Abstract
Growing evidence suggests that the cancer stem cell phenotype in melanoma is dynamically regulated. Therefore, effective therapies have to target simultaneously bulk tumor cells and melanoma stem-like cells. The aim of the present study was to investigate the effects of parthenolide on heterogeneous cancer cell populations from anchorage-independent melanospheres. Cells derived from nodular melanoma specimens were grown under serum-free sphere-forming conditions. The effects of parthenolide on cellular viability, immunophenotype and self-renewing capacity were assessed with cells from dissociated melanospheres. Its penetration capacity was evaluated with intact melanospheres. In melanoma cells that survived treatment with parthenolide, a different immunophenotype than that in untreated control was found. The frequency of cells expressing the ABCB5 transporter was markedly reduced. Most importantly, melanoma cells that survived parthenolide treatment lost their self-renewing capacity. Significantly lower influence of drug on cellular viability and frequency of ABCB5-positive cells was observed in intact melanospheres. The potential clinical significance of our findings is based on the ability of parthenolide to affect both bulk and melanoma stem-like cells with clonogenic capacity and high expression of the ABCB5 transporter. Its low penetration capacity, however, may limit its action to easily accessible melanoma cells, either circulating in the blood or those in the vicinity to blood vessels within the tumor. Because of limited penetration capacity of parthenolide, this drug should be further explored as a part of multimodal therapies rather than as a stand-alone therapeutic agent.
Introduction
Metastatic melanoma is highly resistant to conventional therapeutic regimens, including chemotherapy, radiation and immunotherapy, and prognosis for patients remains poor despite advances in the field. Numerous in vitro and in vivo studies of solid tumors suggest that cancer stem cells (CSCs), including those of melanoma (MSCs), are responsible for tumor resistance and give rise to tumor cells after years of dormancy.Citation1-Citation3 Therefore, it is difficult to completely eradicate a tumor, and its recurrence is an ever-present threat.
As CSCs are more resistant to conventional therapy compared with more differentiated cancer cells, special approaches have to be developed for their elimination, such as targeting molecular markers of CSCs, inhibiting self-renewing capacity or stimulating differentiation.Citation3,Citation4 Recently, it has been shown that acetaminophen-induced differentiation efficiently eradicates breast cancer stem cells.Citation5 Marker-based immunotherapy would require identification of antigens whose expression is restricted to CSCs. Employing genetically engineered cytotoxic T cells, redirected in an antigen-restricted manner by a chimeric receptor, to eliminate CD20-positive melanoma cells is an example of targeting a defined melanoma subpopulation.Citation6 Some studies in melanoma have shown, however, a high level of immunophenotypic variability of cells among melanoma samples.Citation7 In addition, growing evidence indicates that properties of stem cells can be acquired transiently in response to the microenvironment even by highly proliferating, more differentiated cancer cells.Citation3,Citation8-Citation13
We were interested whether parthenolide (PN), a sesquiterpene lactone derived from leaves of the medicinal plant feverfew (Tanacetum parthenium), might target melanoma stem-like cells. PN has demonstrated anti-cancer activity in many preclinical in vitro and in vivo studies of cells from leukemia and from solid tumors,Citation14-Citation18 including melanoma.Citation19,Citation20 A unique feature of PN is its ability to induce cell death in cancer cells while sparing normal ones.Citation21 We have demonstrated that PN was capable of killing melanoma cells without affecting normal melanocytes.Citation20 More interestingly, PN seems to affect CSCs. It selectively reduced the viability of CSCs in acute myelogenous leukemia (AML),Citation21 multiple myeloma (MM),Citation22 and breast tumors.Citation23,Citation24 PN decreased the viability of prostate tumor-initiating cells isolated from cell lines and from patients, and inhibited prostate cancer stem-cell-mediated tumor initiation and progression in mouse xenografts.Citation25 Recently, it has been shown that the combination of PN and inhibitors of the PI3K/mTOR pathway synergized to eradicate both bulk and stem cell populations of AML,Citation26 and the combination of PN with ionizing radiation significantly reduced the viability of both the overall population of osteosarcoma cells and the cancer stem cell subpopulation.Citation27 On the basis of all these findings, it was tempting to speculate that PN would be able to affect melanoma stem-like cells.
In the current study, we investigated the effects of PN on melanoma cells derived from nodular melanoma specimens and grown under conditions forming anchorage-independent melanospheres. Those multicellular structures are considered to portray the original tumor more accurately than monolayer melanoma cell cultures.Citation28
Results
Formation of anchorage-independent melanospheres from nodular melanoma specimens
Only cells from nodular melanomas, the most aggressive type of melanoma, were included in the current study. Five surgical specimens were propagated in serum-free stem cell medium (SCM) supplemented with EGF/bFGF. Melanospheres were formed after 8–10 weeks in four out of five melanoma specimens (DMBC2, DMBC8, DMBC10 and DMBC12). They grew very slowly but continued growth for more than 16 mo when dissociated regularly every few weeks. The fifth cell population (DMBC9) grew in an anchorage-independent manner, but as a single-cell culture. Culturing melanoma cells in SCM on low-adherent plastic generated large melanospheres, some with more than 500 μm in diameter. Small percentage of cells in DMBC2, DMBC8 and DMBC10 populations adhered and grew in monolayer in these experimental settings. To measure the self-renewing potential, one of the hallmarks of CSCs, a clonogenic assay recording sphere generation from single cells in soft agar was used. This in vitro assessment of the self-renewing capacity revealed that up to 95 cells out of 1000 cells in melanospheres retained CSC characteristics.Citation13
Parthenolide (PN) reduced the number of viable cells
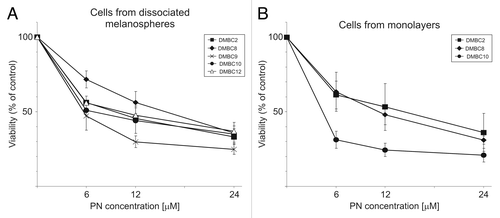
Cells from dissociated melanospheres were treated with various concentrations of PN in 96-well plates for 2 d. Changes in the percentages of viable cells were determined based on the quantification of cytosolic acid phosphatase activity (APA assay). Dose-response curves are shown in . PN at a concentration of 24 μM reduced cell viability to 25–40% of control. When the influence of PN on adherent melanoma cells generated in DMBC2, DMBC8 and DMBC10 populations was investigated, the same concentrations led to a similar reduction in acid phosphatase activity as observed in cells from dissociated melanospheres ().
