Abstract
Skin aging is a complex biological process influenced by a combination of endogenous or intrinsic and exogenous or extrinsic factors. Because of the fact that skin health and beauty is considered one of the principal factors representing overall “well-being” and the perception of “health” in humans, several anti-aging strategies have been developed during the last years. It is the intention of this article to review the most important anti-aging strategies that dermatologists have nowadays in hand, including including preventive measurements, cosmetological strategies, topical and systemic therapeutic agents and invasive procedures.
Introduction
Skin aging is a part of a natural human “aging mosaic” which becomes evident and follows different trajectories in different organs, tissues and cells with time. While the aging signs of internal organs are masked from the ambient “eyes,” the skin provides first obvious marks of the passing time.
Skin aging is a complex biological process influenced by combination of endogenous or intrinsic (genetics, cellular metabolism, hormone and metabolic processes) and exogenous or extrinsic (chronic light exposure, pollution, ionizing radiation, chemicals, toxins) factors.Citation1 These factors lead together to cumulative structural and physiological alterations and progressive changes in each skin layer as well as changes in skin appearance, especially, on the sun-exposed skin areas.Citation2-Citation12 In contrast to thin and atrophic, finely wrinkled and dry intrinsically aged skin, premature photoaged skin typically shows a thickened epidermis, mottled discoloration, deep wrinkles, laxity, dullness and roughness.Citation13-Citation18 Gradual loss of skin elasticity leads to the phenomenon of sagging.Citation19 Slowing of the epidermal turnover rate and cell cycle lengthening coincides with a slower wound healing and less effective desquamation in older adults. This fact is important when esthetic procedures are scheduled.Citation20 On the other side, many of these features are targets to product application or procedures to accelerate the cell cycle, in the belief that a faster turnover rate will yield improvement in skin appearance and will speed wound healing.Citation21 A marked loss of fibrillin-positive structuresCitation22 as well as a reduced content of collagen type VII (Col-7), may contribute to wrinkles by weakening the bond between dermis and epidermis of extrinsically age skin.Citation23 Sun-exposed aged skin is characterized by the solar elastosis. The sparse distribution and decrease in collagen content in photoaged skin can be due to increased collagen degradation by various matrix metalloproteinases, serine, and other proteases irrespective of the same collagen production.Citation24-Citation28 In older skin, collagen looks irregular and disorganized, the ratio of Col-3, to Col-1 has been shown to increase, due, significantly, to a loss of Col-1.Citation29 The overall collagen content per unit area of the skin surface is known to decline approximately 1%/year.Citation30 Glycosaminoglycans (GAGs) are among the primary dermal skin matrix constituents assisting in binding water. In photo-aged skin, GAGs may be associated with abnormal elastotic material and thus be unable to function effectively.Citation31 The total hyaluronic acid (HA) level in the dermis of skin that age intrinsically remains stable; however, epidermal HA diminishes markedly.Citation32
Three primary structural components of the dermis, collagen, elastin and GAGs have been the subjects of the majority of anti-aging research and efforts for aesthetic-anti-aging strategies pertaining to the skin, from ”anti-wrinkle creams” to various filling agents.Citation21
Presentation of aging of the entire face is associated with the gravity impact, muscles action, loss of volume, diminishing and redistribution of superficial and deep fat, loss of bony skeleton support what all together lead to the face sagging, changes in shape and contour. Regardless of the fact that aging is a biological inevitable process and not a pathological condition it is correlated with various skin and body pathologies, including degenerative disorders, benign and malignant neoplasms.
The ‘successful aging’ paradigm, focuses on health and active participation in life, counters traditional conceptualizations of aging as a time of disease and is increasingly equated with minimizing age signs on the skin, face and body.Citation33-Citation35 From this perspective, preventative aesthetic dermatology might supplement the request for healthy aging, treat or prevent certain cutaneous disorders, notably skin cancer, and delay skin aging combining local and systemic methods of therapy, instrumental devices and invasive procedures.Citation36,Citation37 The mainspring of any skin anti-aging therapy is to achieve a healthy, smooth, blemish-free, translucent and resilient skin.Citation38 In clinical practice, “to look better” doesn’t mean to “look younger.” That is why it is so important to understand patients’ wishes and to orientate them to the treatment modality that will give the most satisfying results whereas knowing all available treatment techniques.Citation39 The age, previous procedures or surgery, general health status, type of the skin, style of life and many other factors should be taken into consideration before choosing the strategy for the individual case. The desired therapeutic anti-aging effect of the skin is continuous, step-by step process, which combines various methods of the skin bio-revitalization and rejuvenation, augmentation, restoration of each skin layer individually and in the light of many other factors—from a style of the life to the immune, genetic, emotional and health status in general. This review will emphasize the most important topical and systemic therapeutic agents and trends in the use of invasive procedures.
Skin Aging Prevention and Therapy
The skin anti-aging strategies attempted to reverse the dermal and epidermal signs of photo- and chronological aging can be grouped under the following approaches ().
Table 1. Skin antiaging approaches
Skin Care
Healthy and functioning skin barrier is important protector against dehydration, penetration of various microorganisms, allergens, irritants, reactive oxygen species and radiation. The skin barrier may be specifically adjusted to allow penetration. For this reason daily skin care may increase skin regeneration, elasticity, smoothness, and thus temporarily change the skin condition.Citation40,Citation41 However, it is necessary to stop the degradation of the skin primary structural constituents, such as collagen, elastin, to prevent the formation of wrinkles. Although the technology required to suitably deliver these compounds into the skin has not yet been developed, some products do promote the natural synthesis of these substances except elastin enhancing.Citation42-Citation45 Another integral approach preventing wrinkle formation is the reduction of inflammation by topical or systemic antioxidants which should be used in combination with sunscreens and retinoids to enhance their protective effects.Citation21
Photoprotection and Systemic Antioxidants
Chronic photodamage of the skin manifests itself as extrinsic skin aging (photoageing). DNA photodamage and UV-generated reactive oxygen species (ROS) are the initial molecular events that lead to most of the typical histological and clinical manifestations of chronic photodamage of the skin. Wrinkling and pigmentary changes are directly associated with premature photo-aging and are considered its most important cutaneous manifestations. The strategies aimed at preventing photo-aging include sun avoidance, sun protection using sunscreens to block or reduce skin exposure to UV radiation, retinoids in order to inhibit collagenase synthesis and to promote collagen production, and anti-oxidants, particularly in combination, to reduce and neutralize free radicals (FR).Citation21,Citation46
Interventional studies indicate that it is in fact possible to delay skin aging and to improve skin conditions through administration of selected nutritional supplements. Nutritional antioxidants act through different mechanisms and in different compartments, but are mainly FR scavengers: (1) they directly neutralize FRs, (2) they reduce the peroxide concentrations and repair oxidized membranes, (3) they quench iron to decrease ROS production, (4) via lipid metabolism, short-chain free fatty acids and cholesteryl esters neutralize ROS.Citation47 Endogenous antioxidant defenses are both non-enzymatic (e.g., uric acid, glutathione, bilirubin, thiols, albumin, and nutritional factors, including vitamins and phenols) and enzymatic [e.g., superoxide dismutases, glutathione peroxidases (GSHPx), and catalase]. The most important source of antioxidants is provided by nutrition. To the most known systemic antioxidants belong vitamin C, vitamin E, carotenoids, and from the trace elements copper and selenium.Citation48-Citation50 There are also studies demonstrating that vitamins C and E combined with ferulic acid impart both a sunscreen and an anti-oxidant effect.Citation51
Topical Pharmacological Agents with Anti-Aging Properties
There are two main groups of agents that can be used as anti-aging cream components, the antioxidants and the cell regulators. The antioxidants, such as vitamins, polyphenols and flavonoids, reduce collagen degradation by reducing the concentration of FR in the tissues. The cell regulators, such as retinols, peptides and growth factors (GF), have direct effects on collagen metabolism and influence collagen production.
Vitamins C, B3, and E are the most important antioxidants because of their ability to penetrate the skin through their small molecular weight.Citation52 The water-soluble, heat-labile local L-ascorbic acid (vitamin C) in concentrations between 5 and 15% was proven to have a skin anti-aging effect by inducing the production of Col-1, and Col-3, as well as enzymes important for the production of collagen, and inhibitors of matrixmetalloproteinase (MMP) 1 (collagenase 1).Citation43,Citation53 Clinical studies have proven that the antioxidative protection is higher with the combination of vitamins C and E than with the vitamin C or E alone.Citation54,Citation55 Niacinamide (vitamin B3) regulates cell metabolism and regeneration, and it is used in 5% concentration as an anti-aging agent.Citation56 In some studies, improvement of skin elasticity, erythema and pigmentations after 3 mo of topical treatment has been observed.Citation52,Citation54 Vitamin E (α-tocopherol) used as a component of skin products has anti-inflammatory and antiproliferative effects in concentrations between 2 and 20%. It acts by smoothing the skin and increasing the ability of the stratum corneum to maintain its humidity, to accelerate the epithelialization, and contribute to photoprotection of the skin. The effects are not as strong as with vitamins C and B3.Citation57
An in vivo study has proven that the topical application of green tea polyphenols before UV exposure leads to an increase of the minimal erythema dose, decreases the number of Langerhans cells and reduces DNA damage in the skin.Citation58 Other botanicals that act as antioxidants are for example the isoflavones from soya.
Cell regulators, such as vitamin A derivatives, polypetides and botanicals, act directly on the collagen metabolism and stimulate the production of collagen and elastic fibers.
Vitamin A (retinol) and its derivates (retinaldehyde and tretinoin) are also a group of agents with antioxidant effects. They can induce the biosynthesis of collagen and reduce the expression of MMP 1 (collagenase 1). Retinol is, at the moment, the substance that is most often used as an anti-aging compound and, compared with tretinoin, causes less skin irritation.Citation59,Citation60 It has been shown that retinol has positive effects not only on extrinsic but also on intrinsic skin aging and has a strong positive effect on collagen metabolism.Citation60,Citation61 Tretinoin, a nonaromatic retinoid of the first generation, is approved for application as an anti-aging treatment in a concentration of 0.05% in the United States. It has been shown to be able to reduce the signs of UV-induced early skin aging, such as wrinkles, loss of skin elasticity and pigmentation.
