Abstract
The US Patent and Trademark Office (USPTO) adopts recent patent courts’ opinions (such as KSR In re Fisher and Ariad v. Lilly) in patent examinations, which would certainly create barriers to biotech patent prosecution. To identify the barriers to Candida vaccine patent prosecution, we analyzed 99 US-granted patents from January 2001 to May 2012 related to Candida vaccines. The rejections were based on factors that included obviousness, novelty, indefiniteness, double patenting, enablement, written description and utility. Based on this investigation, we find that some of these rejections were actually avoidable, and then further provide workable solutions to avoid some of the barriers, especially those related to patentability. These principles recited in this study should also be applicable to other fields of vaccines and immunotherapeutics.
Vaccination would be a promising strategy for prevention of Candida infections, which is the fourth most common bloodstream infection in hospitalized patients in the United States.Citation1 However, our studyCitation2 demonstrated that the patenting activity of Candida vaccines seems to have remained stagnant in recent years due to patent prosecution or technical barrier in the field. It would be interesting to realize the patent prosecution barriers on Candida vaccines.
To be patentable, an invention must be of patentable subject matter, and it must be useful, novel and nonobvious. Moreover, the patent applicant(s) must be the original inventor(s) or legal owner(s) and must also avoid statutory time bars, adequately disclose the invention and distinctly claim the invention. These heightened requirements for patentability have become barriers to obtain and enforce biotech patents.Citation3
To identify the barriers to patent prosecution, we searched the patent publication database in the USPTO for Candida vaccines applications from January 2001 to May 2012 (). Among the 940 total applications, we limited our analysis to the 99 cases which the USPTO granted as patents. The total transaction history of these patents included 158 non-final rejections, 73 final rejections, 44 Request for Continued Examinations (RCE), 15 interviews with patent examiners, and 7 appeals to the US Board of Patent Appeals before allowance.
Figure 1. The outcome of the actions of the US Patent Office on Candida vaccines patents from January 2001 to May 2012.
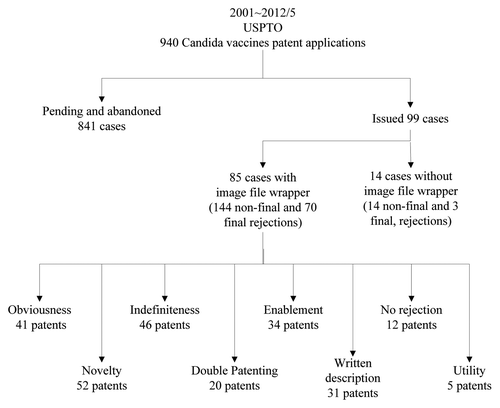
Only 12 Candida vaccines-related patents have been granted without any rejection from the USPTO so far. Thirty-eight patents received final rejections, and another 13 patents received final rejections more than twice. Therefore, to avoid abandoning 29 out of these 51 applications that received final rejections, the applicants had to file a RCE so that they could submit further amendments, such as new claims, arguments, declarations or references.
Court Cases Influence on the US Patent Prosecution
Recent patent cases, such as KSRCitation4,Citation5 In re FisherCitation6 and Ariad v. Lilly,Citation7 may have increased the level of difficulty in obtaining broader protection in biotech-related applications.Citation3,Citation5 Moreover, the USPTO also adopts these courts’ opinions in patent examinations, which would certainly create barriers to vaccine and immunotherapeutic patent prosecutions. To demonstrate the influences of KSR and In re Fisher, we selected the dates of the decisions, April 30, 2007 and September 7, 2005, as cutoff points.
Our analysis showed that the average non-final rejection rate, final rejection rate and RCE rate per patent increased from 1.24 (26 non-final rejections over 21 patents), 0.38 (8 final rejection over 21 patents) and 0.10 (2 RCEs over 21 patents) respectively to 1.69 (132 non final rejections over 78 patents), 0.83 (65 final rejections over 78 patents) and 0.54 (42 RCEs over 78 patents) respectively after In re Fisher; and from 1.47 (44 non-final rejections over 30 patents), 0.47 (14 final rejections over 30 patents) and 0.2 (6 RCEs over 30 patents) respectively to 1.65 (114 non-final rejections over 69 patents), 0.85 (59 final rejections over 69 patents) and 0.55 (38 RCEs over 69 patents) respectively after KRS. The average no-rejection rate per patent decreased from 0.14 (3 no rejections over 21 patents) to 0.12 (9 no rejections over 79 patents) after In re Fisher, but increased slightly from 0.1 (3 no rejections over 30 patents) to 0.13 (9 no rejections over 69 patents) after KSR.