Figure 1. Dose-response curves of melanoma cells treated with parthenolide (PN). Cells from disaggregated melanospheres (A), or their adherent counterparts (B), both growing in SCM, were exposed to PN for 2 d. The number of viable cells was assessed with an acid phosphatase activity (APA) assay. All measurements were normalized to cell viability of DMSO-treated controls. (p < 0.05 vs. DMSO-treated control; n = 3).
Parthenolide (PN) diminished the proliferative potential of melanoma cells
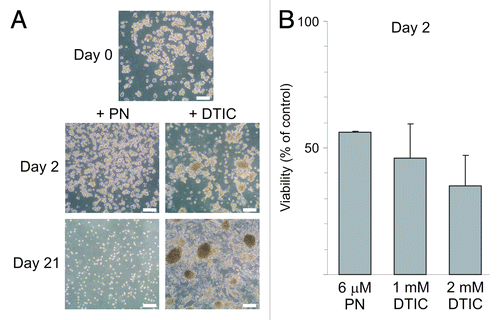
Next, we asked the question whether PN-resistant cells could be selected from the population of PN-treated melanoma cells. In comparison, dacarbazine (DTIC), the first-line treatment agent for advanced melanoma, was used. A fraction of 30% to 40% of melanoma cells treated with DTIC, at 0.5 mM, 1 mM and 2 mM in three consecutive treatments during three weeks, remained viable and was still capable of forming melanospheres during three weeks of selection as described in Materials and Methods, although the proportion of adherent cells was also increased. Data for DMBC2 are shown in . Melanoma cells did not survive two consecutive treatments with 6 μM PN applied within 3 weeks (). Interestingly, when cells were exposed either to 6 μM PN or to 1 or 2 mM DTIC for only two days and the viability was measured immediately after termination of drug exposure, the relative number of viable cells was similar in PN- and DTIC-treated populations (). Moreover, DTIC-treated cells formed melanospheres after three weeks even when DTIC concentration was raised to 4 mM (not shown). These results suggest that PN at 6 μM used repeatedly led to the extinction of the melanoma cell population within three weeks.
Figure 2. Distinct effects of parthenolide (PN) and dacarbazine (DTIC) on melanoma cell survival after long and short treatment. (A) Microphotographs of DMBC2 populations treated with either 6 μM PN or increasing concentration of dacarbazine (DTIC) for three days followed by few day drug-free intervals were taken after 3 weeks. Scale bars represent 100 μm. (B) Relative number of viable cells was assessed after two day exposure to PN or DTIC at indicated concentrations. The results are the mean of three independent experiments ± SD.
Parthenolide (PN) specifically affected the frequency of ABCB5-positive melanoma cells
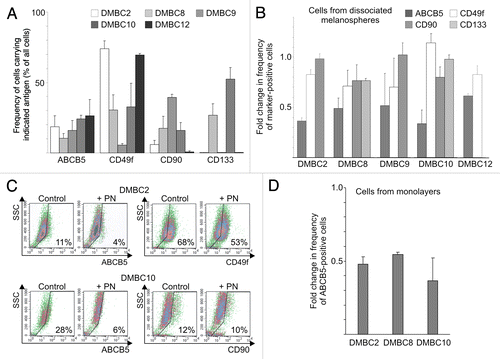
A drug-resistant phenotype of melanoma cells is partially mediated by increased capacity of detoxification.Citation29 ABCB5 transporter, one of the recognized mediators of chemoresistance in melanoma, has been also considered as the marker identifying melanoma stem-like cells.Citation30 Therefore, we evaluated the influence of PN on the frequency of ABCB5-positive cells. Variable frequencies of cells carrying that marker were observed among viable, 7-AAD-negative, melanoma cells from dissociated spheres with the highest frequency observed in DMBC12 (26%) and the lowest (10%) in the DMBC8 cell population (). A few other antigens were also expressed at variable frequencies in different populations as exemplified by CD133 (Prominin-1) expressed only in DMBC8 and DMBC10, CD90 (Thy-1) and CD49f (integrin α6) (). This high immunophenotypic heterogeneity within melanospheres was observed during several months. Treatment with PN at 12 μM for 22 h, followed by a two-day recovery period in SCM without drug, resulted in a significant (p < 0.05) decrease in the frequencies of cells expressing ABCB5 transporter in all treated populations (), with the largest effect found in DMBC2 and DMBC10 populations (). Such a substantial reduction was not observed for the other cell surface markers (). This might indicate that PN affected ABCB5-positive melanosphere-derived cells more efficiently than cells not carrying that marker. Similar results were obtained when changes in the frequency of ABCB5-positive cells were assessed in the monolayers ().
Figure 3. Parthenolide (PN) more efficiently reduces the frequency of cells with a high level of ABCB5 transporter than the frequencies of other subpopulations. (A) The frequency of cells carrying the indicated antigen assessed by flow cytometry showed the high heterogeneity within melanospheres and the variability among populations derived from different nodular melanoma specimens. (B) Treatment with PN decreased the frequency of cells with a high ABCB5 level more efficiently than other subpopulations. (C) Examples of dot plots showing the influence of PN on the frequency of ABCB5-positive cells in DMBC2 and DMBC10 populations. In comparison, changes in the percentage of cells expressing CD49f (DMBC2) and CD90 (DMBC10) were included. (D) Treatment with PN decreased the frequency of ABCB5-positive cells also among adherent counterparts grown in SCM as monolayer culture.
As the ratio of marker-positive to negative melanoma cells was a complex function of the selective killing but also of the proliferation kinetics of variable subpopulations in the recovery period after PN was removed, changes in the frequency of cells carrying a particular marker were related to changes in the number of viable cells. Those relative changes in frequencies (RCFs) were calculated for each experiment, as described in Materials and Methods, and average values for a particular marker in each population and for all tested populations together, are shown in . Although not identical for each population, RCFs for ABCB5 transporter were very close to 0.5, whereas RCFs for other markers (CD133, CD49f, CD90) were either markedly higher or close to 1. We concluded that melanoma cells with a high level of ABCB5 transporter were underrepresented in PN-treated populations as a result of both, the selective killing of ABCB5-positive cells, and overgrowth by ABCB5-negative cells in the period without PN.