Polypeptides or oligopeptides are composed of amino acids and can imitate a peptide sequence of molecules such as collagen or elastin. Through topical application, polypeptides have the ability to stimulate collagen synthesis and activate dermal metabolism.Citation62
Invasive Procedures
There are various in-office procedures, most of which are intended to ‘resurface’ the epidermis: to remove the damaged epidermis and replace the tissue with remodeled skin layers and sometimes spur the formation of new collagen.Citation21,Citation63 It is possible that the potential of GF, cytokines and telomerase will eventually be harnessed via technological advancement and innovation in the burgeoning fields of tissue engineering and gene therapy in the nearest future.Citation64
Chemical Peels
Chemical peels are methods to cause a chemical ablation of defined skin layers to induce an even and tight skin as a result of the regeneration and repair mechanisms after the inflammation of the epidermis and dermis. Chemical peels are classified into three categories.Citation65,Citation66 Superficial peels [α-β-, lipo-hydroxy acids (HA), trichloroacetic acid (TCA) 10–30%] exfoliate epidermal layers without going beyond the basal layer; medium-depth peels (TCA above 30 to 50%) reach the upper reticular dermis; deep peels (TCA > 50%, phenol) penetrate the lower reticular dermis. The depth of peeling depends not on the substance used only, but on its concentration, pH of the solution and time of application.Citation66 A number of skin modifications have been reported after several weeks: epidermal architecture returns to normal, melanocytes are present and distributed uniformly, basal cells contain small melanin grains distributed homogeneously, the thickness of the basal membrane is homogeneous, in the dermis, a new sub epidermal band of collagen appears, elastic fibers form a new network, often parallel to those of collagen.Citation67 If superficial peelings target the corneosomes, cause desquamation, increase epidermal activity of enzymes, lead to epidermolysis and exfoliation,Citation68,Citation69 medium-depth peels cause coagulation of membrane proteins, destroy living cells of the epidermis and, depending on the concentration, the dermis. Deep peels coagulate proteins and produce complete epidermolysis, restructure of the basal layer and restoration of the dermal architecture.Citation69 The depth of peel correlates with the potential side-effects, like hyperpigmentation, solar lentigines, risk of post-operative infections, especially herpetic ones.Citation66,Citation70 The mechanism by which the chemical peel takes effect is not clearly elucidated. An increase in collagen fiber content, water and GAG in the dermis has been reported.Citation71,Citation72 There is a suggestion that improvements in skin elasticity and wrinkles after chemical peeling can be attributed to increase of Col-1 with or without Col-3, elastic fibers, as well of a dense rearrangement of collagen fibers.Citation73-Citation76
Visible Light Devices: IPL, Lasers, RF for the Skin Rejuvenation, Resurfacing and Tightening
Nonablative skin rejuvenation or “subsurfacing” comes as a low risk and short downtime technology which can improve aging structural changes without disruption of cutaneous integrity.Citation77 The mechanism of action is supposed to be a selective, heat induced denaturalization of dermal collagen that leads to subsequent reactive synthesis. Nonablative skin rejuvenation is not a precise term since rejuvenation is a controlled form of skin wounding aimed at achieving a more youthful appearance after the wound heals.Citation39
Treatment of photoaged skin has been divided into treatment of ectatic vessels and erythema, irregular pigmentation, and pilosebaceous changes (Type I) and into the improvement of the dermal and subcutaneous senescence (Type II).Citation77 The epidermis and superficial dermis can be selectively damaged by two basic mechanisms: (a) by targeting discrete chromophores in the dermis or at the dermal-epidermal junction or (b) by utilizing mid infrared (IR) lasers.Citation78
The devices for treatment of vascular and/or pigment irregularities include lasers emitting light at 532-, 585-, 595-, 755-, 800-, and 1064-nm wavelengths as well as filtered light generated by IPL systems equipped with different cut-off filtersCitation39,Citation79(). Lasers emitting 1,320,Citation80 1,450,Citation81 and 1,540 nmCitation82 using interstitial and intracellular water as target chromophores and pulsed dye lasers (PDL)Citation83 using oxyhemoglobin as the primary chromophore are now employed for Type II photo rejuvenation only. The clinical efficacy of these nonablative modalities are weaker than that of the ablative methods, however, new collagen formation and clinically observable improvement in wrinkles can be observed.Citation84,Citation85 Reduction of facial wrinkles by using IPL devices has shown less effect comparing to laser technology,Citation86 but for type I photo rejuvenation, IPL systems have in general shown considerably better results than laser systems operating at subpurpuric energy levels.Citation87-Citation90 Ultrastructural and histological analysis confirmed effectiveness of absorption of light (532, 585, 595, with or without 1064-nm Nd:YAG laser) in the blood vessels of the superficial dermis, resulting in the release of inflammatory mediators and GF into the interstitium followed by stimulated fibroblast activity and initiation of tissue repair and enhanced collagen and elastin neoformation replacing the originally damaged elastic tissue.Citation84,Citation91,Citation92 An increase in grenz zone thickness,Citation91 monoclonal chondroitin sulfate and III procollagen staining as well as quantification of Col-1Citation93 was measured after couple of treatments with PDL. The increase in dermal collagen has also been confirmed by noninvasive ultrasonographic analysisCitation94 and radioimmunoassay.Citation95 Nonablative skin rejuvenation should not yet be considered an alternative for laser resurfacing.Citation39 However there are interesting data showing comparative histological changes between the ablative and nonablative modalities.Citation96
Figure 1. 45-y-old female with signs of photoaged skin: dyschromia of the skin, multiple lentigines. (A) before, (B) after one treatment with IPL with 550 nm cut-off filter.
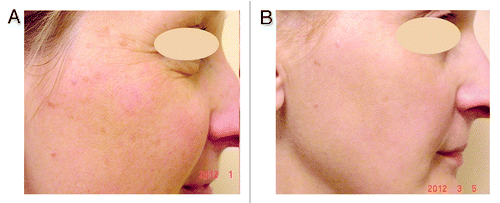
Histological sections of skin before and after treatment with the different IPL devices have shown the formation of new collagen in the papillary and reticular dermis, as well as an increase in the number of fibroblasts and proportional decrease in the amount of solar elastosis is also usually found.Citation92,Citation97-Citation99 If vascular and/or pigment disturbances improvement are immediate, the collagen remodeling response is delayed and maximum results are seen only between 3 and 12 mo after treatment.Citation39
Laser resurfacing has been shown to be effective in counteracting photoaging through entire epidermal ablation, collagen shrinkage, stimulation of neocollagenesis, extensive dermal remodeling, regeneration of cellular organelles and intercellular attachmentsCitation100 but parallelly, results in long recovery time are associated with risks of severe long lasting side effects, such as persistent erythema, hypo- or hyperpigmentation, infection or scarring.Citation101-Citation104
Recently, fractionated CO2-, erbium glass or erbium-YAG lasers have been introduced to reduce downtime and side effects.Citation105 These devices emit light in a pixilated fashion onto the skin, producing an array of microthermal zones in the dermis.Citation105-Citation108 The controlled thermal stress to the epidermis and the dermal compartment is followed by a wound healing response ultimately leading to re-epithelization and dermal remodeling.Citation109
Although the underlying molecular changes induced by different ablative and non-ablative as well as thermal and non-thermal skin rejuvenation treatments are not fully understood, there are investigations suggesting important roles of heat shock proteins (HSP), transforming growth factor β (TGF-β), different MMPs, synthethases, hyals and hyaluronic acid (HA).Citation109-Citation113 Type I and type III procollagen mRNA was also elevated for at least 6 mo.Citation114
Monopolar RF is a noninvasive way to obtain skin tighteningCitation39 and immediate collagen contraction with a single treatment. Unlike lasers, the RF technology produces electric current, which generates heat through resistance in the dermis and as deep as the subcutaneous fat.Citation78 Unfortunately there is a lack of long-term studies of efficacy and analysis of side effects for the skin using this method of skin rejuvenation.
It is obvious that different treatment modalities using visible light devices have resulted in varying clinical effects and allow to select individual treatment parameters for different indications.Citation115 For this reason, careful simultaneous evaluation of any pigment disturbances, vascular abnormalities, wrinkles, and cutaneous sagging as skin layers are all linked is highly recommended.
Injectable Skin Rejuvenation and Dermal Fillers
The goal of skin biorejuvenation is to increase the biosynthetic capacity of fibroblasts, inducing the reconstruction of an optimal physiologic environment, the enhancement of cell activity, hydration, and the synthesis of collagen, elastin and HA (hyalorunic acid). The desired effect could be achieved by the microinjections in the superficial dermis of products containing only one active ingredient or cocktails of different compounds which are perfectly biocompatible and totally absorbable: HA, vitamins, minerals, nutrients, hormones, GF, amino acids, autologous cultured fibroblasts, homeopathic products, etc.Citation116-Citation121 The distinct formulations can induce strikingly divergent molecular and cellular processes in fibroblasts in vitro.Citation122 However, more detailed studies are required to elucidate whether and how the cellular and molecular processes are involved in facial skin rejuvenation in vivo, whether these processes are similarly efficient, independent of the age of the patients. The proof of concept, including long-term efficiency, optimal injecting protocols are still lacking too.Citation123,Citation124
Products injected within or beneath the skin to improve its physical features by soft tissue augmentation are known as fillers.Citation125-Citation129 There are autologous (fat, cultured human fibroblasts), collagen (bovine-derived, human-derived from tissue culture), HA (nonanimal stabilized or viscoelastic HA from bacterial fermentation), synthetic or pseudo-synthetic implants (silicone, polymethacrylate microspheres, poly-L-lactic acid, calcium hydroxylapatite microspheres suspended in aqueous polysaccharide gel, alkyl-imide gel polymer). These may be grouped into temporary, semipermanent (lasting between 1–2 y), or permanent materials (lasting longer than 2 y).
GAG and particularly HA or hyaluronan are major components of the cutaneous extracellular matrix involved in tissue repair of all animal tissues.Citation130-Citation132 HA exhibits no species or tissue specificity. As a physical background material, it has functions in space filling, lubrication, shock absorption, and protein exclusion. In addition, HA has been implicated as a regulator of cell proliferation and locomotion.Citation133-Citation135 Injection of HA is thought to promote skin rejuvenation by increasing both hydration and fibroblast activation.Citation136-Citation140 HA injected into the skin can stimulate fibroblasts to express Col-1, MMP-1 and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1)Citation122,Citation141 as well as is participating in wound healing, modulation of inflammatory cells, interaction with proteoglycans of the extracellular matrix, and scavenging of FR.Citation142 All these features of HA have made it to be useful as an ideal structural compound and have raised injections of HA products to the most acceptable and scientifically investigated “gold standard” procedures for skin rejuvenation and augmentation ().
Figure 2. Patient showing the difference of the nasolabial fold: non-treated left side (with site marks for planned HA injection) and right side straight after injection of only 0.5 ml of nonanimal stabilized cross-linked HA (“Stalagmite” technique on the right cheek).
