Abstract
Virus-like particles (VLPs) are self-assembled structures derived from viral antigens that mimic the native architecture of viruses but lack the viral genome. VLPs have emerged as a premier vaccine platform due to their advantages in safety, immunogenicity, and manufacturing. The particulate nature and high-density presentation of viral structure proteins on their surface also render VLPs as attractive carriers for displaying foreign epitopes. Consequently, several VLP-based vaccines have been licensed for human use and achieved significant clinical and economical success. The major challenge, however, is to develop novel production platforms that can deliver VLP-based vaccines while significantly reducing production times and costs. Therefore, this review focuses on the essential role of plants as a novel, speedy and economical production platform for VLP-based vaccines. The advantages of plant expression systems are discussed in light of their distinctive posttranslational modifications, cost-effectiveness, production speed, and scalability. Recent achievements in the expression and assembly of VLPs and their chimeric derivatives in plant systems as well as their immunogenicity in animal models are presented. Results of human clinical trials demonstrating the safety and efficacy of plant-derived VLPs are also detailed. Moreover, the promising implications of the recent creation of “humanized” glycosylation plant lines as well as the very recent approval of the first plant-made biologics by the U. S. Food and Drug Administration (FDA) for plant production and commercialization of VLP-based vaccines are discussed. It is speculated that the combined potential of plant expression systems and VLP technology will lead to the emergence of successful vaccines and novel applications of VLPs in the near future.
Introduction
Virus-like particles (VLPs) are self-assembled structures from viral antigens that mimic the organization of native viruses but lack the viral genome. They offer many advantages in safety, immunogenicity, and antigen stability and manufacturing over vaccines based on whole pathogen preparations or subunit antigens, and thereby, have gained tremendous momentum as a premier vaccine platform.Citation1 While inactivated or killed pathogens induce strong immune responses and are still the primary source of protection for many infectious diseases, potential reversion of attenuated pathogens or incomplete inactivation of killed pathogen vaccine have remained as a major safety concern. Furthermore, there are still a substantial number of pathogens for which either a safe attenuated strain is unobtainable or there is no available tissue culture system to allow its efficient propagation and manufacture. The development of subunit vaccines through genetic engineering has the potential to phase out whole pathogen vaccines and their associated safety risks. However, vaccines based on individual proteins rarely present epitopes in their native conformation and therefore, are far less effective than whole pathogen preparations. As a result, subunit vaccines often require larger and more frequent doses of antigen, as well as co-delivery of adjuvants to elicit the necessary immune responses.
VLPs combine the best traits of whole-virus and subunit antigens for vaccine development. VLPs lack viral nucleic acid and are noninfectious, therefore, are safer vaccine alternatives than attenuated or inactivated viruses. Furthermore, the potency of VLPs can be significantly enhanced over the native virus when immunosuppressive viral proteins are eliminated from VLP composition. In addition, any unintended epitope modification by the inactivation process of live virus will be avoided for VLP production, further ensuring the VLP’s immunogenicity. Since VLPs structurally mimic infectious viruses, they can induce potent cellular and humoral immune responses without adjuvants and are more effective vaccines than other recombinant antigens.Citation2 Moreover, VLPs are more stable than subunit vaccines and can be manufactured with recombinant technology in expression systems without requiring the capability to support viral replication.Citation1,Citation2
Immunogenic Properties of VLPs
There are two primary reasons that VLPs are far more immunogenic than other subunit vaccines: they are particulate and they display epitopes on their surface in a dense repetitive array. The particulate nature of VLPs allows them to induce potent T-cell mediated immune responses through interaction with antigen-presenting cells (APCs), especially dendritic cells (DCs).Citation1,Citation2 Cytotoxic T cells do not recognize native antigens, but rather their processed peptide products in association with MHC class I molecules.Citation3 It is generally accepted that the best way to induce T-cell activation by a vaccine is to mimic the process of a natural infection including recognition, uptake, and processing of particulate antigens, and presentation of processed peptides to cytotoxic T cells to trigger their activation and proliferation.Citation4,Citation5 Studies have shown that DCs can efficiently carry out these processes by uniquely taking up antigens in the cytosol and presenting processed peptide antigens on MHC class I molecules or receptors through cross-presentation, as well as on MHC class II molecules through the classical antigen-processing pathway.Citation4,Citation5 Thus, in addition to stimulating helper T cells, DCs can also stimulate naïve T cells into cytotoxic T lymphocytes (CTLs) to eliminate intracellular pathogens or cancer cells through antigen presentation on MHC class I, effectively bridging innate and acquired immunity. It was demonstrated that DCs preferentially take up particulate antigens with diameters of 20–300 nm by phagocytosis or macropinocytosis, approximately the size of most viruses.Citation6 Like their cognate viruses, VLPs have a particle size ideal for DC and macrophage uptake and antigen processing to initiate antigen cross-presentation. Due to the high density of epitopes on their surface, uptake of a single VLP feeds thousands of epitopes into the processing and presentation machinery of APCs, further enhancing their potency in CTL induction. Furthermore, it was revealed that particles of 20–200 nm can diffuse rapidly to lymph nodes, allowing VLPs to be presented efficiently to B and T cells.Citation7 Several types of VLPs were also found to directly induce the maturation of DCs, leading to the production of co-stimulatory molecules and cytokines and the activation of CD8+ T cells.Citation8,Citation9 Therefore, the particulate nature of VLPs facilitates their favorable targeting to relevant APCs for optimal induction of T cell-mediated immune responses, which are particularly important for combating non-cytopathic pathogens and eradication of cancers.
In addition to T cell responses, VLPs can be presented efficiently to another crucial component of the immune system, the B cells, and induce strong B cell responses, due to their repetitive and high-density display of epitopes. Like native viruses, their quasi-crystalline surface with arrays of repetitive epitopes is the prime target for B cell recognition and can efficiently crosslink epitope-specific immunoglobulins (Ig) on B cells.Citation10,Citation11 It was hypothesized that particulate antigens with repetitive epitope spacing of 50–100 Å are unique to microbial surfaces, and vertebrate B cells have evolved to specifically recognize and respond vigorously to these types of antigens.Citation12 The dense repetitive antigens can hence trigger the crosslinking of surface membrane-associated Ig on B cells, the B cell receptors (BCR).Citation13 This oligomerization of Igs forms a strong activation signal that leads to B cell proliferation and migration, upregulation of MHC class II molecules, T helper cell activation, IgM and IgG production and secretion, and the generation of long-lived memory B cells.Citation13 Thus, VLPs induce high titer and durable B cell responses in the absence of adjuvants as they can directly activate B cells at much lower concentrations than antigens based on monomeric proteins.
Overall, though they lack the ability to replicate and cause diseases, VLPs can both directly activate B cells, leading to high antibody titers and long-lasting B cell memory even in the absence of adjuvants, and be preferentially taken up by APCs, triggering potent cytotoxic T cell responses. Moreover, the immune system has evolved multiple mechanisms that ensure its vigorous innate and adaptive T and B cell responses to VLPs. Consequently, these inherent advantages have made VLPs one of the most successful recombinant vaccine platforms. Five VLP-based vaccines for hepatitis B virus (HBV) and human papillomavirus (HPV) have been approved by regulatory agencies and licensed commercially, including: HBV VLPs (Recombivax® by Merck and Co., Inc. and Energix® by GlaxoSmithKline (GSK)), assembled from HB major surface antigen (HBsAg); and HPV VLPs (Gardasil® by Merck and Cervarix™ by GSK), consisting of the major capsid protein (CP) L1. These VLP-based vaccines have demonstrated excellent safety profiles, high effectiveness, and the ability to induce long-lasting antibody responses in humans.Citation2 For example, immunization of HBV VLP vaccines induces long-lasting antibody responses that can be observed at least a decade after vaccination.Citation14 Similarly, HPV VLPs elicit stable antibody titers that are10-fold greater than that of natural infection and provide long-term protection from infection.Citation15,Citation16 These successes have encouraged the preclinical and clinical development and testing of VLP-based vaccine candidates for a wide variety of other diseases.
With the major strides of VLPs as a promising vaccine platform, the major challenge is to develop novel production platforms that can overcome issues of the current production systems and can deliver VLP-based vaccines to clinics in a timely manner and at a lower cost. Therefore, this review will summarize the role of plants as an economical and speedy alternative platform for producing VLP vaccines in the light of their ability to provide distinctive posttranslational modifications, cost-effectiveness, production speed and scalability. We will highlight recent achievements in expression and assembly of VLPs in plant systems as well as their immunogenicity in animal models and safety and efficacy in humans.
Limitations of Current VLP Production Platforms
Even though VLP-based vaccines for HPV and HBV have achieved considerable commercial success and many more VLPs have shown promising results as vaccine candidates against many other difficult diseases, commercial production systems are currently limited to yeast, insect and mammalian cell cultures, which have several limitations and cannot be used for the production of many functional VLPs.Citation1 Several other VLP expression platforms such as bacterial (e.g., E. coli) cultures have been explored and found to have their own unique advantages and limitations.Citation1,Citation17,Citation18 The choice of VLP production platforms is generally based on both the structure and function of the resulting VLPs and the scalability and cost of the production process. Interestingly, despite the production of a large number of VLPs by E. coli, no bacterial-derived VLPs have been approved for commercialization and data on their immunogenicity is not always available. This is mostly due to the fact that bacteria are prokaryotes and thereby incapable of performing glycosylation and other post-translational protein modifications which is a key feature in most VLP-based vaccines.Citation1 For example, E. coli cultures cannot be used for the production of HBsAg VLPs, as there is no pathway in bacterial cells to secrete HBsAg for VLP formation.Citation19 Bacteria-derived HBsAg is also non-immunogenic and difficult to purify from the host cell.Citation20
In yeast cells, HBsAg VLPs can be produced, but the antigens are aglycosylated, unlike those found in infected sera.Citation20 In general, glycosylation in yeast cells is limited to inconsistent high mannose glycoforms,Citation21 which may not be optimal for the assembly and function of many VLP vaccines. As for the baculovirus/insect cell system, VLPs can be produced only with simple post-translational modifications (e.g., high mannose glycosylation).Citation22 Furthermore, the coproduction of baculovirus particles in the process may create significant problems in downstream processing, vaccine efficiency, and regulatory approval. Contaminating baculovirus may contribute to the overall immunogenicity of VLPs, which causes safety concerns and regulatory complications. An example came from the production of influenza VLPs by baculovirus/insect cells: the contamination challenge of separating the influenza VLPs from the baculovirus vector particles has to be overcome, a difficult process because both having a similar size range of 80–120 nm.Citation23 As a result, baculovirus particles and their infectivity have to be removed/inactivated by purification steps or chemical treatments to obviate potential side effects. These extra steps not only increase the overall cost of the product but may also impair the quality of the resulting VLPs.
Due to these inherent limitations, mammalian cell cultures provide the optimal environment for appropriate protein post-translational modification and authentic VLP assembly, and therefore, are favorable for VLP production. However, the production cost is significantly higher than other systems and also requires a heavy up-front capital investment to build a manufacturing facility.Citation24-Citation27 In addition, all cell culture-based production systems require the construction of new facilities and fermentation tanks to accommodate larger-scale production, creating challenges in scalability. Therefore, the biology or production costs of current production systems may be too difficult for certain type of VLPs or too prohibitive for resource-poor areas of the world, and may prevent the full realization of the vast health-benefit potential of VLPs. Consequently, the development of alternative VLP production platforms that provide appropriate protein glycosylation, efficient folding and assembly of VLPs, and are versatile, robust, cost-effective, scalable, and safe are urgently needed.
Plants as Production System for VLPs
Plants offer an attractive alternative system for VLP vaccine production owning to their ability to produce large quantities of recombinant protein at low cost, their eukaryotic processing machinery for the post-translational modification and proper assembly of proteins, and the low-risk of introducing adventitious human pathogens.Citation25,Citation28 Plants do not require expensive fermentation facilities for biomass generation or the construction of duplicate facilities for scale-up production. Hence, plant biomass generation and upstream processing capacity can be operated and scaled-up in a flexible, capital-efficient manner that cannot be easily matched by current fermentation-based technologies.Citation29,Citation30 Several VLPs were initially expressed in plants and yielded encouraging results, however, these earlier attempts suffered from several drawbacks including low VLP expression, plant-specific glycosylation of glycoproteins, and the lack of demonstration of producing VLPs with more than one protein.Citation1,Citation27 However, these challenges have all been overcome by the recent development of new plant expression systems and progress in plant glycoengineering. For example, the initial production of VLPs in plants was slow and produced very low yield. This problem reflects the inherent limitations of early expression systems based on stable transgenic plants, including the lack of strong regulatory elements to drive adequate amounts of target protein accumulation as well as the unwanted position effects caused by the randomness of transgene integration in plant genome.Citation24,Citation31 The low production yield made VLP production impractical and greatly reduced the cost-saving benefit of plants.Citation32
The development of plant virus-based transient plant expression systems has overcome the challenges of VLP production speed and yield.Citation33,Citation34 As reported by our group and others, the cloning and high-level transient expression of plant-derived VLPs is simple and can be achieved quickly in 1–2 weeks of vector infiltration with a tobacco mosaic virus (TMV) RNA replicon (the MagnICON) system or a geminiviral DNA replicon system based on bean yellow dwarf virus (BeYDV).Citation27,Citation35-Citation37 Importantly, these new improvements in the speed and yield of VLP production also provide the plant-expression system a critical feature of high versatility for producing VLP vaccines against viruses that mutate their surface antigens rapidly, thus providing a distinct advantage over other production systems in producing vaccines to control potential pandemics (e.g., influenza A) in a timely manner. Similarly, the issue of plant-specific glycans has been successfully resolved by the development of transgenic plant lines with “humanized” glycosylation pathways (see glycosylation section below). Furthermore, successful production and assembly of VLPs with up to three different types of proteins have been demonstrated in plants.Citation38,Citation39
Plant-Derived VLPs May Provide a Novel Vehicle for Oral Delivery of Vaccines
Conventional vaccines are produced by a costly downstream process and require continuous refrigeration, referred to as the “cold-chain,” for their transport and storage.Citation32 Similar to the storage and transport of fruits or dehydrated foods, fresh or dried plant parts containing subunit vaccines may present an ambient, temperature-stable product, thereby, providing a possible solution to the transport, storage and delivery of vaccines.Citation25 At least in theory, oral immunization can be achieved by simply ingesting subunit vaccines in edible plant parts. This approach is appealing because it may eliminate or reduce the need for the costly downstream processing, and allows plants to serve as a novel delivery vehicle for vaccines, in addition to providing a robust production system. Furthermore, the needle-free delivery method and natural preservation of vaccines in plant tissue may circumvent logistic challenges and allow practical implementation of immunization programs in regions where the “cold-chain” and other medical supplies are limited. General concerns for the efficacy of orally delivered subunit vaccines include the possible denaturation and degradation of antigens by the digestion system, poor recognition of certain vaccines at mucosal immune effector sites, and whether edible vaccines would cause inappropriate antigenic tolerance. This is where VLPs could be most valuable. VLPs, especially VLPs derived from viruses that infect the gastrointestinal system such as norovirus and rotavirus are optimal candidates for mucosal immunization and oral delivery of vaccines. With their compact and highly ordered structures, VLPs are more resistant to degradative enzymes in the digestive tract than soluble proteins. They are also naturally recognized at mucosal sites and their particulate nature allows them to be efficiently sampled by the “M” cells of the gut epithelium and thereby transported from the gut lumen across the mucosal barrier into the gut-associated lymphoid tissue (GALT) for antigen processing and presentation.Citation32 Moreover, their structural resemblance to authentic viral particles may present a “danger signal” that can overcome the perception of gut antigens as benign and thus provoke potent immune responses.40These natural mucosally targeted VLPs can be also used as carriers for developing other oral vaccines through genetic fusion or chemical conjugation. As demonstrated by the results of four human clinical trials (), VLPs produced in edible plants represent a novel and cost-effective approach to establishing gut mucosal immunity by oral delivery.Citation41-Citation43 The commercial implementation of this strategy in the developed world, however, may face regulatory challenges as a vaccine candidate is required to have strictly controlled dosage content.Citation24,Citation25,Citation32 Nevertheless, as new expression vectors have allowed more consistent VLP accumulation per unit of plant tissue, this strategy may eventually offer an attractive future option for vaccine delivery in both the developed and developing world.
Table 1. Plant-produced VLP-based vaccines that have reached human clinical trial stage and FDA-approved plant-derived human pharmaceuticals
Hence, current plant expression systems not only offer the traditional advantages of proper eukaryotic protein modification and assembly, low cost, high scalability and increased safety, but they also allow the production of VLPs at unprecedented speed to control potential pandemics or with specific glycoforms for better immunogenicity. These advantages, along with the possibility of a needle-free, oral delivery strategy, offer plants as a superior alternative production system for the broad application of the VLP potential. As a result, diverse VLPs have been expressed in a variety of expression vectors and host plant species. Results from these studies collectively demonstrated that plants are proficient in expressing and assembling both enveloped and non-enveloped VLPs, including those with more than two types of proteins and chimeric proteins. Plant-derived VLPs provide similar structures to commercially licensed or VLPs produced in other systems with an equivalent or superior immunogenicity. Some of these plant-derived VLPs are able to induce protective humoral and cell-mediated immune responses as well as show safety and efficacy in human clinical trials.
