Abstract
Gemcitabine is a deoxycytidine analog used for the treatment of a wide range of solid tumors. Its efficacy is however often reduced due to the development of resistance. Ribonucleotide reductase M1 subunit (RRM1) is a key determinant of gemcitabine resistance, and tumor cells that overexpress RRM1 are resistant to the cytotoxicity of gemcitabine. In the present study, we showed that RRM1-specific small interfering RNA (siRNA), when complexed with polyethylenimine, effectively downregulated the expression of RRM1 protein in mouse tumor cells that overexpress RRM1, both in vitro and in vivo. More importantly, systemic administration of the RRM1-specific siRNA significantly inhibited the growth of RRM1-overexpressing tumors in mice and sensitized the tumors to gemcitabine treatment. These findings suggest that silencing RRM1 expression using siRNA could potentially be an effective strategy to overcome gemcitabine resistance.
Introduction
Gemcitabine (2′,2′-difluorodeoxycytidine, dFdC) is a deoxycytidine analog with antitumor activity against a wide variety of cancers. It is approved as the first line single agent treatment for advanced pancreatic cancer and for combination therapy against non-small cell lung, breast, and ovarian cancers.Citation1 Although gemcitabine is one of the primary standard drugs used to treat various solid tumors, drug resistance often limits its efficacy,Citation2 making it clinically important to understand the mechanisms of gemcitabine resistance and to develop novel strategies to overcome the resistance.
Ribonucleotide reductase (RR) catalyzes the conversion of ribonucleoside 5′-diphosphates into their corresponding 2′-deoxyribonucleotides. This reaction is rate limiting in the production of 2′-deoxyribonucleoside 5′-triphosphates (dNTPs), which are essential for the de novo synthesis of DNA.Citation3 Gemcitabine diphosphate (dFdCDP) binds to the large subunit M1 of RR (RRM1) and inhibits RR, thereby depleting the cellular deoxynucleotide (dNTP) pools.Citation4,Citation5 RRM1 has been identified as the key molecule in determining the efficacy of gemcitabine. The overexpression of RRM1 had been repeatedly reported in gemcitabine-resistant cancer cells both in vitro and in vivo,Citation6-Citation11 and RRM1 overexpression through the transfection of a lung cancer cell line led to gemcitabine resistance as well.Citation9 More importantly, a higher level of RRM1 has been detected in tumors in various cancer patients who were poor responders to gemcitabine.Citation9,Citation12-Citation18 Taken together, these findings demonstrate that RRM1 overexpression plays a key role in gemcitabine resistance. Therefore, the downregulation of RRM1 expression may increase the susceptibility of resistant cancer cells to gemcitabine.
RNA interference (RNAi) represents a powerful method for specific gene silencing.Citation19,Citation20 Previously, we and others have shown that the downregulation of RRM1 expression using small interfering RNA (siRNA) increased the sensitivity of gemcitabine resistant cancer cells to gemcitabine in vitro.Citation10,Citation11,Citation13,Citation14,Citation17,Citation21 In the present study, we tested the feasibility of using RRM1-specific siRNA to downregulate RRM1 expression and thus sensitize RRM1-overexpressing tumor cells to gemcitabine in an animal model. In vivo siRNA delivery remains challenging due to the poor stability of unmodified siRNA molecules and the difficulty in delivering them intracellularly.Citation20,Citation22 It was shown that polyethylenimine (PEI) could protect siRNA from enzymatic and non-enzymatic degradations and efficiently deliver them into target cells in culture.Citation23,Citation24 Moreover, it was shown that PEI can also efficiently deliver siRNA complexed with it to tumors in mice after systemic administration.Citation25,Citation26 We therefore chose to employ PEI as a carrier for RRM1-specific siRNA in this study.
Previously, a gemcitabine resistant lung cancer cell line, TC-1-GR, was developed in our laboratory by continuously culturing TC-1 cells with gradually increasing concentrations of gemcitabine HCl.Citation11 The TC-1-GR cells were found to significantly overexpress RRM1.Citation11 In the present study, using the TC-1-GR tumor cells in a mouse model, we demonstrated that the downregulation of RRM1 overexpression using RRM1-specific siRNAs potentiated the antitumor activity of gemcitabine against the RRM1-overexpressing tumor cells in vivo. Our findings underline the potential of RRM1 as a therapeutic target for chemosensitization, and suggest that the combination of RRM1-specific siRNA with gemcitabine represents a promising strategy for the management of gemcitabine resistant tumors.