Table 1. Relative change in the frequency (RCF) of marker expressing cells in response to parthenolide (PN) treatment
Parthenolide (PN) reduced viability of sorted ABCB5+ melanoma cells
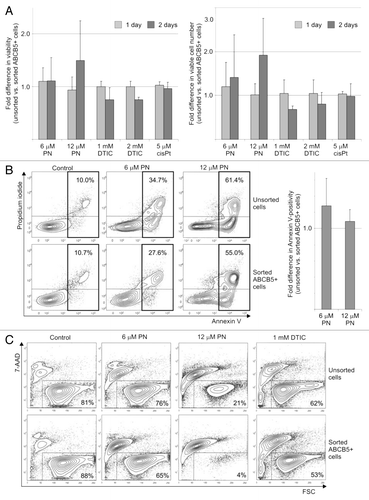
PN reduced viability in all tested populations to a similar level (). ABCB5-positive cells obtained from DMBC12 population by flow-cytometry-based cell-sorting were used to investigate changes in viability after drug treatment. Viable cells were numbered using an automated cell viability analyzer or viability was measured after PI staining. The results from both assays showing the difference in the reduction of viability between sorted and unsorted ABCB5+ populations are presented in . Sorted ABCB5+ cells seemed to be more sensitive to PN than unsorted cells (), however, the difference in the drug-induced reduction of viability did not reach statistical significance. An opposite tendency was observed for DTIC-treated cells, while no difference was evident when cisplatin was used (). One day incubation with PN induced apoptosis in unsorted and sorted ABCB5+ melanoma cell populations to a similar extent (), however, more cells in the late apoptotic phase (Annexin V/PI double positive) were observed in the ABCB5+ subset, especially when PN at 12 μM was used (27.1% and 39.5% in unsorted and sorted populations, respectively). Next, the long-term effect on sorted and unsorted melanoma cells after a very short four-hour incubation with PN was investigated. Viability measured four-days after the drug was removed from the culture was lower in the ABCB5+ subset than in the unsorted population. PN at 12 μM reduced the percentage of viable cells to 21% in the unsorted population whereas in the ABCB5+ subset only 4% cells remained viable (). That 5-fold difference was also observed after additional three days without PN (not shown). On the contrary, a small difference in viability observed on the 4th day after DTIC removal (62% vs. 53%, ) disappeared after three additional days without the drug (44% vs. 41%, not shown).
Figure 4. Parthenolide (PN) reduced viability of sorted ABCB5+ melanoma cells. (A) Fold differences in the drug-reduced viability between unsorted and sorted ABCB5+ populations of DMBC12 were assessed either after PI staining (left panel) or were calculated after viable cells were numbered (right panel). Data are means ± SD of four independent experiments conducted in triplicates using the unsorted population of DMBC12 and the ABCB5-positive subsets obtained in two independent flow-cytometry-based cell-sorting. (p > 0.05). DTIC, dacarbazine; cisPt, cisplatin. (B) PN induced apoptosis in melanoma cells. Typical contour plots are included. Numbers in rectangles indicate the percentages of all Annexin V-positive cells, PI-negative (early apoptosis) and PI-positive (late apoptosis). After sorting of the ABCB5+ subset, no significant changes (p > 0.05) in Annexin V positivity were evident relative to the unsorted populations (right panel). (C) Viability measured four-days after the drug was removed indicate that the ABCB5+ subset was capable of recovering within four days after a short (4 h) treatment with PN less efficiently than the unsorted population. Numbers indicate the percentages of viable, 7-AAD-negative melanoma cells in one of two experiments.
Parthenolide (PN) reduced the self-renewing capacity of melanoma cells
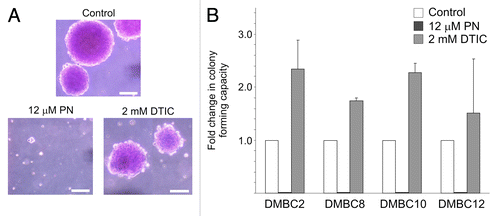
A clonogenic assay was used to determine whether the selective reduction of ABCB5-positive cell frequency was accompanied by changes in self-renewing capacity. Cells were incubated with either 12 μM PN or 2 mM DTIC. Interestingly, a very short-term incubation (4 h) of melanosphere-derived cells with PN completely abolished the ability of melanoma cells to form spheres within the following three weeks (). No sphere formation was evident even when 5 μM PN was used (not shown). These results are consistent with the inability of PN-treated melanoma cells to form a drug-resistant population (). Adherent melanoma cells pre-treated with PN were also not capable of generating colonies in soft agar (not shown). In DTIC-treated cultures, the colonies were similar in size as those in control culture, however, the relative number of cells forming colonies in soft agar was increased (). This might indicate that DTIC was affecting the viability of more differentiated and highly cycling cancer cells, while sparing those with stem cell characteristics, whereas PN efficiently eradicated both cycling melanoma cells and those with self-renewing capacity.
Figure 5. Parthenolide (PN) affects self-renewing capacity of melanoma populations.(A) Images of representative colonies formed by DMBC12 population in soft agar were captured after three weeks. (B) PN-treated melanoma populations lost colony-forming capacity in contrary to dacarbazine (DTIC)-treated populations showing about 2-fold relative increase in the number of colonies formed in soft agar. The results are mean of two independent experiments.
Parthenolide (PN) showed limited capacity to penetrate melanospheres
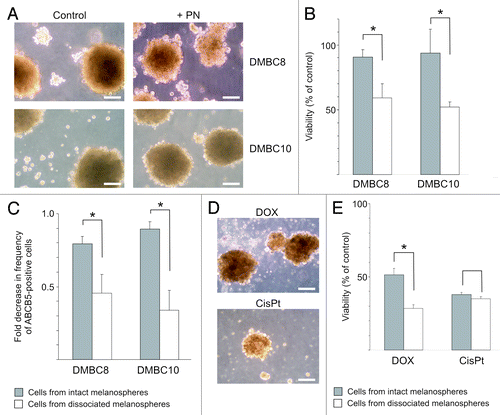
Next, we asked the question about the capacity of PN to penetrate the 3D structure of melanospheres. Two populations, DMBC8 and DMBC10 were chosen since they were forming floating multicellular melanospheres with at least 200 μm in diameter. Cells from dissociated melanospheres were used for comparison. One-day incubation with 12 μM PN only slightly affected melanosphere integrity, especially DMBC10 (). Significant differences in the PN-driven reduction in viability were observed between populations of intact melanospheres and single cells from dissociated melanospheres () suggesting that PN was acting only on the surface of melanospheres. Similarly, significantly lower changes in the frequencies of ABCB5-positive cells in intact spheres were found in comparison with cell populations from dissociated spheres (). If PN was not able to efficiently affect cells inside of spheres, these results might indicate that ABCB5-positive cells were enriched in the center of melanospheres. To further confirm the conclusion that PN was not capable of penetrating melanospheres, two other drugs, cisplatin and doxorubicin which is considered as a drug with low penetration capacity, were used for comparison in DMBC10 culture (). There was no significant difference between the influence of 5 μM cisplatin on intact melanospheres and cells from dissociated spheres, indicating that this drug has a high penetration capacity. As expected, doxorubicin at 1 μM had a higher impact on single cells from dissociated spheres than on intact melanospheres, but still it reduced the viable cell number in melanospheres to about 50% of that in control. Compared with other drugs, PN had the lowest influence on cell viability in intact melanospheres ( vs. E). Both, a viability test and changes in the frequency of ABCB5-positive cells suggested that PN was not capable of penetrating melanospheres.
