Abstract
The epidemiology of Invasive Meningococcal Disease (IMD) is distinct in the United States and Canada compared with other countries. This review describes the incidence, mortality and vaccination strategies relevant to IMD in these countries over the past 65 y. The incidence of IMD has remained consistently low in both countries during this period. Serogroup B and serogroup C have been the most prominent disease-causing serogroups. Notably, serogroup Y has recently become an important cause of IMD in the USA, but has not been as prominent in Canada. Periodic rises in incidence have been characterized by local outbreaks that have raised public concern, especially those caused by serogroup C in Canada, and serogroup B in the USA. Case fatality rates have remained consistent at around 10–20%, but vary by age and serogroup. Recent outbreaks have led to the introduction of vaccination programs for both outbreak control and routine immunization.
Introduction
Invasive Meningococcal Disease (IMD) is a serious bacterial infection caused by Neisseria meningitidis. While in industrialized countries the disease is now considered rare, it may be more common but under ascertained in non-industrialized developing regions of the world, with the African Meningitis belt in sub-Saharan Africa particularly affected by regular large epidemics. Asymptomatic carriage of the bacteria is harmless, but in a small proportion of colonized persons bacteria invade the blood stream and cross the blood-brain barrier, causing meningitis and/or septicemia. The risk of infection is dependent on the balance of the virulence of the strain and the host’s immune response, and on various environmental factors. Crowded living conditions (such as a dormitory) and close contact with an infected person facilitate the transmission of bacteria and acquisition of disease. Recent upper respiratory tract infections, and active and passive smoking also increase the risk of disease. Invasive meningococcal disease is particularly dangerous because of its short incubation period (about 3 to 4 d, with a range of 2 to 10 d), nonspecific early symptoms, fast rate of clinical progression and the potential for long-term sequelae, including deafness, seizures, skin scarring and amputation.Citation1-Citation3
Transmission of bacteria occurs by direct oral contact or through droplets of upper respiratory tract secretions from colonized persons. Asymptomatic carriage is relatively common, and in most people carriage is an immunizing process. Globally, the approximate population carriage prevalence is estimated to be 10%, although this varies with age and is associated with a peak in early adulthood.Citation4 However, although the prevalence of population carriage has been studied relatively extensively in European populations, there are comparatively few carriage data available from North America.Citation4,Citation5
Neisseria meningitidis is categorized into serogroups according to the structure of the capsular polysaccharide. Five serogroups—A, B, C, Y and W-135—account for almost all the disease in the USA and Canada, although at least 13 serogroups exist worldwide.Citation6 Therefore, these five serogroups are the main focus of North American vaccine research and deployment. Currently licensed vaccines provide protection against serogroups A, C, W-135 and Y with monovalent serogroup C, and quadrivalent (A+C+Y+W135 serogroups) conjugate vaccines available. Recently, a combined Haemophilus influenzae type b and Neisseria meningitidis serogroups C and Y has also been licensed in the USA. There are no licensed vaccines currently available in North America that provide broad protection against serogroup B disease although candidates are now entering the late stages of development.Citation7
The current epidemiology of IMD and the serogroups responsible for causing disease vary both globally and within North America.Citation8-Citation12 Recent reviews of epidemiology focus on more contemporary data. However, the objective of the current review is to capture the changes over time in serogroup distribution, incidence, mortality and vaccination coverage relating to IMD in the USA and Canada during the last half century. These data provide a more detailed picture of the long-term history and fluctuating nature of the meningococcal disease epidemiology.
Results
Disease surveillance and trends in overall incidence
The majority of data concerning the incidence of IMD was obtained from government surveillance programs. Surveillance for IMD has been conducted in Canada since 1924.Citation13 Active surveillance has been performed in the USA through the ABCs, established in 1995, which has routinely reported surveillance data since 1997.Citation14 Comprehensive data concerning IMD epidemiology are only widely available for the period from 1970 onwards; there are relatively few data available prior to this period describing the epidemiology in the USA or Canada during the mid-20th century (1940s–1970s).
