Abstract
Radiation therapy is an important treatment approach for esophageal squamous cell carcinoma (ESCC). However, how to promote radiation sensitivity in ESCC remains a challenge. This study aimed to evaluate the effects of berberine, a common used Chinese medicine, on the radiosensitivity of ESCC. ECSS cell line ECA109 and TE13 were subjected to hypoxia and/or ionizing radiation (IR), in the presence or absence of berberine treatment. Cell growth and survival, and apoptosis were evaluated. In addition, ECA109 cells were xenografted into nude mice and subjected to IR and/or berberine treatment. The expression of HIF-1α and VEGF was detected by western blot and immunohistochemical analysis. Our results showed that berberine increased radiosensitivity of ESCC cells and xenografts, and this was associated with the inhibition of HIF-1α and VEGF expression. These data suggest that berberine may be a potential radiotherapy sensitization drugs due to its significant anti-hypoxia activity.
Introduction
Esophageal cancer is one of the most common cancers in the world, and consists of esophageal adenocarcinoma and esophageal squamous cell carcinoma (ESCC), which is more often in China and other developing countries. Radiotherapy is an essential therapy for the patients with inoperable and locally advanced esophageal ESCC. However, a large portion of ESCC tumors develop resistance to radiotherapy, indicating the importance to enhance the radiation sensitivity of ESCC.Citation1
Tumor hypoxia is known as an important factor leading to radiation resistance and poor clinical outcomes.Citation2,Citation3 The oxygen occurred during radiotherapy generates free oxygen radicals that induce DNA damage and kill tumor cells. Hypoxia inducible factor 1 (HIF-1), induced by tumor hypoxia, has recently been implicated in radiation resistance in many preclinical and clinical studies.Citation4 HIF-1 is a heterodimer consisting of an oxygen-sensitive subunit HIF-1α and a constitutively expressed subunit HIF-1β. HIF-1 regulates the expression of over 100 genes involved in cell survival, tumor metabolism, proliferation, invasion, angiogenesis and stimulates cytokines such as vascular endothelial growth factor (VEGF).Citation5 Overexpression of HIF-1α has been reported to be associated with a poor prognosis after radiotherapy in patients with esophageal cancer.Citation6 The suppression of HIF-1α expression may reverse the radioresistant phenotype of hypoxia cancer cells.Citation7,Citation8
Berberine is a common Chinese medicine used to treat gastrointestinal discomfort.Citation9 Recent studies have shown that berberine has anti-tumor activity in various cancers such as hepatoma, glioblastoma, prostate cancer, oral cancer, colorectal cancer, lung cancer, leukemia, and osteosarcoma.Citation10 In particular, berberine could sensitize cancer cells to ionizing radiation (IR) in vitro by inhibiting cell cycle progression and DNA repair, and inducing apoptosis and autophagy.Citation11-Citation13
Interestingly, berberine could inhibit HIF-1α in gastric adenocarcinoma cells, melanoma cells, and neurons.Citation14-Citation16 However, whether berberine suppresses HIF-1α in ESCC cells has not been elucidated. In this study, we reported that berberine could inhibit HIF-1α expression in hypoxic ESCC cell lines and thus sensitize the cells to IR significantly. Furthermore, we demonstrated that berberine could sensitize nude mice bearing ESCC cells to IR by inhibiting HIF-1α and VEGF expression.
Results
Berberine promotes radiosensitivity of hypoxic ESCC cell lines
Berberine treatment inhibited the growth of ESCC cells in a time and dose dependent manner (). At 24 h, the IC50 for ESCC cells ECA109 and TE13 was 59.53 μM and 28.54 μM, respectively. Thus we treated hypoxic ESCC cells for 24 h with low concentration of berberine (5 μM and 15 μM) to investigate the effects of berberine on radiotherapy sensitization of hypoxic ESCC cells. The cells in hypoxia condition (0.8–1.0% pO2) exhibited a significant increase in their ability of forming colonies after IR, indicating their resistance to IR (). However, berberine sensitized hypoxic cancer cells to IR significantly. The dose-survival curves were shown in . The SF data were fitted into the single hit multi target model formula: SF = 1 − (1 − e−D/D0)n. The results showed that SF2 was 0.47 and 0.82 for ECA109 cells, and 0.45 and 0.84 for TE-13 cells in the normoxia and hypoxia conditions, respectively. After treatment with berberine at 5 μM and 15 μM, SF2 of hypoxic cells increased to 0.63 and 0.80 in ECA109 cells, and 0.63 and 0.77 in TE13 cells, respectively ( and ). SF2 of normoxia cells treated by cisplatin was 0.41 for ECA109 cells, and 0.39 for TE-13 cells. These data demonstrated that treating hypoxic ESCC cells with berberine resulted in a significant radiosensitization effect.