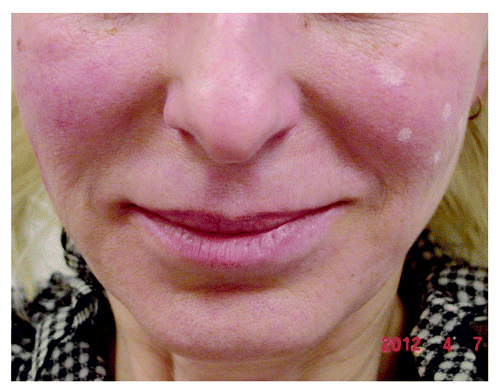
Natural HA has a half-life in tissue of only 1 to 2 d before undergoing aqueous dilution and enzyme degradation in the liver to carbon dioxide and water.Citation143 Produced from bacterial (Staphylococcus equine) fermentation and modified by chemical cross-linking to improve their resistance to enzymatic degradation and prolong their effect, non-animal reticulated HA fillers are more pure, more viscous, usually well tolerated and rarely elicit adverse and immunological reactions.Citation130,Citation144-Citation146 The duration of effect for HA fillers ranges from 3 to 12 mo. The long-lasting dermal fillers maintain the position 1–2 y or even more.Citation147 Modern HA fillers differ in the particulate size, cross-linking and the type of cross-linking agent used in the HA; phasic structure—mono/biphasic, concentration of HA and presence of an anesthetic agent in each syringe.Citation148-Citation151 Besides composition, currently available products differ based on approved indications, duration of aesthetic effect, putative mode of operation, recommended depth of product placement, injection technique, suitability for different facial areas, and common adverse events.Citation152
One of long-lasting synthetic semi-permanent dermal fillers is calcium hydroxyl apatite based filler (CaHA) suspended in an aqueous carboxymethylcelluose gel carrier.Citation150-Citation155 The CaHA particles act as a scaffold for new tissue formation and stimulate collagen formation around the microspheres leading to a thickening of the dermis over time.Citation147 The spherical CaHA particles are gradually phagocytosed, degraded as calcium and phosphate and eliminated via the renal system. CaHA is biocompatible with an identical composition to bones with a low potential for antigenicity, foreign body reaction, and minimal inflammatory response. No osteoblast activity has been observed in soft tissue.Citation155
The application of poly-L-lactic acid (PLA) in soft tissue augmentation exploits a mechanism of action not seen in any other soft tissue filler like a treatment plan, preparation of injection material, and injection technique is distinct as well.Citation156 After the initial response lasting one week or less a delayed but progressive volumizing effect begins.Citation157 The process of hydration, loss of cohesion and molecular weight, and solubilization and phagocytosis of PLA by the host’s macrophages, degrades PLA into lactic acid microspheres and eliminates CO2 by way of respiratory excretion. Crystals are left behind to stimulate collagen and a granulomatous reaction. This inflammatory reaction elicits resorbtion and the formation of fibrous connective tissue about the foreign body, causing dermal fibroplasia that leads to the desired cosmetic effect.Citation158
Although subjective patient satisfaction is high in many of the studies with skin fillers and skin thickness as measured using wrinkle scale ratings of appearance, long-term efficacy and clinical safety data are lacking because patients are likely to continue to undergo subsequent cosmetic interventions.Citation159
Autologous Platelet-Rich Plasma (PRP)
Autologous Platelet-rich Plasma (PRP) has attracted attention for skin rejuvenation. PRP is derived from fresh whole blood, which contains a high concentration of platelets.Citation160 Various GF, including platelet-derived growth factor (PDGF), transforming growth factor (TGF), vascular endothelial growth factor (VEGF), and insulin-like growth factor (IGF), are secreted from the α-granules of concentrated platelets activated by aggregation inducers.Citation161 These factors are known to regulate processes including cell migration, attachment, proliferation and differentiation, and promote extracellular matrix (ECM) accumulation by binding to specific cell surface receptors.Citation162,Citation163 It has been shown that PRP may induce the synthesis of collagen and other matrix components by stimulating the activation of fibroblasts, thus, rejuvenating the skin.Citation164-Citation167 However, the molecular mechanisms underlying PRP-inducing wound healing processes are still largely unknown and experimental studies confirming the effects of PRP on aged fibroblasts are very limited.
Botulinum Toxin
Botulinum toxin (BTX) has no effect on skin texture and cannot discontinue the skin aging process. However, regular BTX injections can slow down the visible aging process by helping in the management of certain dynamic facial lines and wrinklesCitation168-Citation170(). Current treatment options of exaggerated frown lines, glabellar lines or crow feet such as surgery or implants, do not address the underlying cause of these lines, namely the excessive nerve stimulation. The mechanism of action of BTX makes it an ideal agent to target the major cause of these dynamic lines.Citation171
Figure 3. Patient showing glabellar and crow’s feet wrinkles. (A) pre-injection, (B) after injection with botulinum toxin.
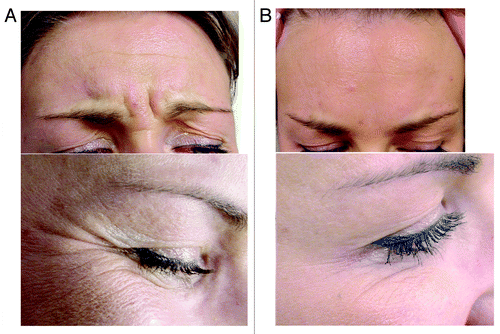
Seven constitutionally similar but antigenically distinct subtypes of neurotoxin (A-G) are produced by different strains of the anaerobic, gram-positive bacterium Clostridium botulinum.Citation172-Citation177 BTX- subtype A (BTX-A) is the most potent. BTX-A produces temporary chemical denervation by blocking the presynaptic release of acetylcholine (Ach) at the neuromuscular junction (NMJ).Citation178 The specific heavy chain is associated with the internalization of the toxin and binds it irreversibly to the motor nerve end-plates with a high affinity to specific receptors (sialoglycoproteins) in the plasma membrane of cholinergic nerve endings. This induces receptor-mediated endocytosis of the toxin. The light chain that is responsible for the toxicity splits off in the cell, and inactivates a synapse-specific protein synaptosomal-associated protein of 25 kDa (SNAP-25) which is one of several proteins required for Ach exocytosis and release into the NMJ.Citation179
The toxin binds to presynaptic neurons of selected muscles rapidly (under an hour) and specifically. Clinically reversible chemical denervation and selective muscle relaxation or paralysis starts after 24 to 48 h and may not be completed for up to 2 weeks.Citation173-Citation177 In muscle, approximately on day 28, nerve sprouts mediate a partial restoration and new neuromuscular junctions are formed in the vicinity of the old junctions. Another factor explaining the regaining of muscle function could be an increase in the area of muscle membrane sensitive to acetylcholine.Citation180 On days 62–91, complete muscle function recovery can be demonstrated.Citation179,Citation181
Muscular changes in the form of atrophy were demonstrated in animal studies, and were completely reversible after 4–6 mo. In human muscle, no lasting atrophy could be detected even after repeated injections, only a predominance of type I fibers.Citation179 The usual duration of effect is 3–6 mo with individual variations.Citation173-Citation177
Dosing of BTX-A is essential in achieving precise and predictable effects. The biological activity given in mouse units (MU) and the weight of the molecule is not associated with the dosage. One MU is equivalent to the amount of toxin at which, after intraperitoneal administration, half of the poisoned Swiss-Webster mice die (50% lethal dose; LD50).Citation182 The amounts of BTX-A used for the treatment are 25–100 times less than the LD50, so that the FDA classifies BTX-A as therapeutically safe.Citation183 BTX-A does not cross the blood-brain barrier or pass through the skin.Citation179
Several commercial preparations of BTX-A products which are produced from different strains of bacteria by different purification methods and therefore have distinct components and properties, requirements of storage, shelf-life, and dose are currently available for aesthetic uses.Citation184,Citation185
A thorough understanding and evaluation of the relevant anatomy and physiology of the muscles and possible alterations in the area to be injected is essential. Dosage for the patients depends on the area, muscle mass, gender and other factors individually. Contraindications include conditions of peripheral motor neuropathic diseases or neuromuscular functional disorders, coadministration with aminoglycoside antibiotics or other agents that interfere with neuromuscular transmission and may potentiate general weakness, treatment of patients with inflammatory skin disorders at the injection site, history of reaction to toxin, pregnancy and lactation, age younger than 12 y, participation in occupations that necessitate a wide range of facial expressions.Citation171,Citation186-Citation188
Given the short-term and localized effects of BTX-A injections, it is reassuring that any potential adverse reactions known to date may also be short lived, localized, and reversible in a dose-dependent period of 6–8 weeks. Systemic or serious side effects in general are rare, immune-mediated disorders or other idiosyncratic reactions are unknown.Citation189,Citation190 The development of antibodies to BTX-A may be related to exposure to high doses of toxin and seems to be related to decreased BTX-A efficacy.Citation191,Citation192 Current batches of BTX-A (manufactured after 1997) have lower albumin concentration and higher toxin-specific activity, which may contribute to reduced clinical antigenicity.Citation182,Citation193,Citation194
The incidence of complications in many cases depends on the proper application and the qualification of the physician. However, it has always to be considered that the benefits of this treatment are transient and repeated injections are necessary for a long-term effect.Citation195
Hormone Replacement Therapy (HRT)
It is well known that there is a progressive decrease of hormone synthesis with age. Levels of growth hormone (GH) and insulin-like growth factor-1 (IGF-1), melatonin (nocturnal), TSH, thyroid hormones (T3), dehydroepiandrosterone (DHEA) (sulphated form and its urinary 17-keto-metabolites), estrogens and testosterone are progressively decreasing. The main hormonal deficits in humans are menopause, andropause and partial androgen deficiency of the aging male.Citation196-Citation199 DHEA substitution has been proven to lead to an improvement of body condition, sexual activity, bone density, and well-being.Citation200
In a randomized and placebo-controlled study of 280 older men and women (60–79 y of age), each subject received 50 mg of DHEA daily for a year. The women showed an improvement of the libido, skin health, and osseous density.Citation201-Citation203Furthermore, another study conducted by Rudman et al. has pointed out that the application of GH decreased the signs of biological aging. The treatment led to an improved body condition, with an increase of muscle mass and osseous density and a decrease of adipose tissue. Moreover, an increase of skin thickness was observed.Citation199
Melatonin has been shown to have a favorable influence on the aging process, because it has an inverse effect with regard to body weight; food restriction raises the levels of melatonin and decreases its age-related decrease. With increasing age comes a decrease of melatonin production, which may have a connection to sleep disorders suffered by elderly people. It also has be shown that melatonin can prevent tumor development and growth. Interestingly, a study showed that patients with tumors had decreased levels of melatonin compared with healthy individuals.Citation204-Citation207
HRT with testosterone is absolutely indicated in older men who are either symptomatic or have a low serum testosterone level. Either a decrease of testosterone or a loss of the circadian rhythm of testosterone secretion has been observed in a high percentage of older men. Clinical symptoms include general weakness, sexual dysfunction, diminished muscle and bone mass, and decreased erythropoiesis. A low testosterone level has been shown in epidemiological studies to lead to a higher morbidity and mortality rate and to a higher prevalence of depression, coronary heart disease, and osteoporosis. Insulin resistance has been shown to play an important role in the development of hypogonadism in older men. Thus, obese men and men with type 2 diabetes, show significantly lower testosterone levels compared with subjects in control groups.Citation199
HRT with estrogen and progesterone has been long considered to have anti-aging effects; results of larger studies though, particularly of the Women’s Health Initiative, have shown that an anti-aging effect is not necessarily to be expected. On the contrary, HRT has been accused to have a higher cardiovascular risk and increase of the risk of breast cancer. However, it has clear, positive preventive effects on osteoporosis, and an early, low-dose estrogen monotherapy can be considered to have advantages.Citation208
Conclusions
While natural aging is genetically determined, extrinsic aging can be prevented. Aesthetic dermatology should contribute to “healthy aging” not only in cosmetic means by trying to erase time vestiges in skin but by also playing a significant part in prevention, regeneration, and delaying of skin aging combining knowledge of possible local and systemic therapy, instrumental devices and invasive procedures, filling the lack of scientific investigations and becoming one of the important focuses of the aging research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Cevenini E, Invidia L, Lescai F, Salvioli S, Tieri P, Castellani G, et al. Human models of aging and longevity. Expert Opin Biol Ther 2008; 8:1393 - 405; http://dx.doi.org/10.1517/14712598.8.9.1393; PMID: 18694357
- Uitto J. Understanding premature skin aging. N Engl J Med 1997; 337:1463 - 5; http://dx.doi.org/10.1056/NEJM199711133372011; PMID: 9358147
- Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science 2006; 312:1059 - 63; http://dx.doi.org/10.1126/science.1127168; PMID: 16645051
- Fisher, G.J. The pathophysiology of photoaging of the skin. Cutis, 75, 5–9 (2005)58–69.
