Abstract
There is currently great interest in the use of mesenchymal stem cells as a therapy for multiple sclerosis with potential to both ameliorate inflammatory processes as well as improve regeneration and repair. Although most clinical studies have used autologous bone marrow-derived mesenchymal stem cells, other sources such as allogeneic umbilical cord-derived cells may provide a more accessible and practical supply of cells for transplantation. In this case report we present the treatment of aggressive multiple sclerosis with multiple allogenic human umbilical cord-derived mesenchymal stem cell and autologous bone marrow-derived mesenchymal stem cells over a 4 y period. The treatments were tolerated well with no significant adverse events. Clinical and radiological disease appeared to be suppressed following the treatments and support the expansion of mesenchymal stem cell transplantation into clinical trials as a potential novel therapy for patients with aggressive multiple sclerosis.
Special Report
Current therapies for Multiple Sclerosis (MS) are effective at reducing the inflammatory component of the disease but do not appear to directly limit axonal degeneration. Mesenchymal stem cells (MSC) were initially targeted as a relatively accessible source of pluripotent cells with the potential to home to sites of injury, suppress inflammation, and rebuild the injured nervous system. While the functional differentiation and integration of MSC-derived neural cells into the brain has not been reliably demonstrated in vivo, evidence of neuro-protection through the production of trophic factors has been consistently observed.Citation1,Citation2 Although pre-clinical studies have shown clear benefits in experimental models of MS,Citation3 clinical translation has been slow and therefore the benefits and risks in MS is mostly unknown. Most pre-clinical and clinical experience with MSCs have involved bone marrow-derived MSCs (BM-MSCs). However, pre-clinical evidence suggests that other sources of MSC such as adipose or umbilical cell-derived MSC (hUC-MSCs) may actually be more efficacious and suitable for clinical translation.Citation8,Citation9 We report a patient treated with hUC-MSC infusions as well as BM-MSCs over a 4 y period.
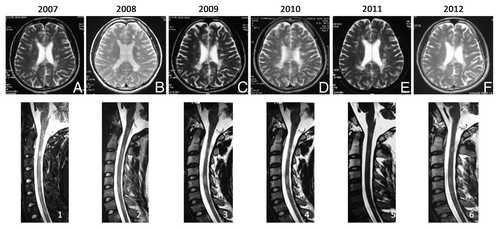
A 25-y-old previously well man from Honghe city, Yunnan Province had an initial episode of right leg weakness and gait ataxia in September 2006. After a 3-d course of intravenous methylprednisolone, symptoms resolved. He subsequently had another relapse in March 2007 treated at a local hospital with good recovery. MRI scans were performed at different time points with representative T2-weighted Fluid-Attenuated Inversion Recovery (FLAIR) axial sequences through the brain (upper panel; ) and T2-weighted sagittal sections through the cervical cord (lower panel; ) presented in . The MRI performed in November 2006 revealed multiple (approximately 12) T2 hyperintense lesions in a typical periventricular distribution perpendicular to the ventricles in combination with subcortical, juxtacortical, infratentorial, and cervical cord lesions. The following year (July 2008) he experienced another relapse resulting in further ataxia, which limited walking to < 500 meters and was accompanied by a right arm tremor. At least five new T2 hyperintense lesions were seen on MRI. After the patient failed to improve with methylprednisolone, he was transferred to Yan’an Hospital where the diagnosis of Multiple Sclerosis was further confirmed with oligoclonal bands detected in the CSF.
Figure 1. Axial (A–E) fluid attenuation inversion recovery (FLAIR) MRI Brain and sagittal T2-weighted MRI of the cervical cord (1–5) demonstrate a stable distribution of lesions from 2007 (A and 1) to 2012 (F and 6).
Informed consent was obtained from the patient for multiple MSC treatments with approval from the ethics committee of Yan’an Hospital of Kunming Medical University. Approximately 200 ml of bone marrow was obtained from the patient’s posterior superior iliac crest under short general anesthesia at Yan’an Hospital. BM mononuclear cells were isolated by Ficoll density centrifugation and cultured, expanded (up to passage 2), characterized, and cryopreserved under good manufacturing practice conditions using conventional standard operating procedures based on the European Group for Blood and Bone Marrow Transplantation developmental committee protocolCitation4 and characterized according to International Society of Cellular Therapy (ICST) recommendationsCitation5 (Fig. S1). In November 2008, 1.3 × 107 BM derived MSCs were infused intravenously and 6.3 × 105 infused intra-thecally with no side effects. Two further infusions of BM-MSC were performed 3 mo apart (19th February 2009, intravenous and intra-thecal, and 7th May 2009, intravenous). Gradual clinical improvement was observed and by May 2009, he was able to walk over 500 meters without rest (see ).
Table 1. Summary of MSC treatment, adverse events and clinical disability score (EDSS)
As the patient stabilized, consideration was given to the use of hUC-MSC. UC-MSCs were extracted from Wharton’s jelly obtained from the placenta of healthy mothers (unrelated to the patient) delivering at Yan’an Hospital following informed consent. Cells were cultured as previously describedCitation6 and cryopreserved at passage 2. Before transplantation, cells were expanded up to Passage 5, examined for MSC phenotype using flow cytometry and karyotype (but not HLA matched) then infused intravenously without pre-medication on the 4th August 2009 (1.2 × 108 cells). The patient experienced minor symptoms of dizziness and headache during and for several hours post-infusion only requiring simple analgesics. Seven months later, the patient received an intra-thecal dose of BM-MSC and in August, 2010 received a second intravenous infusion of over 3 × 108 hUC-MSC. Following this infusion, the patient noted a rash and pain that again resolved within 3 d without medication. A third hUC-MSC infusion (1.4 × 108 cells) was given on the 22nd April, 2011 and the last treatment was given on the 29th December 2011 (1.54 × 108 iv), all with similar mild transient infusion related symptoms.