The increase of average non-final rejection rate, final rejection rate and RCE rate per patent as well as the decrease of average no-rejection rate per patent can indicate the level of difficulty in patent prosecution. However, the differences in rates were not statistically significant by Chi-square test for association. In other words, recent patent cases would create barriers to biotech patent prosecution, but the influence is not significant.
We further reviewed the image file wrappers of the 85 issued patents available online. The actions used by the USPTO office to reject patents included obviousness, novelty, indefiniteness, double patenting, enablement, written description and utility. Because each patent suffered from more than one type of the rejection, the total number of the rejections was much higher the number of issued patents.
Obviousness Rejection
Among the 99 issued Candida vaccines patents, 30 patents were granted before the KSR case of the US Supreme Court dated on April 30, 2007. Our previous studyCitation5 demonstrated that the trends for obvious and non-obvious rulings in terms of the United States Court of Appeals for the Federal Circuit (CAFC) decisions significantly increased and decreased, respectively. The CAFC observed that an obviousness finding was appropriate where the prior art contained detailed enabling methodologies that were used to develop the claimed invention, a suggestion to modify the prior art in order to build the claimed invention, and evidence suggesting that it would be successful. During this image file wrapper review, we also noticed that US patent examiners frequently quoted the KSR case for obviousness rejection.
For determining obviousness, US patent examiners repeatedly cited the factual inquires set forth in the Graham case,Citation8 which include determining the scope and contents of the prior art, ascertaining the difference between the prior art and the claims applied, resolving the level of ordinary skill in the pertinent art, and considering objective evidence present in the application that indicates obviousness or nonobviousness.
For example, a patent application claimed an immunogenic complex comprising at least one ribosomal complex and adhesion of microbe or viral antigen. The ribosomal complex has large and small ribosomal subunits, which were particulate in nature and contained large double-stranded rRNAs. One of the dependent claims recited for adhesion was any protein embedded in or on the surface of any microbe, where said protein was involved in the interaction between the microbe and a host cell.
The first prior art cited by the patent examiner disclosed an immunogenic complex comprising a specific bacterial antigen complex coupled to rRNA or RNA fragments originating from the same or different bacteria, as well as specific membrane proteins as adhesins. However, the first prior art did not specifically teach the viral antigen. The second reference described a recombinant immunogenic composition containing a viral antigen and T-cell as host cell. The rRNA or fragments of RNA may advantageously be used as active conveyors of specific bacterial antigens. Furthermore, a bacterial protein and a viral complex were also demonstrated to stimulate a strong cytotoxic T-cell response and were able to treat a lethal infection with virus. Thus, the patent examiner determined that a common inventor in the art would find it obvious to combine these two references in order to obtain the claimed immunogenic complex when the invention was made. Furthermore, one would have a reasonable expectation of success to obtain the claimed immunogenic complex comprising ribosomal complex and viral antigen.
Based on the obviousness rejection, the applicant amended the claim to focus on very specific subunits. Because obviousness requires a suggestion of all limitations in a claim,Citation9 none of the prior arts cited or further searched included the specific sequence limitation. Thus, the application was allowed for insurance as a patent.
In another example, a patent application claimed a composition comprising a small molecule immune-potentiator (SMIP) compound to enhance the immune response of a subject to an antigen. Administering the claimed SMIP compounds alone or in combination with another agent can treat infectious diseases.
The first prior art cited described cyclic compounds and their use in the treatment of immunologic disorders. The reference also taught that other therapeutic agents such as immune modulators may be administered with the composition claimed. The second reference described a composition comprising spores of Bacillus subtilis as a method of stimulating immune responsiveness in a subject. Furthermore, the reference also demonstrated that antigens can be used as adjuvant in the claimed composition to boost immune response in a mammal. Thus, based on these two prior arts, the invention would have been prima facie obvious to someone of ordinary skill in the art when the invention was made.
