Abstract
Short-term plasticity (STP) comprises several rapid synaptic processes that operate on millisecond-to-minute timescales and modulate synaptic efficacy in an activity-dependent manner. Facilitation and augmentation are two major STP components in central synapses that work to enhance synaptic strength, while various forms of short-term depression work to decrease it. These multiple components of STP interact to perform a variety of synaptic computations. Using a modeling approach in excitatory hippocampal synapses, we recently described the contributions of individual STP components to synaptic operations. In this mini-review, we summarize the recent findings that revealed a wide palette of functions that STP components play in neural operations and discuss their roles in information processing, working memory and decision making.
Short-term synaptic plasticity (STP) represents a rapid, bidirectional and reversible modulation of synaptic strength and is believed to serve as an important mechanism for modifying synaptic and circuit functions during computation.Citation1 STP comprises several rapid synaptic processes that operate on millisecond-to-minute timescales and work to either increase synaptic strength (facilitation, augmentation), or decrease it (various components of depression), as reviewed in references Citation1–Citation4. In most central synapses, these major forms of STP have a presynaptic origin: facilitation and augmentation are thought to arise from elevation in presynaptic calcium levels during repetitive activity, and subsequent increase in the probability of vesicle fusion. Short-term depression, on the other hand, is attributed to several mechanisms that are differentially expressed in various types of synapses, including depletion of release-competent vesicles, inactivation of release sites, reduction in calcium influx due to calcium channel inactivation or calcium-induced calcium channel inhibition.Citation2,Citation3
Synapses in different neural circuits express various combinations of these processes, which determine the types of operations that a given synapse performs. STP is often viewed as a dynamic filter optimized for transmission of specific input frequencies or patterns, i.e., performing low-pass, band-pass or high-pass filtering operations.Citation1,Citation5–Citation8 The expression of specific types of filtering is thought to depend on whether components of short-term enhancement or short-term depression dominate synaptic dynamics. The view of STP as a dynamic filter is supported by numerous experimental studies of cortical and hippocampal circuit operations,Citation8–Citation17 and by a large number of theoretical studies of synaptic computations that are based on computational models of synaptic dynamics.Citation5,Citation15,Citation17–Citation28
Until recently, however, the difficulty of separating contributions from various forms of STP to synaptic dynamics made it difficult to examine the roles of individual types of STP to synaptic operations. This technical limitation has been partially alleviated by the recent development of extensive computational models of STP, which are based on fundamental principals governing synaptic dynamics and provide a close agreement with the experimental data.Citation15,Citation28 These new approaches together with the recent computational and experimental studies have provided strong support for the long hypothesized roles of STP in information processing and suggested importance of STP in other essential brain functions such as decision making and working memory. In this mini-review, we discuss recent findings on the function of individual STP components and overall STP in synaptic and circuit computations.
Roles of Facilitation in Synaptic Computations
Facilitation, the most rapid component of synaptic enhancement, is prominent on the tens to hundreds of milliseconds time scale. Facilitation is probably the most well-studied component of STP that can be easily assessed in many synaptic model systems by a pair of closely spaced stimuli in what's known as the paired-pulse protocol. At some synapses, facilitation has been subdivided into a very rapid component lasting tens of milliseconds and a relative slower component lasting approximately a hundred milliseconds under physiological conditions.Citation3 Facilitation is thought to depend on elevation in presynaptic residual calcium levels that may arise from several mechanisms, including saturation of presynaptic calcium buffers,Citation29–Citation31 enhancement of presynaptic calcium currentsCitation32–Citation35 and possibly changes in sensitivity of calcium sensor,Citation36,Citation37 which, however, remains to be identified.