Figure 6. Parthenolide (PN) has a limited capacity to affect cells inside of melanospheres. (A) Microphotographs showing melanosphere integrity following treatment with 12 μM PN. Scale bars represent 100 μm. (B). Comparison of PN-induced changes in viability between populations of cells treated as intact melanospheres and as single melanoma cells from dissociated spheres. Melanoma cells were exposed to drug for one day, and after additional two days in drug-free medium, viability was measured by flow cytometry as percentage of 7-AAD-negative cells in PN- vs. DMSO-treated cells (control) (* p < 0.05; n = 3). (C). Comparison of PN-induced decrease in the ABCB5-positive cell frequency between DMBC10 populations treated as intact melanospheres and those treated as single-cell culture (*p < 0.05; n = 3). (D). Microphotographs showing DMBC10 melanospheres treated with 1 μM doxorubicin (DOX) and 5 μM cisplatin (cisPt) for one day followed by a recovery period. Scale bars represent 100 μm. (E). Comparison of DOX- or cisPt-induced changes in viability between DMBC10 populations of cells treated as intact melanospheres and as single melanoma cells from dissociated spheres (* p < 0.05; n = 3).
Discussion
Melanospheres derived from surgical melanoma specimens, stage III and IV diseaseCitation13 were employed to evaluate PN influence on heterogeneous melanoma cell populations. This in vitro model has been chosen as it might portray the tumor more accurately than the monolayer cultures.Citation28,Citation31 Our approach overcomes some major problems of using two-dimensional serum-driven monolayer cultures for anti-cancer drug testing including the more homogeneous phenotype than that observed in the original tumor, altered cell-to-cell contacts affecting intracellular signal transduction pathways, and too easy access for drugs to cancer cells. Our in vitro study also excluded potential problems caused by substantial changes in human cells transplanted into mouse microenvironment, such as altered cellular sensitivity toward drugs and variable frequency of tumor-initiating cells, which at least partially might be influenced by the level of immunodeficiency in the recipient mouse.Citation3,Citation7,Citation11 While investigating immunophenotype and clonogenicity, we observed that all populations grown as anchorage-independent melanospheres for numerous generations remained heterogeneous and contained cancer stem-like cells with the self-renewing capacity.
We have previously shown that PN suppressed both constitutive and cisplatin-induced NF-κB activity, inhibited the migration and invasiveness, reduced the viable cell number by the cell cycle arrest in G0/G1 phase and induction of apoptosis associated with loss of mitochondrial membrane potential.Citation19,Citation20 All these effects of PN were observed within two days in the monolayer cultures of three melanoma cell lines, A375, 1205Lu and WM793. In the current study, the viability of cells from dissociated anchorage-independent melanospheres was also significantly reduced within two days. These results indicated that PN killed highly cycling bulk population of melanoma cells. However, as more than 20% of cells remained viable after treatment with PN at 12 μM or 24 μM, we were interested whether PN had some impact on those cells as well. When short incubation with PN was followed by a recovery period, changes in the immunophenotype and capacity to self-renew were observed suggesting that PN was capable of inducing some long-term effects. First, the subpopulation of ABCB5-positive cells was more efficiently eliminated by exposure to PN than other subpopulations. In the sorted ABCB5+ subset, the recovery process was less efficient than in the unsorted population as exerted by the lower percentage of viable cells four-seven days after drug removal. In addition, PN completely abolished self-renewing capacity of melanoma cells, a fundamental property of CSCs. Thus, based on our experimental data, it is plausible to conclude that PN besides reducing the viability of bulk population of melanoma cells also eradicated cells (1) with high self-renewing capacity and (2) carrying ABCB5 transporter. It has been already shown that ABCB5-expressing melanoma cells exerted a high tumorigenic potential,Citation30 and were also more clonogenic in vitro.Citation32 More importantly, some approaches targeting ABCB5 transporter have been already explored in melanoma, e.g., using anti-ABCB5 antibodies.Citation30,Citation33 To our knowledge, the current report is the first one showing the efficacy of a small molecule on the selective reduction of ABCB5-expressing melanoma cells.
The resistance of CSCs to the conventional treatment may be due to several mechanisms, including no/slow cell-cycle progression, high capacity for DNA repair, activated anti-apoptotic pathways and overactivated ABC transporters (for a review see ref. Citation34). Many clinical studies have indicated that CSCs selectively survive conventional therapy. For instance, chemotherapy of primary breast cancer patients increased the level of CD44high/CD24-/low cells in cancer core biopsies and the relative sphere-forming capacity in vitro.Citation35,Citation36 Most recently, it has been demonstrated that ABCB5-expressing cells were more abundant in melanomas from patients treated with dacarbazine and also in vitro, dacarbazine induced an increase in the frequency of ABCB5+ cells.Citation37 Our results, showing that DTIC not only increased the sphere-forming potential of melanoma cells but also led to a melanosphere-enriched DTIC-resistant culture, support these findings. If radio- or chemotherapy fails to eradicate all cancer cells and the residual population is highly enriched for cells that persist in the CSC state, finding a drug to eradicate CSCs is essential for preventing relapse and improving long-term survival. Moreover, as CSCs may be generated from more differentiated tumor cells by phenotype switching,Citation8 therapies employing drugs that specifically target CSCs in combination with drugs with toxicity toward bulk cancer cell populations might be a highly effective strategy for eradicating melanoma tumors. The current study has shown that PN could affect both bulk and melanoma stem-like cells. It supports previously published reports describing similar activity of PN in other cancers.Citation26,Citation27,Citation38 Importantly, PN has already shown safety in Phase I/II clinical trials.Citation39,Citation40 An unresolved problem, however, that was addressed in our study, is the penetration capacity of PN, which may limit its action toward single melanoma cells circulating in the blood flow or to cells in the vicinity of blood vessels within the tumor tissue. However, assuming that metastasis requires the dissemination of melanoma stem-like cells, the activity of PN toward circulating melanoma cells might have potential therapeutic implications. In addition, the penetration capacity of PN could be improved by other chemicals, nanoparticles or polymers. For instance, the encapsulation of PN into stealthy liposomes modified with pegylated-lipid derivative has been used in xenografted mice to enhance its anticancer effect.Citation23 The structure of the drug itself may also be further modified to obtain derivatives with improved pharmacological performance in the clinical applications. The recently published report showing synergism between PN and inhibitors of the PI-3 kinase and mTOR pathway suggested that chemical genomic approaches might be used to predict compounds that are likely to enhance the efficacy of PN and to maximize eradication of heterogeneous tumor populations.Citation26