Figure 1. Berberine sensitizes hypoxic ESCC cancer cells to IR. (A) Berberine inhibited growth of ESCC cells in a time- and dose-dependent manner. (B) Clonogenic assay showing that ESCC cancer cells became radioresistant in hypoxia conditions and that was reversed by berberine. (C and D) Flow cytometric analysis showing that berberine induced apoptosis of hypoxic ECA109 cells. (a) normoxia IR; (b) hypoxia IR; (c) hypoxia IR Ber 5 μM; (d) hypoxia IR Ber 15 μM. The bars presented means ± s.d. of three separate experiments. norm, normoxia; hypo, hypoxia; ber, berberine; DDP, cisplatin. ***P < 0.001; **P < 0.01; *P < 0.05.
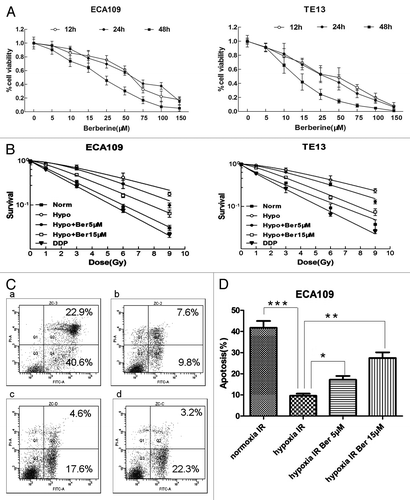
Table 1. The radiosensitization activity of berberine in hypoxia ECA109 cells
Table 2. The radiosensitization activity of berberine in hypoxia TE13 cells
Next we detected the effects of berberine on apoptosis of hypoxic ESCC cells treated by IR. The results showed that the apoptosis rate was significantly lower in hypoxia group than in normoxia group (P < 0.01). After treatment with berberine at 5 μM or 15 μM, the apoptosis rate was significant higher compared to control (P = 0.022 or P < 0.01, respectively) ().
Berberine inhibits hypoxia induced expression of HIF-1α and VEGF in ESCC cells
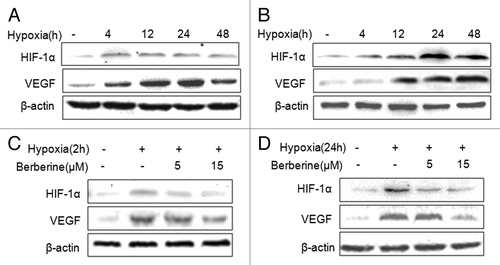
To evaluate HIF-1α and VEGF expression induced by hypoxia, ECA109 and TE-13 cells were treated with hypoxia by incubation in 0.8-1.0% pO2, and we found that hypoxia stimulated HIF-1α and VEGF expression in the two cell lines. HIF-1α level was the highest at 24 h or 4 h after hypoxia in ECA109 cells and TE13 cells, respectively; while VEGF level was the highest at 48 h or 24 h in the two cell lines, respectively (). To determine the effects of berberine on HIF-1α and VEGF expression induced by hypoxia, ECA109 and TE13 cells were treated with 5 μM or 15 μM berberine, and we found that hypoxia stimulated increase of HIF-1α and VEGF expression was inhibited by berberine significantly.
Figure 2. Berberine inhibits HIF-1α and VEGF protein expression in ESCC cells. (A and B) Hypoxia stimulated HIF-1α and VEGF expression in ESCC cell lines. a: ECA109; b: TE13. (C and D) The expression of HIF-1α and VEGF was inhibited by berberine in ESCC cells. c: ECA109; d: TE13. β-actin was loading control.