- Schmuth M, Watson RE, Deplewski D, Dubrac S, Zouboulis CC, Griffiths CE. Nuclear hormone receptors in human skin. Horm Metab Res 2007; 39:96 - 105; http://dx.doi.org/10.1055/s-2007-961808; PMID: 17326005
- Reichrath J, Lehmann B, Carlberg C, Varani J, Zouboulis CC. Vitamins as hormones. Horm Metab Res 2007; 39:71 - 84; http://dx.doi.org/10.1055/s-2007-958715; PMID: 17326003
- Verdier-Sévrain S, Bonté F, Gilchrest B. Biology of estrogens in skin: implications for skin aging. Exp Dermatol 2006; 15:83 - 94; http://dx.doi.org/10.1111/j.1600-0625.2005.00377.x; PMID: 16433679
- Hall G, Phillips TJ. Estrogen and skin: the effects of estrogen, menopause, and hormone replacement therapy on the skin. J Am Acad Dermatol 2005; 53:555 - 68, quiz 569-72; http://dx.doi.org/10.1016/j.jaad.2004.08.039; PMID: 16198774
- Brincat MP, Baron YM, Galea R. Estrogens and the skin. Climacteric 2005; 8:110 - 23; http://dx.doi.org/10.1080/13697130500118100; PMID: 16096167
- Draelos ZD. Topical and oral estrogens revisited for antiaging purposes. Fertil Steril 2005; 84:291 - 2, discussion 295; http://dx.doi.org/10.1016/j.fertnstert.2005.03.033; PMID: 16084864
- Kanda N, Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci 2005; 38:1 - 7; http://dx.doi.org/10.1016/j.jdermsci.2004.10.011; PMID: 15795118
- Shin MH, Rhie GE, Park CH, Kim KH, Cho KH, Eun HC, et al. Modulation of collagen metabolism by the topical application of dehydroepiandrosterone to human skin. J Invest Dermatol 2005; 124:315 - 23; http://dx.doi.org/10.1111/j.0022-202X.2004.23588.x; PMID: 15675949
- Kligman LH. Photoaging. Manifestations, prevention, and treatment. Clin Geriatr Med 1989; 5:235 - 51; PMID: 2646001
- El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol 2002; 11:398 - 405; http://dx.doi.org/10.1034/j.1600-0625.2002.110502.x; PMID: 12366692
- Moragas A, Castells C, Sans M. Mathematical morphologic analysis of aging-related epidermal changes. Anal Quant Cytol Histol 1993; 15:75 - 82; PMID: 8318130
- Lock-Andersen J, Therkildsen P, de Fine Olivarius F, Gniadecka M, Dahlstrøm K, Poulsen T, et al. Epidermal thickness, skin pigmentation and constitutive photosensitivity. Photodermatol Photoimmunol Photomed 1997; 13:153 - 8; http://dx.doi.org/10.1111/j.1600-0781.1997.tb00220.x; PMID: 9453085
- Makrantonaki E, Zouboulis CC. William J. Cunliffe Scientific Awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology 2007; 214:352 - 60; http://dx.doi.org/10.1159/000100890; PMID: 17460411
- Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci 2007; 1119:40 - 50; http://dx.doi.org/10.1196/annals.1404.027; PMID: 18056953
- Escoffier C, de Rigal J, Rochefort A, Vasselet R, Lévêque JL, Agache PG. Age-related mechanical properties of human skin: an in vivo study. J Invest Dermatol 1989; 93:353 - 7; http://dx.doi.org/10.1111/1523-1747.ep12280259; PMID: 2768836
- Yaar M, Gilchrest BA. Aging of skin. In Fitzpatrick’s Dermatology in General Medicine Vol 2, 5th edn. McGraw-Hill:New York, 1999; 1697–1706.
- Baumann L. Skin ageing and its treatment. J Pathol 2007; 211:241 - 51; http://dx.doi.org/10.1002/path.2098; PMID: 17200942
- Watson RE, Craven NM, Kang S, Jones CJ, Kielty CM, Griffiths CE. A short-term screening protocol, using fibrillin-1 as a reporter molecule, for photoaging repair agents. J Invest Dermatol 2001; 116:672 - 8; http://dx.doi.org/10.1046/j.1523-1747.2001.01322.x; PMID: 11348454
- Contet-Audonneau JL, Jeanmaire C, Pauly G. A histological study of human wrinkle structures: comparison between sun-exposed areas of the face, with or without wrinkles, and sun-protected areas. Br J Dermatol 1999; 140:1038 - 47; http://dx.doi.org/10.1046/j.1365-2133.1999.02901.x; PMID: 10354068
- Varani J, Spearman D, Perone P, Fligiel SE, Datta SC, Wang ZQ, et al. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol 2001; 158:931 - 42; http://dx.doi.org/10.1016/S0002-9440(10)64040-0; PMID: 11238041
- Brenneisen P, Oh J, Wlaschek M, Wenk J, Briviba K, Hommel C, et al. Ultraviolet B wavelength dependence for the regulation of two major matrix-metalloproteinases and their inhibitor TIMP-1 in human dermal fibroblasts. Photochem Photobiol 1996; 64:877 - 85; http://dx.doi.org/10.1111/j.1751-1097.1996.tb01851.x; PMID: 8931389
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996; 379:335 - 9; http://dx.doi.org/10.1038/379335a0; PMID: 8552187
- Fisher GJ, Wang ZQ, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. N Engl J Med 1997; 337:1419 - 28; http://dx.doi.org/10.1056/NEJM199711133372003; PMID: 9358139
- Scharffetter-Kochanek K, Wlaschek M, Briviba K, Sies H. Singlet oxygen induces collagenase expression in human skin fibroblasts. FEBS Lett 1993; 331:304 - 6; http://dx.doi.org/10.1016/0014-5793(93)80357-Z; PMID: 8375513
- Oikarinen A. The aging of skin: chronoaging versus photoaging. Photodermatol Photoimmunol Photomed 1990; 7:3 - 4; PMID: 2371168
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol 1975; 93:639 - 43; http://dx.doi.org/10.1111/j.1365-2133.1975.tb05113.x; PMID: 1220811
- Bernstein EF, Underhill CB, Hahn PJ, Brown DB, Uitto J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br J Dermatol 1996; 135:255 - 62; http://dx.doi.org/10.1111/j.1365-2133.1996.tb01156.x; PMID: 8881669
- Elsner P, Maibach HI. Cosmeceuticals and Active Cosmetics: Drugs versus Cosmetics (2nd edn). Marcel Dekker: New York, 2005.