The molecular mechanism(s) of PN toxicity toward cancer stem-like cells remains unclear. PN has been shown to inhibit activity of NF-κBCitation19,Citation41,Citation42 and STAT proteins,Citation43,Citation44 and to induce sustained JNK activityCitation45 and proapoptotic p53 activity via influencing MDM2 and HDAC1 levels.Citation46 At the epigenetic level, PN specifically depleted HDAC1 protein,Citation47 and by inhibiting DNMT1 activity induced global hypomethylation of DNA in vitro and in vivo, which could restore the expression of some suppressor genes.Citation48 The ability of PN for simultaneous targeting NFκB and p53 pathways might be the base for the selective killing of cancer but not normal stem cells.Citation21,Citation25,Citation49 It has been already shown that breast cancer stem-like cells could be preferentially targeted by NFκB pathway inhibitors.Citation24 We have demonstrated that PN affected the NFκB pathway in melanoma cells,Citation19 which might be connected with its influence on melanoma stem-like cells. Most recently, it has been shown that colon cancer stem-like cells having higher levels of phosphorylated STAT3 than cells in the bulk tumor were sensitive to STAT3 inhibitors.Citation50 Therefore, STAT3 is another target, which could be affected by PN in melanoma stem-like cells. PN contains an α-methylene-γ-lactone ring and epoxide moieties that can interact by Michael-type addition with biological nucleophiles, mainly thiol-containing cysteine residues in proteins.Citation51 This mechanism seems to be responsible for the direct influence of PN on the activity of NFκBCitation52 or DNMT1,Citation48 and indirect actions of PN on STAT3Citation43 or NFκB activity.Citation42,Citation51 It would be interesting to analyze a possible interaction of PN with the ABCB5 transporter. In a recently published analysis, it was suggested that a single nucleotide polymorphism (SNP), that changed a cysteine to a tryptophan residue in the nucleotide binding domain, might be deleterious for the structure and function of ABCB5 protein.Citation53 Further studies will be required to characterize the expression and activity of ABCB5 in melanoma and the connections between NF-κB/p53/STAT activities and ABCB5. Based on the current study we can conclude that PN significantly reduced the viability of both the overall population of melanoma cells and the cancer stem-like cell subpopulation.
Materials and Methods
Drugs
Parthenolide (PN) was from BIOMOL (P0270); cisplatin (0.5 mg/ml) was from EBEWE Unterach, Austria; dacarbazine, DTIC (D2390) and doxorubicin from Sigma-Aldrich (D1317). Parthenolide was dissolved in dimethylsulfoxide (DMSO). An equivalent concentration of DMSO was used in the control cultures. Cisplatin and dacarbazine were dissolved in culture medium immediately before use.
Tumor tissues
Nodular melanoma specimens were obtained during surgical procedures. Histopathological analyses were performed to confirm melanocytic characteristics of tumor samples. All samples were from patients with stage III and IV disease (AJCC clinical staging). Patient characteristics of melanoma specimens were published,Citation13 except for DMBC9 which was derived from primary tumor, T3bN1bM0 of male patient, age 52. The study was approved by Ethical Commission of Medical University of Lodz, and informed consent was obtained from each patient.
In vitro cell culture
Tumor samples were minced with scissors into small fragments and incubated in HBSS (Sigma-Aldrich; H9269) supplemented with 3 mM calcium chloride and 1 mg/ml collagenase IV for 2–3 h at 37°C. DNase I (10 μg/ml) was added to reduce clumping and cells were filtered through a 70 μm pore size filter to remove undigested tissue aggregates. Isolated cells were cultured for 1 d in a complete medium (RPMI1640 with 10% FBS) to ensure the removal of dead and nonadherent cells. Then, they were transferred to a stem cell medium (SCM), consisting of DMEM/F12 low osmolality medium (Lonza; BE12–719F) in the presence of B-27 supplement (Gibco; 17504), growth factors: 10 ng/ml bFGF (BD Biosciences; 354060) and 20 ng/ml EGF (BD Biosciences; 354052), insulin (10 µg/ml), heparin (1 ng/ml) and antibiotics (100 IU/ml penicillin, 100 μg/ml streptomycin, 2 µg/ml Fungizone). Melanospheres were maintained in non-adherent flasks at 37°C in a humidified atmosphere containing 5% CO2. Medium was exchanged twice a week. Every few weeks, melanospheres were passaged following enzymatic dissociation into a single cell suspension and replated. For some experiments, DMBC12 population was sorted for ABCB5+ cells with the Becton Dickinson FACSCalibur system. Cells were incubated with unconjugated anti-ABCB5 from Sigma-Aldrich (SAB1300315), then with FITC-conjugated goat anti-rabbit secondary antibody (BD PharMingen; 554020). Single ABCB5+ cells were obtained by gating out cellular aggregates and dead cells were excluded on the basis of propidium iodide staining.
Measurement of cell viability
Melanospheres or adherent cells were first treated with collagenase IV to obtain a suspension of single cells, and then cells were counted after staining with Trypan blue (Sigma-Aldrich; T8154). Melanoma cells were plated at a density of 2–4 × 103 viable cells per well in 96-well plates and cultured for 2 d in 100 μl SCM with vehicle (0.02% DMSO) or with PN at indicated concentrations. An acid phosphatase activity (APA) assay was used to validate viable cell number in the culture. Briefly, the plates were centrifuged, the medium was discarded and replaced with 100 μl assay buffer containing 0.1 M sodium acetate (pH = 5), 0.1% Triton X-100 and 5 mM p-nitrophenyl phosphate, pNPP (Sigma-Aldrich; N4645) and incubated for additional 2 h at 37°C. The reaction was stopped with 10 μl/well of 1 M NaOH, and the absorbance values were measured at the wavelength of 405 nm using a microplate reader (Infinite M200Pro, Tecan). Melanoma cells did not respond to 0.02% DMSO with reduced viability. For each PN concentration, a comparison was made relatively to the value obtained for the control (DMSO-treated cells), and expressed as % of the control.