Possibly because of the difficulty of separating contributions of facilitation from other calcium-dependent forms of short-term synaptic enhancement, such as augmentation, knowledge about the specific roles of facilitation in synaptic computations in different neural circuits is currently limited. The role of facilitation in information processing has been described particularly well in the auditory pathway.Citation4,Citation38 In the cochlear nucleus angularis, which encodes sound intensity information, facilitation was found to be precisely balanced with short-term depression, thereby maintaining the postsynaptic response amplitude throughout the stimulus train at high firing rates.Citation12 This balance serves to linearly convey the input rate information and therefore works to transmit sound intensity information encoded as a rate code. At the calyx of Held synapses, which serve as a highly reliable relay in the sound localization circuit of the auditory brainstem, the interplay between facilitation and depression also plays important roles in auditory information processing.Citation27,Citation39–Citation42 Although these synapses are tonically depressed at lower frequency range corresponding to the rates of spontaneous activity of the auditory neurons in vivo, sudden increases in input frequency during sound-evoked activity induce robust facilitation on top of the tonically depressed state of the synapse.Citation39 Facilitation in this partially depressed state transiently compensates for the effects of depression, thereby stabilizing the transmitter output at the beginning of high-frequency activity. Thus, this facilitation is believed to counteract the delay and the temporal jitter of postsynaptic action potentials seen in these synapses during high-frequency activity and thus works to maintain robust information transmission during sound-evoked activity.Citation40,Citation41
The hippocampal circuit represents another model system in which the roles of facilitation as well as other STP components is starting to emerge. Previous studies have shown that excitatory hippocampal synapses work as an adaptive high-pass filter during natural spike trains, optimized for the transmission of high-frequency spike bursts that carry information about an animal's position in the environment known as place-fields.Citation8 This STP filter enables synapses to perform a highly nonlinear, switch-like operation permitting the passage and amplification of signals with place-field-like characteristics. Our recent computational analysis of STP function using a realistic model of synaptic dynamics at these excitatory synapses, has shown that facilitation is necessary for the rapid increase in synaptic strength in response to place-field discharges.Citation15 This rapid switch-like change in synaptic strength occurs at tens of milliseconds timescale and is essential for the proper tuning of the synaptic response in CA3-CA1 synapses to recognize and amplify activity patterns with place-field-like characteristics.Citation8,Citation15
In addition to these functions of facilitation in synaptic information processing, recent computational and experimental studies revealed potential roles of facilitation as a substrate for working memory and decision making in the cortexCitation43–Citation45 (see below for a detailed discussion). It is important to note, however, that the term facilitation is used broadly in the above studies of working memory and decision making to describe the entire phenomenon of short-term enhancement. The timescale of the described effects reaches several seconds and may include a substantial contribution from augmentation.
Facilitation thus emerges as an important contributor to various types of synaptic information processing and may mediate some of the complex cognitive functions in neocortical networks.
Functions of Augmentation in Synaptic Processing
Augmentation represents a slower component of presynaptic enhancement than facilitation and is also believed to act by potentiating vesicle fusion.Citation46 Augmentation builds up gradually during stimulus trains, and also decays much slower than facilitation, with a time constant of ∼5–8 sCitation2. Thus, augmentation is apparent following sustained, high-frequency stimulation,Citation3,Citation15 but the decay time constant of augmentation is independent of stimulus duration and frequency.Citation15 Similarly to facilitation, augmentation depends on residual calcium buildup in the presynaptic terminals during spike trains.Citation3 Several presynaptic mechanisms are thought to contribute to augmentation buildup in various synapses, including increased calcium influx due to action potential (AP) broadening,Citation47,Citation48 calcium-induced calcium release from internal storesCitation49 (but see a contrasting reportCitation50), increase in quantal size (vesicle-to-vesicle fusion),Citation51 and changes in the size of the readily-releasable pool (RRP).Citation2