Furthermore, we examined the nuclear location of HIF-1α in ECA109 cells by confocal microcopy. We observed that the translocation of HIF-1α into the nucleus in hypoxia condition was inhibited by berberine ().
Figure 3. Berberine inhibits HIF-1α and VEGF protein expression in ESCC in vivo. (A) The nuclear location of HIF-1α was observed by laser scanning confocal microscopy using HIF-1α antibody. Representative images showing nuclear colocalization of HIF-1α (green stain) in cells under hypoxia in the presence or absence of berberine (5 μM and 15 μM). (B) Imumunohistochemical staining of HIF-1α and VEGF in tumor tissues of ECA109 xenograft bearing nude mice.
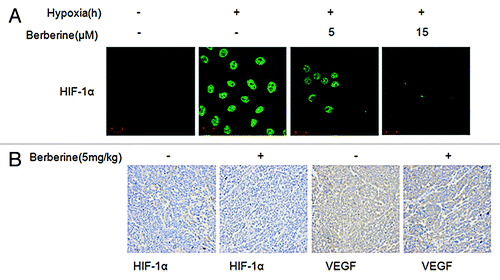
To confirm that berberine inhibits HIF-1α and VEGF protein expression in ESCC in vivo, we performed immunohistochemistry and found that berberine obviously suppressed both VEGF and HIF-1α expression compared to the control group ().
Berberine promotes radiation sensitivity in nude mice
To determine the potential radiosensitization effect of berberine on ESCC tumor in vivo, ECA109 tumor-bearing mice were treated with a single fraction of 8 Gy irradiation. Mice received intraperitoneal injection of berberine (5 mg/kg) for two days before the radiation. Compared to control group, either irradiation or berberine was effective in delaying tumor growth (P < 0.01 for irradiation, P = 0.0062 for berberine on the 25th day) ().
Figure 4. Berberine sensitizes ESCC to IR in vivo. ECA109 xenograft bearing male BALB/c mice were divided into four treatment groups (n = 6): control; berberine alone; irradiation alone; the combination of berberine and irradiation. 10 days after inoculation of ECA109 cells (1 × 106 cells/mouse), the mice were treated with 8 Gy single fraction irradiation. (A) Measurement of tumor size. Data represented average tumor volume; error bars, SD. (B) Representative images of ECA109 xenograft bearing mice.
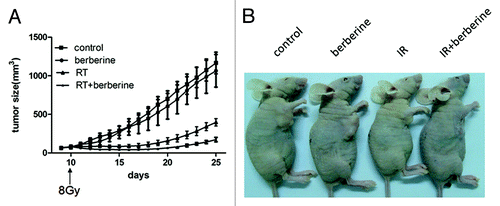
Furthermore, we analyzed the doubling time required for the tumor to grow twice in size under different treatments. Doubling time for ECA109 tumor in control group and berberine alone group was 3.8 ± 0.1 days and 4.0 ± 0.1 days, respectively. For irradiation treated group, the combination treatment extended the doubling time to 14.1 ± 0.8 days, compared to 6.7 ± 0.3 days in irradiation alone group (). Intraperitoneal injection of berberine enhanced response of ECA109 xenografts to irradiation with an enhancement factor of 3.5 calculated by dividing the normalized tumor growth delay ().
Table 3. Effect of berberine on response of ECA109 xenografted tumor to irradiation
Discussion
In this study, we demonstrated for the first time that berberine could significantly inhibit HIF-1α and VEGF expression induced by hypoxia in ESCC cells and promote the radiosensitivity of hypoxic cells. The inhibitory effect of berberine on hypoxia pathway was further confirmed by immunohistochemistry in vivo. In addition, we observed that berberine sensitized ECA109 xenograft tumor to irradiation. These results expand our understanding of the mechanisms of berberine activity and suggest that berberine is a potent radiosensitization agent for ESCC treatment.