Capsid VLPs
VLPs assembled from CPs have been produced for many non-enveloped viruses. Among them, HPV VLPs are the most thoroughly studied. These consist of a 55-nm icosahedral structure, with arrays of 72 pentamers of the major CP, L1.Citation1 These VLPs have been shown to trigger strong protective immune responses against HPV at very low doses even in the absence of adjuvants and consequently developed into commercial vaccines.Citation1 The Merck vaccine (Gardasil®) is produced in yeast cells and targets both cervical cancer-causing HPV types 16 and 18 and subtypes that cause approximately 90% of genital warts (6 and 11).Citation44 The GSK vaccine (Cervarix™) is a bivalent vaccine targeting HPV 16 and 18 manufactured in the baculovirus/insect cell system.Citation45 Both vaccines have been demonstrated to be safe, effective and able to provide long-term protection from infection.Citation44,Citation45
To demonstrate the ability of plant cells in producing VLPs, our laboratory has extensively studied the expression and assembly of VLPs and successfully produced several non-enveloped VLPs including VLPs based on Norwalk virus CP (NVCP) and HBV core antigen (HBcAg). Along with other noroviruses, Norwalk virus (NV) is the major cause of non-bacterial gastroenteritis in the world, responsible for approximately 95% of viral gastroenteritis in the US.Citation46 Studies have revealed that expression of the 58 kDa NVCP alone in insect cells is sufficient to drive the assembly of a non-enveloped icosahedral VLP.Citation46 The NVCP VLPs are composed of 90 dimers of NVCP in a T = 3 symmetry.Citation47 When administered orally in mice, the insect cell-produced VLPs can stimulate mucosal as well as systemic immune response.Citation46 NVCP VLP is one of the most studied VLPs in plants; our research group and collaborators have successfully produced NVCP in several plant species including tobacco, potato, Nicotiana benthamiana, tomato and lettuce, and demonstrated that they assemble into 38 nm virion-sized icosahedral VLPs, similar to those produced in insect cells and to native NV particles.Citation30,Citation35,Citation36,Citation46
The history and current status of plant-made NVCP VLP as a vaccine candidate illustrate the progress and remaining challenges of the plant-made biologics field. As with other early recombinant proteins, NVCP was first expressed in transgenic tobacco and potato plants.Citation46 It required several months to a year to generate and select NVCP-expressing plant lines and the yield of NVCP was generally low [~10 μg/g fresh tissue weight (FW)].Citation40 Nevertheless, assembled VLPs were observed in transgenic tobacco leaves, potato tubers and other plants, although yield and assembly varied depending on the host plant species and targeted tissue of expression.Citation40 For example, the expression level and the degree of assembly of NVCP in potato tubers were relatively poor (25–50%),while higher expression levels and more efficient assembly were observed in tomato fruits.Citation40
Since NV is an enteropathogenic virus, an ideal vaccine candidate should induce NV-specific gut mucosal immunity. Indeed, one of the exciting aspects of plant-derived NVCP VLP is its potential use as an oral mucosal vaccine in the form of minimally processed plant material. Traditionally, oral delivery of vaccines for gut immunity were hindered by concerns of denaturation of antigens by low pH in the stomach, degradation by digestion enzymes, poor transport to GALT for antigen processing and presentation, and potential stimulation of systemic immune tolerance.Citation32,Citation40,Citation46 In contrast to soluble protein antigens, the unique structure of VLPs allows them to potentially overcome these challenges and elicit potent gut immune response upon oral delivery. For example, NVCP VLPs are acid and protease resistant and are stable in the oral-gastrointestinal environment.Citation48,Citation49 The overall structural resemblance of VLP to NV may present a “danger signal” and thus overcome the perception of them as benign. Indeed, specific serum IgG and intestinal IgA responses were stimulated in mice when they were fed with NVCP VLP-expressing potato tubers.Citation40 Similar results were obtained in human volunteers, indicating the immunogenicity and safety of NVCP VLP as vaccines in humans.Citation32 Unlike raw potato tubers, tomato fruits present a better plant material for developing commercial NV vaccines as they are more palatable for ingestion and their production can be readily adapted from the well-established greenhouse culture practice and fruit-processing technologies of the food industry.Citation25 Consequently, NVCP VLPs were produced in transgenic tomato fruits and oral delivery of 4 doses of 0.4 g freeze-dried tomatoes (containing 40 μg VLP) stimulated strong serum anti-NVCP IgG and fecal mucosal IgA responses in more than 80% of mice.Citation50 Moreover, an oral immunization regime with a higher dosage (0.8 g per dose) induced excellent systemic and mucosal antibody responses in all immunized mice.Citation51 Comparative studies indicated that NVCP VLPs orally delivered in freeze-dried tomato were more immunogenic than that in freeze-dried potato tubers.Citation51 Perhaps the relative low phenolic and high antioxidant (i.e., ascorbic acid) content of tomato fruits provided a less oxidative environment than that of potato tubers and resulted in better VLP stability.
Up to this point, the major limitations of NVCP VLP production in plants were the long period of time requirement for generating stable transgenic plants and the accompanying poor antigen yield. To overcome these challenges, we explored the utility of a deconstructed TMV based MagnICON transient expression system for VLP production. Our results showed that this system allows rapid production of NVCP VLPs at very high levels. Specifically, fully assembled VLPs can be produced at a level of 0.8 mg per gram of fresh leaf weight (FLW) within 12 d of infiltration in N. benthamiana plants, at least an 80-fold greater production than in transgenic tobacco and tomato.Citation36 Oral immunization of mice with transiently-expressed VLPs (100 μg dose) induced strong and balanced systemic IgG1/IgG2a response in the absence of any adjuvant.Citation36 Furthermore, oral delivery of VLPs also elicited significant NVCP-specific vaginal and fecal mucosal IgA responses in all immunized mice.Citation36 A significantly enhanced NVCP-specific immune response was observed when cholera toxin was co-delivered in the oral immunization.Citation46 Therefore, this transient expression system has overcome the limitations associated with transgenic plant system and rendered a robust plant system for VLP production. Recently, we developed another robust expression system for NVCP VLPs based on BeYDV DNA replicon vectors and grocery store-bought lettuce.Citation30,Citation52 Lettuce is cultivated readily and can produce large quantities of biomass rapidly. Unlike tobacco and related species of Nicotiana, lettuce is an edible plant and can be consumed raw. Our results demonstrate that the BeYDV replicon system permits NVCP VLP expression and assembly with as high of a level and efficiency in lettuce as those of MagnICON system in N. benthamiana.Citation30 In fact, the highest level of VLP accumulation was observed within 4 d of lettuce infiltration,Citation30 a week faster than the MagnICON system in tobacco. Furthermore, this study demonstrates the feasibility of using commercially produced lettuce for high-level and rapid VLP production.Citation30 This allows our production system to have access to unlimited quantities of inexpensive plant material for industry-scale production. The robustness and scalability of the VLP expression in lettuce, coupled with the unlimited nature of plant material generation, provide a production platform for orally deliverable NVCP VLPs that is low cost, safe, and amenable to large-scale manufacturing.
Other challenges to the commercialization of plant-made vaccines include the lack of scalable downstream processing procedures, the uncertainty of regulatory compliance for production processes, and the lack of demonstration to date of plant-derived products that meet the required safety standards of regulatory agencies.Citation24,Citation25,Citation28 While immunization by eating unprocessed plant parts still presents a viable approach to delivering plant-produced VLPs, considerations of regulatory compliance have necessitated the development of downstream processing technologies to produce VLP vaccines with a defined unit dosage.Citation24 In response to these challenges, our group has successfully developed a novel and scalable extraction and purification scheme for efficiently recovering NVCP VLPs from plant tissue.Citation27 Moreover, we successfully operated the upstream and downstream production processes under current Good Manufacture Practice (cGMP) regulations and produced high quality VLPs that meet the all preset release specifications in identity, purity, potency and safety.Citation27 This provides the first precedent of producing a plant-derived vaccine at scale under cGMP regulations in an academic setting and is an important step for plant-produced VLPs to become a commercial reality. Ongoing research by our group and collaborators is evaluating the effect of various adjuvants including toll-like receptors (TLR) on systemic and mucosal (nasal, bronchoalveolar, salivary, gastrointestinal, fecal and vaginal) immunity when co-delivered orally or nasally with the cGMP-purified VLPs. Our preliminary results indicate that inclusion of a TLR9 agonist in oral or nasal immunization stimulated stronger systemic and mucosal IgG and IgA responses than with VLP alone, with nasal delivery producing the most potent response and being able to induce IgA response in distal mucosa (Chen, manuscript in preparation). NV is a member of the Norovirus genus. There is still no licensed norovirus vaccine for human use on the market. The variability of CPs in different norovirus serotypes requires the development of vaccines that can ideally provide broad protection from all serotypes. Research efforts on NVCP VLPs from our laboratory have demonstrated a robust plant-expression platform for the cost-effective and rapid manufacture of VLP vaccines for noroviruses that can induce strong humoral, mucosal and cellular immune response in animal models.Citation27,Citation30 We anticipate that a new Phase I human clinical trial with NVCP VLPs that are purified from plants will be conducted in the near future.
HBV causes approximately one million deaths per year and has more than 350 million chronically infected carriers in the world with high incidence in the developing world.Citation1 The current recombinant human vaccines for HBV are enveloped VLPs based on HBsAg produced in yeast cells and are delivered by intramuscular injection.Citation53,Citation54 It was observed that HBcAg also self-assembles into sub-viral particles.Citation55 Recombinant HBcAg has been expressed in a variety of expression systems and was observed to assemble into 30 nm VLPs with 180 or 240 subunits arranged in a T = 3 or T = 4 icosahedral symmetry.Citation40,Citation55 HBcAg is highly immunogenic and has been shown to enhance the immunogenicity of HBsAg VLPs when co-delivered together, indicating its potential as a component of a more potent HBV vaccine.Citation56,Citation57 Since plants offer a robust and low cost production platform for antigen proteins, plant-derived HBV VLP vaccines would offer much greater benefit for the developing world. HBcAg was first expressed in transgenic tobacco leaves. Similar to the scenario of NVCP, it took several months to a year to generate and select HBcAg-expressing plants and the expression level was also rather low (up to 24 μg/g FLW).Citation58 Subsequent expression attempts with two full viral vectors based on the potato virus X (PVX) and the cowpea mosaic virus (CPMV) also yielded disappointing results (~10 μg/g FLW).Citation40 In addition to the extremely low-level of HBcAg accumulation, the full virus-based expression vectors also suffer from the drawback of coproduction of plant virus particles, creating problems for downstream processing. Despite these challenges, HBcAg produced in these early plant expression systems did correctly assemble into VLPs as demonstrated by immunosorbent electron microscopy.Citation40,Citation58 Later, our group and collaborators have shown that HBcAg VLPs can be expressed at very high levels in plants when deconstructed viral vectors are employed. For example, transient expression of HBcAg with geminiviral vectors resulted in a high accumulation level of 0.8 mg/g FLW in N. benthamiana within four days of plant infiltration and the assembly of 30 nm particles, indistinguishable from VLPs produced in other host cells.Citation35 Deconstructed TMV-based MagnICON vector allowed even higher-level HBcAg expression (2.38 mg/g FLW) in N. benthamiana, as well as efficient assembly of VLPs.Citation59 Intraperitoneal injection of partially purified HBcAg VLPs (20 μg per dose at weeks zero and two) stimulated strong serum antibody responses in mice, with the same timing and intensity as the E. coli-produced VLPs.Citation59 Furthermore, oral and intranasal delivery (500 μg per dose at weeks zero and two) of these VLPs in the absence of any adjuvant in mice also stimulated HBcAg specific serum IgG and intestinal IgA response.Citation59 These results indicate that plant-expression systems can robustly produce large quantities of immunogenic HBcAg VLPs in a short time period. These VLPs can be potentially formulated with HBsAg VLPs to produce a more potent HBV vaccine than the current yeast cell-derived vaccine that is based on HBsAg alone. Ideally, this new VLP vaccine can be delivered mucosally (oral or intranasal) to eliminate the need of needles. This would greatly improve the practical implementation of vaccine programs in resource-poor countries, where HBV infection is prevalent. Alternatively, plant-derived HBcAg VLPs can be used as an effective carrier for foreign epitope presentation and the mucosal delivery of these epitopes.
The commercial success of HPV L1-based VLP vaccines produced in yeast (Gardasil®) and insect cells (Cervarix™) has encouraged the production of these VLPs in plants. Initial expression with stable transgenic potato and tobacco yielded low to modest levels of VLPs, but they were correctly assembled and similar to the commercial products.Citation60-Citation62 Subsequent expression with MagnICON transient expression vectors or via transplastomic techniques in N. benthamiana has greatly improved the level of VLP accumulation.Citation40,Citation63 Plant-derived HPV16 and 11 VLPs were shown to be as immunogenic as the commercial vaccines in mice and rabbits when administered subcutaneously.Citation61,Citation64-Citation66 Oral delivery of four, 5 g doses of recombinant potato tubers, however, only stimulated weak serum antibody response even in the presence of adjuvant.Citation40,Citation66,Citation67 Nevertheless, subsequent oral or subcutaneous delivery of low doses of purified VLPs significantly boosted the L1-specific response, indicating that the initial feeding did result in the priming and the establishment of L1-specific immune memory.Citation40,Citation66,Citation67 Next generation plant-made HPV vaccines based on chimeric VLPs or chimeric plant virus particles displaying HPV epitopes have been developed. Some of them have shown to be more potent than the current L1 VLPs or to have additional therapeutic efficacy in preventing tumor formation/growth in mice (See chimeric VLPs section below).
One of the early skepticisms of plant expression systems was whether they had the ability to produce and assemble VLPs with more than one protein subunit. However, evidence to support that ability came from the production of VLPs of Rotavirus (RTV) CPs. When RTV CPs VP2 and VP6 were co-expressed in tomato plants, it was observed that VP2/VP6 self-assembled into VLPs in tomato fruits.Citation38 Moreover, expression of VP2, VP6, and VP7 in transgenic tobacco resulted in the assembly of RTV VLPs containing all three CPs with a diameter of 60–80 nm similar to native RTV particles.Citation39 Oral delivery of VLP-containing tobacco protein extracts with cholera toxin as an adjuvant provoked RTV-specific serum IgG and fecal IgA responses comparable to those of attenuated RTV vaccines.Citation39 Since RTV infects the gastrointestinal system and is the leading cause of severe gastroenteritis, plant-produced RTV VLPs are effective yet low-cost vaccine candidates against RTV. Due to the nature of RTV VLPs, their production in plants also provides an ideal candidate carrier for displaying antigens to induce specific gut mucosal immunity against other enteropathogens. Another example is the formation of double-shelled VLPs of rice dwarf virus (RDV). Expression of CP (P8) of RDV alone did not lead to the formation of VLPs. However, when P8 and a major core protein (P3) were co-expressed in transgenic rice plants, the formation of double-shelled VLPs similar to the authentic RDV particles were observed, further demonstrating the ability of plants to express and assemble complicated VLPs.Citation68 Other plant-derived VLPs include particles assembled from hepatitis E virus CP (HEV CP) in potatoCitation69 and Indian peanut clump virus coat protein (IPCV CP) in N. benthamiana.Citation70
Plant-Derived Enveloped VLPs
Enveloped VLPs are shells assembled from the capsid and/or envelope protein(s) of enveloped viruses surrounded with a lipid membrane known as the viral envelope. This envelope is derived from the host cell plasma membrane in a process termed “budding”Citation71. The presence of a host cell-derived membrane provides additional possibilities to integrate heterogonous antigens and adjuvants, either embedded in the membrane, or enclosed inside the lumen, but also potential challenges in regulatory approval due to the uncertainty of host cell components in the envelope.
Since the discovery that HBsAg can self-assemble into 22 nm enveloped VLPs containing approximately 100 HBsAg molecules, vaccines based on HBsAg have served as an excellent example that demonstrated the safety, efficacy, and the high potential for VLP vaccines.Citation72 HBsAg VLPs isolated from plasma are the first licensed VLP vaccine for preventing an infectious disease.Citation73 Furthermore, yeast-produced HBsAg VLPs are the first example of a recombinant vaccine that is effective against a human viral infection and approved by the FDA.Citation53,Citation54 Enveloped VLPs based on HBsAg are also the first VLPs ever produced in plants.Citation32 HBsAg was initially expressed in transgenic tobacco. While the expression level was low (66 μg/g total soluble protein), this study did demonstrate that HBsAg-containing vesicles budded out of endomembrane of tobacco cells to form spherical enveloped VLPs similar to the yeast-produced commercial vaccine antigen and subviral particles of HBV.Citation74 In addition to tobacco, these VLPs have also been successfully produced in transgenic lettuce, tomato, tomatillo, potato, corn, and banana, as well as in cell cultures of soybean and tobacco.Citation75 HBsAg dimer formation, which is required for correct processing and assembly of VLPs, was demonstrated for leaf and plant culture cell-derived HBsAg,Citation40 indicating that plants contain a favorable environment for VLP assembly. Partially purified tobacco-derived HBsAg VLPs were used in intraperitoneal immunization experiments in mice and they were shown to evoke B and T lymphocytic responses similar to the yeast recombinant vaccine.Citation76 To demonstrate that mucosal immune response can also be stimulated by oral administration, mice were fed with 5 g of raw potato tubers containing approximately 42 μg of HBsAg. Surprisingly, the potato-derived VLPs were superior to the yeast-derived antigen in both priming and boosting anti-HBsAg IgG responses in mice.Citation77 These preclinical successes have led to two human clinical trials with plant-derived HBsAg VLPs (See clinical trial section below). Comparison of different plant-derived HBsAg VLPs indicate that M protein-based VLPs induced more potent specific antibody responses than VLPs based on S protein in mice when injected intraperitoneally.Citation50 It is speculated that oral delivery of both VLPs may yield similar results with M-protein (M protein = pre-S2 +S protein) as the more potent immunogen. Since the pre-S2 component produced in yeast was able to recruit T-cell help to overcome non-responsiveness to S protein immunization, the results from plants also suggest that producing a chimeric HBsAg VLP that also displays T-helper epitopes could generate a more potent immunogenicity. Recently, the entire surface antigen (large surface antigen) of HBV has been expressed in transgenic tomato, lettuce and tobacco, and shown to assemble into VLPs.Citation78,Citation79
The recent development of influenza VLP vaccines has demonstrated the superiority of plant expression systems over other manufacturing platforms in their simplicity, speed and cost for controlling potential pandemics of infectious diseases. Influenza VLPs have been produced in mammalian and insect cells with the co-expression of hemagglutinin (HA), neuraminidase (NA), and the matrix (M1) protein.Citation80 While expression of HA alone is able to drive the VLP formation in mammalian cells, NA is still needed to release the VLPs from the producing cells because HA binds to the sialylated glycoproteins on the cell surface.Citation81 These VLPs have been shown to elicit both antibody and cell-mediated immunity as the inactivated native virus, but are superior to soluble HA antigen or inactivated virus in inducing antibodies against a broader panel of distinct influenza isolates.Citation82 However, the recent threat of another deadly influenza pandemic from the rapid, worldwide spread of AH1N1 influenza epidemics has exposed the weakness of the current egg-based, VLP platform, especially in manufacturing speed.Citation83 The capability of responding quickly to flu pandemics is further hindered by the “clustering” of manufacturing facilities in highly developed countries, due to the requirement of heavy capital investments for constructing and operating bioreactor-based cell culture facilities. An effective pandemic flu vaccine needs to be produced in the shortest achievable timeframe to halt the spread of the new strain, preferably by low cost platforms that allow affordable vaccine manufacturing in the developing world. A platform based on transient plant expression is likely to address such cost and time issues. VLPs comprised of HA alone are arguably the simplest and most realistic candidates for flu pandemic vaccines because they require only the HA coding sequence of the pandemic strain for expression, impose fewer constraints on process and product characterization, and lower the risk of failure when production processes need to be adapted for a new viral strain.Citation83 However, producing VLPs based on HA alone is not feasible in mammalian cells because HA binds to the sialylated glycoproteins on the cell surface and cannot be released from the producing cells.Citation81 Plant cells provide a unique advantage for producing VLPs based solely on HA because plant glycoproteins are not sialylated.Citation84,Citation85 HA antigen from two Type A influenza strains (H5N1 and H1N1) was first transiently expressed in N. benthamiana plants. It was found that both H5 and H1 antigens accumulate at a level of 50 μg/g FLW in the apoplast, the space between the plasma membrane and the cell wall.Citation85 A much enhanced HA accumulation level (400–1400 μg/g FLW) was achieved later by using TMV-based transient expression vectors.Citation86 Analysis with differential centrifugation, size-exclusion chromatography, electron microscopy and light scattering techniques all confirmed the assembly of true VLPs with a lipid bilayer envelope supporting the presentation of the expected HA trimers.Citation85 Lipid composition and electron microscopy analyses also revealed that as in its natural animal hosts, plant-derived HA VLPs bud from the plasma membrane of the host cell.Citation85 To date, assembly of plant-derived HA VLPs has been demonstrated for additional type A influenza strains (H2, H3, H6 and H9) and a type B influenza stain, HAB.Citation87 The antigenicity of plant-derived HA VLPs have been tested in animal models, including mouse and ferret. It was shown that two intramuscular doses of as little as 0.1 μg H5-VLPs with or without adjuvant (alum) triggered a strong immune response against the homologous strain, and two doses of 0.5 or 1 μg protected 100% mice from lethal challenges of two heterologous H5N1 strains.Citation85,Citation87 Similarly, intranasal delivery of two doses of H5-VLPs also induced a strong Hemagglutination Inhibition (HI) antibody response in mice, irrespective of the presence of the adjuvant chitosan.Citation85,Citation87 Furthermore, immunization of mice with a single dose (5 μg) of H1N1 (swine flu)-H1 VLPs induced a positive immune response in 100% of animals.Citation88 Plant-derived HA VLPs were further tested in ferrets, which demonstrated that a single dose of 5 μg or two doses of 1 μg adjuvanted (alum) H5-VLPs evoked a strong HI antibody response against a homologous H5N1 strain that meets the protective criteria of influenza vaccine established for product licensure by the European Committee for Medicinal Products for Human use (CHMP).Citation87 The same study also demonstrated the efficacy against heterologous H5N1 virus as two doses of 1 μg plant H5-VLPs elicited a strong cross-reactive immune response against two H5N1 clade 2 viruses; whereas 5 μg doses simulated a response that meets the CHMP protection criteria against a clade 1 heterologous strain.Citation87 Recently, it was demonstrated that co-delivery of two doses of 1.8 μg plant H5-VLPs with alum fully protected ferrets against a heterologous lethal challenge.Citation88
New expression vectors and downstream processing steps have been developed and optimized for rapidly producing HA-based VLPs in plants at commercial scales. Specifically, master and working banks of Agrobacteria strains harboring the H5 and silencing suppressor expression plasmids have been established with working banks having the capacity to support 300 production batches.Citation88 In its current operation the production batch is defined as 25kg of leaf biomass derived from approximately 1,500 N. benthamiana plants and several batches can be agroinfiltrated and processed weekly. Leaf protein extracts are then obtained by mechanical homogenization and removal of insoluble debris by centrifugation. Extracts are further clarified and VLPs are purified by a scheme that consists of ion exchange and affinity chromatography. The purified VLPs are subsequently concentrated and formulated by diafiltration (DF) and ultrafiltration (UF).Citation89 The feasibility of rapid VLP vaccine production in plants to combat a flu pandemic was put to a real-life test in response to an unexpected outbreak of a novel A/H1N1 influenza virus and its rapid development into a pandemic.Citation88 Remarkably, it took only two weeks to obtain infiltrated plants that expressed high levels of HA of the new A/H1N1 strain, and another five days to obtain the first purified lot of this VLP vaccine from the date that the HA sequence of this strain became available. Two doses (5 μg) of injection in mice triggered a potent HI antibody response with a mean HI titer of 1:385 in the presence of the adjuvant (alum) and 1:116 in the absence of alum,Citation88 indicating that the plant platform is not only rapid but also produces efficacious vaccines. This real-life test demonstrated that a highly efficacious pandemic VLP vaccine can be produced in plants within days from the identification of a new influenza strain. Overall, the plant-based platform allows the production of influenza VLPs with unprecedented speed. In addition, large-scale upstream and downstream processing of plant-derived influenza VLP have been successfully developed and yielded cGMP products that demonstrated their efficacy and safety in animal studies.Citation88 The plant-derived pandemic H5N1 VLP vaccine has been tested in a Phase I and a Phase II human clinical trial and an H1N1 VLP seasonal vaccine candidate has been tested in a Phase I trial.