Several diverse roles of augmentation in modulating synaptic function have been proposed. Because the time course of augmentation is comparable in many cases to that of short-term depression,Citation2,Citation3 augmentation was found to work as a main depression-counteracting mechanism to maintain the transmitter release during high levels of neural activity. At the frog neuromuscular junction (NMJ), the model system in which augmentation has been originally described,Citation52 augmentation was shown to sustain transmitter release during prolonged high-frequency activity by increasing the release probability of vesicles within the RRP.Citation53 Although augmentation is fully masked by the dominating depression in the NMJ under physiological conditions, it was found to maintain transmitter release even when depression reduces the RRP sufficiently to completely mask any apparent increase in synaptic strength.Citation53 Similarly, augmentation has been shown to control the fast rebound from depression at excitatory hippocampal synapsesCitation54 and is essential to sustain the elevated levels of release during high-frequency activity in these synapses.Citation15 In addition to augmentation's critical role in maintaining transmitter release, our recent studies at the hippocampal excitatory synapses showed that augmentation is tuned to precisely counterbalance depression during place-field discharges leading to their content-independent processing. As a result, place-field discharges are detected and amplified to a similar extent, independently of the particular temporal pattern within individual discharges.Citation8 Moreover, we found that this augmentation/depression balance controls the dynamic range of the synaptic filter, presumably to allow for robust place-field discharge recognition while limiting resource consumption.Citation15
Although augmentation has also been observed in some types of cortical synapses,Citation55 its contribution to cortical processing remains to be determined. As pointed out above, recent computational studies suggest that some forms of short-term synaptic enhancement operating on the timescale of seconds may function as cellular substrate for working memoryCitation43,Citation44 and decision making.Citation45 Since little is known about augmentation properties in cortical synapses, future studies will be needed to provide experimental support for these functions of augmentation.
Together, the rapidly expanding number of functions revealed for different forms of short-term enhancement emphasizes the importance of these STP components in many fundamental synaptic operations.
Diverse Roles of Depressionin Synaptic Computations
Short-term depression, which is represented by a transient decrease in synaptic strength during and after epochs of high-frequency stimulation, is commonly observed in most central synapses. Unlike components of short-term enhancement that have a predominately presynaptic origin, both pre- and postsynaptic mechanisms of short-term depression have been described. On the presynaptic site, the mechanisms responsible for short-term depression include depletion of the RRP, inactivation of release sites and inhibition or inactivation of calcium channels.Citation1–Citation4,Citation57,Citation58 Presynaptic forms of depression are interdependent with components of short-term enhancement via their common dependence on presynaptic calcium levels that regulate replenishment of vesicle pools, and determine vesicle use by controlling the release probability.Citation4,Citation59–Citation64 A number of postsynaptic processes such as receptor saturation and desensitization have also been found to contribute to short-term depression in several experimental systems.Citation3 However these mechanisms have been found not to play a significant role in STP in some central synapses, including the hippocampal ones.Citation56,Citation65,Citation66
A wide variety of different roles have been described for short-term depression in synaptic computations and information processing.Citation1,Citation4,Citation59 In cortical synapses, short-term depression has been shown to provide a dynamic gain control mechanism that allows equal percentage rate changes for inputs of different frequencies to produce equal postsynaptic responses.Citation67 This gain control mechanism is essential for proper scaling of synaptic inputs, since neurons in the central nervous system receive thousands of synaptic inputs at frequencies ranging from <1 Hz to >200 Hz. In the absence of such a subtle synaptic gain control mechanism, synaptic transmission would lose the dynamic fidelity of input information due to the large number of inputs and their broad spatial and temporal range. Unlike inhibitory and adaptive mechanisms that reduce responsiveness to all inputs, synaptic depression is input-specific, leading to a dramatic increase in the sensitivity of a neuron to subtle changes in the firing patterns of its inputs.Citation67
In the hippocampal excitatory synapses, we used a computational deletion analysis to understand the role of depression in the processing of natural patterns of neural activity.Citation15 We found that in the absence of depression, synaptic strength does not rapidly saturate during place-field discharges, like that observed under normal conditions, but rather continues to increase throughout the discharge. Depression thus controls the dynamic range of this synaptic filter and provides rapid response saturation, thereby preventing runaway synaptic enhancement.Citation15