Results
Silencing of RRM1 sensitizes RRM1-overexpressing, gemcitabine resistant lung cancer cells to gemcitabine
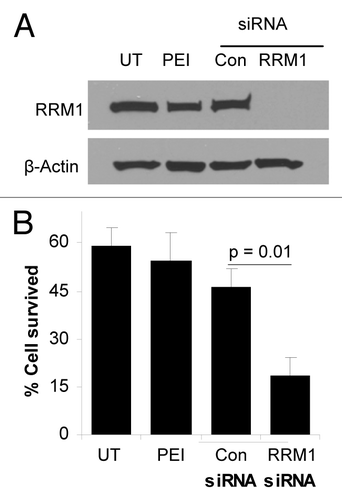
To facilitate the delivery of RRM1-specific siRNA into tumor cells, the siRNA was complexed with PEI. The size of the PEI-RRM1 siRNA complexes was 122 ± 5 nm, with a zeta potential of 15 ± 0.6 mV. As a control, a universal negative control siRNA was also complexed with PEI, resulting in a PEI-control siRNA complex of 119 ± 4 nm, with a zeta potential value of 15 ± 0.5 mV. The polydispersity indices of the complexes were within the range of 0.2 and 0.3, and there was not a significant difference between the sizes (and zeta potentials) of the PEI-RRM1 siRNA complexes and the PEI-control siRNA complexes. We next investigated whether the PEI is able to deliver the siRNA efficiently into cells by determining the level of RRM1 protein after TC-1-GR cells in culture were transfected with either PEI-RRM1 siRNA or PEI-control siRNA complexes. As shown in , RRM1 protein level in cells transfected with the PEI-control siRNA complexes was not different from that in untreated cells or in cells treated with PEI alone. In contrast, RRM1 protein was not detected in cells transfected with the PEI-RRM1 siRNA complexes (), demonstrating that the RRM1-specific siRNA, when delivered using the PEI, was able to significantly inhibit the expression of the RRM1 protein. To evaluate whether silencing RRM1 expression can sensitize the RRM1-overexpressing TC-1-GR tumor cells to gemcitabine, the cytotoxicity of gemcitabine in TC-1-GR cells transfected with PEI-RRM1 siRNA complexes was determined. Incubation of TC-1-GR cells that were pretreated with fresh medium or with PEI alone with 50 µM gemcitabine for 48 h induced approximately 40% cell death (). Cells pretreated with the PEI-control siRNA complexes remained resistant to gemcitabine; after gemcitabine treatment, the viability of the TC-1-GR cells pretreated with the PEI-control siRNA complexes was not different from that of the TC-1-GR cells pretreated with PEI alone (). In contrast, gemcitabine at the same concentration became significantly more cytotoxic to TC-1-GR cells that were pretreated with the PEI-RRM1 siRNA complexes (), indicating that silencing RRM1 overexpression could restore the susceptibility of the TC-1-GR cells to gemcitabine.
Figure 1. RRM1-specific siRNA downregulated RRM1 expression and sensitized TC-1-GR tumor cells to gemcitabine. (A) Immunoblotting analysis of RRM1 in untransfected TC-1-GR cells (UT) or TC-1-GR cells transfected with PEI-RRM1 siRNA or PEI-control siRNA complexes. As a control, cells were also treated with PEI25K alone (PEI). (B) Cytotoxicity of gemcitabine in TC-1-GR cells transfected with PEI-RRM1 siRNA or PEI-control siRNA complexes. Data are presented as a mean ± SEM (n = 3).
Silencing of RRM1 using siRNA shows antitumor activity against RRM1-overexpressing TC-1-GR tumors in mice
Studies have shown that when cells were exposed to progressively higher concentrations of gemcitabine, they subsequently increased the expression of RRM1 gradually. The removal of gemcitabine from culture medium resulted in a relatively rapid return of RRM1 protein level to near baseline.Citation7 Thus, we initially compared RRM1 gene and protein expression in TC-1 and TC-1-GR tumors in nude mice 21 d after tumor cells were injected (s.c.). The overexpression of RRM1 in TC-1-GR tumors in mice was sustained in the 3-week period tested (). RT-PCR analysis revealed a detectable level of RRM1 mRNA only in TC-1-GR tumors (). Western blotting analysis confirmed that there was a clear difference in the expression of RRM1 protein in TC-1 and TC-1-GR tumors (), and the RRM1 protein level in TC-1-GR tumor tissues remained significantly higher than in TC-1 tumor tissues 21 d after tumor cell implantation ().
Figure 2. Systemic administration of RRM1-specific siRNA downregulated RRM1 expression in TC-1-GR tumors in mice and significantly inhibited TC-1-GR tumor growth. (A–B) RT-PCR (A) and immunoblotting (B) analyses of RRM1 expression in TC-1 and TC-1-GR tumor tissues 21 d after tumor cell injection. (C–D) Immunoblotting analysis of RRM1 expression in TC-1-GR tumors after mice were treated with PEI-RRM1 siRNA complexes. TC-1-GR tumor cells were injected (s.c.) in female nude mice on day 0. Mice were injected with PEI-siRNA complexes (0.5 mg/kg siRNA per mouse per injection) every two days for two consecutive weeks, starting on day 7. a The level of RRM1 protein in tumors in mice that were treated with the PEI-RRMi siRNA complexes was significantly lower than that in tumors in mice that were treated with the PEI-control siRNA complexes or PBS. (E-F) RRM1-specific siRNA inhibited the growth of TC-1-GR tumors (E), but not TC-1 tumors (F) in mice. Tumors were injected in mice on day 0. TC-1-GR tumor-bearing mice were injected with PEI-RRM1 siRNA or PEI-control siRNA complexes on days 3–5, 7–12, and 14–16. TC-1 tumor-bearing mice were injected on days 7–11 once daily. The dose of the siRNA was 0.5 mg/kg per mouse per injection. b The values of the RRM1 siRNA group were significantly different from that of the control siRNA group (p < 0.05). Data are presented as a mean ± SEM (n = 2–3 in D, 3–5 in E-F).
