Lau SK, Shields DJ, Murphy EA, Desgrosellier JS, Anand S, Huang M, et al. EGFR-mediated carcinoma cell metastasis mediated by integrin αvβ5 depends on activation of c-Src and cleavage of MUC1. PLoS One 2012; 7:e36753; http://dx.doi.org/10.1371/journal.pone.0036753; PMID: 22586492
- Baldus SE, Mönig SP, Huxel S, Landsberg S, Hanisch FG, Engelmann K, et al. MUC1 and nuclear beta-catenin are coexpressed at the invasion front of colorectal carcinomas and are both correlated with tumor prognosis. Clin Cancer Res 2004; 10:2790 - 6; http://dx.doi.org/10.1158/1078-0432.CCR-03-0163; PMID: 15102686
- Roy LD, Sahraei M, Subramani DB, Besmer D, Nath S, Tinder TL, et al. MUC1 enhances invasiveness of pancreatic cancer cells by inducing epithelial to mesenchymal transition. Oncogene 2011; 30:1449 - 59; http://dx.doi.org/10.1038/onc.2010.526; PMID: 21102519
- Behrens ME, Grandgenett PM, Bailey JM, Singh PK, Yi CH, Yu F, et al. The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF. Oncogene 2010; 29:5667 - 77; http://dx.doi.org/10.1038/onc.2010.327; PMID: 20697347
- Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, et al. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med 1998; 4:1449 - 52; http://dx.doi.org/10.1038/4042; PMID: 9846587
- Bitler BG, Schroeder JA. Anti-cancer therapies that utilize cell penetrating peptides. Recent Pat Anticancer Drug Discov 2010; 5:99 - 108; http://dx.doi.org/10.2174/157489210790936252; PMID: 19961434
- Bitler BG, Menzl I, Huerta CL, Sands B, Knowlton W, Chang A, et al. Intracellular MUC1 peptides inhibit cancer progression. Clin Cancer Res 2009; 15:100 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-08-1745; PMID: 19118037
- Zhang L, Gallup M, Zlock L, Basbaum C, Finkbeiner WE, McNamara NA. Cigarette smoke disrupts the integrity of airway adherens junctions through the aberrant interaction of p120-catenin with the cytoplasmic tail of MUC1. J Pathol 2012; In press http://dx.doi.org/10.1002/path.4070; PMID: 22833523
- Klinge CM, Radde BN, Imbert-Fernandez Y, Teng Y, Ivanova MM, Abner SM, et al. Targeting the intracellular MUC1 C-terminal domain inhibits proliferation and estrogen receptor transcriptional activity in lung adenocarcinoma cells. Mol Cancer Ther 2011; 10:2062 - 71; http://dx.doi.org/10.1158/1535-7163.MCT-11-0381; PMID: 21862684
- Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, et al. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther 2011; 10:806 - 16; http://dx.doi.org/10.1158/1535-7163.MCT-10-1050; PMID: 21421804
- Yin L, Kufe D. MUC1-C Oncoprotein Blocks Terminal Differentiation of Chronic Myelogenous Leukemia Cells by a ROS-Mediated Mechanism. Genes Cancer 2011; 2:56 - 64; http://dx.doi.org/10.1177/1947601911405044; PMID: 21643558
- Hisatsune A, Nakayama H, Kawasaki M, Horie I, Miyata T, Isohama Y, et al. Anti-MUC1 antibody inhibits EGF receptor signaling in cancer cells. Biochem Biophys Res Commun 2011; 405:377 - 81; http://dx.doi.org/10.1016/j.bbrc.2011.01.029; PMID: 21219855
- Schettini J, Kidiyoor A, Besmer DM, Tinder TL, Roy LD, Lustgarten J, et al. Intratumoral delivery of CpG-conjugated anti-MUC1 antibody enhances NK cell anti-tumor activity. Cancer Immunol Immunother 2012; 61:2055 - 65; http://dx.doi.org/10.1007/s00262-012-1264-y; PMID: 22543528
- Wang L, Chen H, Pourgholami MH, Beretov J, Hao J, Chao H, et al. Anti-MUC1 monoclonal antibody (C595) and docetaxel markedly reduce tumor burden and ascites, and prolong survival in an in vivo ovarian cancer model. PLoS One 2011; 6:e24405; http://dx.doi.org/10.1371/journal.pone.0024405; PMID: 21931707
- Oei AL, Moreno M, Verheijen RH, Sweep FC, Thomas CM, Massuger LF, et al. Induction of IgG antibodies to MUC1 and survival in patients with epithelial ovarian cancer. Int J Cancer 2008; 123:1848 - 53; http://dx.doi.org/10.1002/ijc.23725; PMID: 18661524
- Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Drakaki H, Loveland BE, et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835]. [ISRCTN71711835] Breast Cancer Res 2006; 8:R27; http://dx.doi.org/10.1186/bcr1505; PMID: 16776849
- Kamata M, Denda-Nagai K, Kubota N, Aida S, Takeda K, Irimura T. Vaccination of mice with MUC1 cDNA suppresses the development of lung metastases. Clin Exp Metastasis 2002; 19:689 - 96; http://dx.doi.org/10.1023/A:1021332932531; PMID: 12553374
- Lakshminarayanan V, Thompson P, Wolfert MA, Buskas T, Bradley JM, Pathangey LB, et al. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc Natl Acad Sci U S A 2012; 109:261 - 6; http://dx.doi.org/10.1073/pnas.1115166109; PMID: 22171012
- Kovjazin R, Volovitz I, Kundel Y, Rosenbaum E, Medalia G, Horn G, et al. ImMucin: a novel therapeutic vaccine with promiscuous MHC binding for the treatment of MUC1-expressing tumors. Vaccine 2011; 29:4676 - 86; http://dx.doi.org/10.1016/j.vaccine.2011.04.103; PMID: 21570434
- McCarthy N. Running a Muc1. Nat Rev Cancer 2012; 12:317; http://dx.doi.org/10.1038/nrc3274
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science 2011; 331:1559 - 64; http://dx.doi.org/10.1126/science.1203543; PMID: 21436443