Short-term synaptic depression has also been recognized to play important roles in the processing of sensory information. In the mammalian primary visual cortex, for example, short-term depression serves as an important element in the nonlinear temporal dynamics that leads to enhancement of transient responses, nonlinear temporal summation and direction selectivity.Citation9 Synaptic depression of thalamic input to the cortex contributes to the dynamic regulation of neuronal sensitivity during rapid changes in sensory input.Citation10 In the sound localization circuits of the brain stem, the synapses of the timing pathway are also characterized by strong short-term depression,Citation68 and the depression at the synapse onto coincidence-detection neurons in the nucleus laminaris provides an adaptive mechanism for preserving interaural time-delay information (a proxy for the location of sound in space), despite the confounding effects of sound-intensity-related information. This mechanism may help nucleus laminaris neurons pass specific sound-localization information to higher processing centers.Citation11 At the endbulb of Held (the synapse made by auditory-nerve fibers onto bushy cells of the cochlear nucleus), short-term depression enhances response probability or timing under different stimulus conditions.Citation69 The endbulbs of Held are chronically depressed even at low input rates corresponding to spontaneous activity. They exhibit an essentially rate-independent extent of depression during low activity, but are more resistant to further depression when driven by higher-frequency inputs corresponding to sound-evoked activity.Citation70 A somewhat less clear picture on the role of depression, however, emerges in studies of the calyx of Held, the synapse between globular bushy cells in the cochlear nucleus and the medial nucleus of the trapezoid body. This synapse is viewed as a highly reliable relay in the sound localization circuit of the auditory brainstem, with nearly every presynaptic action potential triggering a postsynaptic spike in vivo.Citation40 At high stimulation frequencies associated with sound-evoked activity, however, significant depression occursCitation4,Citation59 accompanied by increases in transmission delay and in the width of the postsynaptic response. Surprisingly, in vivo whole-cell recordings at these synapses show no evidence for a contribution of short-term depression to synaptic responses.Citation40 This observation in vivo could also be recreated in the slice recordings by mimicking the in vivo low release probability conditions through lowering external calcium concentration.Citation40 These recent results thus question the extent and the function of short-term depression in the calyx of Held synapses in vivo. It is possible, however, that depression is indeed present in vivo, but similar to the NMJ, the shift to lower release probabilities in vivo unmasks facilitation,Citation39 which may counterbalance effects of short-term depression under these physiological conditions.Citation39
Together, these recent studies provide evidence for the key roles of short-term depression in the processing of sensory information as well as for controlling a variety of synaptic operations in many neural circuits.
STP and Working Memory
Working memory (also termed short-term memory) is a system for temporarily storing and managing information required to carry out complex cognitive tasks such as learning, reasoning and comprehension.Citation72 Working memory is involved in the selection, initiation and termination of information processing tasks. Studies over the last several decades supported a view that enhanced AP firing activity in the neural networks in the form of persistent reverberation for several seconds serves as the neural correlate of working memory.Citation73 However, recent computational analyses of neural networks' ability to store and recall information have demonstrated that such working memory operations can be mediated instead by calcium-dependent synaptic facilitation in the recurrent connections of neocortical networks.Citation43,Citation44 The short-term increase in residual presynaptic calcium levels is used in this case as a memory buffer that transiently stores the memory of an item, which is then extracted from synaptic to spiking form by appropriately timed external signal. The duration of such working memory can be regulated by modulating the levels of spontaneous network activity. Such a role for STP in working memory is supported by the analysis of single neuron recordings in the prefrontal cortex of monkeys performing a delayed memory task. Population analysis of these recordings are consistent with the model in which the frequency of the stimulus is reflected in the graded facilitation profile of recurrent connections, and this facilitation state is subsequently extracted and translated into the firing rates by increasing excitatory input applied uniformly to the entire network.Citation43 At least from a theoretical standpoint, this STP-dependent type of working memory storage appears to be a more energy-efficient mechanism than network spiking reverberations.Citation74 This idea opens an intriguing possibility that the molecular factors that control presynaptic calcium dynamics (such as presynaptic calcium channels, calcium buffers and calcium sensors) might be involved in the modulation of working memory.