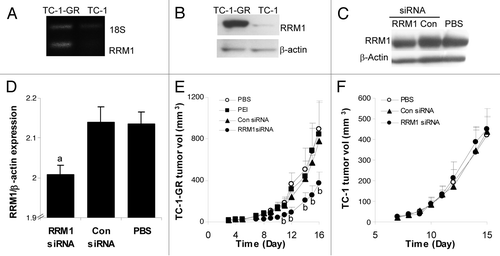
We then determined whether systemic administration of RRM1-specific siRNA in the PEI-siRNA complexes can successfully downregulate RRM1 expression in TC-1-GR tumors pre-established in nude mice. TC-1-GR tumor-bearing nude mice were injected (i.p.) with either 10 µg RRM1-specific siRNA or control siRNA in PEI-siRNA complexes every other day starting 7 d after tumor cells injection. After 2 weeks, tumors were removed to evaluate RRM1 protein expression. As shown in , the RRM1 protein level in TC-1-GR tumors in mice that were injected with the PEI-control siRNA complexes was not different from that in mice that were injected with PBS. However, the RRM1 protein level in tumors in mice that were injected with the PEI-RRM1 siRNA complexes was significantly lower than in mice that were injected with PBS or the PEI-control siRNA complexes (), demonstrating that the PEI-RRM1 siRNA complexes downregulated the expression of the RRM1 protein in TC-1-GR tumor tissues in mice.
To test whether the RRM1-specific siRNA can inhibit TC-1-GR tumor growth in mice, nude mice with pre-established TC-1-GR tumors were injected (i.p.) with PEI-RRM1 siRNA complexes or PEI-control siRNA complexes. TC-1-GR tumors in mice that were injected with PBS grew aggressively (). Both PEI alone and PEI-control siRNA complexes failed to exhibit any significant antitumor activity (). Conversely, the PEI-RRM1 siRNA complexes significantly delayed TC-1-GR tumor growth (), clearly demonstrating that systemic administration of RRM1-specific siRNA inhibited the growth of RRM1-overexpressing TC-1-GR tumors in mice. Finally, in contrast to what was observed in TC-1-GR tumor-bearing mice, the PEI-RRM1 siRNA complexes did not show any significant antitumor activity against the parent TC-1 tumors that do not overexpress RRM1 (), indicating that the RRM1-specific siRNA was only effective against tumor cells that overexpress RRM1.
RRM1-specific siRNA enhances the antitumor activity of gemcitabine in TC-1-GR tumor-bearing mice
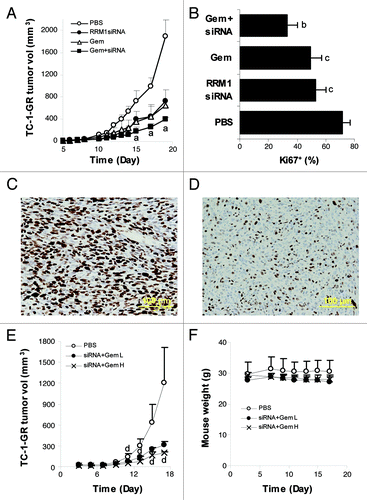
To evaluate whether the RRM1-specific siRNA can increase the antitumor activity of gemcitabine against tumors that overexpress RRM1 in vivo, TC-1-GR tumor-bearing mice were treated with PEI-RRM1 siRNA complexes in combination with gemcitabine. As shown in , PEI-RRM1 siRNA complexes alone or gemcitabine alone significantly delayed TC-1-GR tumor growth. However, combination treatment with both PEI-RRM1 siRNA complexes and gemcitabine was significantly more effective in delaying the tumor growth than either one of them alone (). In addition, a significantly less percent of Ki67-positive cells was detected in TC-1-GR tumors in mice that were treated with both PEI-RRM1 siRNA complexes and gemcitabine than with either of them alone (), indicating that the combination therapy was more anti-proliferative than monotherapy with either of them alone. Finally, data in showed that increasing the doses of siRNA and gemcitabine in the combination therapy significantly further enhanced the resultant antitumor activity. At the highest doses of RRM1 siRNA and gemcitabine tested, the mice had no significant loss in body weight and did not show any other signs of toxicity ().
Figure 3. RRM1-specific siRNA sensitized TC-1-GR tumors to gemcitabine in a mouse model. (A) The antitumor activities of RRM1 siRNA, gemcitabine (Gem), or RRM1 siRNA in combination with gemcitabine (siRNA+Gem) in mice with pre-established TC-1-GR tumors. TC-1-GR tumor cells were injected in nude mice on day 0. On days 5–8, 10–15, and 17, mice were injected with PEI-siRNA complexes (0.5 mg/kg siRNA per mouse per injection). On days 5, 8, 11, 14, and 16, mice were injected with gemcitabine HCl (150 mg/kg per mouse per injection). aThe values of the Gem+siRNA were significantly different from that of the Gem alone or siRNA alone (p < 0.05). (B) Proliferation indices of TC-1-GR tumor cells after different treatments. bThe value of Gem+siRNA was significantly different from that of the Gem alone or siRNA alone. c The values of the Gem alone and siRNA alone were significantly different from that of the PBS (p < 0.05). (C-D) Typical pictures (magnification: 20 x) of TC-1-GR tumor tissues stained against Ki67 cell proliferation marker after mice were treated with PBS (C) or RRM1 siRNAs in combination with gemcitabine (D) (bar = 100 μm). (E) The effect of the dose of gemcitabine on the antitumor activity of RRM1-specific siRNA and gemcitabine combination therapy against TC-1-GR tumors in mice. TC-1-GR tumor cells were injected in nude mice on day 0. On days 3–8 and 10–16, mice were injected with PEI-siRNA complexes. On days 3, 6, 9, 12, and 16, mice were injected with gemcitabine HCl. The dose of siRNA and gemcitabine HCl were 0.5 mg/kg and 150 mg/kg per mouse per injection, respectively, for the siRNA+Gem L group, 1 mg/kg and 300 mg/kg per mouse per injection, respectively, for the siRNA+Gem H group. d The values of siRNA+Gem H and siRNA+Gem L were significantly different (p < 0.05). Data are presented as a mean ± SEM (n = 4–5) in A, B, and E. (F) The body weights of mice treated in (E).