Enveloped VLPs based on the unprocessed major core protein Gag precursor (Pr55gag) of human immunodeficiency retrovirus 1 (HIV-1) have also been produced in transgenic plants. Low Pr55gag expression was detected with nuclear expression of the transgenic gene.Citation90 In contrast, transplastomic expression of Pr55gag in chloroplasts of tobacco has resulted in high levels of accumulation (up to 312–263 μg/g FLW) and assembly of VLPs that are similar to those produced in insect cells.Citation91 Pr55gag VLPs are a promising HIV-1 vaccine candidate as they can stimulate both humoral and cellular immune responses in the complete absence of adjuvant.Citation92 Indeed, plant-derived VLPs were able to boost both humoral and cellular immune responses in mice primed with a Gag DNA vaccine,Citation90 suggesting that a T-cell stimulating HIV vaccine based on plant-derived VLPs is becoming possible.
Our laboratory has explored the plant production of enveloped VLPs as vaccine candidates for flaviviruses. We constructed a molecular construct containing plant-codon optimized genes for the premembrane (prM) and the envelop (E) protein of West Nile virus (WNV) New York 1999 strain and transiently expressed them in N. benthamiana with the deconstructed TMV vectors. Western blot analyses showed that PrM and E proteins were expressed at the expected sizes in plants (). Furthermore, the processed mature membrane (M) protein was also observed (). Interestingly, the relative band intensity of plant-derived prM and M on western blots () is comparable to that in the purified WNV virion,Citation93 indicating prM to M processing was similar between plant-derived recombinant antigen and virion protein. Results of sucrose gradient centrifugation demonstrated the assembly of VLPs containing both E and prM/M proteins of WNV (Chen, manuscript in preparation).Similarly, a plasmid encoding part of the capsid, complete prM and truncated E protein of dengue virus (DV) 3 was introduced into lettuce chloroplasts by another group; results of transmission electron microscopy showed that structures resembling VLPs were observed in transplastomic lettuce samples, but not in wild-type lettuce, suggesting a possibility of VLP assembly.Citation94 Other plant-produced enveloped VLPs include a bivalent vaccine for HIV and HBV expressed in tobacco and Arabidopsis,Citation95 HBsAg VLPs displaying GFP as antigen,Citation96 HBsAg VLPs displaying hepatocyte receptor binding epitope,Citation97 HBsAg VLPs displaying HIV-1 ENV and GAG epitopesCitation98 and HIV-1 Gag VLPs produced in N. benthamianaCitation99 ().
Figure 1. Production of West Nile virus enveloped VLP based on the prM/M and the E protein in N. benthamiana plants. Leaf tissue was infiltrated with Agrobacterium harboring the WNV prM-E construct. Leaf proteins were extracted 7 d post infiltration. PrM/M-E VLP was isolated by PEG precipitation and analyzed on 4–12% SDS-PAGE gels and transferred onto PVDF membranes. The membranes were incubated with an anti-WNV E antibody (Lanes 1–3) or an anti-WNV M-E antibody (Lane 4). Lane1, Protein sample from buffer-infiltrated leaves; Lane 2, Purified WNV E protein as positive control; Lanes 3–4, Samples from prM-E construct-infiltrated plants. *: E protein; **: Unprocessed prM protein; ***: Processed M protein.
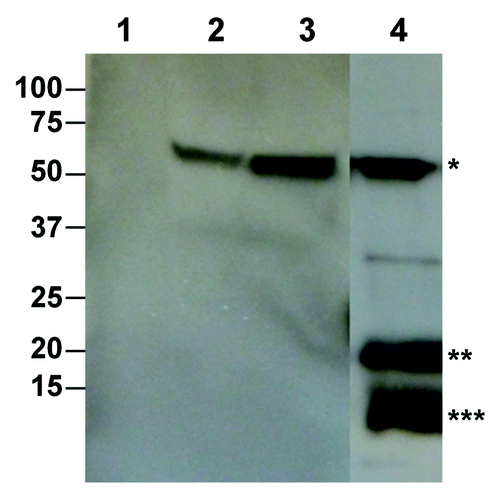
Table 2. Examples of plant-derived enveloped VLPs and cVLPs
Plant-Produced Chimeric VLPs
The particulate nature and high-density presentation of CP on their surface make VLPs an attractive carrier for displaying foreign epitopes. Consequently, a forthcoming application of VLPs is to display heterologous antigens by either genetic fusion or chemical conjugation to generate more immunogenic vaccines.Citation100 The mode of action that benefits the displayed antigen may occur at multiple levels as the heterogonous antigen is anchored in the VLP and presented in a high-density repetitive array, thereby, protecting antigen and enhancing immune cell uptake and stimulation. The carrier VLP type and the foreign antigen density and accessibility on/within the VLP significantly dictate the direction and intensity of the immune response, favoring either a humoral or cell-based immune response, or both.Citation101 Moreover, pre-existing immunity against the epitopes of the carrier VLP may significantly impact the response against the heterogonous antigen.Citation102 Several of these recombinant VLPs, termed chimeric VLPs (cVLPs), have entered clinical trials, including HBcAg VLPs displaying M2 epitope of influenza A,Citation103 yeast transposon Ty VLPs displaying HIV p17/p24 antigens,Citation104 and HBcAg VLPs displaying malaria epitopes.Citation105
Generation of cVLPs by genetic fusion
Genetic fusion offers several advantages including a stable bond between VLPs and the fused antigen and a less complex manufacturing process for the target cVLPs. However, only protein-based antigens can be attached to VLPs by genetic fusion and the outcome of the genetically created cVLPs can be unpredictable depending on many factors including the length and net charge of the target antigen peptide.Citation106 In general, many antigens are incompatible with VLP assembly and only small peptides shorter than 30 amino acids can be displayed without interfering with the proper assembly of VLPs, although there are rare exceptions to this rule.Citation100 Other potential issues include the inappropriate folding of displayed antigens and/or the formation of cVLPs with heterogeneous size.Citation107 To avoid inappropriate folding and/or assembly problems, extensive structural studies have been performed for various VLPs including HBcAg, HBsAg and HIV Gag that have identified domains that are dispensable for VLP assembly and also allow insertion of foreign antigens.Citation72 For single component cVLPs, the simplest way is to fuse the peptide to the N- or C-terminus of chimeric VLPs. Multiple fusion positions, however, have to be identified for generating multi-component cVLPs. For example, parts of the matrix and CPs or the carboxyl-terminal p6 moiety of HIV-1 Gag protein are found to be amenable for deletion and subsequent insertion of antigen sequences without affecting VLP assembly, allowing the display of multiple epitopes either from other regions of the cognate virus or from a foreign virus in the Gag particle context.Citation72 Platforms like this facilitate the development of multi-epitope vaccines that have been shown to be more potent in inducing broad immune responses.
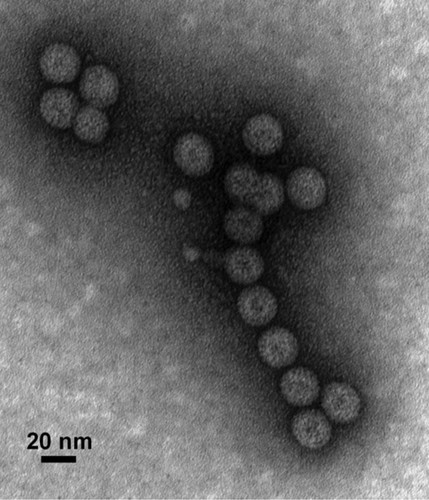
Our laboratory has been actively involved in the development of cVLPs in plants. One of the latest examples is the development of a vaccine candidate against WNV infection. In this construct, the domain III (DIII) of the WNV E protein was genetically fused to HBcAg. Expression of this construct with geminiviral transient expression vectors in N. benthamiana rendered robust high-level accumulation of the HBcAg-DIII fusion antigen in plant leaves.Citation52 Analyses with electron microscopy confirmed the assembly of the cVLPs (). Immunological studies in mice demonstrated that these cVLPs provoked strong B and T-cell responses that are superior to that of the non-fused DIII antigen (Chen, manuscript in preparation). Similar results were obtained when this cVLP was expressed with MagnICON vectors. HBcAg was also explored to display the influenza virus M2 epitope. Transient expression via PVX viral vectors resulted in the expression and assembly of HBcAg-M2 cVLPs.Citation108 Intraperitoneal delivery of this cVLP induced a strong M2-specific serum antibody response and protected 90% mice from a lethal influenza challenge.Citation108 HBcAg was also used to display a neutralizing epitope of HPV16 L2 protein. Expression and assembly of HBcAg-HPV16 L2 epitope cVLPs were observed in tobacco and nasal delivery of cVLPs triggered antigen specific antibody response in mice.Citation40 In another example, an HPV 16 L1-based chimeric VLP was produced in transgenic tomato to display several T-cell epitopes from HPV16 E6 and E7 proteins.Citation109 The HPV L1-E6/E7 VLPs were found to be assembled similar to the parental L1 VLPs and induced a neutralizing antibody response comparable to that from an equivalent amount of the commercial vaccine (Gardasil®) in mice.Citation109 Moreover, the chimeric VLP also elicited CTLs activities against the E6 and E7 epitopes.Citation109 Chimeric HPV L1 VLPs have also been created by genetic fusion to display epitopes of influenza M2 protein.Citation110
Figure 2. Production of chimeric HBcAg VLPs displaying West Nile virus Domain III of the envelope protein in plants. N. benthamiana leaves were infiltrated with Agrobacterium carrying the HBcAg and WNV DIII fusion construct. HBcAg-DIII cVLPs were purified from the infiltrated leaf tissue, stained with 0.2% aqueous uranyl acetate, and analyzed by transmission electron microscopy.
Chimeric VLPs have been employed to develop animal vaccines. One example is to use the VLP of cucumber mosaic virus (CMV) CP to display several epitopes of Newcastle Disease Virus (NDV). Epitopes from fusion (F), hemagglutinin-neuraminidase (NH) protein and the tandem F-NH peptide were genetically fused to CP and expressed via PVX vectors in N. benthamiana, which resulted in the production of chimeric CMV VLPs that are morphologically indistinguishable from wild type CMV particles.Citation111 Chickens immunized with purified F-NH VLPs developed antigen-specific antibody response.Citation111
The presence of the lipid bilayer envelope provides these VLPs additional opportunities to incorporate foreign antigens, either by anchoring to the membrane, or packaging inside the lumen. Several enveloped chimeric VLPs have been produced in plants. For example, the 239 amino-acid full length GFP was genetically fused to HBsAg and the fusion protein was shown to fold correctly and form VLPs in plants, demonstrating the feasibility of using HBsAg-VLPs as a carrier to display large protein antigens on its surface and the use of plants as a platform for the robust production of this type of cVLPs.Citation96 Moreover, leaf co-expression of the GFP-HBsAg fusion with unmodified HBsAg resulted in disulfide cross-linking to create heterodimers, suggesting the possibility of creating multivalent cVLPs with this carrier.Citation112 Indeed, expression of a fusion protein consisting of amino acids 21–47 of the hepatocyte receptor-binding presurface 1 region (preS1) fused to the C-terminus of HBsAg resulted in the formation of cVLPs in rice seeds.Citation97 This plant-produced cVLP was shown to induce antibody responses against both HBsAg and preS1 in mice, indicating it is a potential candidate for a more potent HBV vaccine as anti-preS1 antibodies can prevent HBV from binding to hepatocytes.Citation97 In another example, a polyepitope identified in five major HIV proteins was fused to HBsAg and expressed in both tobacco and Arabidopsis plants. Enveloped VLPs containing the polyepitope-HBsAg fusion protein were identified in these transgenic plants.Citation95 Similarly, chimeric HBsAg VLPs displaying HIV-1 ENV and GAG epitopes were produced in transgenic tomato.Citation98 These chimeric VLPs have the potential to serve as vaccines for inducing mucosal immune response against HIV since oral delivery of plant-derived HBsAg VLPs elicited potent immune response against HBsAg in both mice and humans.Citation77,Citation113 Indeed, feeding of recombinant dried tomato induced high levels of serum and mucosal (fecal) HIV- and HBV-specific antibodies in mice.Citation98 Furthermore, oral boosting with lyophilized recombinant tobacco tissue after DNA vaccine priming in mice provoked strong anti-HIV-1 specific CD8+ cell activation.Citation114 These VLPs could also be used to develop bivalent vaccines for HIV and HBV. A cVLP consisting of the envelope protein gp41 and Gag of HIV-1 was also produced in N. benthamiana. It was shown that transient expression of Gag with MagnICON vectors resulted in the accumulation of 100 nm Gag VLPs in leaves.Citation99 Transient expression of a “deconstructed” version of gp41 in stable Gag-expressing transgenic N. benthamiana plants suggested that the two proteins may assemble into chimeric enveloped VLPs.Citation99 Since gp41 plays a critical role in HIV mucosal transmission and infection of CD4+ cells, this cVLP may be considered as a potential mucosal vaccine candidate against HIV-1.
Generation of cVLPs by chemical conjugation
To overcome the antigen size, conformation and VLP assembly constraints associated with genetic fusion, chemical attachment approaches have been extensively explored to produce cVLPs. In this strategy target antigens and native VLPs are produced separately and subsequently linked together by attaching the antigen to the surface of the preassembled VLPs. Two major advantages of this approach are: (1) diverse sizes and types of antigens, including nonprotein-based antigens, can be displayed and (2) the antigen-VLP binding site can be manipulated to maximize the exposure of the conjugated antigen. For example, it is now possible to use VLPs to display full-length and correctly folded proteins, such as interleukin-17.Citation115,Citation116 This ability is crucial for developing effective vaccines against pathogens with antigenic variations, as larger proteins are more potent than short peptides in provoking antibodies that recognize a broad range of liner and conformational epitopes on the pathogen. The conjugation approach has also allowed the creation of chimeric VLPs that display nonprotein antigens. For example, an antismoking therapeutic vaccine was successfully developed by conjugating nicotine to VLPs of the bacteriophage Qb.Citation117 The power of this approach was further demonstrated by the results of human Phase I and II clinical trials: nicotine-Qb VLPs are well tolerated and elicit high anti-nicotine antibody titers and consequently promoted abstinence from smoking up to 12 mo in immunized volunteers.Citation117
Antigens can be linked to VLPs through either covalent or noncovalent bonds. The most common covalent method is via the use of hetero-bifunctional chemical cross-linkers with amine- and sulfhydryl-reactive arms.Citation118 For example, cysteine-containing antigens can be conjugated to VLPs with surface lysine residues at a high density of up to three peptides per coat protein molecules.Citation117,Citation119 The noncovalent conjugation strategy includes the use of streptavidin as linkers to attach biotinylated antigens and VLPs via their specific and strong interaction.Citation119 Potentially, the specific interaction between protein A from Staphylococcus aureus and Ig Fc fragment could also be exploited for such purpose. The successful assembly of chimeric turnip vein clearing virus (TVCV) particles displaying a functional fragment of protein A demonstrated the feasibility of this application.Citation120 The success of chemically conjugated chimeric VLPs as effective vaccines has been demonstrated extensively.Citation1,Citation72,Citation118 Epitopes from a variety of pathogens have been chemically conjugated to plant viruses (PV) and the resulting chimeric plant virus particles have been shown to be immunogenic and even protective in animal models (See chimeric plant virus particles section below). While the application of chemical conjugation of antigens to plant-produced non-PV VLPs has not been reported, there is no doubt that plant-derived VLPs will play an important role for this technology due to the low-cost, safe, and enormous manufacturing capacity of the plant-production platform.