STP and Decision Making
Decision making is commonly viewed as a cognitive process resulting in the selection of a course of action among several alternatives.Citation75 During decision making, a common task involves comparing two sets of stimuli that occur sequentially at different times. Understanding the cellular and network basis of decision making then involves elucidating what operations are performed by synapses and neural networks to store the first set of stimuli in memory and then compare it with the second one. A recent study proposed that neurons encode the stimuli by the pattern of AP firing, and then use synaptic facilitation not only to temporarily store the first set of stimuli during the delay period (which would represent working memory) but also during the presentation of the second set of stimuli.Citation45 In this case, neurons could respond to a combination of both stimuli, as was observed for so-called “partial differential” neurons in the premotor cortex during vibrotactile decision making.Citation76 In other words, during the decision making process, facilitation-based working memory in partial differential neurons is involved in storing information about the first set of stimuli, thus allowing comparison with the second set of stimuli, which is then read out by applying an external non-specific stimulation to all neurons in the network. The resulting combination of the first and second stimuli provides important input to a subsequent attractor decision-making network that compares it with inputs from other neurons that respond only to the second stimulus.Citation45 While the facilitation-based model of decision making will require experimental verification, these recent studies argue for the importance of STP not only in certain forms of synaptic information processing, but also in fundamental cognitive processes including working memory and decision making. As pointed out above, however, the models that use facilitation to account for working memory and decision making require short-term enhancement on the timescale of several seconds and may thus include both facilitation and augmentation.
Summary
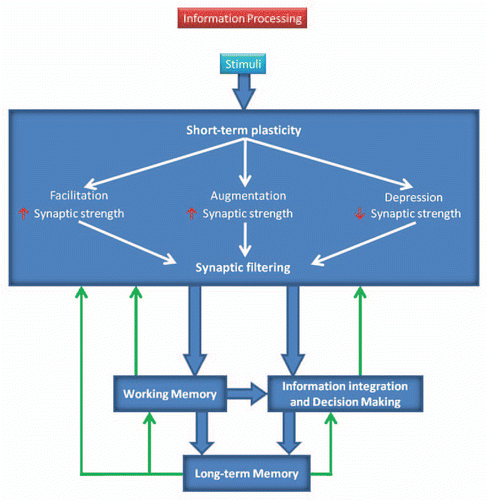
In summary, the diverse set of STP components and their interactions provide synapses with a powerful toolbox to perform synaptic computations. The differential expression and integration of STP components in different synapses enhances and sustains the synaptic input filtering functions and are used in various information processing operations. This information can be held as working memory by the neural circuits, and then can serve in the decision-making process. STP may thus be involved in the wide range of synaptic operations from sensory stimuli processing to decision making, as summarized in . Despite significant advances in understanding the roles of STP in synaptic computation and information processing, less is known about the precise molecular mechanisms responsible for regulating STP, such as, for example, modulation of presynaptic calcium channels, identity of calcium sensor(s), regulation and functional significance of heterogeneity of synaptic vesicles and release sites. Further studies will be needed to determine the significance of the regulatory factors that may control the presynaptic dynamic and tuning of the STP properties during computations.