Throughout the 4 y treatment period (November 2008-present), the patient remained completely free of clinical and radiological disease activity with no treatment other than BM and UC-MSC. He made a good recovery from the severe relapse in 2008 and remains able to walk unaided for > 500 meters (although not unlimited). No new lesions were reported on the MRI performed in July 2012 and in fact, many lesions had resolved (see ). It was also interesting to note that oligoclonal bands which were present in July 2008 were not detected when tested after the first four MSC transplants.
This case study illustrates a number of interesting points with regard to hUC-MSC transplantation. First, it demonstrates that large numbers of allogeneic hUC-MSC can be obtained more easily than BM-MSC. It is not particularly desirable for a patient with aggressive MS to be subjected to a painful large volume BM aspirate procedure. Even starting with 200 ml of bone marrow and expanding to passage 2, less than 30 × 106 MSC could be obtained. In comparison, 110x106 UC-MSC cells were obtained from one umbilical cord after only 2 passages. Animal studies have suggested that higher doses (> 1–2 × 106) of MSC are more effective.Citation7 Allogeneic UC-MSC could also be readily available “off-the-shelf” for any patient who required them. This case suggests that the systemic infusion of a large number of non-HLA matched hUC-MSC is probably safe without significant graft-vs-host disease in the absence of pre-conditioning. MSCs lack HLA class II antigens and T-cell co-stimulatory molecules and have actually been shown to reduce graft-vs-host rejection following allogeneic bone marrow transplantation for hematological malignancy.Citation4 Despite quite an aggressive early disease course with many poor prognostic indicators (male, spinal cord involvement, and early disability), inflammatory activity was significantly reduced following the MSC treatments with no further clinical relapses or new MRI lesions. The EDSS improvement from 3.5 to 2.0 over the duration of the treatment period was probably due to natural recovery of function associated with suppression of inflammatory disease as observed with NatalizumabCitation10 however secreted trophic factors promoting repair is a possibility based on in vitro data.Citation1 Randomized and blinded clinical trials involving a large number of MS patients is required to fully assess the safety and efficacy of MSC transplantation as described by Freedman et al.Citation7 Case reports such as this illustrate the feasibility and potential of MSC therapy in MS, an important first step toward clinical trials.
In conclusion, this is a unique case of a patient with MS transplanted with multiple allogeneic hUC-MSC and autologous BM-MSC infusions. Allogeneic hUC-MSC may be a safe, effective, and more practical source of stem cells for the treatment of MS.
Additional material
Download Zip (3.2 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Hou ZL was supported by grants from Key Scientific and Technological projects of Yunnan province (Grant No. 2010CD211). Short M was supported by a fellowship from Biogen Idec Australia. Xiao ZC was supported by the Talent Program, Yunnan Province, China, and The Monash Professorial Fellowship, Monash University, Australia. Bernard CCA was supported by grants from the Eva and Les Erdi AUSiMED Fellowship in Neurological Diseases and the National Health and Medical Research Council of Australia.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/celladhesion/article/26941/
References
- Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol 2011; 24:59 - 64; http://dx.doi.org/10.1016/j.beha.2011.01.004; PMID: 21396593
- Dalous J, Larghero J, Baud O. Transplantation of umbilical cord-derived mesenchymal stem cells as a novel strategy to protect the central nervous system: technical aspects, preclinical studies, and clinical perspectives. Pediatr Res 2012; 71:482 - 90; http://dx.doi.org/10.1038/pr.2011.67; PMID: 22430384
- Payne N, Siatskas C, Barnard A, Bernard CCA. The prospect of stem cells as multi-faceted purveyors of immune modulation, repair and regeneration in multiple sclerosis. Curr Stem Cell Res Ther 2011; 6:50 - 62; http://dx.doi.org/10.2174/157488811794480735; PMID: 20955155
- Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, et al, Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet 2008; 371:1579 - 86; http://dx.doi.org/10.1016/S0140-6736(08)60690-X; PMID: 18468541
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315 - 7; http://dx.doi.org/10.1080/14653240600855905; PMID: 16923606
- Meng MY, Pang W, Jiang LH, Liu YH, Wei CY, Xie YH, Yu HD, Hou ZL. Stemness gene expression profile analysis in human umbilical cord mesenchymal stem cells. Exp Biol Med (Maywood) 2012; 237:709 - 19; http://dx.doi.org/10.1258/ebm.2012.011429; PMID: 22728706
- Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, Scolding N, Slavin S, Le Blanc K, Uccelli A, MSCT Study Group. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler 2010; 16:503 - 10; http://dx.doi.org/10.1177/1352458509359727; PMID: 20086020
- Payne NL, Sun G, McDonald C, Layton D, Moussa L, Emerson-Webber A, Veron N, Siatskas C, Herszfeld D, Price J, et al. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant 2013; 22:1409 - 25; http://dx.doi.org/10.3727/096368912X657620; PMID: 23057962
- Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011; 13:675 - 85; http://dx.doi.org/10.3109/14653249.2010.549122; PMID: 21231804
- Phillips JT, Giovannoni G, Lublin FD, O’Connor PW, Polman CH, Willoughby E, Aschenbach W, Pace A, Hyde R, Munschauer FE. Sustained improvement in Expanded Disability Status Scale as a new efficacy measure of neurological change in multiple sclerosis: treatment effects with natalizumab in patients with relapsing multiple sclerosis. Mult Scler 2011; 17:970 - 9; http://dx.doi.org/10.1177/1352458511399611; PMID: 21421809