To avoid an obviousness rejection during patent prosecution, the patent applicant should demonstrate evidence of unexpected results or an indication that prior art describes results contradictory to the claimed invention. Furthermore, evidence of secondary considerations, such as long-felt-unsolved needs or a failure of others to solve the issue at hand, should be used to legitimize the invention for patenting.Citation5
Novelty Rejection
An invention must be new to be patentable. Hence, an important part of patent prosecution involves comparing the claimed invention to prior art in order to determine whether the claims are novel. The prior art includes knowledge or use of the invention by others in the United States, patents, patent applications and publications of the invention in any country.
Under 35 USC §102, every limitation of a claim must appear in identical from in a single prior art reference to anticipate the claim.Citation10 For biotech-related inventions, an anticipatory must clearly and unequivocally disclose the claimed compound, or those directly skilled in the art must disclose the compound without any need for picking, choosing or combining various disclosures that are not directly related to one another by the information cited in the references.Citation11 Furthermore, the discovery of a previously unappreciated property or function in an existing composition, or of a scientific explanation for function of the prior art, does not render the old composition newly patentable based on the discovery.Citation12
For example, a patent application claimed an immunogenic composition depleted of mannoprotein by treatment of a fungal cell wall with a protease. However, a prior art disclosed polysaccharide protein carrier conjugates and utilized ethanol extraction to remove mannoproteins. The patent examiner considered that the purification or production of a product by a particular process does not impart novelty to a product taught by the prior art. To overcome the novelty rejection, the applicant amended the claimed composition comprising a glucan polymer conjugated to a bacteria toxin. The prior art cited in the office action failed to teach or suggest such a composition.
Based on above analysis, a comprehensive search of all available patents and publications can greatly reduce novelty rejection during patent prosecution. To comprehensively explore all the patent documents, we developed a thought process to precisely target and completely cover the relevant patent documents.Citation13 Helpful methods in the process include expanding the keywords searches with synonyms and equivalents, limiting the search to a short period of time, excluding irrelevant keywords, identifying other possible keywords to screen any patents owned by the leading assignees or inventors, and inspecting other possible International Patent Classification or United States Patent Classification.
Furthermore, chemical and biological entities may refer to different nomenclature systems, such as the International Union of Pure and Applied Chemistry and Chemical Abstracts Service, for organic molecules. Some known chemical entities may also be described by one or more common names or even brand names within patent documents to identify the source of goods. However, it should be noted that the use of a trademark or trade name in a claim to describe a material or product not only would render a claim indefinite, but also would constitute an improper use of the trademark or trade name.
In addition to patent documents, publications also place technological developments into the public domain. PubMed (www.ncbi.nlm.nih.gov/pubmed/), the ISI Web of knowledge (apps.isiknowledge.com), and even Goggle and Yahoo searches are useful for searching for available life science literature or information. During our miRNA image file wrapper review, we noticed that much scientific literatures was cited in the office actions for novelty rejection. Moreover, some US patent examiners even directly cited the prior art listed in the information disclosure statement (IDS), describing all relevant prior art references known to the applicant, for novelty rejections. Therefore, a patent applicant should distinguish the prior art from the current invention, especially for those listed in the IDS, to avoid a novelty rejection.
Indefiniteness Rejection
To allow for patentability over prior art during prosecution, the scope of a claim should recite and define the structure or acts of an invention in precise, logical and exact terms. This requirement is recited in the second paragraph of 35 USC §112, which states that “specification shall conclude with one or more claims particularly pointing out and distinctly claiming the subject matter which the applicant regards as his invention.”