To evaluate the influence of PN and DTIC on proliferative potential, melanoma cells from dissociated melanospheres were treated with these drugs for three days and then allowed to recover in the absence of drugs for 4 d (DTIC) or 7 d (PN). DTIC was used at increasing concentrations of 0.5 mM, 1 mM and 2 mM, whereas PN concentration was kept at 6 μM in two consecutive treatments. Microphotographs were taken to record the changes in morphology of melanoma cells after dissociation of melanospheres (day 0), the first 2 d of treatment and 3 weeks of three-day treatments interrupted by recovery periods.
Drug-induced changes in viability were also assessed by propidium iodide staining or by using an automated cell viability analyzer according to standard procedures. In both assays, cells were analyzed using a FACSVerse flow cytometer (Becton Dickinson). Briefly, unsorted and sorted ABCB5+cells were exposed to drugs for one or two days. Fold difference in viability between sorted and unsorted populations was calculated using the formula: viable cells in unsorted drug-treated population (% of control)/viable cells in sorted ABCB5+ drug-treated population (% of control).
Annexin V/PI staining was employed to assess the percentages of apoptotic cells in unsorted and sorted ABCB5+ melanoma cultures after one day incubation with PN at indicated concentrations. Briefly, after treatment melanoma cells were washed with cold PBS and incubated for 15 min with 100 µl staining solution containing Annexin V and PI (Roche Diagnostics). Cells were analyzed by flow cytometry using a FACSVerse flow cytometer (Becton Dickinson). The results were processed using FACSuite software (Becton Dickinson).
Soft agar colony formation assay
Cell suspensions obtained by enzymatic dissociation of melanospheres were first incubated with either 12 μM PN or 2 mM DTIC for 4 h in SCM, followed by 2 h incubation without drugs. Then, viability was determined by Trypan blue staining and 1 or 2 × 103 single, viable melanoma cells were transferred to 350 μl top agar medium mixture (SCM, 0.35% (w/v) agar) and the obtained cell suspensions were overlaid onto 12-well culture plates coated with 350 μl solidified bottom agar mixture (SCM, 0.5% (w/v) agar). Each well was checked under the microscope, and only wells containing a single cell suspension with no cell clusters were retained. The plates were then incubated at 37°C in a humidified incubator for 3 weeks. Cells were stained with 250 μl of 0.005% crystal violet for 1 h, and spheres were counted under the microscope. Colony-forming capacity (CFC) was expressed as the number of spheres at least 50 μm in diameter, generated by the 1 × 103 cells seeded before. Fold change in CFC was calculated by dividing CFC obtained in the presence of a drug by CFC obtained in control conditions.
Flow cytometry analysis of the expression of selected markers
The frequency of cells expressing particular markers was assessed by flow cytometry. Collagenase IV was used to digest melanospheres. The following primary antibodies were used: FITC-conjugated anti-CD90 from BD PharMingen (555595), PE-conjugated anti-CD133 from Miltenyi Biotec (130–080–801), FITC-conjugated anti-CD49f from R&D Systems (MAB1350), and unconjugated anti-ABCB5 from Sigma-Aldrich (SAB1300315) along with FITC-conjugated goat anti-rabbit secondary antibody (BD PharMingen; 554020). Typically, 30 000 cells were analyzed per sample. Appropriate isotype controls were included in each experiment. To exclude dead cells from the analysis, 7-aminoactinomycin D staining (7-AAD; eBiosciences; 00–6993–50) was used. Flow cytometric acquisition was performed using FACSCalibur (BD Biosciences) and analyzed using BD Cell Quest software. To evaluate PN-induced changes in immunophenotype, intact melanospheres or cell suspensions from melanospheres after acute dissociation, or cells from adherent monolayers, were cultured with or without 12 μM PN for one day. Flow cytometric analysis was performed after additional two days of culture in drug-free medium. Relative changes in frequencies (RCFs) were calculated using the formula
RCF = FPN/FC / VPN/VC, where FPN and FC were the frequencies of marker-positive cells in PN-treated and DMSO-treated control populations, respectively, and VPN and VC were the percentages of viable cells (7-AAD-negative) in those populations.
Cell morphology
Changes in cell morphology were registered with a digital Olympus camera (C-5050) attached to an inverted Olympus phase contrast microscope (CKX41).
Statistical analysis
All values are expressed as the mean ± SD from three independent experiments, unless otherwise indicated. Comparisons between values were performed using a two-tailed Student’s t-test. For all statistical analyses, the level of significance was set at a probability of p < 0.05.
Disclosure of Potential Conflicts of Interest
None of the authors has declared any conflict of interest regarding the material presented in the manuscript.
Acknowledgments
The authors wish to thank Dr. Justyna Jakubowska for isolation and culturing melanoma cells, Dr. Marta Stasiak for her help with flow cytometric analysis, Dr. Markus Düchler and Prof. Ewa Zietkiewicz for critically reviewing the manuscript, and Karolina Niewinna for her excellent technical assistance. This research was supported by Grant N N401 020235 from the Ministry of Scientific Research and Higher Education and Grant 2011/01/B/NZ4/04921 from the National Science Centre.