Figures and Tables
Note and Acknowledgments
We apologize to those whose closely related studies could not be cited in this review due to space limitations. We thank Dr. V. Cavalli and D. Owyoung, for helpful comments on the manuscript. This work was supported in part by grants to V.K. from the Whitehall Foundation and the Mallinckrodt Foundation.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature 2004; 431:796 - 803
- Fioravante D, Regehr WG. Short-term forms of presynaptic plasticity. Curr Opin Neurobiol 2011; 21:269 - 274
- Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol 2002; 64:355 - 405
- von Gersdorff H, Borst JG. Short-term plasticity at the calyx of held. Nat Rev Neurosci 2002; 3:53 - 64
- Dittman JS, Kreitzer AC, Regehr WG. Interplay between facilitation, depression and residual calcium at three pre-synaptic terminals. J Neurosci 2000; 20:1374 - 1385
- Fortune ES, Rose GJ. Short-term synaptic plasticity as a temporal filter. Trends Neurosci 2001; 24:381 - 385
- Lewis JE, Maler L. Dynamics of electrosensory feedback: short-term plasticity and inhibition in a parallel fiber pathway. J Neurophysiol 2002; 88:1695 - 1706
- Klyachko VA, Stevens CF. Excitatory and feed-forward inhibitory hippocampal synapses work synergistically as an adaptive filter of natural spike trains. PLoS Biol 2006; 4:207
- Chance FS, Nelson SB, Abbott LF. Synaptic depression and the temporal response characteristics of V1 cells. J Neurosci 1998; 18:4785 - 4799
- Chung S, Li X, Nelson SB. Short-term depression at thalamocortical synapses contributes to rapid adaptation of cortical sensory responses in vivo. Neuron 2002; 34:437 - 446
- Cook DL, Schwindt PC, Grande LA, Spain WJ. Synaptic depression in the localization of sound. Nature 2003; 421:66 - 70
- MacLeod KM, Horiuchi TK, Carr CE. A role for short-term synaptic facilitation and depression in the processing of intensity information in the auditory brain stem. J Neurophysiol 2007; 97:2863 - 2874
- Taschenberger H, von Gersdorff H. Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics and synaptic plasticity. J Neurosci 2000; 20:9162 - 9173
- DeWeese MR, Hromadka T, Zador AM. Reliability and representational bandwidth in the auditory cortex. Neuron 2005; 48:479 - 488
- Kandaswamy U, Deng PY, Stevens CF, Klyachko VA. The role of presynaptic dynamics in processing of natural spike trains in hippocampal synapses. J Neurosci 2010; 30:15904 - 15914
- Silberberg G, Wu C, Markram H. Synaptic dynamics control the timing of neuronal excitation in the activated neocortical microcircuit. J Physiol 2004; 556:19 - 27
- Markram H, Pikus D, Gupta A, Tsodyks M. Potential for multiple mechanisms, phenomena and algorithms for synaptic plasticity at single synapses. Neuropharmacology 1998; 37:489 - 500
- Goldman MS, Maldonado P, Abbott LF. Redundancy reduction and sustained firing with stochastic depressing synapses. J Neurosci 2002; 22:584 - 591
- Varela JA, Sen K, Gibson J, Fost J, Abbott LF, Nelson SB. A quantitative description of short-term plasticity at excitatory synapses in layer 2/3 of rat primary visual cortex. J Neurosci 1997; 17:7926 - 7940
- Maass W, Zador AM. Dynamic stochastic synapses as computational units. Neural Comput 1999; 11:903 - 917
- Natschlager T, Maass W, Zador A. Efficient temporal processing with biologically realistic dynamic synapses. Network 2001; 12:75 - 87
- Zador A. Impact of synaptic unreliability on the information transmitted by spiking neurons. J Neurophysiol 1998; 79:1219 - 1229
- Tsodyks M, Pawelzik K, Markram H. Neural networks with dynamic synapses. Neural Comput 1998; 10:821 - 835
- Tsodyks MV, Markram H. The neural code between neocortical pyramidal neurons depends on neurotransmitter release probability. Proc Natl Acad Sci USA 1997; 94:719 - 723