The test for definiteness compliance is whether a person skilled in the art would understand what is claimed in light of the specification.Citation14 Thus, vague or unclear terms that cause confusion over the intended scope of a claim would render an indefiniteness rejection. For example, a dependent claim claimed “the immunogenic complex of claim 1, wherein said ribosomal complex retains sufficient integrity to substantially preserve said double-stranded large rRNA’s contained in said ribosomal subunits.” The terms “sufficient” and “substantially” were not defined in claims nor did the specification provide a standard for ascertaining the requisite degree, which rendered the claim indefinite. Someone of ordinary skill in the art would not be apprised of the scope of the invention.
During the review, we found that US examiners pointed to vague words such as “at least about,” “substantially complementary,” “associated,” “from about 20% to about 30%” and “larger,” where the boundaries of the terms were not defined in the specifications. Furthermore, the US Manual of Patent Examining Procedure also points out that exemplary claim language, including “for example,” or “such as,” is indefinite because the intended scope of the claim is unclear.
To avoid indefiniteness rejection during patent prosecution, the patent applicant should allow a person skilled in the art to understand what is claimed in light of the specification. Patent examiners may allow those inherently inexact terms if the terms are understood in the industry or if the bounds of the terms are defined in the specifications.
Non-statutory Double Patenting Rejection
To prevent unjustified or improper timewise extension of a patent exclusive period, a nonstatutory obviousness type double rejection is appropriate when the conflicting claims may not be identical, but at least one examined application claim is either anticipated by or obvious based on the reference claim/s.Citation15
Nonstatutory obviousness type double rejection has become another barrier since the USPTO offered inventors the option of filing a provisional patent application (PPA) on June 8, 1995. Applicants are entitled to claim the benefit of a PPA filing date in a corresponding regular patent application filed within 12 mo, if the written description and any drawing(s) of the PPA adequately support the subject matter. However, some applicants may be too optimistic with respect to a PPA and thus apply for many regular patent applications. During this review, we noticed that several applicants even sent up to five or seven patent applications based on the same PPA.
A timely filed terminal disclaimer can overcome a nonstatutory obviousness type double patenting rejection by showing the conflicting applications to be commonly owned or by claiming the invention to be the result of a joint research agreement. Moreover, to show candor and good faith toward the Patent Office, every inventor has the duty disclosing all information that is material to patentability. A patent applicant should distinguish the difference between all related applications based on the same PPA to avoid the obviousness type double patenting rejection.
For example, a patent application claimed “a composition comprising a vaccine protein that comprises an unnatural amino acid, wherein the composition additionally comprises a recombinant glycosytransferase that glycosylates the unnatural amino acid.” The claim was provisionally rejected on the ground of nonstatutory obviousness type double patenting. The conflicting claims were not identical, but not patentably distinct from each other because of overlapping scope. Thus, the applicant needed to file a terminal disclaimer in response to the double patenting rejection.
Enablement Rejection
The enablement requirement dictates that the patent specification describes the invention in clear and exact terms such that persons skilled in the art can make and use the invention without undue experimentation. The enablement can bar inoperable invention from patent protection and can guard against overly broad claims and serve the disclosure role of patent law.
Factors that may determine whether a disclosure meets the enablement requirement of 35 USC §112 include the quantity of experiment necessary, the amount of direction or guidance presented, the presence or absence of working examples, the nature of the invention, the state of the prior art, the relative skill of those in the art, the predictability or unpredictability of the art and the breadth of the claims.Citation16
For example, a patent specification, while being enabling for immunogenic compositions consisting of antigens bound to lectins, does not reasonably provide enablement for vaccines compositions consisting of antigens bound to lectins. First of all, the patent examiner considered the nature of the invention, which comprising an antigen chemically derived with a lectin and a polysaccharide coating. Next, the examiner checked the state of the prior art. At the time the invention was made, people skilled in the art were not sure whether a single protein derived from a pathogen will elicit protective immunity. Third, the examiner considered the breadth of the claim, which was extremely broad and encompassed any antigen derived with a lectin. Then, the examiner judged whether the specification provided enough guidance to enable the scope of the claim. The specification did reveal to select antigens from among commercially available vaccines. However, no limitations for the structure, function or identity were recited in the claims.