References
- Schatton T, Frank MH. Cancer stem cells and human malignant melanoma. Pigment Cell Melanoma Res 2008; 21:39 - 55; http://dx.doi.org/10.1111/j.1755-148X.2007.00427.x; PMID: 18353142
- Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest 2010; 120:41 - 50; http://dx.doi.org/10.1172/JCI41004; PMID: 20051635
- Girouard SD, Murphy GF. Melanoma stem cells: not rare, but well done. Lab Invest 2011; 91:647 - 64; http://dx.doi.org/10.1038/labinvest.2011.50; PMID: 21445060
- Eramo A, Haas TL, De Maria R. Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene 2010; 29:4625 - 35; http://dx.doi.org/10.1038/onc.2010.207; PMID: 20531299
- Takehara M, Hoshino T, Namba T, Yamakawa N, Mizushima T. Acetaminophen-induced differentiation of human breast cancer stem cells and inhibition of tumor xenograft growth in mice. Biochem Pharmacol 2011; 81:1124 - 35; http://dx.doi.org/10.1016/j.bcp.2011.02.012; PMID: 21371442
- Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc Natl Acad Sci U S A 2011; 108:2474 - 9; http://dx.doi.org/10.1073/pnas.1009069108; PMID: 21282657
- Quintana E, Shackleton M, Foster HR, Fullen DR, Sabel MS, Johnson TM, et al. Phenotypic heterogeneity among tumorigenic melanoma cells from patients that is reversible and not hierarchically organized. Cancer Cell 2010; 18:510 - 23; http://dx.doi.org/10.1016/j.ccr.2010.10.012; PMID: 21075313
- Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res 2010; 23:746 - 59; http://dx.doi.org/10.1111/j.1755-148X.2010.00757.x; PMID: 20726948
- Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell 2010; 141:583 - 94; http://dx.doi.org/10.1016/j.cell.2010.04.020; PMID: 20478252
- Abel EV, Aplin AE. Finding the root of the problem: the quest to identify melanoma stem cells. Front Biosci (Schol Ed) 2011; 3:937 - 45; http://dx.doi.org/10.2741/198; PMID: 21622243
- Fukunaga-Kalabis M, Roesch A, Herlyn M. From cancer stem cells to tumor maintenance in melanoma. J Invest Dermatol 2011; 131:1600 - 4; http://dx.doi.org/10.1038/jid.2011.159; PMID: 21654838
- Ghislin S, Deshayes F, Lauriol J, Middendorp S, Martins I, Al-Daccak R, et al. Plasticity of melanoma cells induced by neural cell crest conditions and three-dimensional growth. Melanoma Res 2012; 22:184 - 94; http://dx.doi.org/10.1097/CMR.0b013e328351e7c4; PMID: 22454190
- Sztiller-Sikorska M, Koprowska K, Jakubowska J, Zalesna I, Stasiak M, Duechler M, et al. Sphere formation and self-renewal capacity of melanoma cells is affected by the microenvironment. Melanoma Res 2012; 22:215 - 24; http://dx.doi.org/10.1097/CMR.0b013e3283531317; PMID: 22495670
- Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem 2002; 277:38954 - 64; http://dx.doi.org/10.1074/jbc.M203842200; PMID: 12151389
- Zunino SJ, Ducore JM, Storms DH. Parthenolide induces significant apoptosis and production of reactive oxygen species in high-risk pre-B leukemia cells. Cancer Lett 2007; 254:119 - 27; http://dx.doi.org/10.1016/j.canlet.2007.03.002; PMID: 17470383
- Duechler M, Stańczyk M, Czyz M, Stepnik M. Potentiation of arsenic trioxide cytotoxicity by Parthenolide and buthionine sulfoximine in murine and human leukemic cells. Cancer Chemother Pharmacol 2008; 61:727 - 37; http://dx.doi.org/10.1007/s00280-007-0527-3; PMID: 17594095
- Suvannasankha A, Crean CD, Shanmugam R, Farag SS, Abonour R, Boswell HS, et al. Antimyeloma effects of a sesquiterpene lactone parthenolide. Clin Cancer Res 2008; 14:1814 - 22; http://dx.doi.org/10.1158/1078-0432.CCR-07-1359; PMID: 18347184
- Dai Y, Guzman ML, Chen S, Wang L, Yeung SK, Pei XY, et al. The NF (Nuclear factor)-κB inhibitor parthenolide interacts with histone deacetylase inhibitors to induce MKK7/JNK1-dependent apoptosis in human acute myeloid leukaemia cells. Br J Haematol 2010; 151:70 - 83; http://dx.doi.org/10.1111/j.1365-2141.2010.08319.x; PMID: 20701602
- Czyz M, Lesiak-Mieczkowska K, Koprowska K, Szulawska-Mroczek A, Wozniak M. Cell context-dependent activities of parthenolide in primary and metastatic melanoma cells. Br J Pharmacol 2010; 160:1144 - 57; http://dx.doi.org/10.1111/j.1476-5381.2010.00749.x; PMID: 20590608
- Lesiak K, Koprowska K, Zalesna I, Nejc D, Düchler M, Czyz M. Parthenolide, a sesquiterpene lactone from the medical herb feverfew, shows anticancer activity against human melanoma cells in vitro. Melanoma Res 2010; 20:21 - 34; http://dx.doi.org/10.1097/CMR.0b013e328333bbe4; PMID: 19949351
- Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood 2005; 105:4163 - 9; http://dx.doi.org/10.1182/blood-2004-10-4135; PMID: 15687234
- Gunn EJ, Williams JT, Huynh DT, Iannotti MJ, Han C, Barrios FJ, et al. The natural products parthenolide and andrographolide exhibit anti-cancer stem cell activity in multiple myeloma. Leuk Lymphoma 2011; 52:1085 - 97; http://dx.doi.org/10.3109/10428194.2011.555891; PMID: 21417826
- Liu Y, Lu WL, Guo J, Du J, Li T, Wu JW, et al. A potential target associated with both cancer and cancer stem cells: a combination therapy for eradication of breast cancer using vinorelbine stealthy liposomes plus parthenolide stealthy liposomes. J Control Release 2008; 129:18 - 25; http://dx.doi.org/10.1016/j.jconrel.2008.03.022; PMID: 18466993
- Zhou J, Zhang H, Gu P, Bai J, Margolick JB, Zhang Y. NF-kappaB pathway inhibitors preferentially inhibit breast cancer stem-like cells. Breast Cancer Res Treat 2008; 111:419 - 27; http://dx.doi.org/10.1007/s10549-007-9798-y; PMID: 17965935
- Kawasaki BT, Hurt EM, Kalathur M, Duhagon MA, Milner JA, Kim YS, et al. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: An integrated molecular profiling approach. Prostate 2009; 69:827 - 37; http://dx.doi.org/10.1002/pros.20931; PMID: 19204913
- Hassane DC, Sen S, Minhajuddin M, Rossi RM, Corbett CA, Balys M, et al. Chemical genomic screening reveals synergism between parthenolide and inhibitors of the PI-3 kinase and mTOR pathways. Blood 2010; 116:5983 - 90; http://dx.doi.org/10.1182/blood-2010-04-278044; PMID: 20889920
- Zuch D, Giang AH, Shapovalov Y, Schwarz E, Rosier R, O'Keefe R, et al. Targeting radioresistant osteosarcoma cells with parthenolide. J Cell Biochem 2012; 113:1282 - 91; PMID: 22109788