Hypoxia tumor microenvironment results from the imbalance between increasing oxygen consumption by extensive growth of tumor cells and poor oxygen delivery by disorganized tumor blood vessels.Citation17 It has been known that tumor in hypoxia microenvironment is resistant to radiation therapy by reducing the generation of oxygen radicals. Radiation was shown to stimulate HIF-1α expression, leading to the production of many angiogenic cytokines such as VEGF which may protect the endothelial cells from radiation damage.Citation18 Moreover, the perinecrotic cells, which did not express HIF-1α originally, tend to acquire HIF-1α activity after surviving radiation to cause tumor recurrence.Citation19 HIF-1α has been known as an important determinant factor of the clinical response and a potential target of radiation therapy including predicting the prognosis of ESCC.Citation6 Moreover, enhancement of tumor radiation sensitivity by inhibiting HIF-1α expression has been demonstrated in various preclinical studies.Citation20
Berberine is a major alkaloid derived from Huanglian (Coptis chinensis) which is one of the most widely used herbs in traditional Chinese medicine. Berberine has strong antimicrobial and anti-inflammatory activities with mild side effects in clinical trials.Citation9 Berberine has anti-tumor activities via the inhibition of the transcriptional activity of cyclooxygenase-2 and the induction of oxygen species and apoptosis, it also inhibits the expression of DNA repair protein RAD51, which would promote radiation sensitivity.Citation11-Citation13
Berberine has been shown to inhibit HIF-1α expression in gastric cancer and melanoma cells.Citation13-Citation15 Therefore, we investigated the prospect of berberine as a potent HIF-1α inhibitor for ESCC, and found that HIF-1α and VEGF expression in both ESCC cells and tumor xenografts was suppressed by berberine. These data suggest that berberine could sensitize hypoxic cancer cells to radiotherapy by inhibiting HIF-1α and VEGF expression.
In conclusion, we provided both in vitro and in vivo evidence that berberine enhances radiosensitivity of ESCC under hypoxia condition, and this is associated with the downregulation of HIF-1α expression. Berberine appears to be a potential radiotherapy sensitization agent due to its significant anti-hypoxia activity.
Materials and Methods
Cell culture
ESCC cell lines ECA109 and TE13 were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 μg/mL penicillin, and 100 units/mL streptomycin, under conditions of 5% CO2 in an incubator at 37 °C. For growth under hypoxia condition, the cells were incubated in a modular chamber flushed with a complex air of 1% O2, 94% N2, and 5% CO2 at 37 °C.
Reagents and antibodies
Berberine and cisplatin were provided by Sigma. The antibodies were as follows: HIF-1α antibody (NB100-105, Novus Biologicals; 1:500); VEGF antibody (A-20, Santa Cruz Biotechnology; 1:250); β-actin antibody (C-4, Santa Cruz Biotechnology; 1:500), fluorescein (FITC) conjugated donkey anti-mouse IgG, biotinylated anti-mouse IgG and biotinylated goat anti-rabbit IgG (Jackson ImmunoResearch; 1:100).
Western blot analysis
Cultured cells were lysed in RIPA lysis buffer (Beyotime Biotechnology) and kept on ice for 20 min. Amount of protein in the lysates was quantified by BCA kit (Beyotime). Equal amounts of proteins were separated by SDS–PAGE and transferred to PVDF membranes, which were probed with the antibodies described above. The signals were visualized using the SuperSignal West Femto kit (Pierce).
MTT assay
Cells were plated at the concentration of 5 × 103 cells/well in 96-well plates. The medium was removed and replaced with fresh medium with or without berberine 24 h later. Cell viability was measured by using MTT cell proliferation and cytotoxicity assay kit (Beyotime) at 12, 24, and 48 h later. The absorbance was measured at a wavelength of 490 nm. All experiments were repeated three times.
Clonogenic assay
ESCC cells were seeded onto 6-well dishes. After overnight culture, the cells were treated in normoxia and hypoxia conditions with berberine (5 μM or 15 μM) or control for 24 h, the cells treated by cisplatin at 10 μM were used as positive controls. Cells were then irradiated at a dose of 0, 1, 3, 6, and 9 Gy with 6MV X-rays, 4.5 Gy/min. The cells were then cultured in 5% CO2 incubator at 37 °C for 10 days. The colonies were fixed and stained with crystal violet for counting the number of colonies (>50 cells/colony).