Chimeric Plant Virus Particles as Vaccines
One of the unique aspects of plant expression systems is the possibility of using plant virus particles (PVPs) as a carrier to display foreign epitopes. While PVs are distinct from human or animal pathogens and cannot replicate in animal cells, the high-density, quasi-crystalline array of CP and the particulate nature of their virions are ideal for the resulting chimeric PVPs (cPVPs) to stimulate B-cell response, as well as dendritic cell antigen uptake.Citation121-Citation123 The ability of a TMV-displayed peptide vaccine in breaking B-cell tolerance clearly demonstrates that cPVPs are at least equivalent in their capacity to induce potent immune responses as other VLP platforms, such as HPV VLPs and bacteriophage particles.Citation124 In general, cPVPs have been shown to offer many similar advantages as VLPs in enhancing antigen immunogenicity, safety, as well as in enhancing the production level and stability of the conjugated antigen.Citation121,Citation122 Similar to VLPs, cPVPs can be created by genetic fusion of epitopes to CP of PVs, or by chemical conjugation of antigens to preassembled PVPs.
For example, a chimeric peptide containing determinants of glycoprotein and nucleoprotein of rabies virus (RV) was fused to the N-terminus of the alfalfa mosaic virus (AlMV) CP and cloned into AlMV and TMV-based viral vectors. The transfection of this fusion protein gene in tobacco, N. benthamiana and spinach plants resulted in high-level accumulation (0.4 mg/g FLW) of recombinant virus particles that displaying the chimeric RV epitopes.Citation43 The RV cPVPs can be isolated easily with polyethylene glycol precipitation. Intraperitoneal injection of three doses of 250 μg RV cPVPs (35 μg peptide) at 2-week intervals induced a strong systemic neutralizing antibody response in mice and protected them against a lethal challenge of RV for at least 120 d.Citation43 This research demonstrated for the first time the longevity of protective immune responses elicited by plant-derived antigens and suggested that immunological memory to the RV epitopes was established by the immunogen. As predicted, the chimeric peptide displayed on the RV cPVPs was more immunogenic than the non-conjugated peptide co-delivered with adjuvant in mice.Citation43 Oral delivery of four doses of purified RV cPVPs (250 μg per dose) or raw spinach leaves containing the RV cPVPs (1 g per dose) stimulated relatively strong mucosal RV-specific IgA response as well as serum IgG and IgA immunity.Citation125 Interestingly, mice fed with RV cPVPs-containing spinach leaves produced a stronger mucosal IgA response than those receiving purified chimeric virus. Similar encouraging results were also obtained in humans.Citation43 RV still poses a significant threat to human health and causes > 50,000 death each year, with most of the fatal cases occurring in developing countries.Citation126 In contrast to the current inactivated RV vaccine produced in infected human diploid cells, plant-derived chimeric VLPs or cPVPs displaying RV epitopes may provide an affordable, safer, and needle-free vaccine alternative for the developing world.
The activation of cell-mediated immune responses by cPVPs has also been demonstrated. A chimeric CP of PVX was created to display the H-2D(b)-restricted epitope of influenza nucleoprotein (NP).Citation127 Infection of N. benthamiana plants by the recombinant PVX vector resulted in the robust production of chimeric PVX particles. Subcutaneous immunization of mice with an endotoxin-free preparation indicated that 167 μg of chimeric PVX particles in the absence of adjuvant or 50 μg in the presence of incomplete Freund’s adjuvant activated epitope-specific CD8+ IFN-γ secreting cells.Citation127 This suggests the potential utility of cPVPs as vaccine components in activating cell-mediated immune responses.
The ability of stimulating protective immunity has been demonstrated by many cPVPs. For example, a chimeric CPMV displaying a 17 amino acid epitope of the VP2 CP of canine parvovirus (CPV) was produced in cowpea plants.Citation128 Subcutaneous injection of two doses (7.5 mg chimeric CPMV particle, 150 μg peptide per dose) of adjuvanted (Quil-A/alum) CPMV-CPV VP2 protected all vaccinated dogs from a lethal challenge of CPV and totally abolished shedding of virus.Citation129 Since the epitope is shared by CPV, mink enteritis virus (MEV) and feline panleukopenia virus, this plant-derived vaccine perhaps can be also effective in mink and cats. Indeed, a single subcutaneous injection of 1 mg of the chimeric particles protected 100% mink from a lethal challenge of MEV and eliminated all clinical signs of the disease.Citation128 Subsequently, protective immunity was also demonstrated for cPVPs that display epitopes of urine hepatitis coronavirus,Citation130 foot-and mouth disease virus (FMDV),Citation131,Citation132 and bacterial origin.Citation133,Citation134 Many other cPVP-based vaccine candidates have been developed and demonstrated immunogenicity or protection in animal models ().
Table 3. Examples of chimeric plant virus particles as vaccine candidates
Like VLPs, CP of PVPs was believed to only tolerate genetic insertions of short peptides. Increasing evidence, however, suggests that this assumption may not be true. One such example comes from a recombinant Tobamovirus in which the CP was fused to a functional protein A fragment of the same size.Citation120 In spite of the doubling of its CP size, viral replication, particle assembly, and systemic movement of the recombinant virus were found adequately active.Citation120 This result suggests that it is possible for cPVPs and VLPs to display larger conformational epitopes through genetic fusion in addition to the linear ones. Since most pathogen-neutralizing antibodies are elicited by conformational epitopes, this evidence indicated a much broader utility for cPVPs and VLPs as antigen-displaying carriers.
CPVPs can also be created by chemical conjugation with similar strategies as VLPs, namely by using bifunctional cross-linkers, or exploring the specific interaction between biotin and streptavidin, or between Fc of Ig and protein A. PVPs with surface lysine or cysteine residuals can be directly conjugated with cysteine or lysine-containing antigens. Otherwise, CP of PVPs can be genetically engineered to have exposed lysine residues on the surface to enable conjugation. Since the introduction of a lysine residue may affect the expression, assembly and stability of the recombinant CP, careful consideration has to be given to balance the need for such reactive residues and the optimal expression and assembly of the modified CP.
For example, a lysine was genetically engineered onto the surface of TMV for chemical conjugation of antigens.Citation121 It was found that when a cysteine-containing 15 amino acid HPV L2 peptide was incubated with the recombinant TMV, 95% of CP was conjugated with the target peptide.Citation121 Immunization data suggested that while this cPVP induced strong B-cell response, it was not as potent as an equivalent cPVP created by genetic fusion.Citation121 Since the antigen composition of the two cPVPs is almost identical, the immunogenic disparity was perhaps caused by the difference in the bond that connected PVPs and the target peptide. It was suggested that cPVPs and cVLPs with stable bonds would have a more stable crystalline array of antigen on their surface, and thereby, more likely to induce the maximum humoral immune response.Citation121 To further demonstrate the utility of chemical conjugated cPVPs, a whole protein antigen, the canine oral papillomavirus L2 (COPV.L2) antigen, was conjugated to TMV through biotin and streptavidin interaction.Citation135 Approximately 30% of TMV CP subunits per TMV particle were found to associate with the antigen. Immunization of mice and guinea pigs with this cPVP rendered potent specific antibody responses which were far superior to those in animals that were immunized with the non-conjugated COPV.L2 antigen.Citation135 This application demonstrates the feasibility and utility of PVPs in displaying whole protein antigens, especially ones that cannot be made by genetic means. Similar to cPVPs generated by genetic fusion, cPVPs chemically-conjugated with T-cell epitopes also stimulated potent CTL responses. One of the examples came from the same recombinant TMV that displayed the T-cell epitope of murine melanoma peptides.Citation136 In contrast to results from antibody stimulating chimeric TMVs, where genetic fusions induced higher titer antibodies, chemically conjugated chimeric TMVs were found far superior in eliciting IFN-γ-producing T cells.Citation136
Overall, tremendous success has been achieved with cPVPs as carriers for displaying and enhancing the immunogenicity of foreign antigens. However, in comparison to VLPs, cPVPs do suffer from several inherent disadvantages. For example, conformational epitopes may not be presented as correctly as by native virus or corresponding VLPs. The biggest challenge, however, may come from the regulatory aspect of this technology. Unlike VLPs, there is no precedent of approved products made by this technology; therefore, it will have to overcome a series of regulatory hurdles before becoming an accepted manufacturing platform for vaccines.
Plant Protein Glycosylation and VLP Functions
Glycosylation of viral surface antigens has a major impact on the efficacy of vaccine antigens as it is critical for immune recognition, receptor binding, inflammation, and pathogenicity.Citation137,Citation138 For example, mutation of one of the two N-linked envelope glycoprotein 2 (GP) glycosylation sites of Ebola virus is detrimental to the immunogenicity of GP.Citation138 Alterations of glycosylation of the viral HA due to egg-adaptation have also been shown to severely affect the antigenicity of influenza virus.Citation139 It was also demonstrated that N-glycosylation is crucial for the correct folding of viral glycoproteins, and hence the structure and function of VLPs that assemble from glycosylated viral structural proteins. For example, HCV VLPs produced in the presence of α-glucosidase inhibitors contain misfolded glycoproteins and have impaired binding to hepatoma cells.Citation140 E protein glycosylation is a molecular determinant of the neuro-invasiveness of the WNVCitation141 and is critical for the secretion of VLPs of tick-borne encephalitis virus.Citation142
Therefore, the glycosylation status of VLP proteins is critical for their structure and function, as well as determines the choice of platforms for their production. An example of this was demonstrated by the production of VLPs of Lassa virus (LASV) based on glycoprotein subunits of GP1 and GP2.Citation143 Since the glycosylation pattern of GP1 and GP2 has been shown to play a critical structural and functional role in preserving protein stability and allowing binding and fusion to host cells, LASV VLPs cannot be easily produced in bacterial, or insect cell-based production systems.Citation143 As prokaryotic cells, E. coli do not have the ability to glycosylate nor to process the precursor protein into GP1 and GP2 subunits. Glycosylation in insect cells is mostly limited to high mannose glycoforms and these cells also lack the processing enzyme for the precursor glycoprotein. Even though mammalian cells have the critical subtilases and efficiently process the precursor glycoprotein into GP1 and GP2 subunits, the glycosylation profile of GP1 and GP2 in mammalian-cell produced VLPs is highly heterogeneous and more so than in the native virions.Citation143 The presence of mixed and inconsistent glycosylation types is a common phenomenon for most mammalian cell-produced glycoproteins. Thus, none of the current popular production systems is ideal for the production of LASV VLPs and other VLPs based on glycoproteins. This is where plant expression systems may play another important role in providing a solution to this common problem.
N-glycosylation of proteins in plants is generally similar to that of mammalian cells. However, wild-type (WT) plants add plant-specific β -1,2-xylose and core α-1,3-fucose residues to complex N-linked glycans and lack terminal β1,4-gal and N-acetylneuraminic acid (Neu5Ac) residues.Citation144 The unique plant glycosylation pattern can be advantageous for certain pharmaceutical applications, as in the case of glucocerebrosidase (GCD).Although not a VLP-based vaccine, this therapeutic enzyme for treating Gaucher disease is the first plant-derived pharmaceutical ever approved by FDA (May 1, 2012) and demonstrates the advantage of plant glycoforms for its efficacy.Citation145 Mammalian cell-produced GCD requires in vitro N-glycan processing to achieve the desired efficacy, while the carrot-cell produced GCD (commercial name: ELEYSOTM) already has the required glycoform, eliminating the costly N-glycan processing, that may have resulted in better and more consistent efficacy.Citation28 Also as discussed earlier, plant-specific glycans provide a unique advantage for producing flu VLPs vaccines based solely on HA as plant glycoproteins are not sialylated.Citation84,Citation85 However, the minor differences in protein glycosylation between WT plant and mammalian cells used to be one of the major issues of plant-expression platforms, because they may produce improper glycoforms reducing efficacy, but there is also the possibility of inducing plant-glycan specific antibodies that accelerate protein clearance from plasma or cause potential adverse effects through immune complex formation. Fortunately, there has been tremendous progress in glycoengineering to create “humanized” plant lines by knocking out enzyme genes for synthesis of plant specific glycans and/or introducing mammalian glycosylation genes into plant cells.Citation144 As a result, glycoengineering has resulted in a portfolio of “humanized” plant lines that lack plant-specific glycans and that produce pharmaceutical proteins with various specific and defined mammalian glycoforms including high mannose, GnGn, G0-G2 galactose, bisected GlcNAc, fucosylated and nonfucosylated, and full complex form with terminal sialic acid addition.Citation146-Citation151 Glycoproteins produced from these glycoengineered plants not only have defined and specific mammalian glycoforms, but also possess a high degree of glycan uniformity that cannot be produced by mammalian cells or achieved by in vitro treatments.Citation146,Citation152This portfolio of plant lines, therefore, provides a superior system for producing VLPs with defined and uniform carbohydrate constituents. With these plant lines, in theory, we can ultimately custom design VLPs with a tailor-made glycoform that is best suited for the assembly and immunogenicity needed for a particular clinical application. Thus, the creation of these glycoengineered plants provides an additional advantage for plant-based expression systems to become a desirable VLP production platform.
Downstream Processing of VLPs from Plants
The ability of large-scale production of VLPs in plants and other heterologous expression systems promises a broad application of this vaccine platform. While unprocessed or partially processed VLP-containing plant materials still represent a viable opportunity to deliver vaccines by ingestion, regulatory concerns motivate the development of processing technologies to produce VLPs with a more defined unit dosage.Citation24,Citation25 Consequently, successful application of VLPs depends on the availability of robust and scalable downstream processing methodologies that can effectively recover and purify VLPs at low cost.
Downstream process development should aim to increase manufacturing productivity, reduce production costs, enhance scalability, preserve the integrity of the VLPs, and ensure the compliance of the manufacturing procedures with the FDA’s cGMP regulations. As with other recombinant proteins, an optimized downstream process should yield highly purified VLPs with the highest possible percentage of product recovery and a minimal number of purification steps. Due to the diverse structural and architectural nature of VLPs, downstream processing for a particular VLP depends on the size, shape and architecture of the assembled VLPs, as well as on the production system and the production process. Typically, the initial design of processing steps depends on the structural nature of the target VLPs (e.g., enveloped or nonenveloped) and the cellular nature of the production host. The processing scheme can then be further refined based on the size and shape of the VLP, and the site of VLP accumulation within or outside the cell. For example, purification of enveloped VLPs can be more difficult than nonenveloped VLPs as the envelope is labile and sensitive to shear and osmotic pressure variations.Citation153 Since the lipid bilayer is often targeted for their purification, separation of enveloped VLPs from host cell-derived membrane-bound vesicles is often difficult. As for production systems, VLP production in bacterial cells often encounters difficulty in producing soluble proteins and VLPs free of contamination by endotoxins.Citation154,Citation155 As a result, special attention has to be paid to protein solubilization and toxin removal. In contrast, the major challenge for insect cell-produced VLP processing is the separation of the target VLPs from baculovirus particles that are coproduced and share a similar molecular weight.Citation1 In both cases, these processing problems may cause a substantial increase in product cost.
Similar to other vaccines, the purification of VLPs consists of the basic capture, purification and polishing steps, but with special attention given to preserving the integrity of the assembled VLPs. The objective of the capture step is to remove the most abundant contaminants, and consequently, high throughput and volumetric operations are typically employed. Highly selective operations are desirable for the purification stage for removing all impurities and obtaining highly purified VLPs. On the other hand, the polishing step should use even more selective methodologies to achieve higher purity by separating assembled VLPs from non- or partial-assembled VLPs, or VLPs with non-targeted architecture. This step is crucial for obtaining VLPs with the targeted conformation as many proteins tend to adopt improper yet stable aggregate structures. Despite the production of many VLPs in a diversity of production systems, VLPs have been mostly purified by a few methods based on variations of centrifugation and precipitation.Citation36,Citation48,Citation156,Citation157 The centrifugation methods were originally developed for virus particle isolation, but were subsequently applied to VLP purification. Similar to their cognate viruses, VLPs can be purified on the basis of their size and density using ultracentrifugation techniques, with sucrose, cesium chloride (CsCl) and potassium bromide as the most common media for gradient generation.Citation158 While ultracentrifugation and density gradient methods are widely used for characterizing VLP size and assembly,Citation36,Citation49,Citation157,Citation159 their application to large-scale commercial VLP manufacturing is limited because they are time-consuming, difficult to scale up, and produce poor yields.Citation52,Citation160 Furthermore, the hyperosmotic nature of density gradient agents and the high centrifugation force often shear and disrupt the integrity of assembled VLPs, causing their degradation.Citation161
To meet the demand for more selective and scalable methods of VLP purification, sophisticated methods like tangential flow filtration, ion exchange, affinity, and size exclusion chromatography have been explored.Citation162-Citation165 Purification methods based on chromatography are favorable for VLP production due to their high selectivity, high recovery rate and high scalability for large-scale production. For example, high purity of insect cell-produced parvovirus B19 VLPs is effectively achieved by a series of diethylaminoethyl (DEAE) chromatography steps in large-scale purifications.Citation166 Similarly, HPV16 L1 VLPs can be purified with heparin or cation-exchange chromatography with reasonable recoveries.Citation167 So far, a wide variety of classical chromatographic resins including those for ion-exchange, hydrophobic interaction, and affinity and size exclusion chromatography have been shown to be effective for the purification of different VLPs.Citation153,Citation163,Citation168,Citation169 Due to the unusually large size of VLPs, traditional chromatography resins may not be optimal for their purification due to poor binding capacity, low resolution and VLP recovery. These flaws are caused by the surface adsorption and pore exclusion effects as the relatively small pore sizes of the traditional resin limit the adsorption of VLPs to their surface, while smaller impurities can bind to all areas of the beads including those inside the pores.Citation170-Citation173 To further optimize the recovery and binding capacities of VLPs, novel chromatographic matrices and strategies that can accommodate their unusually large size are being developed including membranes, monoliths and tentacle supports. Membrane chromatography based on newer, disposable membrane technology, for example, is gaining momentum as the leading alternative to traditional beads for VLP purification.Citation162,Citation174-Citation178 Membrane chromatography is particularly useful for purification of VLPs and other large particles with low diffusivities. Because the interaction of the binding sites on the membrane and the target molecules occurs in convective flow-through pores, it overcomes the pore diffusional issue of conventional resins and allows membrane chromatography to maintain high efficiency even at high flow rates.Citation52,Citation176 Membrane-based separation strategies also facilitate the purification of enveloped VLPs that are sensitive to shear and osmotic shock, and prone to degradation. An example is the use of hollow-fiber cartridge membranes in purification of a HIV VLPs. The tangential flow of the feed stream across the membrane allows the separation to occur under low-stress conditions, leading to efficient purification of HIV particles with 95% recovery.Citation179 In addition to their promising applications in the purification step, membrane-based separation methods have already gained increasing importance in the capture and polishing steps including microfiltration for media clarification and product sterilization, UF for VLP concentration and fractionation, and DF for buffer exchange.Citation153 These membrane-based filtration steps require simple equipment and are particularly efficient in eliminating aggregates and partially assembled VLPs, providing a convenient means to integrate purification and size separation into a single step to achieve both purity and size uniformity.Citation180 Additional advantages of membrane-based operations such as high scalability and cGMP compliance further justify their increasing importance for VLP downstream processing.Citation153
The development of virus-based transient expression systems has significantly increased the yield of VLPs in plant expression systems.Citation35,Citation52,Citation181-Citation183 Consequently, cost of downstream processing has become an increasingly significant proportion (> 80%) of the total cost, demanding more scalable and low cost technologies for VLP recovery from plants.Citation24,Citation184,Citation185 The unique properties of plant tissues present both challenges and opportunities for downstream processing. For example, some plants produce mucilages and are rich in aromatic compounds, which may complicate product purification. On the other hands, plant-derived VLPs have low contamination risks by human or animal pathogens, and therefore, reduce safety concerns and have the potential of simplifying downstream operations. For instance, the tedious viral validation step required for purifying mammalian cell-derived therapeutics could be eliminated, providing a potential time and cost saving opportunity.Citation25 While the majority of plant-derived VLPs are still purified by the traditional precipitation and centrifugation methods, a new trend has started toward more robust and scalable methods such as filtration and chromatography.Citation25 For example, our group has successfully developed a novel and scalable extraction and purification scheme for efficiently recovering NVCP VLPs from plant tissue. This is a three-step process consisting of low pH precipitation, UF/DF with tangential flow filtration (TFF) membranes, and DEAE anion-exchange chromatography.Citation27 Our results showed that low pH precipitation removed the most abundant plant host protein, RuBisCo, and DEAE chromatography eliminated the remaining contaminants and enriched NVCP VLPs to > 95% purity.Citation27 We also demonstrated that our process is highly scalable and produces NVCP VLPs with consistent high purity and recovery among batches of different scales.Citation27 Compared with the laborious and time-consuming methods of gradient centrifugation, our method is robust, more scalable and can shorten the operation time from several days to ≤ 12 h.Citation27,Citation52 NVLP purified by this process was shown to maintain VLP structure and immunogenicity in mice following mucosal administration.Citation27 Moreover, our upstream and downstream production processes can be successfully operated under cGMP regulations and produce high quality VLPs that meet the preset release specifications in identity, purity, potency and safety.Citation27 Our studies thereby provided the first precedent of producing a plant-derived vaccine at scale under cGMP regulations in an academic setting, an important step for plant-produced VLPs to become a commercial reality.