- Loebel A, Tsodyks M. Computation by ensemble synchronization in recurrent networks with synaptic depression. J Comput Neurosci 2002; 13:111 - 124
- Fuhrmann G, Segev I, Markram H, Tsodyks M. Coding of temporal information by activity-dependent synapses. J Neurophysiol 2002; 87:140 - 148
- Hermann J, Grothe B, Klug A. Modeling short-term synaptic plasticity at the calyx of held using in vivo-like stimulation patterns. J Neurophysiol 2009; 101:20 - 30
- Pan B, Zucker RS. A general model of synaptic transmission and short-term plasticity. Neuron 2009; 62:539 - 554
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 2003; 38:79 - 88
- Matveev V, Zucker RS, Sherman A. Facilitation through buffer saturation: constraints on endogenous buffering properties. Biophys J 2004; 86:2691 - 2709
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 1998; 20:389 - 399
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron 2008; 59:882 - 901
- Inchauspe CG, Martini FJ, Forsythe ID, Uchitel OD. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of held presynaptic terminal. J Neurosci 2004; 24:10379 - 10383
- Ishikawa T, Kaneko M, Shin HS, Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J Physiol 2005; 568:199 - 209
- Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic Ca(V)2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron 2008; 57:210 - 216
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci 1996; 16:5661 - 5671
- Tang Y, Schlumpberger T, Kim T, Lueker M, Zucker RS. Effects of mobile buffers on facilitation: experimental and computational studies. Biophys J 2000; 78:2735 - 2751
- Macleod KM. Short-term synaptic plasticity and intensity coding. Hear Res 2011; 279:13 - 21
- Muller M, Goutman JD, Kochubey O, Schneggenburger R. Interaction between facilitation and depression at a large CNS synapse reveals mechanisms of short-term plasticity. J Neurosci 2010; 30:2007 - 2016
- Lorteije JA, Rusu SI, Kushmerick C, Borst JG. Reliability and precision of the mouse calyx of Held synapse. J Neurosci 2009; 29:13770 - 13784
- Tolnai S, Englitz B, Scholbach J, Jost J, Rubsamen R. Spike transmission delay at the calyx of Held in vivo: rate dependence, phenomenological modeling and relevance for sound localization. J Neurophysiol 2009; 102:1206 - 1217
- Hennig MH, Postlethwaite M, Forsythe ID, Graham BP. Interactions between multiple sources of short-term plasticity during evoked and spontaneous activity at the rat calyx of Held. J Physiol 2008; 586:3129 - 3146
- Barak O, Tsodyks M, Romo R. Neuronal population coding of parametric working memory. J Neurosci 2010; 30:9424 - 9430
- Mongillo G, Barak O, Tsodyks M. Synaptic theory of working memory. Science 2008; 319:1543 - 1546
- Deco G, Rolls ET, Romo R. Synaptic dynamics and decision making. Proc Natl Acad Sci USA 2010; 107:7545 - 7549
- Stevens CF, Wesseling JF. Augmentation is a potentiation of the exocytotic process. Neuron 1999; 22:139 - 146
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci 1997; 17:3425 - 3435
- Wang LY, Kaczmarek L. High-frequency firing helps replenish the readily releasable pool of synaptic vesicles. Nature 1998; 394:384 - 388
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry and spontaneous transmitter release. Neuron 2001; 29:197 - 208
- Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci 2002; 22:21 - 28
- He L, Xue L, Xu J, McNeil BD, Bai L, Melicoff E, et al. Compound vesicle fusion increases quantal size and potentiates synaptic transmission. Nature 2009; 459:93 - 97
- Magleby KL, Zengel JE. Augmentation: A process that acts to increase transmitter release at the frog neuromuscular junction. J Physiol 1976; 257:449 - 470