At the fifth step, the examiner checked the predictability of the art. A vaccine must trigger protective immunity in the vaccinated host, but a mere immune response is not enough. Finally, the patent examiner considered the amount of experimentation necessary. Since the prior art taught the unpredictability of using a single antigen for vaccination, it would be an undue burden and require extensive experimentation to isolate polysaccharide-coated antigen which is capable of inducing protective immune response. Thus, the patent examiner made the enablement rejection.
Similarly, another application claimed an immunogenic composition comprising glucan polymers on the fungal cell wall to conjugate to a carrier protein. However, the specification only disclosed several methods for linking polysaccharides with proteins. These methods would destroy the fungal cell and not necessarily result in the glucan-protein conjugate. Furthermore, the prior art did not demonstrate any means to conjugate a protein carrier with a glucan on the fungal cell wall. Thus, neither the specification nor the prior art provided sufficient guidance in making and using the claimed conjugates. The patent examiner made the enablement rejection in this case, indicating that someone skilled in the art would have to conduct undue experimentation to perform the claimed method.
As the two examples mentioned above indicate, the most typical enablement question arises when the patent claims are substantially broader than the embodiments disclosed in the specification. Patent systems only allow that the disclosure in the specification shall be commensurate in scope with,Citation17 or even greater than,Citation18 the breadth of the claims. Especially for vaccine and immunotherapeutics applications, even a small change in the structure of a molecule may have a great and unanticipated effect. Thus, a patent applicant would need to draft claims that are more limited in scope and closer to the specific embodiments disclosed in the specification. In the two examples for which an enablement rejection was given as mentioned above, a applicant had to amend the claim to refer to a specific group consisting of vaccines, microorganisms, and infectious agents; the other one needed to amend the immunogenic component conjugated to bacterial toxin but not to any pharmaceutically acceptable protein carrier, before the applications were allowed for insurance as patents.
Written Description Rejection
According to the US Statute, a patent specification must describe the invention sought to be patented, enable one skilled in the art to make or use the invention, and set forth the best mode of carrying out the invention.Citation19 However, the written description requirement is easily confused with the enablement requirement and is one of the more nebulous concepts in US patent law.Citation20 More recently, the CAFC en banc courtCitation7 attempted to sort out the confusion and determined that the first paragraph of 35 U.S.C. §112 does contain a written description requirement separate from the enablement requirement. Actually, the US Supreme Court clearly indicated that “the patent application must describe, enable, and set forth the best mode of carrying out the invention” in the Festo case.Citation21
Thus, it has been more than clear in the US that the written description requirement in the first paragraph of 35 U.S.C. §112 ensures that when a patent claims a genus by virtue of its function or effect, the specification must include a description of the materials sufficient to accomplish that function. In the case of vaccine and immunotherapeutics applications, without disclosing the structure or physical properties of any of the biologics required to practice the claimed methods, an inventor cannot lay claim to the subject matter. Moreover, if the specification does not disclose a variety of species that accomplish the result, then the assignee cannot claim to have methods encompassing a genus of materials that achieve the stated effects.Citation19
The guidelinesCitation22 for the written description requirement outline the steps of analysis of claims to determine whether adequate written description is present; first to determine the full scope of the claim, then to compare the scope of the claim with that of the description and finally to resolve whether the application was in possession of the claimed invention as a whole at the time of filing. The factors considered by the examiner encompass actual reduction to practice; disclosure of drawings or structural chemical formulas; sufficient relevant identifying characteristics such as structure, physical and/or chemical properties and functional characteristics when coupled with known or disclosed correlation between function and structure; a method of making the claimed invention; the required level of skilled and knowledge in the art; and the predictability of the art.
For example, a patent application claimed “An expression vector comprising (a) a DNA sequence encoding a nuclear-anchoring protein operatively linked to a heterologous promoter, said nuclear-anchoring protein comprising a DNA binding domain which binds to a specific DNA sequence and a functional domain that binds to a nuclear component; and (b) a multimerized DNA sequence forming a binding site for the nuclear anchoring protein, wherein said vector lacks an origin of replication that can function in mammalian cell.” A dependent claim thus claimed “the vector of claim 1, wherein said vector further comprises one or more expression cassettes of a DNA sequence of interest.”