Affinity chromatography is a powerful separation technique that may reduce the number of purification steps and increase yield and purity. It has been explored to purify plant-derived VLPs including monoclonal antibody conjugated sepharose to purify Vicia faba VLPsCitation186 and fetuin-agarose to purified influenza VLPs from N. benthamiana plants (see below). The commonly used affinity ligands are biologics which are expensive to make, prone to degradation, easily denatured by sanitizing agents, and likely to leech into the purified products. As a result, applications of affinity chromatography are often limited to small scale VLP purification. In response to this challenge, we have employed new technologies such as microarrays to develop synthetic ligands for affinity purification of VLPs.Citation52 For example, we have developed a system to screen 20mer peptide ligands with specific affinity to NVCP VLPs in a microarray containing a library of 10,000 randomly generated peptides. It is noteworthy that our peptides are immobilized in a configuration that mimics the conformation they have when conjugated to affinity beads. This strategy is superior to other library selection approaches such as phage display because it ensures the identification of peptides that will bind the target protein with equivalent specificity and affinity when conjugated to chromatographic matrix. Indeed, we obtained highly purified NVCP VLPs from N. benthamiana plant extract with these affinity ligands (Chen Q, Diehnelt C, and Arntzen C, manuscript in preparation). Our approach offers the advantage of being entirely synthetic and therefore insensitive to ligand denaturation or degradation. Coupled with the rapid ligand discovery process, the low cost of peptide production will likely allow the large-scale application of this technology and its adaptation for the purification of other VLPs.
The feasibility of efficiently extracting and purifying enveloped VLPs from plants at a commercial scale has been demonstrated by the production of HA-based influenza VLPs vaccines.Citation89 In this weekly operation, several batches of 1,500 N. benthamiana plants can be agroinfiltrated. After 6 d of incubation for VLP expression, 25 kg of leaf biomass will be harvested from each production batch and mechanically homogenized to obtain protein extracts, which are clarified, concentrated and buffer exchanged sequentially by centrifugation, microfiltration, TFF and DF.Citation88 HA VLPs are then purified from the clarified extracts by a series of chromatographic steps including anion-exchange with Poros HQ, cation exchange with Poros HS and affinity chromatography with fetuin-agarose resins.Citation89 The purified VLPs are subsequently concentrated and formulated by TFF, and DF and sterilized by microfiltration.Citation89 Results have shown that this process can produce highly purified HA VLPs with the expected size and architecture, and that induced potent immune responses in animals.Citation89 Furthermore, this downstream process has been successfully implemented in large-scale operations under cGMP regulations and produced human clinical grade HA VLP vaccines from N. benthamiana with high purity and consistent recovery.Citation89 The cGMP VLPs have been tested in human clinical trials and their efficacy and safety in human studies has been demonstrated.Citation89
Like in other systems, it is critical to prevent plant host contaminants from entering processing feed streams at an early stage to simplify the overall purification process and ensure the regulatory compliance of the final VLP product. Due to the diversity of VLP architecture, size and epitopes they are designed to display, as well as the diversity of production plant hosts, it is impossible to have one universal downstream process that can fit the purification needs of all plant-derived VLPs. However, more versatile purification strategies are being developed, which can be readily applied or adapted to the purification of various VLPs that share similar sizes, architecture or epitopes with common physiochemical properties.Citation153,Citation163 General purification procedures are also being established for removing unique contaminants of popular plant production hosts. Innovations in separation materials and technologies will continue to drive the further improvement of downstream processes for plant-produced VLPs.
Human Clinical Trials of Plant-Produced VLPs
Successes in the production, purification and demonstration of strong systemic and mucosal immunogenicity have prompted several clinical studies examining the safety and immunogenicity of plant-produced VLP-based vaccines in humans ().
For example, results of preclinical studies in mice and ferrets encouraged a randomized, double-blind, and placebo-controlled Phase I human trial with plant-made enveloped VLPs based on HA of an H5N1 avian pandemic influenza (A/Indonesia/5/05).Citation88 In this study, 48 adult volunteers between 18–60 y of age were given two intramuscular doses of 5, 10 or 20 μg of adjuvanted (alum) H5 VLPs vaccine or placebo. The immunogenicity was evaluated by three independent assays including HI, single radial hemolysis (SRH) and microneutralization (MN). After the second dose, 75%, 75%, and 92% of individuals in the 20 μg group produced detectable antibody titers as measured by HI, SRH and MN respectively, with 16.7%, 25% and 50% of volunteers in the 5, 10 and 20 μg groups developed HI titers of > 40, while no HI antibodies were detected in the placebo group.Citation89 The results also show that two doses of 20 μg vaccination of plant-derived VLPs elicited an immune response that meets all three protective criteria (seroprotection > 70%, seroconversion > 40%, and geometric mean increase (GMI) > 2.5) established by CHMP for influenza vaccines.Citation89 Importantly, this trial demonstrated the safety of the plant-derived VLP vaccine in humans as it was well tolerated at all doses and the adverse events were mild-to-moderate and self-limited. Furthermore, this plant-made vaccine did not significantly increase the level of naturally occurring antibodies to plant-specific glycans and no allergic reactions were observed.Citation89 Based on these results, a two-part Phase II human clinical trial was conducted. In Phase II part A, 135 volunteers received two doses of 20, 30 and 45 μg H5 VLP vaccine in the presence of alum or 45 μg without adjuvant or placebo 21 d apart. Interestingly, 20 μg with alum proved to be the optimal dosage to induce a strong immune response. In the part B study, an additional 120 volunteers were further tested. The results showed that older and younger volunteers responded similarly to the plant-made VLP vaccine, a differential advantage over similar vaccines produced by other vaccine technologies.Citation187 While no further details have been released, the sponsor of the trial did report that “The vaccine induced a solid immune response and was found to be safe, well tolerated.”Citation188
A Phase I clinical trial has also been completed for a seasonal flu vaccine candidate based on plant-derived HA VLPs of an H1N1 strain, also known as swine flu.Citation189 One hundred healthy adult volunteers 18–49 y of age were given a single dose of intramuscular administration of 5, 13, and 28 μg H1-VLPs or placebo. As for the H5 VLP vaccine, this plant-derived VLP was also found to be safe and well tolerated at all dosages. Moreover, even a single dose of 5 µg triggered a strong immune response that meets the CHMP protective criteria.Citation189 Based on these results, the sponsor is planning a Phase II human trial for its seasonal trivalent vaccine with the recommended H1N1, H3N2 and B influenza strains.Citation189 Overall, these encouraging results suggest that plant-made VLPs are viable human vaccine candidates against both seasonal and pandemic influenza viruses.
To demonstrate the safety and efficacy of orally-delivered VLPs in plant materials, a Phase I clinical trial was conducted with potato-produced NVCP VLPs. Twenty human volunteers were fed with two or three doses of 150 g uncooked NVCP-transgenic potato tubers which containing 215–751 μg of VLPs.Citation190 An increase in the number of IgA-secreting cells was observed in 19 of 20 human subjects. Furthermore, four of the volunteers developed NVCP-specific serum IgG and six developed specific stool IgA.Citation190 The ingestion of VLP-producing potato tubers appeared safe as the incidence rates of nausea, vomiting, mild cramps, fever or diarrhea were similar among volunteers who ate recombinant or control tubers.Citation190 Together, these results indicate the immunogenicity and safety of using edible VLP-containing plant parts as oral vaccines in humans. The overall antibody response was, however, weaker than that obtained in a clinical trial in which purified insect cell-derived NVCP VLPs (250 μg per dose) were used.Citation157 It is likely that the inconsistent NVCP content and poor VLP assembly in potato tubers caused variable effective VLP dosage and contributed to the weak antigenicity. It is also possible that VLPs were not effectively released from the potato tissue in the gut lumen, further reducing the effectiveness of the antigen. These results suggest that purified NVCP VLP is a better oral vaccine candidate than that in unprocessed plant tissue. In light of this, we are planning a new human clinical trial with purified plant-derived NVCP VLPs in collaboration with our industry partners.Citation27
Preclinical studies have demonstrated the potency of plant-derived HBsAg VLPs in evoking both B and T-cell mediated immunity by both systemic and mucosal delivery. These successes have led to two human Phase I clinical trials with plant-produced HBsAg VLPs. In one of these trials, two or three doses of 100 g raw potato tuber containing 850 μg HBsAg were orally ingested by 33 human volunteers who were previously vaccinated by the commercial yeast-derived HBV vaccine.Citation113 HBV seropositive subjects were selected for this trial in consideration of the possibility that HBV naïve participants might experience antigenic tolerance after oral delivery of HBsAg. Significant increases in serum anti-HBsAg titers were observed in 10 of 16 volunteers who consumed three doses of antigen-containing tubers and in 9 of 17 participants who ate two doses of antigen.Citation113 These results provide a clear indication that oral delivery of plant-derived HBsAg VLPs can activate systemic memory cells and thereby can be used as an effective oral booster for HBV vaccines. In a separate trial, three HBV naïve volunteers were given two oral doses of transgenic lettuce containing 1 μg of HBsAg VLP. Two subjects developed high titers of serum anti-HBsAg IgG that exceeded the protective level.Citation42,Citation191 This result suggests that plant-derived HBsAg VLPs can also effectively prime immune response through oral delivery in addition to working as a booster. The protective anti-HBsAg level for humans has been defined as > 10 mIU/ml. The two clinical trials above have demonstrated that such antibody responses can be stimulated by oral delivery of plant-derived HBsAg VLPs, indicating their promising potential as HBV vaccines. Furthermore, since no adjuvant was employed in either trial, the efficacy of oral delivery can be further optimized.
Due to the success of preclinical studies in mice, cPVPs that display RV epitopes were tested in humans. The first study focused on the safety and immunogenicity of this vaccine candidate in 10 human subjects that had been previously immunized with a conventional rabies vaccine in consideration of the potential issue of antigenic tolerance in immunologically naïve subjects.Citation43 Five volunteers were fed three doses of 20 g chimeric viral-producing raw spinach leaves at biweekly intervals with each dose containing 0.6 mg of recombinant virus or 84 μg of rabies peptide, while the five control individuals received 20 g of control spinach leaves. Three of the five antigen-fed individuals developed significant boosting of systemic anti-rabies IgG levels.Citation43 In contrast, elevation of serum anti-rabies antibodies was not observed in any of the volunteers in the control group. This study also indicated the safety of this vaccine candidate for human use, as no adverse effects such as nausea, vomiting, fever, mild cramps or diarrhea were observed among members of volunteers.Citation43 Since no evidence of untoward effects on pre-existing immunity to RV was observed in this study, the trial was extended to include 14 naïve human volunteers who had no rabies antibodies in their serum. Nine volunteers ingested three doses of raw rabies chimeric virus-producing spinach leaves (150 g per dose) and five control subjects ate an equal amount of control leaves. All 14 participants then received a single injection of commercial human diploid cell rabies vaccine (HDCV) seven days after the last feeding. Six out of the nine participants who were fed with rabies recombinant spinach showed significant increase in rabies-specific serum IgG or IgA levels.Citation43 Furthermore, three antigen-consumed subjects, but none of the control volunteers, developed neutralizing antibodies against rabies following the single-dose administration of HDCV.Citation43 While the response rate in these two studies was not ideal, they nevertheless demonstrated the safety and the potential utility of cPVPs as successful oral vaccines in humans.
Overall, the clinical trials to date have examined and demonstrated both the safety and immunogenicity of plant produced VLPs or cPVPs derived from influenza HA, NVCP, HBsAg, and rabies glycoprotein (). Human volunteers who consumed potato tubers, lettuce or spinach leaves containing 0.3 to 1 mg antigens developed specific antibody responses in all these trials, while no adverse effects were observed. In addition, participants who were injected with purified tobacco-produced HA VLPs developed protective antibody responses against seasonal or pandemic influenza strains. It is notable that these antigens cover a broad range of pathogens including enteric (NV) and nonenteric (HBV, RV and influenza virus) viruses. Protection of infection was suggested by the titers of systemic and mucosal antibodies in the influenza trials and in some of the test volunteers of other trials. Along with the recent development of adjuvant and understanding of mucosal immunology, these results definitely warrant further testing of plant-derived VLP antigens in humans.
Conclusions
Since the discovery of HBsAg particles 25 y ago,Citation192 VLPs have gained significant momentum in the past decade as premier vaccine platforms and have achieved remarkable economical success: five VLP-based vaccines are already on the global vaccine market with over $4.4 billion combined accumulative revenue for the two HPV VLP vaccines (through year end 2009), and average annual revenue of $ 993 million, $64 million, and $26 million for the three HBV vaccines.Citation1 These vaccines would offer much greater benefit for the developing world due to higher incidence of these infectious diseases in these countries. The urgent need of vaccines in resource-poor countries and the encouraging revenue numbers have attracted more interest than ever from the pharmaceutical industry in employing novel robust VLP production platforms that can deliver vaccines with a significant reduction in production time and cost.
In recent years, plants have emerged as a commercially attractive system for manufacturing biologics with superior scalability, safety, time and cost-saving benefits, along with significant progress in addressing technical and regulatory issues.Citation25,Citation28 Recent developments in novel plant-expression vectors, especially those based on “deconstructed” plant viruses, have significantly enhanced expression levels and allowed plant systems to compete with microbial or mammalian cell fermentation for production of VLPs.Citation52,Citation181 The creation of “humanized” glycosylation plant lines by glycoengineering provides plants a further advantage over other production systems in producing VLPs with a tailor-made glycoform that is optimal for their assembly and immunogenicity.Citation144 Indeed, examples in this review collectively demonstrate that plants can produce VLPs of various origins and a diversity of sizes and architectural characteristics, and that displaying various heterologous antigens by genetic fusion or chemical conjugation can be achieved with low cost and high scalability. Development of downstream processing has allowed the efficient, scalable, economic and cGMP-compliant recovery of VLPs from plants. When administered properly, plant-derived VLPs induce a potent immune response in animal models and have demonstrated efficacy and safety in several human clinical trials. Despite these achievements, a lingering criticism of plant-based production platforms has been the absence of approved human products in the US after two and a half decades of active research and development.Citation26 Excitingly, this last remaining hurdle has been overcome by the recent approval of a plant-produced GCD by the FDA for treating Gaucher disease, heralding a new era in the field of plant-made pharmaceutics.Citation145 We speculate that many successful cases and novel applications of plant-made VLPs, including as vessels for the delivery of small therapeutics, DNA fragments, and adjuvants will emerge in the near future, allowing us to explore the tremendous combined potential of the plant-expression system and VLP technology.