- Calasanti TM, Slevin KF, King N. Ageism and feminism: from ‘et cetera’ to center. NWSA J 2006; 18:13 - 30; http://dx.doi.org/10.2979/NWS.2006.18.1.13
- Bayer K. Cosmetic surgery and cosmetics: redefining the appearance of age. Generations 2005; 13 - 8
- Holstein MB, Minkler M. Self, society, and the “new gerontology”. Gerontologist 2003; 43:787 - 96; http://dx.doi.org/10.1093/geront/43.6.787; PMID: 14704376
- Mykytyn C. Anti-aging medicine: Predictions, moral obligations, and biomedical intervention. Anthropol Q 2006; 79:5 - 32; http://dx.doi.org/10.1353/anq.2006.0010
- Wollina U, Payne CR. Aging well--the role of minimally invasive aesthetic dermatological procedures in women over 65. J Cosmet Dermatol 2010; 9:50 - 8; http://dx.doi.org/10.1111/j.1473-2165.2010.00475.x; PMID: 20367673
- Vedamurthy M.. Antiaging therapies. Indian J Dermatol Venereol Leprol 2006; 72:183 - 6
- Dierickx CC, Anderson RR. Visible light treatment of photoaging. Dermatol Ther 2005; 18:191 - 208; http://dx.doi.org/10.1111/j.1529-8019.2005.05019.x; PMID: 16229721
- Tabata N, O’Goshi K, Zhen YX, Kligman AM, Tagami H. Biophysical assessment of persistent effects of moisturizers after their daily applications: evaluation of corneotherapy. Dermatology 2000; 200:308 - 13; http://dx.doi.org/10.1159/000018393; PMID: 10894961
- Lübbe J. Evidence-based corneotherapy. Dermatology 2000; 200:285 - 9; http://dx.doi.org/10.1159/000018388; PMID: 10960243
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000; 114:480 - 6; http://dx.doi.org/10.1046/j.1523-1747.2000.00902.x; PMID: 10692106
- Nusgens BV, Humbert P, Rougier A, Colige AC, Haftek M, Lambert CA, et al. Topically applied vitamin C enhances the mRNA level of collagens I and III, their processing enzymes and tissue inhibitor of matrix metalloproteinase 1 in the human dermis. J Invest Dermatol 2001; 116:853 - 9; http://dx.doi.org/10.1046/j.0022-202x.2001.01362.x; PMID: 11407971
- Kockaert M, Neumann M. Systemic and topical drugs for aging skin. J Drugs Dermatol 2003; 2:435 - 41; PMID: 12884471
- Margelin D, Medaisko C, Lombard D, Picard J, Fourtanier A. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. J Invest Dermatol 1996; 106:505 - 9; http://dx.doi.org/10.1111/1523-1747.ep12343819; PMID: 8648184
- Trautinger F. Mechanisms of photodamage of the skin and its functional consequences for skin ageing. Clin Exp Dermatol 2001; 26:573 - 7; http://dx.doi.org/10.1046/j.1365-2230.2001.00893.x; PMID: 11696060
- Berger MM. Can oxidative damage be treated nutritionally?. Clin Nutr 2005; 24:172 - 83; http://dx.doi.org/10.1016/j.clnu.2004.10.003; PMID: 15784476
- Marini A. [Beauty from the inside. Does it really work?]. Hautarzt 2011; 62:614 - 7; http://dx.doi.org/10.1007/s00105-011-2138-5; PMID: 21732161
- Fusco D, Colloca G, Lo Monaco MR, Cesari M. Effects of antioxidant supplementation on the aging process. Clin Interv Aging 2007; 2:377 - 87; PMID: 18044188
- Terada A, Yoshida M, Seko Y, Kobayashi T, Yoshida K, Nakada M, et al. Active oxygen species generation and cellular damage by additives of parenteral preparations: selenium and sulfhydryl compounds. Nutrition 1999; 15:651 - 5; http://dx.doi.org/10.1016/S0899-9007(99)00119-7; PMID: 10467607
- Lin FH, Lin JY, Gupta RD, Tournas JA, Burch JA, Selim MA, et al. Ferulic acid stabilizes a solution of vitamins C and E and doubles its photoprotection of skin. J Invest Dermatol 2005; 125:826 - 32; http://dx.doi.org/10.1111/j.0022-202X.2005.23768.x; PMID: 16185284
- Bissett DL, Miyamoto K, Sun P, Li J, Berge CA. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin. Int J Cosmet Sci 2004; 26:231 - 8; http://dx.doi.org/10.1111/j.1467-2494.2004.00228.x; PMID: 18492135
- Haftek M, Mac-Mary S, Le Bitoux MA, Creidi P, Seité S, Rougier A, et al. Clinical, biometric and structural evaluation of the long-term effects of a topical treatment with ascorbic acid and madecassoside in photoaged human skin. Exp Dermatol 2008; 17:946 - 52; http://dx.doi.org/10.1111/j.1600-0625.2008.00732.x; PMID: 18503551
- Kerscher M, Buntrock H. [Anti-aging creams. What really helps?]. Hautarzt 2011; 62:607 - 13; http://dx.doi.org/10.1007/s00105-011-2137-6; PMID: 21755353
- Murray JC, Burch JA, Streilein RD, Iannacchione MA, Hall RP, Pinnell SR. A topical antioxidant solution containing vitamins C and E stabilized by ferulic acid provides protection for human skin against damage caused by ultraviolet irradiation. J Am Acad Dermatol 2008; 59:418 - 25; http://dx.doi.org/10.1016/j.jaad.2008.05.004; PMID: 18603326
- Draelos ZD. The latest cosmeceutical approaches for anti-aging. J Cosmet Dermatol 2007; 6:2 - 6; http://dx.doi.org/10.1111/j.1473-2165.2007.00313.x; PMID: 17348987
- Zhai H, Behnam S, Villarama CD, Arens-Corell M, Choi MJ, Maibach HI. Evaluation of the antioxidant capacity and preventive effects of a topical emulsion and its vehicle control on the skin response to UV exposure. Skin Pharmacol Physiol 2005; 18:288 - 93; http://dx.doi.org/10.1159/000088014; PMID: 16145283
- Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar S, Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J Am Acad Dermatol 2001; 44:425 - 32; http://dx.doi.org/10.1067/mjd.2001.112919; PMID: 11209110
- Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, Hamilton TA, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol 2007; 143:606 - 12; http://dx.doi.org/10.1001/archderm.143.5.606; PMID: 17515510
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, et al. Vitamin A antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol 2000; 114:480 - 6; http://dx.doi.org/10.1046/j.1523-1747.2000.00902.x; PMID: 10692106
- Bhawan J. Short- and long-term histologic effects of topical tretinoin on photodamaged skin. Int J Dermatol 1998; 37:286 - 92; http://dx.doi.org/10.1046/j.1365-4362.1998.00433.x; PMID: 9585903
- Lupo MP, Cole AL. Cosmeceutical peptides. Dermatol Ther 2007; 20:343 - 9; http://dx.doi.org/10.1111/j.1529-8019.2007.00148.x; PMID: 18045359
- Nelson BR, Majmudar G, Griffiths CE, Gillard MO, Dixon AE, Tavakkol A, et al. Clinical improvement following dermabrasion of photoaged skin correlates with synthesis of collagen I. Arch Dermatol 1994; 130:1136 - 42; http://dx.doi.org/10.1001/archderm.1994.01690090060008; PMID: 8085868
- Ostler EL, Wallis CV, Aboalchamat B, Faragher RG. Telomerase and the cellular lifespan: implications of the aging process. J Pediatr Endocrinol Metab 2000; 13:Suppl 6 1467 - 76; PMID: 11202223
- Monheit GD, Chastain MA. Chemical peels. Facial Plast Surg Clin North Am 2001; 9:239 - 55, viii; PMID: 11457690
- Fischer TC, Perosino E, Poli F, Viera MS, Dreno B, Cosmetic Dermatology European Expert Group. Chemical peels in aesthetic dermatology: an update 2009. J Eur Acad Dermatol Venereol 2010; 24:281 - 92; http://dx.doi.org/10.1111/j.1468-3083.2009.03409.x; PMID: 19744174
- Brown AM, Kaplan LM, Brown ME. Phenol-induced histological skin changes: hazards, technique, and uses. Br J Plast Surg 1960; 13:158 - 69; http://dx.doi.org/10.1016/S0007-1226(60)80032-X; PMID: 13804881
- Fartasch M, Teal J, Menon GK. Mode of action of glycolic acid on human stratum corneum: ultrastructural and functional evaluation of the epidermal barrier. Arch Dermatol Res 1997; 289:404 - 9; http://dx.doi.org/10.1007/s004030050212; PMID: 9248619
- Deprez P. Textbook of Chemical Peels. Superficial, Medium, and Deep Peels in Cosmetic Practice. Informa UK, London, 2007.
- Baker TJ, Gordon HL. The ablation of rhytides by chemical means; a preliminary report. J Fla Med Assoc 1961; 48:541
- Behin F, Feuerstein SS, Marovitz WF. Comparative histological study of mini pig skin after chemical peel in an animal model after chemical peel and dermabrasion. Arch Otolaryngol 1977; 103:271; http://dx.doi.org/10.1001/archotol.1977.00780220065006; PMID: 856133
- Vagotis FL, Brundage SR. Histologic study of dermabrasion and chemical peel in an animal model after pretreatment with Retin-A. Aesthetic Plast Surg 1995; 19:243 - 6; http://dx.doi.org/10.1007/BF00451097; PMID: 7668170
- Han SH, Kim HJ, Kim SY, Kim YC, Choi GS, Shin JH. Effects of chemical peeling in photoaged hairless mice. Int J Dermatol 2011; 50:1075 - 82; http://dx.doi.org/10.1111/j.1365-4632.2010.04712.x; PMID: 22126868
- Butler PE, Gonzalez S, Randolph MA, Kim J, Kollias N, Yaremchuk MJ. Quantitative and qualitative effects of chemical peeling on photo-aged skin: an experimental study. Plast Reconstr Surg 2001; 107:222 - 8; http://dx.doi.org/10.1097/00006534-200101000-00036; PMID: 11176627
- Nelson BR, Fader DJ, Gillard M, Majmudar G, Johnson TM. Pilot histologic and ultrastructural study of the effects of medium-depth chemical facial peels on dermal collagen in patients with actinically damaged skin. J Am Acad Dermatol 1995; 32:472 - 8; http://dx.doi.org/10.1016/0190-9622(95)90072-1; PMID: 7868719
- El-Domyati MB, Attia SK, Saleh FY, Ahmad HM, Uitto JJ. Trichloroacetic acid peeling versus dermabrasion: a histometric, immunohistochemical, and ultrastructural comparison. Dermatol Surg 2004; 30:179 - 88; http://dx.doi.org/10.1111/j.1524-4725.2004.30061.x; PMID: 14756647
- Sadick NS. Update on non-ablative light therapy for rejuvenation: a review. Lasers Surg Med 2003; 32:120 - 8; http://dx.doi.org/10.1002/lsm.10127; PMID: 12561045
- Hardaway CA, Ross EV. Nonablative laser skin remodeling. Dermatol Clin 2002; 20:97 - 111, ix; http://dx.doi.org/10.1016/S0733-8635(03)00049-4; PMID: 11859598
- Bjerring P, Christiansen K, Troilius A, Dierickx C. Facial photo rejuvenation using two different intense pulsed light (IPL) wavelength bands. Lasers Surg Med 2004; 34:120 - 6; http://dx.doi.org/10.1002/lsm.20000; PMID: 15004823
- Goldberg DJ. Full-face nonablative dermal remodeling with a 1320 nm Nd:YAG laser. Dermatol Surg 2000; 26:915 - 8; http://dx.doi.org/10.1046/j.1524-4725.2000.026010915.x; PMID: 11050492