- Thurber AE, Douglas G, Sturm EC, Zabierowski SE, Smit DJ, Ramakrishnan SN, et al. Inverse expression states of the BRN2 and MITF transcription factors in melanoma spheres and tumour xenografts regulate the NOTCH pathway. Oncogene 2011; 30:3036 - 48; http://dx.doi.org/10.1038/onc.2011.33; PMID: 21358674
- Chen KG, Valencia JC, Gillet JP, Hearing VJ, Gottesman MM. Involvement of ABC transporters in melanogenesis and the development of multidrug resistance of melanoma. Pigment Cell Melanoma Res 2009; 22:740 - 9; http://dx.doi.org/10.1111/j.1755-148X.2009.00630.x; PMID: 19725928
- Schatton T, Murphy GF, Frank NY, Yamaura K, Waaga-Gasser AM, Gasser M, et al. Identification of cells initiating human melanomas. Nature 2008; 451:345 - 9; http://dx.doi.org/10.1038/nature06489; PMID: 18202660
- Perego M, Tortoreto M, Tragni G, Mariani L, Deho P, Carbone A, et al. Heterogeneous phenotype of human melanoma cells with in vitro and in vivo features of tumor-initiating cells. J Invest Dermatol 2010; 130:1877 - 86; http://dx.doi.org/10.1038/jid.2010.69; PMID: 20376064
- Keshet GI, Goldstein I, Itzhaki O, Cesarkas K, Shenhav L, Yakirevitch A, et al. MDR1 expression identifies human melanoma stem cells. Biochem Biophys Res Commun 2008; 368:930 - 6; http://dx.doi.org/10.1016/j.bbrc.2008.02.022; PMID: 18279661
- Frank NY, Margaryan A, Huang Y, Schatton T, Waaga-Gasser AM, Gasser M, et al. ABCB5-mediated doxorubicin transport and chemoresistance in human malignant melanoma. Cancer Res 2005; 65:4320 - 33; http://dx.doi.org/10.1158/0008-5472.CAN-04-3327; PMID: 15899824
- Maugeri-Saccà M, Vigneri P, De Maria R. Cancer stem cells and chemosensitivity. Clin Cancer Res 2011; 17:4942 - 7; http://dx.doi.org/10.1158/1078-0432.CCR-10-2538; PMID: 21622723
- Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci U S A 2009; 106:13820 - 5; http://dx.doi.org/10.1073/pnas.0905718106; PMID: 19666588
- Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 2007; 131:1109 - 23; http://dx.doi.org/10.1016/j.cell.2007.10.054; PMID: 18083101
- Chartrain M, Riond J, Stennevin A, Vandenberghe I, Gomes B, Lamant L, et al. Melanoma chemotherapy leads to the selection of ABCB5-expressing cells. PLoS One 2012; 7:e36762; http://dx.doi.org/10.1371/journal.pone.0036762; PMID: 22675422
- Kim YR, Eom JI, Kim SJ, Jeung HK, Cheong JW, Kim JS, et al. Myeloperoxidase expression as a potential determinant of parthenolide-induced apoptosis in leukemia bulk and leukemia stem cells. J Pharmacol Exp Ther 2010; 335:389 - 400; http://dx.doi.org/10.1124/jpet.110.169367; PMID: 20699435
- Murphy JJ, Heptinstall S, Mitchell JR. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet 1988; 2:189 - 92; http://dx.doi.org/10.1016/S0140-6736(88)92289-1; PMID: 2899663
- Curry EA 3rd, Murry DJ, Yoder C, Fife K, Armstrong V, Nakshatri H, et al. Phase I dose escalation trial of feverfew with standardized doses of parthenolide in patients with cancer. Invest New Drugs 2004; 22:299 - 305; http://dx.doi.org/10.1023/B:DRUG.0000026256.38560.be; PMID: 15122077
- Hehner SP, Heinrich M, Bork PM, Vogt M, Ratter F, Lehmann V, et al. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J Biol Chem 1998; 273:1288 - 97; http://dx.doi.org/10.1074/jbc.273.3.1288; PMID: 9430659
- Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb Feverfew directly binds to and inhibits IkappaB kinase. Chem Biol 2001; 8:759 - 66; http://dx.doi.org/10.1016/S1074-5521(01)00049-7; PMID: 11514225
- Carlisi D, D’Anneo A, Angileri L, Lauricella M, Emanuele S, Santulli A, et al. Parthenolide sensitizes hepatocellular carcinoma cells to TRAIL by inducing the expression of death receptors through inhibition of STAT3 activation. J Cell Physiol 2011; 226:1632 - 41; http://dx.doi.org/10.1002/jcp.22494; PMID: 21413021
- Sobota R, Szwed M, Kasza A, Bugno M, Kordula T. Parthenolide inhibits activation of signal transducers and activators of transcription (STATs) induced by cytokines of the IL-6 family. Biochem Biophys Res Commun 2000; 267:329 - 33; http://dx.doi.org/10.1006/bbrc.1999.1948; PMID: 10623619
- Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene 2004; 23:7330 - 44; http://dx.doi.org/10.1038/sj.onc.1207995; PMID: 15286701
- Gopal YN, Chanchorn E, Van Dyke MW. Parthenolide promotes the ubiquitination of MDM2 and activates p53 cellular functions. Mol Cancer Ther 2009; 8:552 - 62; http://dx.doi.org/10.1158/1535-7163.MCT-08-0661; PMID: 19276167
- Gopal YN, Arora TS, Van Dyke MW. Parthenolide specifically depletes histone deacetylase 1 protein and induces cell death through ataxia telangiectasia mutated. Chem Biol 2007; 14:813 - 23; http://dx.doi.org/10.1016/j.chembiol.2007.06.007; PMID: 17656318
- Liu Z, Liu S, Xie Z, Pavlovicz RE, Wu J, Chen P, et al. Modulation of DNA methylation by a sesquiterpene lactone parthenolide. J Pharmacol Exp Ther 2009; 329:505 - 14; http://dx.doi.org/10.1124/jpet.108.147934; PMID: 19201992
- Zhou J, Zhang Y. Cancer stem cells: Models, mechanisms and implications for improved treatment. Cell Cycle 2008; 7:1360 - 70; http://dx.doi.org/10.4161/cc.7.10.5953; PMID: 18418062
- Lin L, Liu Y, Li H, Li PK, Fuchs J, Shibata H, et al. Targeting colon cancer stem cells using a new curcumin analogue, GO-Y030. Br J Cancer 2011; 105:212 - 20; http://dx.doi.org/10.1038/bjc.2011.200; PMID: 21694723
- Skalska J, Brookes PS, Nadtochiy SM, Hilchey SP, Jordan CT, Guzman ML, et al. Modulation of cell surface protein free thiols: a potential novel mechanism of action of the sesquiterpene lactone parthenolide. PLoS One 2009; 4:e8115; http://dx.doi.org/10.1371/journal.pone.0008115; PMID: 19956548
- García-Piñeres AJ, Castro V, Mora G, Schmidt TJ, Strunck E, Pahl HL, et al. Cysteine 38 in p65/NF-kappaB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem 2001; 276:39713 - 20; http://dx.doi.org/10.1074/jbc.M101985200; PMID: 11500489
- Moitra K, Scally M, McGee K, Lancaster G, Gold B, Dean M. Molecular evolutionary analysis of ABCB5: the ancestral gene is a full transporter with potentially deleterious single nucleotide polymorphisms. PLoS One 2011; 6:e16318; http://dx.doi.org/10.1371/journal.pone.0016318; PMID: 21298007