Acknowledgments
The authors wish to thank J. Caspermeyer and C. Brock for their critical reading of the manuscript and Dr K. Steel for her helpful suggestions. We also acknowledge Dr J. He for her assistance in microscopy. This work was supported in part by NIH grants U01 AI075549 and 1R21AI101329 to Q. Chen.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Roldão A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines 2010; 9:1149 - 76; http://dx.doi.org/10.1586/erv.10.115; PMID: 20923267
- Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines 2007; 6:381 - 90; http://dx.doi.org/10.1586/14760584.6.3.381; PMID: 17542753
- Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol 1989; 7:601 - 24; http://dx.doi.org/10.1146/annurev.iy.07.040189.003125; PMID: 2469442
- Kovacsovics-Bankowski M, Clark K, Benacerraf B, Rock KL. Efficient major histocompatibility complex class I presentation of exogenous antigen upon phagocytosis by macrophages. Proc Natl Acad Sci USA 1993; 90:4942 - 6; http://dx.doi.org/10.1073/pnas.90.11.4942; PMID: 8506338
- Bachmann MF, Lutz MB, Layton GT, Harris SJ, Fehr T, Rescigno M, et al. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur J Immunol 1996; 26:2595 - 600; http://dx.doi.org/10.1002/eji.1830261109; PMID: 8921944
- Fifis T, Gamvrellis A, Crimeen-Irwin B, Pietersz GA, Li J, Mottram PL, et al. Size-dependent immunogenicity: therapeutic and protective properties of nano-vaccines against tumors. J Immunol 2004; 173:3148 - 54; PMID: 15322175
- Roozendaal R, Mebius RE, Kraal G. The conduit system of the lymph node. Int Immunol 2008; 20:1483 - 7; http://dx.doi.org/10.1093/intimm/dxn110; PMID: 18824503
- Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, et al. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol 2001; 166:5346 - 55; PMID: 11313370
- Bosio CM, Moore BD, Warfield KL, Ruthel G, Mohamadzadeh M, Aman MJ, et al. Ebola and Marburg virus-like particles activate human myeloid dendritic cells. Virology 2004; 326:280 - 7; http://dx.doi.org/10.1016/j.virol.2004.05.025; PMID: 15302213
- Bachmann MF, Zinkernagel RM. The influence of virus structure on antibody responses and virus serotype formation. Immunol Today 1996; 17:553 - 8; http://dx.doi.org/10.1016/S0167-5699(96)10066-9; PMID: 8991286
- Fehr T, Skrastina D, Pumpens P, Zinkernagel RM. T cell-independent type I antibody response against B cell epitopes expressed repetitively on recombinant virus particles. Proc Natl Acad Sci USA 1998; 95:9477 - 81; http://dx.doi.org/10.1073/pnas.95.16.9477; PMID: 9689105
- Bachmann MF, Rohrer UH, Kündig TM, Bürki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science 1993; 262:1448 - 51; http://dx.doi.org/10.1126/science.8248784; PMID: 8248784
- Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol 1997; 15:235 - 70; http://dx.doi.org/10.1146/annurev.immunol.15.1.235; PMID: 9143688
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al, Taiwan Childhood Hepatoma Study Group. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N Engl J Med 1997; 336:1855 - 9; http://dx.doi.org/10.1056/NEJM199706263362602; PMID: 9197213
- Mao C, Koutsky LA, Ault KA, Wheeler CM, Brown DR, Wiley DJ, et al. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol 2006; 107:18 - 27; http://dx.doi.org/10.1097/01.AOG.0000192397.41191.fb; PMID: 16394035
- Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al, HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247 - 55; http://dx.doi.org/10.1016/S0140-6736(06)68439-0; PMID: 16631880
- Pattenden LK, Middelberg APJ, Niebert M, Lipin DI. Towards the preparative and large-scale precision manufacture of virus-like particles. Trends Biotechnol 2005; 23:523 - 9; http://dx.doi.org/10.1016/j.tibtech.2005.07.011; PMID: 16084615
- Grgacic EVL, Anderson DA. Virus-like particles: passport to immune recognition. Methods 2006; 40:60 - 5; http://dx.doi.org/10.1016/j.ymeth.2006.07.018; PMID: 16997714
- Edman JC, Hallewell RA, Valenzuela P, Goodman HM, Rutter WJ. Synthesis of hepatitis B surface and core antigens in E. coli. Nature 1981; 291:503 - 6; http://dx.doi.org/10.1038/291503a0; PMID: 6262658
- Michel ML, Tiollais P. Hepatitis B vaccines: protective efficacy and therapeutic potential. Pathol Biol (Paris) 2010; 58:288 - 95; http://dx.doi.org/10.1016/j.patbio.2010.01.006; PMID: 20382485
- Wildt S, Gerngross TU. The humanization of N-glycosylation pathways in yeast. Nat Rev Microbiol 2005; 3:119 - 28; http://dx.doi.org/10.1038/nrmicro1087; PMID: 15685223
- Harrison RL, Jarvis DL, Bryony C. Bonning KMaAJS. Protein N-Glycosylation in the Baculovirus-Insect Cell Expression System and Engineering of Insect Cells to Produce “Mammalianized” Recombinant Glycoproteins. Advances in Virus Research: Academic Press, 2006:159-91.
- Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine 2005; 23:5751 - 9; http://dx.doi.org/10.1016/j.vaccine.2005.07.098; PMID: 16143432
- Chen Q. Expression and Purification of Pharmaceutical Proteins in Plants Biological Engineering 2008; 1:291 - 321
- Chen Q. Expression and manufacture of pharmaceutical proteins in genetically engineered horticultural plants. In: Mou B, Scorza R, eds. Transgenic Horticultural Crops: Challenges and Opportunities - Essays by Experts. Boca Raton: Taylor & Francis 2011:86-126.
- Chen Q. Turning a new leaf. European Biopharm Rev 2011; 2:64 - 8
- Lai H, Chen Q. Bioprocessing of plant-derived virus-like particles of Norwalk virus capsid protein under current Good Manufacture Practice regulations. Plant Cell Rep 2012; 31:573 - 84; http://dx.doi.org/10.1007/s00299-011-1196-6; PMID: 22134876
- Faye L, Gomord V. Success stories in molecular farming-a brief overview. Plant Biotechnol J 2010; 8:525 - 8; http://dx.doi.org/10.1111/j.1467-7652.2010.00521.x; PMID: 20500680
- Lai H, Engle M, Fuchs A, Keller T, Johnson S, Gorlatov S, et al. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci USA 2010; 107:2419 - 24; http://dx.doi.org/10.1073/pnas.0914503107; PMID: 20133644
- Lai H, He J, Engle M, Diamond MS, Chen Q. Robust production of virus-like particles and monoclonal antibodies with geminiviral replicon vectors in lettuce. Plant Biotechnol J 2012; 10:95 - 104; http://dx.doi.org/10.1111/j.1467-7652.2011.00649.x; PMID: 21883868
- Davies HM. Review article: commercialization of whole-plant systems for biomanufacturing of protein products: evolution and prospects. Plant Biotechnol J 2010; 8:845 - 61; http://dx.doi.org/10.1111/j.1467-7652.2010.00550.x; PMID: 20731788
- Chen Q, Tacket CO, Mason H, Mor T, Cardineau GA, Arntzen C. Subunit vaccines produced using plant biotechnology. In: Levine MM, ed. New Generation Vaccines. New York: Informa Healthcare USA, Inc., 2009:306-15.
- Komarova TV, Baschieri S, Donini M, Marusic C, Benvenuto E, Dorokhov YL. Transient expression systems for plant-derived biopharmaceuticals. Expert Rev Vaccines 2010; 9:859 - 76; http://dx.doi.org/10.1586/erv.10.85; PMID: 20673010
- Lico C, Chen Q, Santi L. Viral vectors for production of recombinant proteins in plants. J Cell Physiol 2008; 216:366 - 77; http://dx.doi.org/10.1002/jcp.21423; PMID: 18330886
- Huang Z, Chen Q, Hjelm B, Arntzen C, Mason H. A DNA replicon system for rapid high-level production of virus-like particles in plants. Biotechnol Bioeng 2009; 103:706 - 14; http://dx.doi.org/10.1002/bit.22299; PMID: 19309755
- Santi L, Batchelor L, Huang Z, Hjelm B, Kilbourne J, Arntzen CJ, et al. An efficient plant viral expression system generating orally immunogenic Norwalk virus-like particles. Vaccine 2008; 26:1846 - 54; http://dx.doi.org/10.1016/j.vaccine.2008.01.053; PMID: 18325641
- He J, Lai H, Brock C, Chen Q.. A Novel System for Rapid and Cost-Effective Production of Detection and Diagnostic Reagents of West Nile Virus in Plants. J Biomedicine and Biotechnology 2012; 2012:1 - 10
- Saldaña S, Esquivel Guadarrama F, Olivera Flores TdeJ, Arias N, López S, Arias C, et al. Production of rotavirus-like particles in tomato (Lycopersicon esculentum L.) fruit by expression of capsid proteins VP2 and VP6 and immunological studies. Viral Immunol 2006; 19:42 - 53; http://dx.doi.org/10.1089/vim.2006.19.42; PMID: 16553549
- Yang Y, Li X, Yang H, Qian Y, Zhang Y, Fang R, et al. Immunogenicity and virus-like particle formation of rotavirus capsid proteins produced in transgenic plants. Sci China Life Sci 2011; 54:82 - 9; http://dx.doi.org/10.1007/s11427-010-4104-3; PMID: 21104033
- Santi L, Huang Z, Mason H. Virus-like particles production in green plants. Methods 2006; 40:66 - 76; http://dx.doi.org/10.1016/j.ymeth.2006.05.020; PMID: 16997715
- Tacket CO, Pasetti MF, Edelman R, Howard JA, Streatfield S. Immunogenicity of recombinant LT-B delivered orally to humans in transgenic corn. Vaccine 2004; 22:4385 - 9; http://dx.doi.org/10.1016/j.vaccine.2004.01.073; PMID: 15474732
- Kapusta J, Modelska A, Figlerowicz M, Pniewski T, Letellier M, Lisowa O, et al. A plant-derived edible vaccine against hepatitis B virus. FASEB J 1999; 13:1796 - 9; PMID: 10506582
- Yusibov V, Hooper DC, Spitsin SV, Fleysh N, Kean RB, Mikheeva T, et al. Expression in plants and immunogenicity of plant virus-based experimental rabies vaccine. Vaccine 2002; 20:3155 - 64; http://dx.doi.org/10.1016/S0264-410X(02)00260-8; PMID: 12163267
- Villa LL, Costa RLR, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271 - 8; http://dx.doi.org/10.1016/S1470-2045(05)70101-7; PMID: 15863374
- Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al, GlaxoSmithKline HPV Vaccine Study Group. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 2004; 364:1757 - 65; http://dx.doi.org/10.1016/S0140-6736(04)17398-4; PMID: 15541448
- Herbst-Kralovetz M, Mason HS, Chen Q. Norwalk virus-like particles as vaccines. Expert Rev Vaccines 2010; 9:299 - 307; http://dx.doi.org/10.1586/erv.09.163; PMID: 20218858
- Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science 1999; 286:287 - 90; http://dx.doi.org/10.1126/science.286.5438.287; PMID: 10514371
- Jiang X, Wang M, Graham DY, Estes MK. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol 1992; 66:6527 - 32; PMID: 1328679
- Ausar SF, Foubert TR, Hudson MH, Vedvick TS, Middaugh CR. Conformational stability and disassembly of Norwalk virus-like particles. Effect of pH and temperature. J Biol Chem 2006; 281:19478 - 88; http://dx.doi.org/10.1074/jbc.M603313200; PMID: 16675449
- Huang Z, Elkin G, Maloney BJ, Beuhner N, Arntzen CJ, Thanavala Y, et al. Virus-like particle expression and assembly in plants: hepatitis B and Norwalk viruses. Vaccine 2005; 23:1851 - 8; http://dx.doi.org/10.1016/j.vaccine.2004.11.017; PMID: 15734055
- Zhang X, Buehner NA, Hutson AM, Estes MK, Mason HS. Tomato is a highly effective vehicle for expression and oral immunization with Norwalk virus capsid protein. Plant Biotechnol J 2006; 4:419 - 32; http://dx.doi.org/10.1111/j.1467-7652.2006.00191.x; PMID: 17177807
- Chen Q, He J, Phoolcharoen W, Mason HS. Geminiviral vectors based on bean yellow dwarf virus for production of vaccine antigens and monoclonal antibodies in plants. Hum Vaccin 2011; 7:331 - 8; http://dx.doi.org/10.4161/hv.7.3.14262; PMID: 21358270
- Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 2003; 63:1021 - 51; http://dx.doi.org/10.2165/00003495-200363100-00006; PMID: 12699402
- Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Expert Rev Vaccines 2004; 3:119 - 29; http://dx.doi.org/10.1586/14760584.3.2.119; PMID: 15056038
- Wynne SA, Crowther RA, Leslie AGW. The crystal structure of the human hepatitis B virus capsid. Mol Cell 1999; 3:771 - 80; http://dx.doi.org/10.1016/S1097-2765(01)80009-5; PMID: 10394365
- Lobaina Y, Palenzuela D, Pichardo D, Muzio V, Guillén G, Aguilar JC. Immunological characterization of two hepatitis B core antigen variants and their immunoenhancing effect on co-delivered hepatitis B surface antigen. Mol Immunol 2005; 42:289 - 94; http://dx.doi.org/10.1016/j.molimm.2004.09.005; PMID: 15589316
- Aguilar JC, Lobaina Y, Muzio V, García D, Pentón E, Iglesias E, et al. Development of a nasal vaccine for chronic hepatitis B infection that uses the ability of hepatitis B core antigen to stimulate a strong Th1 response against hepatitis B surface antigen. Immunol Cell Biol 2004; 82:539 - 46; http://dx.doi.org/10.1111/j.0818-9641.2004.01278.x; PMID: 15479440
- Tsuda S, Yoshioka K, Tanaka T, Iwata A, Yoshikawa A, Watanabe Y, et al. Application of the human hepatitis B virus core antigen from transgenic tobacco plants for serological diagnosis. Vox Sang 1998; 74:148 - 55; http://dx.doi.org/10.1046/j.1423-0410.1998.7430148.x; PMID: 9595641
- Huang Z, Santi L, LePore K, Kilbourne J, Arntzen CJ, Mason HS. Rapid, high-level production of hepatitis B core antigen in plant leaf and its immunogenicity in mice. Vaccine 2006; 24:2506 - 13; http://dx.doi.org/10.1016/j.vaccine.2005.12.024; PMID: 16417953
- Warzecha H, Mason HS, Lane C, Tryggvesson A, Rybicki E, Williamson AL, et al. Oral immunogenicity of human papillomavirus-like particles expressed in potato. J Virol 2003; 77:8702 - 11; http://dx.doi.org/10.1128/JVI.77.16.8702-8711.2003; PMID: 12885889
- Varsani AWA-L, Williamson AL, Rose RC, Jaffer M, Rybicki EP. Expression of Human papillomavirus type 16 major capsid protein in transgenic Nicotiana tabacum cv. Xanthi. Arch Virol 2003; 148:1771 - 86; http://dx.doi.org/10.1007/s00705-003-0119-4; PMID: 14505089
- Varsani A, Williamson A-L, Stewart D, Rybicki EP. Transient expression of Human papillomavirus type 16 L1 protein in Nicotiana benthamiana using an infectious tobamovirus vector. Virus Res 2006; 120:91 - 6; http://dx.doi.org/10.1016/j.virusres.2006.01.022; PMID: 16530873
- Fernández-San Millán A, Ortigosa SM, Hervás-Stubbs S, Corral-Martínez P, Seguí-Simarro JM, Gaétan J, et al. Human papillomavirus L1 protein expressed in tobacco chloroplasts self-assembles into virus-like particles that are highly immunogenic. Plant Biotechnol J 2008; 6:427 - 41; http://dx.doi.org/10.1111/j.1467-7652.2008.00338.x; PMID: 18422886
- Maclean J, Koekemoer M, Olivier AJ, Stewart D, Hitzeroth II, Rademacher T, et al. Optimization of human papillomavirus type 16 (HPV-16) L1 expression in plants: comparison of the suitability of different HPV-16 L1 gene variants and different cell-compartment localization. J Gen Virol 2007; 88:1460 - 9; http://dx.doi.org/10.1099/vir.0.82718-0; PMID: 17412974
- Šmídková M, Müller M, Thönes N, Piuko K, Angelisová P, Velemínský J, et al. Transient expression of human papillomavirus type 16 virus-like particles in tobacco and tomato using a tobacco rattle virus expression vector. Biol Plant 2010; 54:451 - 60; http://dx.doi.org/10.1007/s10535-010-0081-4
- Biemelt S, Sonnewald U, Galmbacher P, Willmitzer L, Müller M. Production of human papillomavirus type 16 virus-like particles in transgenic plants. J Virol 2003; 77:9211 - 20; http://dx.doi.org/10.1128/JVI.77.17.9211-9220.2003; PMID: 12915537
- Warzecha H, Mason HS, Lane C, Tryggvesson A, Rybicki E, Williamson AL, et al. Oral immunogenicity of human papillomavirus-like particles expressed in potato. J Virol 2003; 77:8702 - 11; http://dx.doi.org/10.1128/JVI.77.16.8702-8711.2003; PMID: 12885889
- Zheng H, Yu L, Wei C, Hu D, Shen Y, Chen Z, et al. Assembly of double-shelled, virus-like particles in transgenic rice plants expressing two major structural proteins of rice dwarf virus. J Virol 2000; 74:9808 - 10; http://dx.doi.org/10.1128/JVI.74.20.9808-9810.2000; PMID: 11000259
- Maloney BJ , Takeda N, Suzaki Y, Ami Y, Li TC, Miyamura T, Arntzen CJ, et al. Challenges in creating a vaccine to prevent hepatitis E. Vaccine 2005; 23:1870 - 4; PMID: 15734058
- Bragard C, Duncan GH, Wesley SV, Naidu RA, Mayo MA. Virus-like particles assemble in plants and bacteria expressing the coat protein gene of Indian peanut clump virus. J Gen Virol 2000; 81:267 - 72; PMID: 10640566
- Patzer EJ, Nakamura GR, Yaffe A. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells. J Virol 1984; 51:346 - 53; PMID: 6748160
- Ludwig C, Wagner R. Virus-like particles-universal molecular toolboxes. Curr Opin Biotechnol 2007; 18:537 - 45; http://dx.doi.org/10.1016/j.copbio.2007.10.013; PMID: 18083549
- Krugman S, Overby LR, Mushahwar IK, Ling CM, Frösner GG, Deinhardt F. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med 1979; 300:101 - 6; http://dx.doi.org/10.1056/NEJM197901183000301; PMID: 758598
- Mason HS, Lam DM, Arntzen CJ. Expression of hepatitis B surface antigen in transgenic plants. Proc Natl Acad Sci USA 1992; 89:11745 - 9; http://dx.doi.org/10.1073/pnas.89.24.11745; PMID: 1465391
- Streatfield SJ. Mucosal immunization using recombinant plant-based oral vaccines. Methods 2006; 38:150 - 7; http://dx.doi.org/10.1016/j.ymeth.2005.09.013; PMID: 16431131
- Thanavala Y, Yang YF, Lyons P, Mason HS, Arntzen C. Immunogenicity of transgenic plant-derived hepatitis B surface antigen. Proc Natl Acad Sci USA 1995; 92:3358 - 61; http://dx.doi.org/10.1073/pnas.92.8.3358; PMID: 7724566
- Kong Q, Richter L, Yang YF, Arntzen CJ, Mason HS, Thanavala Y. Oral immunization with hepatitis B surface antigen expressed in transgenic plants. Proc Natl Acad Sci USA 2001; 98:11539 - 44; http://dx.doi.org/10.1073/pnas.191617598; PMID: 11553782
- Lou XM, Yao QH, Zhang Z, Peng RH, Xiong AS, Wang HK. Expression of the human hepatitis B virus large surface antigen gene in transgenic tomato plants. Clin Vaccine Immunol 2007; 14:464 - 9; http://dx.doi.org/10.1128/CVI.00321-06; PMID: 17314228
- Pniewski T, Kapusta J, Bociąg P, Kostrzak A, Fedorowicz-Strońska O, Czyż M, et al. Plant expression, lyophilisation and storage of HBV medium and large surface antigens for a prototype oral vaccine formulation. Plant Cell Rep 2012; 31:585 - 95; http://dx.doi.org/10.1007/s00299-011-1223-7; PMID: 22246107
- Kang SM, Song JM, Quan FS, Compans RW. Influenza vaccines based on virus-like particles. Virus Res 2009; 143:140 - 6; http://dx.doi.org/10.1016/j.virusres.2009.04.005; PMID: 19374929
- Chen BJ, Leser GP, Morita E, Lamb RA. Influenza virus hemagglutinin and neuraminidase, but not the matrix protein, are required for assembly and budding of plasmid-derived virus-like particles. J Virol 2007; 81:7111 - 23; http://dx.doi.org/10.1128/JVI.00361-07; PMID: 17475660
- Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine 2007; 25:3871 - 8; http://dx.doi.org/10.1016/j.vaccine.2007.01.106; PMID: 17337102
- D’Aoust MA, Couture MM, Charland N, Trépanier S, Landry N, Ors F, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 2010; 8:607 - 19; http://dx.doi.org/10.1111/j.1467-7652.2009.00496.x; PMID: 20199612
- Saint-Jore-Dupas C, Faye L, Gomord V. From planta to pharma with glycosylation in the toolbox. Trends Biotechnol 2007; 25:317 - 23; http://dx.doi.org/10.1016/j.tibtech.2007.04.008; PMID: 17493697
- D’Aoust MA, Lavoie PO, Couture MM, Trépanier S, Guay JM, Dargis M, et al. Influenza virus-like particles produced by transient expression in Nicotiana benthamiana induce a protective immune response against a lethal viral challenge in mice. Plant Biotechnol J 2008; 6:930 - 40; http://dx.doi.org/10.1111/j.1467-7652.2008.00384.x; PMID: 19076615
- Shoji Y, Farrance CE, Bautista J, Bi H, Musiychuk K, Horsey A, et al. A plant-based system for rapid production of influenza vaccine antigens. Influenza Other Respi Viruses 2012; 6:204 - 10; http://dx.doi.org/10.1111/j.1750-2659.2011.00295.x; PMID: 21974811
- D'Aoust M, Couture M, Ors F, Trépanier S, Lavoie P-O, Dargis M, et al. Recombinant influenza virus-like particles (VLPs) produced in transgenic plants expressing hemagglutinin. International Patent application WO2009/076778 2009.