- Goldberg DJ, Rogachefsky AS, Silapunt S. Non-ablative laser treatment of facial rhytides: a comparison of 1450-nm diode laser treatment with dynamic cooling as opposed to treatment with dynamic cooling alone. Lasers Surg Med 2002; 30:79 - 81; http://dx.doi.org/10.1002/lsm.10011; PMID: 11870784
- Fournier N, Dahan S, Barneon G, Rouvrais C, Diridollou S, Lagarde JM, et al. Nonablative remodeling: a 14-month clinical ultrasound imaging and profilometric evaluation of a 1540 nm Er:Glass laser. Dermatol Surg 2002; 28:926 - 31; http://dx.doi.org/10.1046/j.1524-4725.2002.02078.x; PMID: 12410677
- Bjerring P, Clement M, Heickendorff L, Egevist H, Kiernan M. Selective non-ablative wrinkle reduction by laser. J Cutan Laser Ther 2000; 2:9 - 15; PMID: 11446096
- Zelickson BD, Kilmer SL, Bernstein E, Chotzen VA, Dock J, Mehregan D, et al. Pulsed dye laser therapy for sun damaged skin. Lasers Surg Med 1999; 25:229 - 36; http://dx.doi.org/10.1002/(SICI)1096-9101(1999)25:3<229::AID-LSM7>3.0.CO;2-D; PMID: 10495300
- Goldberg D, Tan M, Dale Sarradet M, Gordon M. Nonablative dermal remodeling with a 585-nm, 350-microsec, flashlamp pulsed dye laser: clinical and ultrastructural analysis. Dermatol Surg 2003; 29:161 - 3, discussion 163-4; http://dx.doi.org/10.1046/j.1524-4725.2003.29040.x; PMID: 12562346
- Prieto VG, Sadick NS, Lloreta J, Nicholson J, Shea CR. Effects of intense pulsed light on sun-damaged human skin, routine, and ultrastructural analysis. Lasers Surg Med 2002; 30:82 - 5; http://dx.doi.org/10.1002/lsm.10042; PMID: 11870785
- Tanghetti E, Sherr E. Treatment of telangiectasia using the multi-pass technique with the extended pulse width, pulsed dye laser (Cynosure V-Star). J Cosmet Laser Ther 2003; 5:71 - 5; http://dx.doi.org/10.1080/14769170310001249; PMID: 12850799
- Alam M, Dover JS, Arndt KA. Treatment of facial telangiectasia with variable-pulse high-fluence pulsed-dye laser: comparison of efficacy with fluences immediately above and below the purpura threshold. Dermatol Surg 2003; 29:681 - 4, discussion 685; http://dx.doi.org/10.1046/j.1524-4725.2003.29181.x; PMID: 12828690
- Negishi K, Wakamatsu S, Kushikata N, Tezuka Y, Kotani Y, Shiba K. Full-face photorejuvenation of photodamaged skin by intense pulsed light with integrated contact cooling: initial experiences in Asian patients. Lasers Surg Med 2002; 30:298 - 305; http://dx.doi.org/10.1002/lsm.10036; PMID: 11948600
- Negishi K, Tezuka Y, Kushikata N, Wakamatsu S. Photorejuvenation for Asian skin by intense pulsed light. Dermatol Surg 2001; 27:627 - 31, discussion 632; http://dx.doi.org/10.1046/j.1524-4725.2001.01002.x; PMID: 11442612
- Omi T, Kawana S, Sato S, Honda M. Ultrastructural changes elicited by a non-ablative wrinkle reduction laser. Lasers Surg Med 2003; 32:46 - 9; http://dx.doi.org/10.1002/lsm.10119; PMID: 12516070
- Zelickson B, Kist D. Effect of pulsed dye laser and intense pulse light source in dermal extracellular matrix remodeling. Lasers Surg Med 2000; S12:68
- Hsu TS, Zelickson B, Dover JS, Kilmer S, Burns J, Hruza G, et al. Multicenter study of the safety and efficacy of a 585 nm pulsed-dye laser for the nonablative treatment of facial rhytides. Dermatol Surg 2005; 31:1 - 9; PMID: 15720087
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585-nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg 2003; 29:997 - 9, discussion 999-1000; http://dx.doi.org/10.1046/j.1524-4725.2003.29290.x; PMID: 12974694
- Bjerring P, Clement M, Heickendorff L, Lybecker H, Kiernan M. Dermal collagen production following irradiation by dye laser and broadband light source. J Cosmet Laser Ther 2002; 4:39 - 43; http://dx.doi.org/10.1080/147641702320602555; PMID: 12470517
- Dahiya R, Lam SM, Williams EF 3rd. A systematic histologic analysis of nonablative laser therapy in a porcine model using the pulsed dye laser. Arch Facial Plast Surg 2003; 5:218 - 23; http://dx.doi.org/10.1001/archfaci.5.3.218; PMID: 12756114
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med 2000; 26:196 - 200; http://dx.doi.org/10.1002/(SICI)1096-9101(2000)26:2<196::AID-LSM10>3.0.CO;2-9; PMID: 10685092
- Goldberg DJ. New collagen formation after dermal remodeling with an intense pulsed light source. J Cutan Laser Ther 2000; 2:59 - 61; PMID: 11360318
- Hernández-Pérez E, Ibiett EV. Gross and microscopic findings in patients submitted to nonablative full-face resurfacing using intense pulsed light: a preliminary study. Dermatol Surg 2002; 28:651 - 5; http://dx.doi.org/10.1046/j.1524-4725.2002.02010.x; PMID: 12174053
- Alster TS, Garg S. Treatment of facial rhytides with a high-energy pulsed carbon dioxide laser. Plast Reconstr Surg 1996; 98:791 - 4; http://dx.doi.org/10.1097/00006534-199610000-00005; PMID: 8823015
- Lowe NJ, Lask G, Griffin ME, Maxwell A, Lowe P, Quilada F. Skin resurfacing with the Ultrapulse carbon dioxide laser. Observations on 100 patients. Dermatol Surg 1995; 21:1025 - 9; http://dx.doi.org/10.1016/1076-0512(96)82352-1; PMID: 7496669
- Fitzpatrick RE, Goldman MP, Satur NM, Tope WD. Pulsed carbon dioxide laser resurfacing of photo-aged facial skin. Arch Dermatol 1996; 132:395 - 402; http://dx.doi.org/10.1001/archderm.1996.03890280047007; PMID: 8629842
- Teikemeier G, Goldberg DJ. Skin resurfacing with the erbium:YAG laser. Dermatol Surg 1997; 23:685 - 7; http://dx.doi.org/10.1016/S1076-0512(97)00179-9; PMID: 9256915
- Biesman BS. Fractional ablative skin resurfacing: complications. Lasers Surg Med 2009; 41:177 - 8; http://dx.doi.org/10.1002/lsm.20764; PMID: 19291748
- Manstein D, Herron GS, Sink RK, Tanner H, Anderson RR. Fractional photothermolysis: a new concept for cutaneous remodeling using microscopic patterns of thermal injury. Lasers Surg Med 2004; 34:426 - 38; http://dx.doi.org/10.1002/lsm.20048; PMID: 15216537
- Farkas JP, Richardson JA, Hoopman J, Brown SA, Kenkel JM. Micro-island damage with a nonablative 1540-nm Er:Glass fractional laser device in human skin. J Cosmet Dermatol 2009; 8:119 - 26; http://dx.doi.org/10.1111/j.1473-2165.2009.00441.x; PMID: 19527336
- Laubach HJ, Tannous Z, Anderson RR, Manstein D. Skin responses to fractional photothermolysis. Lasers Surg Med 2006; 38:142 - 9; http://dx.doi.org/10.1002/lsm.20254; PMID: 16392146
- Helbig D, Paasch U. Molecular changes during skin aging and wound healing after fractional ablative photothermolysis. Skin Res Technol 2011; 17:119 - 28; http://dx.doi.org/10.1111/j.1600-0846.2010.00477.x; PMID: 21226879
- Hantash BM, Bedi VP, Kapadia B, Rahman Z, Jiang K, Tanner H, et al. In vivo histological evaluation of a novel ablative fractional resurfacing device. Lasers Surg Med 2007; 39:96 - 107; http://dx.doi.org/10.1002/lsm.20468; PMID: 17311274
- Arany PR, Nayak RS, Hallikerimath S, Limaye AM, Kale AD, Kondaiah P. Activation of latent TGF-beta1 by low-power laser in vitro correlates with increased TGF-beta1 levels in laser-enhanced oral wound healing. Wound Repair Regen 2007; 15:866 - 74; http://dx.doi.org/10.1111/j.1524-475X.2007.00306.x; PMID: 18028135
- Souil E, Capon A, Mordon S, Dinh-Xuan AT, Polla BS, Bachelet M. Treatment with 815-nm diode laser induces long-lasting expression of 72-kDa heat shock protein in normal rat skin. Br J Dermatol 2001; 144:260 - 6; http://dx.doi.org/10.1046/j.1365-2133.2001.04010.x; PMID: 11251556
- Wilmink GJ, Opalenik SR, Beckham JT, Abraham AA, Nanney LB, Mahadevan-Jansen A, et al. Molecular imaging-assisted optimization of hsp70 expression during laser-induced thermal preconditioning for wound repair enhancement. J Invest Dermatol 2009; 129:205 - 16; http://dx.doi.org/10.1038/jid.2008.175; PMID: 18580963
- Ravanti L, Kähäri VM. Matrix metalloproteinases in wound repair (review). Int J Mol Med 2000; 6:391 - 407; PMID: 10998429
- Orringer JS, Kang S, Johnson TM, Karimipour DJ, Hamilton T, Hammerberg C, et al. Connective tissue remodeling induced by carbon dioxide laser resurfacing of photodamaged human skin. Arch Dermatol 2004; 140:1326 - 32; http://dx.doi.org/10.1001/archderm.140.11.1326; PMID: 15545540
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med 2003; 32:78 - 87; http://dx.doi.org/10.1002/lsm.10145; PMID: 12561039
- Iorizzo M, De Padova MP, Tosti A. Biorejuvenation: theory and practice. Clin Dermatol 2008; 26:177 - 81; http://dx.doi.org/10.1016/j.clindermatol.2007.09.011; PMID: 18472058
- Rotunda AM, Kolodney MS. Mesotherapy and phosphatidylcholine injections: historical clarification and review. Dermatol Surg 2006; 32:465 - 80; http://dx.doi.org/10.1111/j.1524-4725.2006.32100.x; PMID: 16681654
- Caruso MK, Roberts AT, Bissoon L, Self KS, Guillot TS, Greenway FL. An evaluation of mesotherapy solutions for inducing lipolysis and treating cellulite. J Plast Reconstr Aesthet Surg 2008; 61:1321 - 4; http://dx.doi.org/10.1016/j.bjps.2007.03.039; PMID: 17954040
- Matarasso A, Pfeifer TM, Plastic Surgery Educational Foundation DATA Committee. Mesotherapy for body contouring. Plast Reconstr Surg 2005; 115:1420 - 4; http://dx.doi.org/10.1097/01.PRS.0000162227.94032.ED; PMID: 15809611
- Rotunda AM, Avram MM, Avram AS. Cellulite: Is there a role for injectables?. J Cosmet Laser Ther 2005; 7:147 - 54; http://dx.doi.org/10.1080/14764170500430234; PMID: 16414902
- Lacarrubba F, Tedeschi A, Nardone B, Micali G. Mesotherapy for skin rejuvenation: assessment of the subepidermal low-echogenic band by ultrasound evaluation with cross-sectional B-mode scanning. Dermatol Ther 2008; 21:Suppl 3 S1 - 5; http://dx.doi.org/10.1111/j.1529-8019.2008.00234.x; PMID: 19076625
- Jager C, Brenner C, Habicht J, Wallic R. Bioactive reagents used in mesotherapy for skin rejuvenation in vivo induce diverse physiological processes in human skin fibroblasts in vitro – a pilot study. Exp Dermatol 2011; 21:70 - 80; PMID: 22082171