- Kalkstein JM, Magleby KL. Augmentation increases vesicular release probability in the presence of masking depression at the frog neuromuscular junction. J Neurosci 2004; 24:11391 - 11403
- Garcia-Perez E, Wesseling JF. Augmentation controls the fast rebound from depression at excitatory hippocampal synapses. J Neurophysiol 2008; 99:1770 - 1786
- Hempel CM, Hartman KH, Wang XJ, Turrigiano GG, Nelson SB. Multiple forms of short-term plasticity at excitatory synapses in rat medial prefrontal cortex. J Neurophysiol 2000; 83:3031 - 3041
- Klyachko VA, Stevens CF. Temperature-dependent shift of balance among the components of short-term plasticity in hippocampal synapses. J Neurosci 2006; 26:6945 - 6957
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron 1998; 20:797 - 807
- Xu J, Wu LG. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron 2005; 46:633 - 645
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci 2002; 25:206 - 212
- Wilkinson RS, Lin MY. Endocytosis and synaptic plasticity: might the tail wag the dog?. Trends Neurosci 2004; 27:171 - 174
- Xu J, He L, Wu LG. Role of Ca(2+) channels in short-term synaptic plasticity. Curr Opin Neurobiol 2007; 17:352 - 359
- Calakos N, Schoch S, Sudhof TC, Malenka RC. Multiple roles for the active zone protein RIM1alpha in late stages of neurotransmitter release. Neuron 2004; 42:889 - 896
- Schoch S, Castillo PE, Jo T, Mukherjee K, Geppert M, Wang Y, et al. RIM1alpha forms a protein scaffold for regulating neurotransmitter release at the active zone. Nature 2002; 415:321 - 326
- Hosoi N, Sakaba T, Neher E. Quantitative analysis of calcium-dependent vesicle recruitment and its functional role at the calyx of Held synapse. J Neurosci 2007; 27:14286 - 14298
- Wesseling JF, Lo DC. Limit on the role of activity in controlling the release-ready supply of synaptic vesicles. J Neurosci 2002; 22:9708 - 9720
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, et al. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 2008; 320:201 - 205
- Abbott LF, Sen K, Varela JA, Nelson SB. Synaptic depression and cortical gain control. Science 1997; 275:220 - 222
- Zhang S, Trussell LO. Voltage clamp analysis of excitatory synaptic transmission in the avian nucleus magnocellularis. J Physiol 1994; 480:123 - 136
- Yang H, Xu-Friedman MA. Impact of synaptic depression on spike timing at the endbulb of Held. J Neurophysiol 2009; 102:1699 - 1710
- Wang Y, Ren C, Manis PB. Endbulb synaptic depression within the range of presynaptic spontaneous firing and its impact on the firing reliability of cochlear nucleus bushy neurons. Hear Res 2010; 270:101 - 109
- Haustein MD, Reinert T, Warnatsch A, Englitz B, Dietz B, Robitzki A, et al. Synaptic transmission and short-term plasticity at the calyx of Held synapse revealed by multielectrode array recordings. J Neurosci Methods 2008; 174:227 - 236
- Baddeley A,, Hitch G. Bower GH. Working memory. Recent Advances in Learning and Motivation 1974; New York Academic Press 47 - 90
- Goldman-Rakic PS. Cellular basis of working memory. Neuron 1995; 14:477 - 485
- Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J Cereb Blood Flow Metab 2001; 21:1133 - 1145
- Wang XJ. Decision making in recurrent neuronal circuits. Neuron 2008; 60:215 - 234
- Romo R, Hernandez A, Zainos A. Neuronal correlates of a perceptual decision in ventral premotor cortex. Neuron 2004; 41:165 - 173
- Lisman JE. Bursts as a unit of neural information: making unreliable synapses reliable. Trends Neurosci 1997; 20:38 - 43
- Zador A, Dobrunz L. Dynamic synapses in the cortex. Neuron 1997; 19:1 - 4
- Fortune ES, Rose GJ. Short-term synaptic plasticity contributes to the temporal filtering of electrosensory information. J Neurosci 2000; 20:7122 - 7130