- D’Aoust MA, Couture MM, Charland N, Trépanier S, Landry N, Ors F, et al. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J 2010; 8:607 - 19; http://dx.doi.org/10.1111/j.1467-7652.2009.00496.x; PMID: 20199612
- Landry N, Ward BJ, Trépanier S, Montomoli E, Dargis M, Lapini G, et al. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 2010; 5:e15559; http://dx.doi.org/10.1371/journal.pone.0015559; PMID: 21203523
- Meyers A, Chakauya E, Shephard E, Tanzer FL, Maclean J, Lynch A, et al. Expression of HIV-1 antigens in plants as potential subunit vaccines. BMC Biotechnol 2008; 8:53; http://dx.doi.org/10.1186/1472-6750-8-53; PMID: 18573204
- Scotti N, Alagna F, Ferraiolo E, Formisano G, Sannino L, Buonaguro L, et al. High-level expression of the HIV-1 Pr55gag polyprotein in transgenic tobacco chloroplasts. Planta 2009; 229:1109 - 22; http://dx.doi.org/10.1007/s00425-009-0898-2; PMID: 19234717
- Deml L, Speth C, Dierich MP, Wolf H, Wagner R. Recombinant HIV-1 Pr55gag virus-like particles: potent stimulators of innate and acquired immune responses. Mol Immunol 2005; 42:259 - 77; http://dx.doi.org/10.1016/j.molimm.2004.06.028; PMID: 15488613
- Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, et al. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol 2001; 75:4040 - 7; http://dx.doi.org/10.1128/JVI.75.9.4040-4047.2001; PMID: 11287553
- Kanagaraj AP, Verma D, Daniell H. Expression of dengue-3 premembrane and envelope polyprotein in lettuce chloroplasts. Plant Mol Biol 2011; 76:323 - 33; http://dx.doi.org/10.1007/s11103-011-9766-0; PMID: 21431782
- Greco R, Michel M, Guetard D, Cervantes-Gonzalez M, Pelucchi N, Wain-Hobson S, et al. Production of recombinant HIV-1/HBV virus-like particles in Nicotiana tabacum and Arabidopsis thaliana plants for a bivalent plant-based vaccine. Vaccine 2007; 25:8228 - 40; http://dx.doi.org/10.1016/j.vaccine.2007.09.061; PMID: 17976876
- Huang Z, Mason HS. Conformational analysis of hepatitis B surface antigen fusions in an Agrobacterium-mediated transient expression system. Plant Biotechnol J 2004; 2:241 - 9; http://dx.doi.org/10.1111/j.1467-7652.2004.00068.x; PMID: 17147615
- Qian B, Shen H, Liang W, Guo X, Zhang C, Wang Y, et al. Immunogenicity of recombinant hepatitis B virus surface antigen fused with preS1 epitopes expressed in rice seeds. Transgenic Res 2008; 17:621 - 31; http://dx.doi.org/10.1007/s11248-007-9135-6; PMID: 17882531
- Shchelkunov SN, Salyaev RK, Pozdnyakov SG, Rekoslavskaya NI, Nesterov AE, Ryzhova TS, et al. Immunogenicity of a novel, bivalent, plant-based oral vaccine against hepatitis B and human immunodeficiency viruses. Biotechnol Lett 2006; 28:959 - 67; http://dx.doi.org/10.1007/s10529-006-9028-4; PMID: 16794774
- Kessans S, Frater J. Plant expression of chimeric Gag/gp41 virus-like particles as a mucosally-targeted subunit vaccine against HIV-1. Retrovirology 2009; 6:15; http://dx.doi.org/10.1186/1742-4690-6-S3-P15; PMID: 19216757
- Ramqvist T, Andreasson K, Dalianis T. Vaccination, immune and gene therapy based on virus-like particles against viral infections and cancer. Expert Opin Biol Ther 2007; 7:997 - 1007; http://dx.doi.org/10.1517/14712598.7.7.997; PMID: 17665989
- Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur J Immunol 2002; 32:3305 - 14; http://dx.doi.org/10.1002/1521-4141(200211)32:11<3305::AID-IMMU3305>3.0.CO;2-J; PMID: 12555676
- Jegerlehner A, Wiesel M, Dietmeier K, Zabel F, Gatto D, Saudan P, et al. Carrier induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine 2010; 28:5503 - 12; http://dx.doi.org/10.1016/j.vaccine.2010.02.103; PMID: 20307591
- Fiers W, De Filette M, El Bakkouri K, Schepens B, Roose K, Schotsaert M, et al. M2e-based universal influenza A vaccine. Vaccine 2009; 27:6280 - 3; http://dx.doi.org/10.1016/j.vaccine.2009.07.007; PMID: 19840661
- Peters BS, Cheingsong-Popov R, Callow D, Foxall R, Patou G, Hodgkin K, et al. A pilot phase II study of the safety and immunogenicity of HIV p17/p24:VLP (p24-VLP) in asymptomatic HIV seropositive subjects. J Infect 1997; 35:231 - 5; http://dx.doi.org/10.1016/S0163-4453(97)92814-0; PMID: 9459393
- Buonaguro L, Tagliamonte M, Tornesello ML, Buonaguro FM. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev Vaccines 2011; 10:1569 - 83; http://dx.doi.org/10.1586/erv.11.135; PMID: 22043956
- Bendahmane M, Koo M, Karrer E, Beachy RN. Display of epitopes on the surface of tobacco mosaic virus: impact of charge and isoelectric point of the epitope on virus-host interactions. J Mol Biol 1999; 290:9 - 20; http://dx.doi.org/10.1006/jmbi.1999.2860; PMID: 10388554
- Murawski MR, McGinnes LW, Finberg RW, Kurt-Jones EA, Massare MJ, Smith G, et al. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J Virol 2010; 84:1110 - 23; http://dx.doi.org/10.1128/JVI.01709-09; PMID: 19889768
- Ravin NV, Kotlyarov RY, Mardanova ES, Kuprianov VV, Migunov AI, Stepanova LA, et al. Plant-produced recombinant influenza vaccine based on virus-like HBc particles carrying an extracellular domain of M2 protein. Biochemistry (Mosc) 2012; 77:33 - 40; http://dx.doi.org/10.1134/S000629791201004X; PMID: 22339631
- Paz De la Rosa G, Monroy-García A, Mora-García MdeL, Peña CG, Hernández-Montes J, Weiss-Steider B, et al. An HPV 16 L1-based chimeric human papilloma virus-like particles containing a string of epitopes produced in plants is able to elicit humoral and cytotoxic T-cell activity in mice. Virol J 2009; 6:2; http://dx.doi.org/10.1186/1743-422X-6-2; PMID: 19126233
- Matić S, Rinaldi R, Masenga V, Noris E. Efficient production of chimeric human papillomavirus 16 L1 protein bearing the M2e influenza epitope in Nicotiana benthamiana plants. BMC Biotechnol 2011; 11:106; http://dx.doi.org/10.1186/1472-6750-11-106; PMID: 22085463
- Natilla A, Nemchinov LG. Improvement of PVX/CMV CP expression tool for display of short foreign antigens. Protein Expr Purif 2008; 59:117 - 21; http://dx.doi.org/10.1016/j.pep.2008.01.011; PMID: 18280751
- Mason H, Ball J, Shi JJ, Jiang X, Estes MK, Arntzen CJ. Expression and Immunogenicity of Norwalk virus capsid protien from transgenic tobacco and potato. Proc Natl Acad Sci USA 1996; 93:5335 - 40; http://dx.doi.org/10.1073/pnas.93.11.5335; PMID: 8643575
- Thanavala Y, Mahoney M, Pal S, Scott A, Richter L, Natarajan N, et al. Immunogenicity in humans of an edible vaccine for hepatitis B 10.1073/pnas.0409899102. Proceedings of the National Academy of Sciences 2005; 102:3378-82.
- Guetard D, Greco R, Cervantes Gonzalez M, Celli S, Kostrzak A, Langlade-Demoyen P, et al. Immunogenicity and tolerance following HIV-1/HBV plant-based oral vaccine administration. Vaccine 2008; 26:4477 - 85; http://dx.doi.org/10.1016/j.vaccine.2008.06.059; PMID: 18601967
- Röhn TA, Jennings GT, Hernandez M, Grest P, Beck M, Zou Y, et al. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur J Immunol 2006; 36:2857 - 67; http://dx.doi.org/10.1002/eji.200636658; PMID: 17048275
- Jennings GT, Bachmann MF. The coming of age of virus-like particle vaccines. Biol Chem 2008; 389:521 - 36; http://dx.doi.org/10.1515/BC.2008.064; PMID: 18953718
- Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, et al. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and Phase I safety and immunogenicity. Eur J Immunol 2005; 35:2031 - 40; http://dx.doi.org/10.1002/eji.200526285; PMID: 15971275
- Lechner F, Jegerlehner A, Tissot AC, Maurer P, Sebbel P, Renner WA, et al. Virus-like particles as a modular system for novel vaccines. Intervirology 2002; 45:212 - 7; http://dx.doi.org/10.1159/000067912; PMID: 12566703
- Chackerian B, Rangel M, Hunter Z, Peabody DS. Virus and virus-like particle-based immunogens for Alzheimer’s disease induce antibody responses against amyloid-beta without concomitant T cell responses. Vaccine 2006; 24:6321 - 31; http://dx.doi.org/10.1016/j.vaccine.2006.05.059; PMID: 16806604
- Werner S, Marillonnet S, Hause G, Klimyuk V, Gleba Y. Immunoabsorbent nanoparticles based on a tobamovirus displaying protein A. Proc Natl Acad Sci USA 2006; 103:17678 - 83; http://dx.doi.org/10.1073/pnas.0608869103; PMID: 17090664
- McCormick AA, Palmer KE. Genetically engineered Tobacco mosaic virus as nanoparticle vaccines. Expert Rev Vaccines 2008; 7:33 - 41; http://dx.doi.org/10.1586/14760584.7.1.33; PMID: 18251692
- Sainsbury F, Cañizares MC, Lomonossoff GP. Cowpea mosaic virus: the plant virus-based biotechnology workhorse. Annu Rev Phytopathol 2010; 48:437 - 55; http://dx.doi.org/10.1146/annurev-phyto-073009-114242; PMID: 20455698
- Cañizares MC, Nicholson L, Lomonossoff GP. Use of viral vectors for vaccine production in plants. Immunol Cell Biol 2005; 83:263 - 70; http://dx.doi.org/10.1111/j.1440-1711.2005.01339.x; PMID: 15877604
- Fitchen J, Beachy RN, Hein MB. Plant virus expressing hybrid coat protein with added murine epitope elicits autoantibody response. Vaccine 1995; 13:1051 - 7; http://dx.doi.org/10.1016/0264-410X(95)00075-C; PMID: 7491811
- Modelska A, Dietzschold B, Sleysh N, Fu ZF, Steplewski K, Hooper DC, et al. Immunization against rabies with plant-derived antigen. Proc Natl Acad Sci USA 1998; 95:2481 - 5; http://dx.doi.org/10.1073/pnas.95.5.2481; PMID: 9482911
- Plotkin SA. Rabies. Clin Infect Dis 2000; 30:4 - 12; http://dx.doi.org/10.1086/313632; PMID: 10619725
- Lico C, Mancini C, Italiani P, Betti C, Boraschi D, Benvenuto E, et al. Plant-produced potato virus X chimeric particles displaying an influenza virus-derived peptide activate specific CD8+ T cells in mice. Vaccine 2009; 27:5069 - 76; http://dx.doi.org/10.1016/j.vaccine.2009.06.045; PMID: 19563889
- Dalsgaard K, Uttenthal A, Jones TD, Xu F, Merryweather A, Hamilton WD, et al. Plant-derived vaccine protects target animals against a viral disease. Nat Biotechnol 1997; 15:248 - 52; http://dx.doi.org/10.1038/nbt0397-248; PMID: 9062924
- Langeveld JP, Brennan FR, Martínez-Torrecuadrada JL, Jones TD, Boshuizen RS, Vela C, et al. Inactivated recombinant plant virus protects dogs from a lethal challenge with canine parvovirus. Vaccine 2001; 19:3661 - 70; http://dx.doi.org/10.1016/S0264-410X(01)00083-4; PMID: 11395200
- Koo M, Bendahmane M, Lettieri GA, Paoletti AD, Lane TE, Fitchen JH, et al. Protective immunity against murine hepatitis virus (MHV) induced by intranasal or subcutaneous administration of hybrids of tobacco mosaic virus that carries an MHV epitope. Proc Natl Acad Sci USA 1999; 96:7774 - 9; http://dx.doi.org/10.1073/pnas.96.14.7774; PMID: 10393897
- Jiang L, Li Q, Li M, Zhou Z, Wu L, Fan J, et al. A modified TMV-based vector facilitates the expression of longer foreign epitopes in tobacco. Vaccine 2006; 24:109 - 15; http://dx.doi.org/10.1016/j.vaccine.2005.09.060; PMID: 16337317
- Wu L, Jiang L, Zhou Z, Fan J, Zhang Q, Zhu H, et al. Expression of foot-and-mouth disease virus epitopes in tobacco by a tobacco mosaic virus-based vector. Vaccine 2003; 21:4390 - 8; http://dx.doi.org/10.1016/S0264-410X(03)00428-6; PMID: 14505922
- Brennan FR, Gilleland LB, Staczek J, Bendig MM, Hamilton WD, Gilleland HE Jr.. A chimaeric plant virus vaccine protects mice against a bacterial infection. Microbiology 1999; 145:2061 - 7; http://dx.doi.org/10.1099/13500872-145-8-2061; PMID: 10463172
- Rennermalm A, Li YH, Bohaufs L, Jarstrand C, Brauner A, Brennan FR, et al. Antibodies against a truncated Staphylococcus aureus fibronectin-binding protein protect against dissemination of infection in the rat. Vaccine 2001; 19:3376 - 83; http://dx.doi.org/10.1016/S0264-410X(01)00080-9; PMID: 11348701
- Smith ML, Lindbo JA, Dillard-Telm S, Brosio PM, Lasnik AB, McCormick AA, et al. Modified tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology 2006; 348:475 - 88; http://dx.doi.org/10.1016/j.virol.2005.12.039; PMID: 16466765
- McCormick AA, Corbo TA, Wykoff-Clary S, Nguyen LV, Smith ML, Palmer KE, et al. TMV-peptide fusion vaccines induce cell-mediated immune responses and tumor protection in two murine models. Vaccine 2006; 24:6414 - 23; http://dx.doi.org/10.1016/j.vaccine.2006.06.003; PMID: 16860441
- Rudd PM, Wormald MR, Stanfield RL, Huang M, Mattsson N, Speir JA, et al. Roles for glycosylation of cell surface receptors involved in cellular immune recognition. J Mol Biol 1999; 293:351 - 66; http://dx.doi.org/10.1006/jmbi.1999.3104; PMID: 10529350
- Dowling W, Thompson E, Badger C, Mellquist JL, Garrison AR, Smith JM, et al. Influences of glycosylation on antigenicity, immunogenicity, and protective efficacy of ebola virus GP DNA vaccines. J Virol 2007; 81:1821 - 37; http://dx.doi.org/10.1128/JVI.02098-06; PMID: 17151111
- Robertson JS. Sequence analysis of the haemagglutinin of A/Taiwan/1/86, a new variant of human influenza A(H1N1) virus. J Gen Virol 1987; 68:1205 - 8; http://dx.doi.org/10.1099/0022-1317-68-4-1205; PMID: 3572359
- Chapel C, Garcia C, Roingeard P, Zitzmann N, Dubuisson J, Dwek RA, et al. Antiviral effect of alpha-glucosidase inhibitors on viral morphogenesis and binding properties of hepatitis C virus-like particles. J Gen Virol 2006; 87:861 - 71; http://dx.doi.org/10.1099/vir.0.81503-0; PMID: 16528036
- Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, et al. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol 2004; 85:3637 - 45; http://dx.doi.org/10.1099/vir.0.80247-0; PMID: 15557236
- Goto A, Yoshii K, Obara M, Ueki T, Mizutani T, Kariwa H, et al. Role of the N-linked glycans of the prM and E envelope proteins in tick-borne encephalitis virus particle secretion. Vaccine 2005; 23:3043 - 52; http://dx.doi.org/10.1016/j.vaccine.2004.11.068; PMID: 15811651
- Branco LM, Grove JN, Geske FJ, Boisen ML, Muncy IJ, Magliato SA, et al. Lassa virus-like particles displaying all major immunological determinants as a vaccine candidate for Lassa hemorrhagic fever. Virol J 2010; 7:279; http://dx.doi.org/10.1186/1743-422X-7-279; PMID: 20961433
- Gomord V, Fitchette AC, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, et al. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J 2010; 8:564 - 87; http://dx.doi.org/10.1111/j.1467-7652.2009.00497.x; PMID: 20233335
- FDA. FDA approves new orphan drug to treat a form of Gaucher disease. http://wwwfdagov/NewsEvents/Newsroom/PressAnnouncements/ucm302549htm 2012; (accessed July 05 2012).