Discussion
RRM1 has been identified as a key molecule in determining the sensitivity of tumor cells to gemcitabine. The involvement of RRM1 overexpression in gemcitabine resistance had been documented in various cancers, including non-small cell lung cancer, pancreatic cancer, breast cancer, and biliary tract cancer.Citation9,Citation12-Citation18 Thus, it was proposed that knocking down of RRM1 expression can potentiate the chemosensitivity of gemcitabine resistant cancer cells, and previous in vitro data, including ours, confirmed that the downregulation of the expression of RRM1 could restore the sensitivity of gemcitabine resistant cancer cells to gemcitabine.Citation10,Citation11,Citation13,Citation14,Citation17,Citation21 However, to our best knowledge, the feasibility of enhancing the chemosensitivity of cancer cells to gemcitabine using RRM1-specific siRNA has not yet been tested in an animal model.
In the present study, we showed that knocking down of RRM1 expression in tumor cells that overexpress RRM1 using RRM1-specific siRNA significantly delayed the growth of the tumors (), indicating that RRM1 may be a potential target for cancer therapy. Data from previous studies also demonstrated the benefits of target RRM1 overexpression. For example, Reid et al. (2009) reported that the inhibition of RRM1 using siRNA decreased cell proliferation.Citation21 In addition, GTI-2501, an antisense DNA oligo targeting RRM1, was also able to inhibit tumor growth in vitro and in vivo.Citation27 It however should be noted that RRM1-specific siRNA is only effective in tumors that overexpress RRM1 (), but not in gemcitabine-sensitive parent TC-1 tumors that do not overexpress RRM1 (). The RRM1-specific siRNA was not effective against B16-F10 tumors that do not overexpress RRM1 as well (data not show). More experiments will have to be completed to understand why the RRM1-specific siRNA was active only to tumors that overexpress RRM1, but not effective against gemcitabine-sensitive tumor cells that do not overexpress RRM1. Nonetheless, the specific antitumor activity of RRM1-specific siRNA only against tumors that overexpress RRM1 may actually be desired because it would not likely be cytotoxic to normal non-tumor cells that do not overexpress RRM1. Moreover, Fan et al. (1997) reported that RRM1 is a tumor suppressor, and the loss of RRM1 could potentially initiate tumor development.Citation28
In addition to its own antitumor activity against RRM1-expressing tumors, RRM1-specific siRNA also sensitized the RRM1-overexpressing tumor cells to gemcitabine in vitro and in vivo (Fig. One and 3). As shown in , frequent high doses of RRM1-specific siRNA alone were able to significantly inhibit the growth of the RRM1-overexpressing TC-1-GR tumors. The TC-1-GR tumors are resistant to gemcitabine,Citation11 but frequent high doses of gemcitabine HCl alone (150 mg/kg) were also able to significantly inhibit its growth (). However, combination treatment with both RRM1-specific siRNA and gemcitabine HCl was more effective than any of them alone, likely because the downregulation of the RRM1 expression by the siRNA made the otherwise resistant TC-1-GR tumor cells less resistant to gemcitabine. In fact, tranilast, an antiallergic drug, that can decrease RRM1 protein expression was recently found to strongly sensitize pancreatic cancer cells to gemcitabine as well.Citation29 As mentioned previously, RR catalyzes the production of dNTPs, which are required for DNA synthesis. It has been suggested that RRM1 overexpression promotes gemcitabine resistance primarily through increasing the dNTP pools.Citation30 Because dNTPs directly compete with dFdCTP for incorporation into DNA, an increase in the cellular concentration of dNTPs can decrease the incorporation of dFdCTP into DNA.Citation5,Citation31-Citation33 In addition, dNTPs can also inhibit the activity deoxycytidine kinase (dCK),Citation34 a key enzyme in the phosphorylation of gemcitabine.Citation32 In other words, an increase in the dNTP pool decreases the phosphorylation of gemcitabine. Furthermore, dNTPs are also required for the activity of deoxycytidine deaminase (dCDA),Citation34 the rate limiting enzyme for the deamination (i.e., deactivation) of gemcitabine.Citation32 Thereby, an increase in the cellular level of dNTPs could enhance the rate of gemcitabine deamination. Taken together, it is likely that the downregulation of RRM1 expression using siRNA decreased the activity of RR and thus reduced the dNTP level in tumor cells, which in turn may have increased the incorporation of dFdCTP into DNA and increased the cellular concentration of gemcitabine metabolites by enhancing gemcitabine activation and inhibiting gemcitabine deactivation. Additional studies to identify the effect of siRNA-mediated RRM1 silencing on the level of dNTPs are planned.