- Amin SP, Phelps RG, Goldberg DJ. Mesotherapy for facial skin rejuvenation: a clinical, histologic, and electron microscopic evaluation. Dermatol Surg 2006; 32:1467 - 72; http://dx.doi.org/10.1111/j.1524-4725.2006.32353.x; PMID: 17199654
- Atiyeh BS, Ibrahim AE, Dibo SA. Aesthetic Plast Surg 2008: 32: 842–849. 12 Brown S A. Aesthet Surg J 2006; 26:95 - 8; PMID: 19338891
- Eppley BL, Dadvand B. Injectable soft-tissue fillers: clinical overview. Plast Reconstr Surg 2006; 118:98e - 106e; http://dx.doi.org/10.1097/01.prs.0000232436.91409.30; PMID: 16980841
- Haneke E. Skin rejuvenation without a scalpel. I. Fillers. J Cosmet Dermatol 2006; 5:157 - 67; http://dx.doi.org/10.1111/j.1473-2165.2006.00243.x; PMID: 17173591
- Homicz MR, Watson D. Review of injectable materials for soft tissue augmentation. Facial Plast Surg 2004; 20:21 - 9; http://dx.doi.org/10.1055/s-2004-822955; PMID: 15034810
- Kanchwala SK, Holloway L, Bucky LP. Reliable soft tissue augmentation: a clinical comparison of injectable soft-tissue fillers for facial-volume augmentation. Ann Plast Surg 2005; 55:30 - 5, discussion 35; http://dx.doi.org/10.1097/01.sap.0000168292.69753.73; PMID: 15985788
- Klein AW. Techniques for soft tissue augmentation: an ‘a to z’. Am J Clin Dermatol 2006; 7:107 - 20; http://dx.doi.org/10.2165/00128071-200607020-00004; PMID: 16605291
- Laurent TC, Fraser JR. Hyaluronan. FASEB J 1992; 6:2397 - 404; PMID: 1563592
- Tzellos TG, Klagas I, Vahtsevanos K, Triaridis S, Printza A, Kyrgidis A, et al. Extrinsic ageing in the human skin is associated with alterations in the expression of hyaluronic acid and its metabolizing enzymes. Exp Dermatol 2009; 18:1028 - 35; http://dx.doi.org/10.1111/j.1600-0625.2009.00889.x; PMID: 19601984
- Alberts B, Johnson A, Lewis J, et al., eds. 2002. The molecular biology of the cell. Cell junctions, cell adhesion, and the extracellular matrix. 1065. New York: Garland Science.
- Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol 1994; 6:726 - 33; http://dx.doi.org/10.1016/0955-0674(94)90100-7; PMID: 7530464
- Turley EA. Hyaluronan and cell locomotion. Cancer Metastasis Rev 1992; 11:21 - 30; http://dx.doi.org/10.1007/BF00047600; PMID: 1380898
- Papakonstantinou E, Karakiulakis G, Eickelberg O, Perruchoud AP, Block L-H, Roth M. A 340 kDa hyaluronic acid secreted by human vascular smooth muscle cells regulates their proliferation and migration. Glycobiology 1998; 8:821 - 30; http://dx.doi.org/10.1093/glycob/8.8.821; PMID: 9639543
- Tammi MI, Day AJ, Turley EA. Hyaluronan and homeostasis: a balancing act. J Biol Chem 2002; 277:4581 - 4; http://dx.doi.org/10.1074/jbc.R100037200; PMID: 11717316
- Fligiel SE, Varani J, Datta SC, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photodamaged skin in vivo and after exposure to matrix metalloproteinase-1 in vitro. J Invest Dermatol 2003; 120:842 - 8; http://dx.doi.org/10.1046/j.1523-1747.2003.12148.x; PMID: 12713591
- Kuroda K, Shinkai H. Gene expression of types I and III collagen, decorin, matrix metalloproteinases and tissue inhibitors of metalloproteinases in skin fibroblasts from patients with systemic sclerosis. Arch Dermatol Res 1997; 289:567 - 72; http://dx.doi.org/10.1007/s004030050241; PMID: 9373715
- Uitto J, Bernstein EF. Molecular mechanisms of cutaneous aging: connective tissue alterations in the dermis. J Investig Dermatol Symp Proc 1998; 3:41 - 4; PMID: 9732056
- Yoneda M, Shimizu S, Nishi Y, Yamagata M, Suzuki S, Kimata K. Hyaluronic acid-dependent change in the extracellular matrix of mouse dermal fibroblasts that is conducive to cell proliferation. J Cell Sci 1988; 90:275 - 86; PMID: 3073165
- Gao F, Liu Y, He Y, Yang C, Wang Y, Shi X, et al. Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol 2010; 29:107 - 16; http://dx.doi.org/10.1016/j.matbio.2009.11.002; PMID: 19913615
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol 2007; 23:435 - 61; http://dx.doi.org/10.1146/annurev.cellbio.23.090506.123337; PMID: 17506690
- Fink RM, Lengfelder E. Hyaluronic acid degradation by ascorbic acid and influence of iron. Free Radic Res Commun 1987; 3:85 - 92; http://dx.doi.org/10.3109/10715768709069773; PMID: 3508446
- Carruthers A, Carruthers J. Non-animal-based hyaluronic acid fillers: scientific and technical considerations. Plast Reconstr Surg 2007; 120:Suppl 33S - 40S; http://dx.doi.org/10.1097/01.prs.0000248808.75700.5f; PMID: 18090341
- Balazs EA, Bland PA, Denlinger JL, Goldman AI, Larsen NE, Leshchiner EA, et al. Matrix engineering. Blood Coagul Fibrinolysis 1991; 2:173 - 8; http://dx.doi.org/10.1097/00001721-199102000-00026; PMID: 1772987
- Andre P. New trends in face rejuvenation by hyaluronic acid injections. J Cosmet Dermatol 2008; 7:251 - 8; http://dx.doi.org/10.1111/j.1473-2165.2008.00402.x; PMID: 19146600
- Eppley BL, Dadvand B. Injectable soft-tissue fillers: clinical overview. Plast Reconstr Surg 2006; 118:98e - 106e; http://dx.doi.org/10.1097/01.prs.0000232436.91409.30; PMID: 16980841
- Gold MH. Use of hyaluronic acid fillers for the treatment of the aging face. Clin Interv Aging 2007; 2:369 - 76; PMID: 18044187
- Robinson JK, Hanke CW, Sengelmann RD, Siegel DM. Surgery of the skin. Philadelphia: Elsevier Mosby, 2005.
- Alam M, Gladstone H, Kramer EM, Murphy JP Jr., Nouri K, Neuhaus IM, et al, American Society for Dermatologic Surgery. ASDS guidelines of care: injectable fillers. Dermatol Surg 2008; 34:Suppl 1 S115 - 48; http://dx.doi.org/10.1111/j.1524-4725.2008.34253.x; PMID: 18547175
- Lizzul PF, Narurkar VA. The role of calcium hydroxylapatite (Radiesse) in nonsurgical aesthetic rejuvenation. J Drugs Dermatol 2010; 9:446 - 50; PMID: 20480786
- Goldberg DJ. Breakthroughs in US dermal fillers for facial soft-tissue augmentation. J Cosmet Laser Ther 2009; 11:240 - 7; http://dx.doi.org/10.3109/14764170903341731; PMID: 19951196
- Ghersetich I, Lotti T, Campanile G, Grappone C, Dini G. Hyaluronic acid in cutaneous intrinsic aging. Int J Dermatol 1994; 33:119 - 22; http://dx.doi.org/10.1111/j.1365-4362.1994.tb01540.x; PMID: 8157393
- Graivier MH, Bass LS, Busso M, Jasin ME, Narins RS, Tzikas TL. Calcium hydroxylapatite (Radiesse) for correction of the mid- and lower face: consensus recommendations. Plast Reconstr Surg 2007; 120:Suppl 55S - 66S; http://dx.doi.org/10.1097/01.prs.0000285109.34527.b9; PMID: 18090343
- Tzikas TL. A 52-month summary of results using calcium hydroxylapatite for facial soft tissue augmentation. Dermatol Surg 2008; 34:Suppl 1 S9 - 15; http://dx.doi.org/10.1111/j.1524-4725.2008.34237.x; PMID: 18547188
- Burgess CM, Lowe NJ. NewFill for skin augmentation: a new filler or failure?. Dermatol Surg 2006; 32:1530 - 2, author reply 1532; http://dx.doi.org/10.1111/j.1524-4725.2006.32369.x; PMID: 17199668
- Mest D.. Experience with injectable poly-L-lactic acid in clinicalpractice. Cosmetic Dermatol 2005; 18:5 - 8
- Burgess CM, Quiroga RM. Assessment of the safety and efficacy of poly-L-lactic acid for the treatment of HIV-associated facial lipoatrophy. J Am Acad Dermatol 2005; 52:233 - 9; http://dx.doi.org/10.1016/j.jaad.2004.08.056; PMID: 15692467
- Sturm LP, Cooter RD, Mutimer KL, Graham JC, Maddern GJ. A systematic review of dermal fillers for age-related lines and wrinkles. ANZ J Surg 2011; 81:9 - 17; http://dx.doi.org/10.1111/j.1445-2197.2010.05351.x; PMID: 21299793
- Zimmermann R, Jakubietz R, Jakubietz M, Strasser E, Schlegel A, Wiltfang J, et al. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion 2001; 41:1217 - 24; http://dx.doi.org/10.1046/j.1537-2995.2001.41101217.x; PMID: 11606819
- Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004; 62:489 - 96; http://dx.doi.org/10.1016/j.joms.2003.12.003; PMID: 15085519
- Freymiller EG. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004; 62:1046 - , author reply 1047-8; http://dx.doi.org/10.1016/j.joms.2004.05.205; PMID: 15278876
- Wrotniak M, Bielecki T, Gaździk TS. Current opinion about using the platelet-rich gel in orthopaedics and trauma surgery. Ortop Traumatol Rehabil 2007; 9:227 - 38; PMID: 17721419
- Kim DH, Je YJ, Kim CD, Lee YH, Seo YJ, Lee JH, et al. Can Platelet-rich Plasma Be Used for Skin Rejuvenation? Evaluation of Effects of Platelet-rich Plasma on Human Dermal Fibroblast. Ann Dermatol 2011; 23:424 - 31
- Krasna M, Domanovic D, Tomsic A, Svajger U, Jeras M.. Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Acta Dermatovenerol Alp Panonica Adriat 2007; 16:105 - 10
- Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 2003; 24:3095 - 100; http://dx.doi.org/10.1016/S0142-9612(03)00114-5; PMID: 12895582
- Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Platelet-rich plasma enhances human osteoblast-like cell proliferation and differentiation. J Oral Maxillofac Surg 2005; 63:362 - 9; http://dx.doi.org/10.1016/j.joms.2004.07.016; PMID: 15742288
- Dessy LA, Mazzocchi M, Rubino C, Mazzarello V, Spissu N, Scuderi N. An objective assessment of botulinum toxin A effect on superficial skin texture. Ann Plast Surg 2007; 58:469 - 73; http://dx.doi.org/10.1097/01.sap.0000244968.16977.1f; PMID: 17452827
- Cheng CM. Cosmetic use of botulinum toxin type A in the elderly. Clin Interv Aging 2007; 2:81 - 3; http://dx.doi.org/10.2147/ciia.2007.2.1.81; PMID: 18044078
- Rzany B, Dill-Müller D, Grablowitz D, Heckmann M, Caird D, German-Austrian Retrospective Study Group. Repeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4,103 treatments in 945 patients. Dermatol Surg 2007; 33:1 Spec No. S18 - 25; http://dx.doi.org/10.1111/j.1524-4725.2006.32327.x; PMID: 17241409
- Böni R, Kreyden OP, Burg G. Revival of the use of botulinum toxin: application in dermatology. Dermatology 2000; 200:287 - 91; http://dx.doi.org/10.1159/000018389; PMID: 10894957
- Coffield JA, Considine RB, Simpson LL. The site and mechanism of action of botulinum neurotoxin; in Jankovic J, Hallett M (eds): Therapy with Botulinum Toxin. New York, Dekker, 1994, pp 3–14.