Immunocytochemical staining
ECA109 cells were grown on glass coverslips. The cells were fixed with acetone and permeabilized with 0.1% Triton X-100 in PBS for 5 min at room temperature. The cells were incubated with HIF-1α antibody (diluted 1:500) at 4 °C overnight and then incubated with FITC conjugated secondary antibody (diluted 1:100) for 1 h at room temperature. After washing in PBS, cells were incubated with 0.25 mg/mL DAPI for 3 min. The images were examined by using a laser scanning confocal microscope (Zeiss LSM510).
Flow cytometric analysis of apoptosis
ECA109 cells were fixed in 2% paraformaldehyde and then stained with Annexin V-FITC Apoptosis Kit (Keygene). The cells were analyzed by flow cytometry using a FACSan with Cell-Quest software (BD Bioscience). All experiments were repeated for three times.
Tumor xenograft mouse models
Animal experiments were approved by Ethics Committee of Nanjing Medical University. Five- to six week-old male BALB/C nude mice were provided by Nanjing Medical University Animal Center. Mice were then subcutaneously inoculated with 0.1 mL of ECA109 cells (1 × 107 cells/mL) at one site of the right leg. When tumors grew to 50–100 mm3 after inoculation on day 9, the animals were randomly grouped into 4 different groups (n = 6): (1) vehicle (PBS), (2) berberine, (3) radiation, and (4) berberine plus radiation. The mice in control group were treated with vehicle control, whereas the mice in the second and fourth groups were given daily intraperitoneal injection of 5 mg/kg berberine on day 9 and day 10. Tumors were irradiated by RS-2000 biological irradiator at a dose of 8 Gy with X-rays, 2 Gy/min for 2 h on day 10. Tumor growth was measured every day till day 25, the tumor volume was calculated according to the formula: Tumor volume = (length [L] × width [W])2/2.
Immunohistochemical staining
At the end of experiments, the mice were killed and the tumor tissues were fixed in 10% formalin, deparaffinized, and cut into 4 mm thick sections. After microwave pretreatment in a citrate buffer (pH 6.0; for antigen retrieval), the slides were immersed in 3% hydrogen peroxide for 20 min to block endogenous peroxidase activity. After intensive washing with PBS, the slides were incubated with HIF-1α and VEGF antibodies and then incubated with HRP-conjugated antibodies. The slides were visualized with DAB Horseradish Peroxidase Color Development Kit (Beyotime), and counterstained with hematoxylin. The images were analyzed by IPP (Image-Pro Plus 6.0) software.
Statistical analysis
Data were expressed as mean ± standard deviation (SD) of at least three independent experiments, and analyzed by SPSS statistical software system for Windows version 16.0 (SPSS Inc.). Statistical significance was determined by using the Student t test or a two-way analysis of variance (ANOVA). P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
This study was supported by the Natural Science Foundation of China (81272504), the Innovation Team (LJ201123-EH11), Jiangsu Provincial Science and Technology Projects BK2011854 (DA11), and “333” Project of Jiangsu Province BRA2012210 (RS12).
References
- Shridhar R, Almhanna K, Meredith KL, Biagioli MC, Chuong MD, Cruz A, Hoffe SE. Radiation therapy and esophageal cancer. Cancer Control 2013; 20:97 - 110; PMID: 23571700
- Bayer C, Vaupel P. Acute versus chronic hypoxia in tumors: Controversial data concerning time frames and biological consequences. Strahlenther Onkol 2012; 188:616 - 27; http://dx.doi.org/10.1007/s00066-012-0085-4; PMID: 22454045
- Babar IA, Czochor J, Steinmetz A, Weidhaas JB, Glazer PM, Slack FJ. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther 2011; 12:908 - 14; http://dx.doi.org/10.4161/cbt.12.10.17681; PMID: 22027557