Besides gemcitabine, it was shown that the level of RRM1 also significantly affect the efficacies of other cytotoxic agents including 5-fluorouracil, methotrexate, pemetrexed, and platinum in several types of cancer.Citation9,Citation35 Therefore, it is possible that the attenuation of RRM1 expression using siRNA technology may also be beneficial to overcoming chemoresistance to those anticancer drugs as well.
The RRM1-specific siRNA was less efficient in downregulating the RRM1 expression in TC-1-GR tumor cells in vivo than in vitro ( vs. −D), likely because the PEI/siRNA complexes were not as effective in delivering the siRNA into tumor cells in vivo as in vitro. We plan to further optimize the siRNA delivery system to improve its ability to more effectively deliver the siRNA into tumors pre-established in mice.
In conclusion, data in the present study clearly demonstrated that RRM1 overexpression in gemcitabine resistant cancer cells can be effectively downregulated using RRM1-specific siRNA both in vitro and in vivo, and simply downregulating RRM1 expression in tumor cells that overexpress RRM1 using the RRM1-specific siRNA significantly inhibited the tumor growth and sensitized the tumor cells to gemcitabine as well. RRM1-specific siRNA-mediated downregulation of RRM1 may represent an effective strategy to overcome RRM1 overexpression-related tumor cell resistance to gemcitabine and to other cytotoxic antitumor agents whose efficacy is also significantly affected by the level of RRM1 expression.
Materials and Methods
Materials and cell lines
Bovine serum albumin (BSA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), ethidium bromide, glycine, PEI (branched, MW 25 kDa), sodium dodecyl sulfate (SDS), sodium hydrochloride, and tris(hydroxymethyl)aminomethane hydrochloride were from Sigma-Aldrich (St. Louis, MO). Agarose I was from Amresco. Gemcitabine hydrochloride (gemcitabine HCl) was from US. Pharmacopia. Nitrocellulose membranes, western blotting filter papers and CL-XPosureTM film were from Thermo Scientific. Blotting-grade blocker, laemmli sample buffer, precision plus protein standards were from Bio-Rad. The duplex small interfering RNA (siRNA) oligonucleotides specific to RRM1 (UUAAUAACUGGGCUUCUGGGCUCUC and GAGAGCCCAGAAGCCCAGUUAUUAA) and the universal negative control siRNA were from Invitrogen. Mouse lung cancer cell line (TC-1, ATCC #CRL-2785) were cultured in complete RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin and 100 µg/ml of streptomycin, all from Invitrogen. The previously established TC-1-GR cells were cultured in similar RPMI 1640 medium further supplemented with 1 µM gemcitabine HCl.
Preparation of PEI-siRNA complexes
PEI25K/siRNA complexes were prepared as described previously with slight modification.Citation36 Briefly, 10 µg of siRNAs was dissolved in 150 µl of 150 mM NaCl and incubated for 10 min. Thirty-three microliters of PEI25K (5 mg/ml) in 150 mM NaCl was added to the siRNA solution, resulting in a nitrogen-to-phosphorus (N/P) ratio of 33.Citation23 After vortexing, the mixture was incubated at room temperature for 30 min prior to further use. The particle size and zeta potential of the complexes were measured in triplicates with at least 12 runs for each measurement at 25°C using a Malvern Zetasizer Nano ZS. The complexes were diluted 200-fold with purified water before applying to the Zetasizer.
In vitro downregulation of RRM1 expression and sensitization of TC-1-GR cells to gemcitabine
TC-1-GR (2 × 104) cells were plated in 96-well plates in RPMI 1640 medium supplemented with 10% FBS. When reached 80% confluent, cells were transfected with 60 pmol PEI-RRM1 siRNA complexes or PEI-control siRNA complexes. Forty-eight hours after the transfection, the cell culture medium was refreshed. The cells were then treated with 50 µM gemcitabine HCl for another 48 h before the cytotoxicity was evaluated using the MTT assay. Cells were also harvested 48 h after the transfection to prepare total proteins for western blotting assay.
Animal studies
All animal procedures were performed in accordance with the guideline established by the National Institutes of Health for humane treatment of animals. Animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas at Austin. Female C57BL/6 and nu/nu mice (18–20 g) were from Charles River. TC-1 or TC-1-GR tumors were established in the right flank of mice by subcutaneous (s.c.) injection of 5 × 105 cells.
To find out whether the TC-1-GR tumor cells continue to overexpress RRM1 when injected in mice, TC-1-GR or TC-1 tumors were injected into nude mice (n = 3) on day 0. Tumor tissues were harvested 21 d later to extract total proteins and total RNA for western blotting and RT-PCR analyses.