- Coleman WP, Hanke CW, Alt TH. Cosmetic Surgery of the Skin, 2nd edn. St Louis, Missouri: Mosby Year Book Inc., 1997: 231-235.
- Garcia A, Fulton JE Jr.. Cosmetic denervation of the muscles of facial expression with botulinum toxin. A dose-response study. Dermatol Surg 1996; 22:39 - 43; http://dx.doi.org/10.1016/1076-0512(95)00464-5; PMID: 8556256
- Carruthers A, Kiene K, Carruthers J. Botulinum A exotoxin use in clinical dermatology. J Am Acad Dermatol 1996; 34:788 - 97; http://dx.doi.org/10.1016/S0190-9622(96)90016-X; PMID: 8632076
- Guyuron B, Huddleston SW. Aesthetic indications for botulinum toxin injection. Plast Reconstr Surg 1994; 93:913 - 8; http://dx.doi.org/10.1097/00006534-199404001-00003; PMID: 8134483
- Brooks GF, Butel JS, Ornston LN. Jawetz, Melnick and Adelbergs Medical Microbiology, 20th edn. Connecticut: Appleton and Lange, 1995: 174±176.
- Aoki RK. Immunologic and other properties of therapeutic botulinum toxin serotypes. In: BrinMF, Hallett M, Jankovic J, eds. Scientific and Therapeutic Aspects of Botulinum Toxin. Philadelphia, PA: Lippincott Williams & Wilkins; 2002.
- Becker-Wegerich P, Rauch L, Ruzicka T. Botulinum toxin A in the therapy of mimic facial lines. Clin Exp Dermatol 2001; 26:619 - 30; http://dx.doi.org/10.1046/j.1365-2230.2001.00901.x; PMID: 11696067
- Ganong WF. Review of Medical Physiology, 16th edn. East Norwalk, Connecticut: Appleton and Lange, 1993: 99±102.
- de Paiva A, Meunier FA, Molgó J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci U S A 1999; 96:3200 - 5; http://dx.doi.org/10.1073/pnas.96.6.3200; PMID: 10077661
- Product Information Package Insert. (71390US12J). Irvine, CA: Allergan, Inc.; 2002.
- Hatheway CG. Immunology of Botulinum Toxin. New York: Marcel-Dekker; 1993.
- Huang W, Foster JA, Rogachefsky AS. Pharmacology of botulinum toxin. J Am Acad Dermatol 2000; 43:249 - 59; http://dx.doi.org/10.1067/mjd.2000.105567; PMID: 10906647
- Rzany B, Ascher B, Monheit GD. Treatment of glabellar lines with botulinum toxin type A (Speywood Unit): a clinical overview. J Eur Acad Dermatol Venereol 2010; 24:Suppl 1 1 - 14; http://dx.doi.org/10.1111/j.1468-3083.2009.03475.x; PMID: 19930409
- Botox (Package Insert). Allergan, Inc.
- Carruthers J, Fagien S, Matarasso SL. the Botox Consensus Group. Botulinum toxin and facial aesthetics. Plast Reconstr Surg 2004; 114:1S - 22S; http://dx.doi.org/10.1097/01.PRS.0000144795.76040.D3; PMID: 15507786
- Shetty MK, IADVL Dermatosurgery Task Force. Guidelines on the use of botulinum toxin type A. Indian J Dermatol Venereol Leprol 2008; 74:Suppl S13 - 22; PMID: 18688099
- Sommer B, Sattler G. Botulinumtoxin in der aÈsthetischen Medizin. Berlin; Blackwell Vissenschafts-Verlag, 2001.
- Carruthers J, Carruthers A. Cosmetic use of botulinum A exotoxin. In: Advances in Dermatology (Dzubow L, ed.) 1996: 280-303.
- Lange DJ, Rubin M, Greene PE, Kang UJ, Moskowitz CB, Brin MF, et al. Distant effects of locally injected botulinum toxin: a double-blind study of single fiber EMG changes. Muscle Nerve 1991; 14:672 - 5; http://dx.doi.org/10.1002/mus.880140711; PMID: 1922173
- Goschel H, et al. Botulinum toxin therapy, neutralizing and non-neutralizing antibodies. Exp Neurol 1997; 147:96; http://dx.doi.org/10.1006/exnr.1997.6580; PMID: 9294406
- Blitzer A, Binder WJ, Boyd JB, Carruthers A. Management of Facial Lines and Wrinkles. Philadelphia, PA: Lippincott Williams & Wilkins; 2000.
- Matarasso A, Deva AK, American Society of Plastic Surgeons DATA Committee. Botulinum toxin. Plast Reconstr Surg 2002; 109:1191 - 7; http://dx.doi.org/10.1097/00006534-200203000-00066; PMID: 11884859
- Fagien S. Botox for the treatment of dynamic and hyperkinetic facial lines and furrows: adjunctive use in facial aesthetic surgery. Plast Reconstr Surg 1999; 103:701 - 13; http://dx.doi.org/10.1097/00006534-199902000-00055; PMID: 9950563
- Hertoghe T. The “multiple hormone deficiency” theory of aging: is human senescence caused mainly by multiple hormone deficiencies?. Ann N Y Acad Sci 2005; 1057:448 - 65; http://dx.doi.org/10.1196/annals.1322.035; PMID: 16399912
- Cranney A, Papaioannou A, Zytaruk N, Hanley D, Adachi J, Goltzman D, et al, Clinical Guidelines Committee of Osteoporosis Canada. Parathyroid hormone for the treatment of osteoporosis: a systematic review. CMAJ 2006; 175:52 - 9; http://dx.doi.org/10.1503/cmaj.050929; PMID: 16818910
- Vitetta L, Anton B. Lifestyle and nutrition, caloric restriction, mitochondrial health and hormones: scientific interventions for anti-aging. Clin Interv Aging 2007; 2:537 - 43; PMID: 18225453
- Heutling D, Lehnert H. [Hormone therapy and anti-aging: is there an indication?]. Internist (Berl) 2008; 49:570 - 80, 572-6, 578-9; http://dx.doi.org/10.1007/s00108-008-2110-3; PMID: 18389196
- Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab 1994; 78:1360 - 7; http://dx.doi.org/10.1210/jc.78.6.1360; PMID: 7515387
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, et al. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A 2000; 97:4279 - 84; http://dx.doi.org/10.1073/pnas.97.8.4279; PMID: 10760294
- Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, et al. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab 2006; 91:2986 - 93; http://dx.doi.org/10.1210/jc.2005-2484; PMID: 16735495
- Nair KS, Rizza RA, O’Brien P, Dhatariya K, Short KR, Nehra A, et al. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med 2006; 355:1647 - 59; http://dx.doi.org/10.1056/NEJMoa054629; PMID: 17050889
- Wetterberg L, Bergiannaki JD, Paparrigopoulos T, von Knorring L, Eberhard G, Bratlid T, et al. Normative melatonin excretion: a multinational study. Psychoneuroendocrinology 1999; 24:209 - 26; http://dx.doi.org/10.1016/S0306-4530(98)00076-6; PMID: 10101729
- Roth GS, Lesnikov V, Lesnikov M, Ingram DK, Lane MA. Dietary caloric restriction prevents the age-related decline in plasma melatonin levels of rhesus monkeys. J Clin Endocrinol Metab 2001; 86:3292 - 5; http://dx.doi.org/10.1210/jc.86.7.3292; PMID: 11443203
- Ferrari E, Cravello L, Falvo F, Barili L, Solerte SB, Fioravanti M, et al. Neuroendocrine features in extreme longevity. Exp Gerontol 2008; 43:88 - 94; http://dx.doi.org/10.1016/j.exger.2007.06.010; PMID: 17764865
- Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine 2005; 27:179 - 88; http://dx.doi.org/10.1385/ENDO:27:2:179; PMID: 16217131
- Heutling D, Lehnert H. [Hormone therapy and menopause]. Dtsch Med Wochenschr 2005; 130:829 - 34; http://dx.doi.org/10.1055/s-2005-865097; PMID: 15789305