While the specification described that the vectors may contain a gene or genes or a DNA sequence or DNA sequences from fungi such as Candida albicans, no sufficient recitation of distinguishing characteristics was provided to support the breadth of the numerous species .In certain embodiments, a vector of the invention comprising an expression cassette of a DNA sequence of interest is administered to a subject to induce an immune response. Specifically, the DNA sequence of interest encodes a protein (for example, a peptide or polypeptide), which induces a specific immune response upon its expression. However, the applicant did not utilize structure, formula, chemical name or physical properties to define the vectors. Thus, the patent examiner rejected the claim under the 35 USC §112 first paragraph, as failing to meet the written description. Based on the rejection, the applicant had to amend the dependent claim to recite “DNA sequence of interest encodes a protein or peptide that simulates an immune response specific to the protein or peptide before the applications were allowed for insurance as patents.”
Utility Rejection
The CAFC previously addressed the utility requirement for DNA expressed sequence tags (ESTs), where an EST sequence lacked specific and substantial utility when the function of the genes represented by the ESTs were not yet identified.Citation6 In a previous study,Citation23 the authors expressed concern that the miRNA patent applications would encounter a fate similar to the failure of EST patents. Fortunately, only seven out of 99 Candida vaccine patents have suffered from utility rejections so far. Moreover, all of the rejections could be overcome by citing a prior reference, filing a declaration or emphasizing the disclosure in the specification.
For example, a patent application claimed “a method of identifying a Replikin Scaffold in a virus or organism comprising identifying a series of Replikin Scaffold peptides comprising about 16 to 30 amino acids comprising (1) a terminal lysine and a lysine immediately adjacent to said terminal lysine; (2) a terminal histidine and a histidine immediately adjacent to said terminal histidine; (3) a lysine within about 6 to 10 amino acids from another lysine; and (4) at least 6% lysine.” The method only involved limitation to define Replikin Scaffold peptides, but not any active method steps. As such, the claim did not recite any tie to a particular machine or apparatus, nor involved a transformation to particular article. Thus, the patent examiner issued a utility rejection. In responding to the rejection, the applicant amended the claim to recite the steps of isolating a peptide or polypeptide, analyzing the sequence of the peptide or polypeptide and identifying the peptide sequence within the peptide or polypeptide as a Replikin Scaffold peptide.
Conclusion
Recent patent cases, such as KSR,Citation4,Citation5 In re FisherCitation6 and Ariad v. Lilly,Citation7 may have increased the level of difficulties in obtaining the broader protection in vaccines and immunotherapeutics.Citation3,Citation5 Moreover, the USPTO also adopts these courts’ opinions in patent examinations, which would certainly create barriers to patent prosecution. To identify the barriers, we analyzed 99 issued patents out of 940 Candida vaccine-related applications from January 2001 to May 2012 that are found in the USPTO patent application database. Our analysis showed that these recent patent cases would create barriers to patent prosecution, but the influence is not significant.
We further reviewed the image file wrapper of the 85 issued patents available online to find the rejection office actions. The actions used by the USPTO to reject patents included obviousness, novelty, indefiniteness, double patenting, enablement, written description and utility. Since these applications were allowed after amendment, we believe that some of these rejections were actually avoidable. For example, the patent applicant should demonstrate evidence of unexpected results or an indication that prior art describes results contradictory to the claimed invention to avoid an obviousness rejection during patent prosecution.Citation5
Moreover, in addition to conducting a comprehensive searchCitation13 of all available patents and publications, a patent applicant should also distinguish the prior art from the current invention, especially for those listed in the IDS, to avoid the novelty rejection.
To avoid the indefiniteness rejection, the patent applicant should clearly define the boundaries of the terms to allow a person skilled in the art to understand the terms, and the applicant should avoid vague or unclear terms in the specification. Furthermore, a patent applicant should distinguish the difference between all related applications based on the same PPA to avoid the obviousness type double patenting rejection.