- Moeller BJ, Dewhirst MW. HIF-1 and tumour radiosensitivity. Br J Cancer 2006; 95:1 - 5; http://dx.doi.org/10.1038/sj.bjc.6603201; PMID: 16735998
- Liao D, Johnson RS. Hypoxia: a key regulator of angiogenesis in cancer. Cancer Metastasis Rev 2007; 26:281 - 90; http://dx.doi.org/10.1007/s10555-007-9066-y; PMID: 17603752
- Sohda M, Ishikawa H, Masuda N, Kato H, Miyazaki T, Nakajima M, Fukuchi M, Manda R, Fukai Y, Sakurai H, et al. Pretreatment evaluation of combined HIF-1alpha, p53 and p21 expression is a useful and sensitive indicator of response to radiation and chemotherapy in esophageal cancer. Int J Cancer 2004; 110:838 - 44; http://dx.doi.org/10.1002/ijc.20215; PMID: 15170665
- Moon SY, Chang HW, Roh JL, Kim GC, Choi SH, Lee SW, Cho KJ, Nam SY, Kim SY. Using YC-1 to overcome the radioresistance of hypoxic cancer cells. Oral Oncol 2009; 45:915 - 9; http://dx.doi.org/10.1016/j.oraloncology.2009.04.005; PMID: 19457706
- Staab A, Fleischer M, Loeffler J, Said HM, Katzer A, Plathow C, Einsele H, Flentje M, Vordermark D. Small interfering RNA targeting HIF-1α reduces hypoxia-dependent transcription and radiosensitizes hypoxic HT 1080 human fibrosarcoma cells in vitro. Strahlenther Onkol 2011; 187:252 - 9; http://dx.doi.org/10.1007/s00066-011-2167-0; PMID: 21437769
- Singh IP, Mahajan S. Berberine and its derivatives: a patent review (2009 - 2012). Expert Opin Ther Pat 2013; 23:215 - 31; http://dx.doi.org/10.1517/13543776.2013.746314; PMID: 23231038
- Tillhon M, Guamán Ortiz LM, Lombardi P, Scovassi AI. Berberine: new perspectives for old remedies. Biochem Pharmacol 2012; 84:1260 - 7; http://dx.doi.org/10.1016/j.bcp.2012.07.018; PMID: 22842630
- Hur JM, Hyun MS, Lim SY, Lee WY, Kim D. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J Cell Biochem 2009; 107:955 - 64; http://dx.doi.org/10.1002/jcb.22198; PMID: 19492307
- Liu Q, Jiang H, Liu Z, Wang Y, Zhao M, Hao C, Feng S, Guo H, Xu B, Yang Q, et al. Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS One 2011; 6:e23427; http://dx.doi.org/10.1371/journal.pone.0023427; PMID: 21858113
- Peng PL, Kuo WH, Tseng HC, Chou FP. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: the contribution of autophagic cell death. Int J Radiat Oncol Biol Phys 2008; 70:529 - 42; http://dx.doi.org/10.1016/j.ijrobp.2007.08.034; PMID: 18207031
- Lin S, Tsai SC, Lee CC, Wang BW, Liou JY, Shyu KG. Berberine inhibits HIF-1alpha expression via enhanced proteolysis. Mol Pharmacol 2004; 66:612 - 9; PMID: 15322253
- Hamsa TP, Kuttan G. Antiangiogenic activity of berberine is mediated through the downregulation of hypoxia-inducible factor-1, VEGF, and proinflammatory mediators. Drug Chem Toxicol 2012; 35:57 - 70; http://dx.doi.org/10.3109/01480545.2011.589437; PMID: 22145808
- Zhang Q, Qian Z, Pan L, Li H, Zhu H. Hypoxia-inducible factor 1 mediates the anti-apoptosis of berberine in neurons during hypoxia/ischemia. Acta Physiol Hung 2012; 99:311 - 23; http://dx.doi.org/10.1556/APhysiol.99.2012.3.8; PMID: 22982719
- Yoshimura M, Itasaka S, Harada H, Hiraoka M. Microenvironment and radiation therapy, Biomed Res Int (2013).
- Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci 2012; 33:207 - 14; http://dx.doi.org/10.1016/j.tips.2012.01.005; PMID: 22398146
- Harada H, Inoue M, Itasaka S, Hirota K, Morinibu A, Shinomiya K, Zeng L, Ou G, Zhu Y, Yoshimura M, et al. Cancer cells that survive radiation therapy acquire HIF-1 activity and translocate towards tumour blood vessels. Nat Commun 2012; 3:783; http://dx.doi.org/10.1038/ncomms1786; PMID: 22510688
- Meijer TW, Kaanders JH, Span PN, Bussink J. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res 2012; 18:5585 - 94; http://dx.doi.org/10.1158/1078-0432.CCR-12-0858; PMID: 23071360