To understand whether systemic administration of RRM1 siRNA can downregulate RRM1 protein expression, TC-1-GR tumors were injected into nude mice on day 0. Starting on day 7 after tumor cell implantation (tumors reached 4–5 mm), mice were randomized and injected intraperitoneally (i.p.) with PEI-RRM1 siRNA or PEI-control siRNA complexes (0.5 mg/kg siRNA per mouse per injection) every two days for two consecutive weeks. As a control, mice were injected with PBS. The PEI-siRNA complexes were i.p. injected because data from a previous study showed that i.p. injection of similar PEI-siRNA complexes successfully downregulated the expression of the target protein.Citation25 Tumor tissues were harvested 21 d later to extract total proteins for western blotting analysis
To evaluate the in vivo antitumor activity of the PEI-RRM1 siRNA complexes, tumor cells were injected (s.c.) in mice on day 0. When tumors became visible (2–4 mm), TC-1-GR tumor-bearing nude mice (n = 5) were injected (i.p.) with PEI-RRM1 siRNA complexes or PEI-control siRNA complexes on days 3–5, 7–12, and 14–16. TC-1 tumor-bearing C57BL/6 mice (n = 5) were treated from days 7–11 once daily; the treatment was stopped after day 11 due to lack of apparent antitumor activity. The dose of the siRNA was 0.5 mg/kg per mouse per injection (in 150 μl of sterile PBS). As controls, mice were injected with PBS or PEI alone. Tumor volume was measured three times a week with a caliper and calculated based on the following equation: tumor volume (mm3) = 1/2 [length × (width)Citation2].
To evaluate the effect of treatment with RRM1-specific siRNA on the antitumor activity of gemcitabine, TC-1-GR tumors were established in the right flank of nude mice (n = 4–5) on day 0. Tumors reached 3–5 mm in diameter on day 5. On days 5–8, 10–15 and 17, mice were injected (i.p.) with PEI-RRM1 siRNA complexes (0.5 mg/kg siRNA per mouse per injection, in 150 μl of PBS). On days 5, 8, 11, 14 and 16, mice were injected (i.p.) with gemcitabine HCl (150 mg/kg per mouse per injection, in 150 μl of PBS). Mice in the siRNA and gemcitabine combination treatment group were injected with both siRNA and gemcitabine HCl, according to the above dosing schedule. Tumor size was monitored as mentioned above. To evaluate the effect of the doses of the RRM1 siRNA and gemcitabine in the combination on the resultant antitumor activity, TC-1-GR tumor cells were injected in female nude mice (n = 3–4) on day 0. Tumors reached 2–4 mm on day 3. Mice were then injected daily with PEI-siRNA complexes on days 3–8 and 10–16 and with gemcitabine HCl on days 3, 6, 9, 12 and 16. The dose of siRNA and gemcitabine HCl were 0.5 mg/kg and 150 mg/kg per mouse per injection, respectively, for the low dose group (siRNA+Gem L), 1 mg/kg and 300 mg/kg per mouse per injection, respectively, for the high dose group (siRNA+Gem H).
Western blot analysis
Cell or tumor tissue lysates were prepared by homogenizing cells or tumor tissues in Pierce® RIPA lysis buffer (Thermo Scientific) containing the HaltTM protease inhibitor cocktail (Thermo Scientific). Protein concentration of supernatants obtained after centrifugation at 14,000 g for 10 min was determined by a microplate assay with the Bio-Rad DC Protein assay reagents (Bio-Rad) and bovine serum albumin as a standard. Fifty micrograms of TC-1-GR protein lysates were separated on a 7.5% Mini-Protean® TGXTM precast gel (Bio-Rad). Immunoblotting for RRM1 protein was performed using a rabbit polyclonal antibody against RRM1 (Aviva System Biology) and a polyclonal anti-rabbit IgG horseradish peroxidase conjugated secondary antibody (Aviva). β-actin (mouse monoclonal antibody, Santa Cruz Biotechnology) was used as a control. Protein bands were detected using the enhanced chemiluminescence method (Pierce ECL Western Blotting Substrate, Thermo Scientific) and quantified using the G-Box system from Syngene using the Genetool quantitation software. The levels of the RRM1 protein was normalized to the levels of the corresponding β-actin protein.
RT-PCR
Total RNA was isolated from TC-1/TC-1-GR tumor tissues using RNeasy Lipid Tissue Mini kit (QIAGEN). Isolated RNA was reversed transcribed with random hexamers using the SuperScript first-strand synthesis system (Invitrogen). PCR was performed in an Eppendorf Mastercycler with the following cycling conditions: 95°C for 30 sec, 58°C for 1 min and 68°C for 1 min for a total of 21 cycles. Primer sequences for RRM1 were 5′-CCC AAT GAG TGT CCT GGT CT-3′ (forward) and 5′-TTC TGC TGG TTG CTC TTC C-3′ (reverse). QuantumRNATM 18S internal standards using Ambion’s competimer technology (Applied Biosystems/Ambion) were co-amplified in individual reaction tubes. Reaction products were visualized on a 1.5% agarose gel containing ethidium bromide, and the intensity of each band determined by densitometric analysis using the Genetool quantitation software (Syngene).
Histology
Tumor tissues were processed to generate 5 µm tissue slides. Sections were stained with antibodies against Ki67, a marker of cell proliferation, and examined under a light microscope. The average % of Ki67 positive cells (i.e., proliferation index) was determined from 20 microscopic pictures per treatment.
Statistical analysis
The statistical significance of differences among groups were evaluated by a one-way analysis of variance (ANOVA) followed by a Bonferroni/Dunn post-hoc comparison test. Differences were considered to be significant when p ≤ 0.05.
Disclosure of Potential Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
This work was supported in part by a National Cancer Institute grant (CA135274) to Z.C.