The patent system demands that the disclosure in the specification shall be commensurate in scope withCitation17 or even greater thanCitation18 the breadth of the claims. Thus, a vaccine and immunotherapeutics patent applicant would need to draft claims that are more limited in scope and closer to the specific embodiments disclosed in the specification. Moreover, when a patent claims a genus by virtue of its function or effect, the specification should include sufficient materials by which to accomplish that function. For vaccines and immunotherapeutics, without disclosing the structure or physical properties of any of the biologics required to practice the claimed methods, an inventor cannot lay claim to the subject matter.
Even though these rejections may be amendable or arguable during the patent prosecution, an application should still avoid some of these barriers, especially those related to patentability. Because any amendment can relate to patentability by adding limitation on the element, the prosecution history estoppel would bar the application of the doctrine equivalents as to that element.Citation24 Such amendments triggering the bar of doctrine equivalents would simply be a waste of money and resources.
Although the target of this analysis focused on Candida vaccines, the principles recited here are also applicable to other filed of biotech.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Perlroth J, Choi B, Spellberg B. Nosocomial fungal infections: epidemiology, diagnosis, and treatment. Med Mycol 2007; 45:321 - 46; http://dx.doi.org/10.1080/13693780701218689; PMID: 17510856
- Wang S-J. Candida vaccines development from point view of US patent application. Human Vaccines 2011; 7:1-4.
- Simon BM, Scott CT. Unsettled expectations: how recent patent decisions affect biotech. Nat Biotechnol 2011; 29:229 - 30; http://dx.doi.org/10.1038/nbt.1795; PMID: 21390025
- KSR Int’l Co. v. Teleflex, Inc.: US Supreme Court, 2007.
- Wang S-J. The obviousness rejection as a barrier to biotech patent prosecution. Nat Biotechnol 2009; 27:1125 - 6; http://dx.doi.org/10.1038/nbt1209-1125; PMID: 20010590
- In re Fisher. the United States Court of Appeals for the Federal Circuit 2005.
- Ariad Pharmaceuticals, Inc. v. Eli Lilly and Co.: the United States Court of Appeals for the Federal Circuit 2010.
- Graham v. John Deere Co.: US Supreme Court, 1966.
- CFMT, Inc. v. Yieldup Intern. Corp.: United States Court of Appeals for the Federal Circuit, 2003.
- Gechter v. Davidson. United States Court of Appeals for the Federal Circuit, 1997.
- In re Arkley. the United States Court of Customs and Patent Appeals, 1972.
- Atlas Power Co. v. Ireco Inc.: the United States Court of Appeals for the Federal Circuit, 1999.
- Wang S-J. The state of art patent search with an example of human vaccines. Hum Vaccin 2011; 7:265 - 8; http://dx.doi.org/10.4161/hv.7.2.14004; PMID: 21307657
- Beachcombers v. Wildwood Creative products, Inc.: the United States Court of Appeals for the Federal Circuit 1994.
- In re Berg. the United States Court of Appeals for the Federal Circuit 1998.
- In re Wands. the United States Court of Appeals for the Federal Circuit, 1988.
- Amgen, Inc. v. Chugai Pharmaceutical Co.: the United States Court of Appeals for the Federal Circuit, 1991.
- Toro Co. v. White Consolidated Industries, Inc.: the United States Court of Appeals for the Federal Circuit, 2004.
- Wang S-J. The written description rejection as a barrier to biotech patent prosecution. Hum Vaccin 2011; 7:569 - 73; http://dx.doi.org/10.4161/hv.7.5.14358; PMID: 21552000
- Durham AL. Patent law essentials. Wesport, CT: Quorum Books, 1999.
- Festo Corp. v. Shoketsu Kinzoku Kogyo Kabushiki Co.: The US Supreme Court, 2002.
- Guidelines for Examination of Patent Applications Under the 35 U.S.C. 112, Written Description Requirement. In: Commerce Do, ed.: Federal Register, 1991:1099.
- McLeod BW, Hayman ML, Purcell AL, Marcus JS, Veitenheimer E. The ‘real world’ utility of miRNA patents: lessons learned from expressed sequence tags. Nat Biotechnol 2011; 29:129 - 33; http://dx.doi.org/10.1038/nbt.1765; PMID: 21301436
- Warner Jenkinson Co., Inc. v. Hilton Davis Chemical Co.: The US Supreme Court, 1997.