- Strasser R, Castilho A, Stadlmann J, Kunert R, Quendler H, Gattinger P, et al. Improved virus neutralization by plant-produced anti-HIV antibodies with a homogeneous β1,4-galactosylated N-glycan profile. J Biol Chem 2009; 284:20479 - 85; http://dx.doi.org/10.1074/jbc.M109.014126; PMID: 19478090
- Castilho A, Gattinger P, Grass J, Jez J, Pabst M, Altmann F, et al. N-glycosylation engineering of plants for the biosynthesis of glycoproteins with bisected and branched complex N-glycans. Glycobiology 2011; 21:813 - 23; http://dx.doi.org/10.1093/glycob/cwr009; PMID: 21317243
- Castilho A, Pabst M, Leonard R, Veit C, Altmann F, Mach L, et al. Construction of a functional CMP-sialic acid biosynthesis pathway in Arabidopsis. Plant Physiol 2008; 147:331 - 9; http://dx.doi.org/10.1104/pp.108.117572; PMID: 18326787
- Castilho A, Strasser R, Stadlmann J, Grass J, Jez J, Gattinger P, et al. In planta protein sialylation through overexpression of the respective mammalian pathway. J Biol Chem 2010; 285:15923 - 30; http://dx.doi.org/10.1074/jbc.M109.088401; PMID: 20305285
- Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J 2008; 6:392 - 402; http://dx.doi.org/10.1111/j.1467-7652.2008.00330.x; PMID: 18346095
- Schähs M, Strasser R, Stadlmann J, Kunert R, Rademacher T, Steinkellner H. Production of a monoclonal antibody in plants with a humanized N-glycosylation pattern. Plant Biotechnol J 2007; 5:657 - 63; http://dx.doi.org/10.1111/j.1467-7652.2007.00273.x; PMID: 17678502
- Cox KM, Sterling JD, Regan JT, Gasdaska JR, Frantz KK, Peele CG, et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol 2006; 24:1591 - 7; http://dx.doi.org/10.1038/nbt1260; PMID: 17128273
- Pedro L, Soares SS, Ferreira GNM. Purification of Bionanoparticles. Chem Eng Technol 2008; 31:815 - 25; http://dx.doi.org/10.1002/ceat.200800176
- Lai WB, Middelberg AP. The production of human papillomavirus type 16 L1 vaccine product from Escherichia coli inclusion bodies. Bioprocess Biosyst Eng 2002; 25:121 - 8; http://dx.doi.org/10.1007/s00449-002-0289-6; PMID: 14505012
- Zhang W, Carmichael J, Ferguson J, Inglis S, Ashrafian H, Stanley M. Expression of human papillomavirus type 16 L1 protein in Escherichia coli: denaturation, renaturation, and self-assembly of virus-like particles in vitro. Virology 1998; 243:423 - 31; http://dx.doi.org/10.1006/viro.1998.9050; PMID: 9568041
- Venkataram Prasad BV, Hardy ME, Estes MK. Structural studies of recombinant Norwalk capsids. J Infect Dis 2000; 181:Suppl 2 S317 - 21; http://dx.doi.org/10.1086/315576; PMID: 10804144
- Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol 2003; 108:241 - 7; http://dx.doi.org/10.1016/S1521-6616(03)00120-7; PMID: 14499247
- Estes MK. Virus like particle (VLP) vaccines. In: Levine MM, ed. New generation vaccines. New York: Academic Press, 2004:283-94.
- Ausar SF, Foubert TR, Hudson MH, Vedvick TS, Middaugh CR. Conformational stability and disassembly of Norwalk virus-like particles. Effect of pH and temperature. J Biol Chem 2006; 281:19478 - 88; http://dx.doi.org/10.1074/jbc.M603313200; PMID: 16675449
- Rolland D, Gauthier M, Dugua JM, Fournier C, Delpech L, Watelet B, et al. Purification of recombinant HBc antigen expressed in Escherichia coli and Pichia pastoris: comparison of size-exclusion chromatography and ultracentrifugation. J Chromatogr B Biomed Sci Appl 2001; 753:51 - 65; http://dx.doi.org/10.1016/S0378-4347(00)00538-7; PMID: 11302448
- Rodrigues T, Carvalho A, Roldão A, Carrondo MJ, Alves PM, Cruz PE. Screening anion-exchange chromatographic matrices for isolation of onco-retroviral vectors. J Chromatogr B Analyt Technol Biomed Life Sci 2006; 837:59 - 68; http://dx.doi.org/10.1016/j.jchromb.2006.03.061; PMID: 16697280
- Morenweiser R. Downstream processing of viral vectors and vaccines. Gene Ther 2005; 12:Suppl 1 S103 - 10; http://dx.doi.org/10.1038/sj.gt.3302624; PMID: 16231042
- Vicente T, Roldão A, Peixoto C, Carrondo MJT, Alves PM. Large-scale production and purification of VLP-based vaccines. J Invertebr Pathol 2011; 107:Suppl S42 - 8; http://dx.doi.org/10.1016/j.jip.2011.05.004; PMID: 21784230
- Vicente T, Sousa MFQ, Peixoto C, Mota JPB, Alves PM, Carrondo MJT. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J Membr Sci 2008; 311:270 - 83; http://dx.doi.org/10.1016/j.memsci.2007.12.021
- Wolff MW, Siewert C, Hansen SP, Faber R, Reichl U. Purification of cell culture-derived modified vaccinia ankara virus by pseudo-affinity membrane adsorbers and hydrophobic interaction chromatography. Biotechnol Bioeng 2010; 107:312 - 20; http://dx.doi.org/10.1002/bit.22797; PMID: 20506129
- Shelly D, Cleave V. Parvovirus B19 VLP vaccine manufacturing. Genet Eng Biotechnol News 2009; 29:1 - 4
- Kim HJ, Lim SJ, Kwag HL, Kim HJ. The choice of resin-bound ligand affects the structure and immunogenicity of column-purified human papillomavirus type 16 virus-like particles. PLoS ONE 2012; 7:e35893; http://dx.doi.org/10.1371/journal.pone.0035893; PMID: 22563414
- Cook JC, Joyce JG, George HA, Schultz LD, Hurni WM, Jansen KU, et al. Purification of virus-like particles of recombinant human papillomavirus type 11 major capsid protein L1 from Saccharomyces cerevisiae. Protein Expr Purif 1999; 17:477 - 84; http://dx.doi.org/10.1006/prep.1999.1155; PMID: 10600468
- Kee GS, Jin J, Balasundaram B, Bracewell DG, Pujar NS, Titchener-Hooker NJ. Exploiting the intracellular compartmentalization characteristics of the S. cerevisiae host cell for enhancing primary purification of lipid-envelope virus-like particles. Biotechnol Prog 2010; 26:26 - 33; PMID: 19856403
- Ferreira GN, Cabral JM, Prazeres DM. Anion exchange purification of plasmid DNA using expanded bed adsorption. Bioseparation 2000; 9:1 - 6; http://dx.doi.org/10.1023/A:1008134822673; PMID: 10840595
- Ferreira GN, Cabral JM, Prazeres DM. Studies on the batch adsorption of plasmid DNA onto anion-exchange chromatographic supports. Biotechnol Prog 2000; 16:416 - 24; http://dx.doi.org/10.1021/bp0000196; PMID: 10835244
- Lyddiatt A. Process chromatography: current constraints and future options for the adsorptive recovery of bioproducts. Curr Opin Biotechnol 2002; 13:95 - 103; http://dx.doi.org/10.1016/S0958-1669(02)00293-8; PMID: 11950558
- Vicente T, Mota JP, Peixoto C, Alves PM, Carrondo MJ. Analysis of adsorption of a baculovirus bioreaction bulk on an ion-exchange surface by surface plasmon resonance. J Biotechnol 2010; 148:171 - 81; http://dx.doi.org/10.1016/j.jbiotec.2010.05.005; PMID: 20566345
- Peixoto C, Ferreira TB, Sousa MF, Carrondo MJ, Alves PM. Towards purification of adenoviral vectors based on membrane technology. Biotechnol Prog 2008; 24:1290 - 6; http://dx.doi.org/10.1002/btpr.25; PMID: 19194943
- Palomares LA, Ramírez OT. Challenges for the production of virus-like particles in insect cells: The case of rotavirus-like particles. Biochem Eng J 2009; 45:158 - 67; http://dx.doi.org/10.1016/j.bej.2009.02.006
- Vicente T, Sousa MFQ, Peixoto C, Mota JPB, Alves PM, Carrondo MJT. Anion-exchange membrane chromatography for purification of rotavirus-like particles. J Membr Sci 2008; 311:270 - 83; http://dx.doi.org/10.1016/j.memsci.2007.12.021
- Vicente T, Peixoto C, Carrondo MJ, Alves PM. Purification of recombinant baculoviruses for gene therapy using membrane processes. Gene Ther 2009; 16:766 - 75; http://dx.doi.org/10.1038/gt.2009.33; PMID: 19340018
- Vicente T, Mota JP, Peixoto C, Alves PM, Carrondo MJ. Rational design and optimization of downstream processes of virus particles for biopharmaceutical applications: current advances. Biotechnol Adv 2011; 29:869 - 78; http://dx.doi.org/10.1016/j.biotechadv.2011.07.004; PMID: 21784144
- Hammonds J, Chen X, Zhang X, Lee F, Spearman P. Advances in methods for the production, purification, and characterization of HIV-1 Gag-Env pseudovirion vaccines. Vaccine 2007; 25:8036 - 48; http://dx.doi.org/10.1016/j.vaccine.2007.09.016; PMID: 17936444
- Sweeney SF, Woehrle GH, Hutchison JE. Rapid purification and size separation of gold nanoparticles via diafiltration. J Am Chem Soc 2006; 128:3190 - 7; http://dx.doi.org/10.1021/ja0558241; PMID: 16522099
- Giritch A, Marillonnet S, Engler C, van Eldik G, Botterman J, Klimyuk V, et al. Rapid high-yield expression of full-size IgG antibodies in plants coinfected with noncompeting viral vectors. Proc Natl Acad Sci USA 2006; 103:14701 - 6; http://dx.doi.org/10.1073/pnas.0606631103; PMID: 16973752
- Huang Z, Phoolcharoen W, Lai H, Piensook K, Cardineau G, Zeitlin L, et al. High-level rapid production of full-size monoclonal antibodies in plants by a single-vector DNA replicon system. Biotechnol Bioeng 2010; 106:9 - 17; PMID: 20047189
- Sainsbury F, Lomonossoff GP. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol 2008; 148:1212 - 8; http://dx.doi.org/10.1104/pp.108.126284; PMID: 18775971
- Platis D, Labrou NE. Affinity chromatography for the purification of therapeutic proteins from transgenic maize using immobilized histamine. J Sep Sci 2008; 31:636 - 45; http://dx.doi.org/10.1002/jssc.200700481; PMID: 18307162
- Roque ACA, Lowe CR, Taipa MA. Antibodies and genetically engineered related molecules: production and purification. Biotechnol Prog 2004; 20:639 - 54; http://dx.doi.org/10.1021/bp030070k; PMID: 15176864
- Desvoyes B, Dulieu P. Purification by monoclonal antibody affinity chromatography of virus-like particles associated with the '447' cytoplasmic male sterility of Vicia faba and investigation of their antigenic composition. Plant Sci 1996; 116:239 - 46; http://dx.doi.org/10.1016/0168-9452(96)04390-7
- Potera C. Vaccine Manufacturing Gets Boost from Tobacco Plants: Canada-Based Medicago Opens U.S. Facility to Exploit Its Influenza Vaccine Production Method. Genetic Engineering & Biotechnology News 2012; 32:8 - 10; http://dx.doi.org/10.1089/gen.32.6.02
- Medicago. Clinical Trials Update: Avian flu vaccine. Genetic Engineering & Biotechnology News 2011; 31:61; http://dx.doi.org/10.1089/gen.31.14.28
- Medicago. Medicago reports positive U.S. clinical trial results for its H1N1 / seasonal influenza vaccine. http://wwwmedicagocom/English/news/News-Releases/News-ReleaseDetails/2011/Medicago-reports-positive-US-clinical-trial-results-for-its-H1N1–seasonal-influenza-vaccine1125593/defaultaspx 2011; (accessed on July 04 2012).
- Tacket CO, Mason HS, Losonsky G, Estes MK, Levine MM, Arntzen CJ. Human immune responses to a novel norwalk virus vaccine delivered in transgenic potatoes. J Infect Dis 2000; 182:302 - 5; http://dx.doi.org/10.1086/315653; PMID: 10882612
- Kapusta J, Modelska A, Pniewski T, Figlerowicz M, Jankowski K, Lisowa O, et al. Oral immunization of human with transgenic lettuce expressing hepatitis B surface antigen. Adv Exp Med Biol 2001; 495:299 - 303; http://dx.doi.org/10.1007/978-1-4615-0685-0_41; PMID: 11774582
- Bayer ME, Blumberg BS, Werner B. Particles associated with Australia antigen in the sera of patients with leukaemia, Down’s Syndrome and hepatitis. Nature 1968; 218:1057 - 9; http://dx.doi.org/10.1038/2181057a0; PMID: 4231935
- Fernández-Fernández MR, Martínez-Torrecuadrada JL, Casal JI, García JA. Development of an antigen presentation system based on plum pox potyvirus. FEBS Lett 1998; 427:229 - 35; http://dx.doi.org/10.1016/S0014-5793(98)00429-3; PMID: 9607317
- Palmer KE, Benko A, Doucette SA, Cameron TI, Foster T, Hanley KM, et al. Protection of rabbits against cutaneous papillomavirus infection using recombinant tobacco mosaic virus containing L2 capsid epitopes. Vaccine 2006; 24:5516 - 25; http://dx.doi.org/10.1016/j.vaccine.2006.04.058; PMID: 16725236
- Usha R, Rohll JB, Spall VE, Shanks M, Maule AJ, Johnson JE, et al. Expression of an animal virus antigenic site on the surface of a plant virus particle. Virology 1993; 197:366 - 74; http://dx.doi.org/10.1006/viro.1993.1598; PMID: 7692669
- Wigdorovitz A, Pérez Filgueira DM, Robertson N, Carrillo C, Sadir AM, Morris TJ, et al. Protection of mice against challenge with foot and mouth disease virus (FMDV) by immunization with foliar extracts from plants infected with recombinant tobacco mosaic virus expressing the FMDV structural protein VP1. Virology 1999; 264:85 - 91; http://dx.doi.org/10.1006/viro.1999.9923; PMID: 10544132
- Andrianova EP, Krementsugskaia SR, Lugovskaia NN, Mayorova TK, Borisov VV, Eldarov MA, et al. Foot and mouth disease virus polyepitope protein produced in bacteria and plants induces protective immunity in guinea pigs. Biochemistry (Mosc) 2011; 76:339 - 46; http://dx.doi.org/10.1134/S0006297911030072; PMID: 21568869
- Nuzzaci M, Bochicchio I, De Stradis A, Vitti A, Natilla A, Piazzolla P, et al. Structural and biological properties of Cucumber mosaic virus particles carrying hepatitis C virus-derived epitopes. J Virol Methods 2009; 155:118 - 21; http://dx.doi.org/10.1016/j.jviromet.2008.10.005; PMID: 18992770
- Piazzolla G, Nuzzaci M, Tortorella C, Panella E, Natilla A, Boscia D, et al. Immunogenic properties of a chimeric plant virus expressing a hepatitis C virus (HCV)-derived epitope: new prospects for an HCV vaccine. J Clin Immunol 2005; 25:142 - 52; http://dx.doi.org/10.1007/s10875-005-2820-4; PMID: 15821891
- Nemchinov LG, Liang TJ, Rifaat MM, Mazyad HM, Hadidi A, Keith JM. Development of a plant-derived subunit vaccine candidate against hepatitis C virus. Arch Virol 2000; 145:2557 - 73; http://dx.doi.org/10.1007/s007050070008; PMID: 11205105
- Joelson T, Akerblom L, Oxelfelt P, Strandberg B, Tomenius K, Morris TJ. Presentation of a foreign peptide on the surface of tomato bushy stunt virus. J Gen Virol 1997; 78:1213 - 7; PMID: 9191910
- McLain L, Durrani Z, Wisniewski LA, Porta C, Lomonossoff GP, Dimmock NJ. Stimulation of neutralizing antibodies to human immunodeficiency virus type 1 in three strains of mice immunized with a 22 amino acid peptide of gp41 expressed on the surface of a plant virus. Vaccine 1996; 14:799 - 810; http://dx.doi.org/10.1016/0264-410X(95)00229-T; PMID: 8817828
- McLain L, Porta C, Lomonossoff GP, Durrani Z, Dimmock NJ. Human immunodeficiency virus type 1-neutralizing antibodies raised to a glycoprotein 41 peptide expressed on the surface of a plant virus. AIDS Res Hum Retroviruses 1995; 11:327 - 34; http://dx.doi.org/10.1089/aid.1995.11.327; PMID: 7786579
- Marusic C, Rizza P, Lattanzi L, Mancini C, Spada M, Belardelli F, et al. Chimeric plant virus particles as immunogens for inducing murine and human immune responses against human immunodeficiency virus type 1. J Virol 2001; 75:8434 - 9; http://dx.doi.org/10.1128/JVI.75.18.8434-8439.2001; PMID: 11507188
- Cerovska N, Hoffmeisterova H, Moravec T, Plchova H, Folwarczna J, Synkova H, et al. Transient expression of Human papillomavirus type 16 L2 epitope fused to N- and C-terminus of coat protein of Potato virus X in plants. J Biosci 2012; 37:125 - 33; http://dx.doi.org/10.1007/s12038-011-9177-z; PMID: 22357210
- Meshcheriakova IuA, El’darov MA, Migunov AI, Stepanova LA, Repko IA, Kiselev OI, et al. [Cowpea mosaic virus chimeric particles bearing ectodomain of matrix protein 2 (M2E) of influenza A virus: production and characteristics]. Mol Biol (Mosk) 2009; 43:741 - 50; PMID: 19807038
- Brennan FR, Jones TD, Gilleland LB, Bellaby T, Xu F, North PC, et al. Pseudomonas aeruginosa outer-membrane protein F epitopes are highly immunogenic in mice when expressed on a plant virus. Microbiology 1999; 145:211 - 20; http://dx.doi.org/10.1099/13500872-145-1-211; PMID: 10206701
- Gilleland HE, Gilleland LB, Staczek J, Harty RN, García-Sastre A, Palese P, et al. Chimeric animal and plant viruses expressing epitopes of outer membrane protein F as a combined vaccine against Pseudomonas aeruginosa lung infection. FEMS Immunol Med Microbiol 2000; 27:291 - 7; http://dx.doi.org/10.1111/j.1574-695X.2000.tb01442.x; PMID: 10727884
- Staczek J, Bendahmane M, Gilleland LB, Beachy RN, Gilleland HE Jr.. Immunization with a chimeric tobacco mosaic virus containing an epitope of outer membrane protein F of Pseudomonas aeruginosa provides protection against challenge with P. aeruginosa. Vaccine 2000; 18:2266 - 74; http://dx.doi.org/10.1016/S0264-410X(99)00571-X; PMID: 10717347
- Belanger H, Fleysh N, Cox S, Bartman G, Deka D, Trudel M, et al. Human respiratory syncytial virus vaccine antigen produced in plants. FASEB J 2000; 14:2323 - 8; http://dx.doi.org/10.1096/fj.00-0144com; PMID: 11053254
- Yusibov V, Mett V, Mett V, Davidson C, Musiychuk K, Gilliam S, et al. Peptide-based candidate vaccine against respiratory syncytial virus. Vaccine 2005; 23:2261 - 5; http://dx.doi.org/10.1016/j.vaccine.2005.01.039; PMID: 15755607