References
- Wong A, Soo RA, Yong WP, Innocenti F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev 2009; 41:77 - 88; http://dx.doi.org/10.1080/03602530902741828; PMID: 19514966
- Long J, Zhang Y, Yu X, Yang J, LeBrun DG, Chen C, et al. Overcoming drug resistance in pancreatic cancer. Expert Opin Ther Targets 2011; 15:817 - 28; http://dx.doi.org/10.1517/14728222.2011.566216; PMID: 21391891
- Thelander L, Reichard P. Reduction of ribonucleotides. Annu Rev Biochem 1979; 48:133 - 58; http://dx.doi.org/10.1146/annurev.bi.48.070179.001025; PMID: 382982
- Baker CH, Banzon J, Bollinger JM, Stubbe J, Samano V, Robins MJ, et al. 2′-Deoxy-2′-methylenecytidine and 2′-deoxy-2′,2′-difluorocytidine 5′-diphosphates: potent mechanism-based inhibitors of ribonucleotide reductase. J Med Chem 1991; 34:1879 - 84; http://dx.doi.org/10.1021/jm00110a019; PMID: 2061926
- Heinemann V, Xu YZ, Chubb S, Sen A, Hertel LW, Grindey GB, et al. Inhibition of ribonucleotide reduction in CCRF-CEM cells by 2′,2′-difluorodeoxycytidine. Mol Pharmacol 1990; 38:567 - 72; PMID: 2233693
- Jordheim LP, Guittet O, Lepoivre M, Galmarini CM, Dumontet C. Increased expression of the large subunit of ribonucleotide reductase is involved in resistance to gemcitabine in human mammary adenocarcinoma cells. Mol Cancer Ther 2005; 4:1268 - 76; http://dx.doi.org/10.1158/1535-7163.MCT-05-0121; PMID: 16093443
- Davidson JD, Ma L, Flagella M, Geeganage S, Gelbert LM, Slapak CA. An increase in the expression of ribonucleotide reductase large subunit 1 is associated with gemcitabine resistance in non-small cell lung cancer cell lines. Cancer Res 2004; 64:3761 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-03-3363; PMID: 15172981
- Bergman AM, Eijk PP, Ruiz van Haperen VW, Smid K, Veerman G, Hubeek I, et al. In vivo induction of resistance to gemcitabine results in increased expression of ribonucleotide reductase subunit M1 as the major determinant. Cancer Res 2005; 65:9510 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-05-0989; PMID: 16230416
- Bepler G, Kusmartseva I, Sharma S, Gautam A, Cantor A, Sharma A, et al. RRM1 modulated in vitro and in vivo efficacy of gemcitabine and platinum in non-small-cell lung cancer. J Clin Oncol 2006; 24:4731 - 7; http://dx.doi.org/10.1200/JCO.2006.06.1101; PMID: 16966686
- Oguri T, Achiwa H, Sato S, Bessho Y, Takano Y, Miyazaki M, et al. The determinants of sensitivity and acquired resistance to gemcitabine differ in non-small cell lung cancer: a role of ABCC5 in gemcitabine sensitivity. Mol Cancer Ther 2006; 5:1800 - 6; http://dx.doi.org/10.1158/1535-7163.MCT-06-0025; PMID: 16891466
- Chung WG, Sandoval MA, Sloat BR, Lansakara-P DS, Cui Z. Stearoyl gemcitabine nanoparticles overcome resistance related to the over-expression of ribonucleotide reductase subunit M1. J Control Release 2012; 157:132 - 40; http://dx.doi.org/10.1016/j.jconrel.2011.08.004; PMID: 21851843
- Gong W, Zhang X, Wu J, Chen L, Li L, Sun J, et al. RRM1 expression and clinical outcome of gemcitabine-containing chemotherapy for advanced non-small-cell lung cancer: A meta-analysis. Lung Cancer 2011; PMID: 21889227
- Nakahira S, Nakamori S, Tsujie M, Takahashi Y, Okami J, Yoshioka S, et al. Involvement of ribonucleotide reductase M1 subunit overexpression in gemcitabine resistance of human pancreatic cancer. Int J Cancer 2007; 120:1355 - 63; http://dx.doi.org/10.1002/ijc.22390; PMID: 17131328
- Ohtaka K, Kohya N, Sato K, Kitajima Y, Ide T, Mitsuno M, et al. Ribonucleotide reductase subunit M1 is a possible chemoresistance marker to gemcitabine in biliary tract carcinoma. Oncol Rep 2008; 20:279 - 86; PMID: 18636187
- Akita H, Zheng Z, Takeda Y, Kim C, Kittaka N, Kobayashi S, et al. Significance of RRM1 and ERCC1 expression in resectable pancreatic adenocarcinoma. Oncogene 2009; 28:2903 - 9; http://dx.doi.org/10.1038/onc.2009.158; PMID: 19543324
- Reynolds C, Obasaju C, Schell MJ, Li X, Zheng Z, Boulware D, et al. Randomized phase III trial of gemcitabine-based chemotherapy with in situ RRM1 and ERCC1 protein levels for response prediction in non-small-cell lung cancer. J Clin Oncol 2009; 27:5808 - 15; http://dx.doi.org/10.1200/JCO.2009.21.9766; PMID: 19884554
- Rodriguez J, Boni V, Hernández A, Bitarte N, Zarate R, Ponz-Sarvisé M, et al. Association of RRM1 -37A>C polymorphism with clinical outcome in colorectal cancer patients treated with gemcitabine-based chemotherapy. Eur J Cancer 2011; 47:839 - 47; http://dx.doi.org/10.1016/j.ejca.2010.11.032; PMID: 21220199
- Jordheim LP, Sève P, Trédan O, Dumontet C. The ribonucleotide reductase large subunit (RRM1) as a predictive factor in patients with cancer. Lancet Oncol 2011; 12:693 - 702; http://dx.doi.org/10.1016/S1470-2045(10)70244-8; PMID: 21163702
- Burnett JC, Rossi JJ, Tiemann K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol J 2011; 6:1130 - 46; http://dx.doi.org/10.1002/biot.201100054; PMID: 21744502
- de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther 2008; 19:125 - 32; http://dx.doi.org/10.1089/hum.2008.928; PMID: 18257677
- Reid G, Wallant NC, Patel R, Antonic A, Saxon-Aliifaalogo F, Cao H, et al. Potent subunit-specific effects on cell growth and drug sensitivity from optimised siRNA-mediated silencing of ribonucleotide reductase. J RNAi Gene Silencing 2009; 5:321 - 30; PMID: 19771229
- Xie FY, Woodle MC, Lu PY. Harnessing in vivo siRNA delivery for drug discovery and therapeutic development. Drug Discov Today 2006; 11:67 - 73; http://dx.doi.org/10.1016/S1359-6446(05)03668-8; PMID: 16478693
- Werth S, Urban-Klein B, Dai L, Höbel S, Grzelinski M, Bakowsky U, et al. A low molecular weight fraction of polyethylenimine (PEI) displays increased transfection efficiency of DNA and siRNA in fresh or lyophilized complexes. J Control Release 2006; 112:257 - 70; http://dx.doi.org/10.1016/j.jconrel.2006.02.009; PMID: 16574264
- Aigner A. Gene silencing through RNA interference (RNAi) in vivo: strategies based on the direct application of siRNAs. J Biotechnol 2006; 124:12 - 25; http://dx.doi.org/10.1016/j.jbiotec.2005.12.003; PMID: 16413079
- Urban-Klein B, Werth S, Abuharbeid S, Czubayko F, Aigner A. RNAi-mediated gene-targeting through systemic application of polyethylenimine (PEI)-complexed siRNA in vivo. Gene Ther 2005; 12:461 - 6; http://dx.doi.org/10.1038/sj.gt.3302425; PMID: 15616603
- Ibrahim AF, Weirauch U, Thomas M, Grünweller A, Hartmann RK, Aigner A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res 2011; 71:5214 - 24; http://dx.doi.org/10.1158/0008-5472.CAN-10-4645; PMID: 21690566
- Lee Y, Vassilakos A, Feng N, Jin H, Wang M, Xiong K, et al. GTI-2501, an antisense agent targeting R1, the large subunit of human ribonucleotide reductase, shows potent anti-tumor activity against a variety of tumors. Int J Oncol 2006; 28:469 - 78; PMID: 16391803
- Fan H, Huang A, Villegas C, Wright JA. The R1 component of mammalian ribonucleotide reductase has malignancy-suppressing activity as demonstrated by gene transfer experiments. Proc Natl Acad Sci U S A 1997; 94:13181 - 6; http://dx.doi.org/10.1073/pnas.94.24.13181; PMID: 9371820
- Mitsuno M, Kitajima Y, Ohtaka K, Kai K, Hashiguchi K, Nakamura J, et al. Tranilast strongly sensitizes pancreatic cancer cells to gemcitabine via decreasing protein expression of ribonucleotide reductase 1. Int J Oncol 2010; 36:341 - 9; PMID: 20043067
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. RNA interference targeting the M2 subunit of ribonucleotide reductase enhances pancreatic adenocarcinoma chemosensitivity to gemcitabine. Oncogene 2004; 23:1539 - 48; http://dx.doi.org/10.1038/sj.onc.1207272; PMID: 14661056
- Gandhi V, Plunkett W. Modulatory activity of 2′,2′-difluorodeoxycytidine on the phosphorylation and cytotoxicity of arabinosyl nucleosides. Cancer Res 1990; 50:3675 - 80; PMID: 2340517
- Heinemann V, Hertel LW, Grindey GB, Plunkett W. Comparison of the cellular pharmacokinetics and toxicity of 2′,2′-difluorodeoxycytidine and 1-beta-D-arabinofuranosylcytosine. Cancer Res 1988; 48:4024 - 31; PMID: 3383195
- Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2′,2′-difluorodeoxycytidine on DNA synthesis. Cancer Res 1991; 51:6110 - 7; PMID: 1718594
- Bouffard DY, Laliberté J, Momparler RL. Kinetic studies on 2′,2′-difluorodeoxycytidine (Gemcitabine) with purified human deoxycytidine kinase and cytidine deaminase. Biochem Pharmacol 1993; 45:1857 - 61; http://dx.doi.org/10.1016/0006-2952(93)90444-2; PMID: 8494545
- Zhou J, Oliveira P, Li X, Chen Z, Bepler G. Modulation of the ribonucleotide reductase-antimetabolite drug interaction in cancer cell lines. J Nucleic Acids 2010; 2010:597098; http://dx.doi.org/10.4061/2010/597098; PMID: 20976259
- Dimitrova M, Affolter C, Meyer F, Nguyen I, Richard DG, Schuster C, et al. Sustained delivery of siRNAs targeting viral infection by cell-degradable multilayered polyelectrolyte films. Proc Natl Acad Sci U S A 2008; 105:16320 - 5; http://dx.doi.org/10.1073/pnas.0800156105; PMID: 18922784