Overall, the incidence of IMD in the USA remained relatively stable during the second half of the twentieth century (incidence rates 0.5–1.8 cases per 100,000 population).Citation15–Citation17 No major disease epidemics (substantially increased incidence across a large region or country) were reported during this period, although state-wide and localized outbreaks were observed. In contrast, surveillance data indicate that the incidence of IMD in the USA declined sharply during the early 21st century and has remained low (falling from 0.8 cases per 100,000 population (2,200 estimated cases) in 2000 to 0.28 cases per 100,000 population (850 estimated cases in 2009)Citation14 ().
Figure 1. The recent decline in incidence of IMD in the USA and Canada.Citation13,Citation14,Citation16,Citation18,Citation22,Citation29 Footnote: The introduction of routine vaccination programs are indicated by the dashed vertical lines; however, some provinces in Canada introduced routine vaccination after 2002.
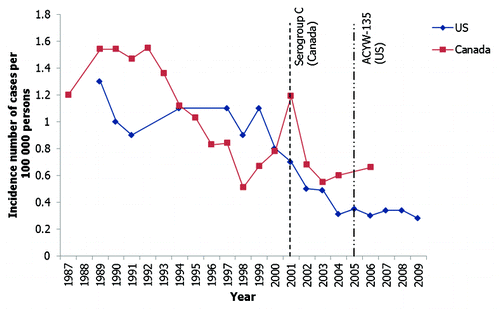
As observed in the USA, the incidence of IMD in Canada remained relatively stable following the end of an epidemic during the 2nd World War period (at around 1.5 cases per 100,000 population).Citation18,Citation19 Since 1945, incidence peaked at slightly higher rates (2 cases per 100,000 population) in Canada than in the USA. Incidence has also been observed to decline in Canada as well as the USA since 2000;Citation20,Citation21 from a peak incidence of 1.2 per 100,000 in 2001 the incidence rates of IMD in Canada fell to 0.66 per 100,000 in 2006.
The recent decline in IMD incidence within Canada is at least partly attributable to the mass vaccination campaigns followed by routine immunization programs initiated after a series of serogroup C outbreaks at the end of the 20th century.Citation22,Citation23 However, the cause for the observed drop in IMD incidence in the USA, which predates the routine use of quadrivalent vaccine, is uncertain. Current expert opinion suggests that the reduction in IMD incidence may be due to population immunity to the currently circulating strains of Neisseria meningitidis, changes in the prevalence of behavioral risk factors such as smoking and crowding, or other unknown variables, in addition to the impact of vaccination programs employed.Citation20 However, alternatively this longer term decrease in IMD incidence observed may also reflect a natural trend in the epidemiology of the disease.
Serogroup fluctuation with time
1945–1979
Both the USA and Canada experienced an epidemic of serogroup A disease during the 2nd World War.Citation18,Citation24
Following this epidemic, serogroup B became the primary strain responsible for the majority of IMD cases in the USA).Citation25 This dominance was short-lived as serogroup C replaced serogroup B as the most frequent cause of the disease in the USA at the end of the 1960s. During the early 1970s, serogroup Y grew in importance as a major cause of IMD, with this strain accounting for 18% of all cases from 1973 to 1975.Citation26 Similarly, the proportion of disease caused by serogroup W-135 increased in the USA from < 3% during 1964–1976 to 10% during 1975–1980,Citation27 paralleling the increase in the annual attack rate from 0.7 per 100,000 population in 1975 to 1.3 per 100,000 population in 1980. It is possible that the significant increase in cases of disease caused by W-135 observed during this period may have been inflated by the preferential submission from state health department laboratories to the CDC of isolates where serogroup could not be confirmed with more readily available antisera. However, it is also feasible that many of the isolates classified as nongroupable by the state health department laboratories may actually have been group W135, supporting an increase in prevalence of W135 disease during this period.Citation27
In contrast, serogroup A and serogroup C were responsible for the majority of disease in Canada during the 1960s and early 1970s.Citation28 Post 1975, serogroup B became the dominant serogroup (accounting for about half of the infections from 1979 to1982, when rate of IMD reached 0.8 cases per 100,000 population) in Canada although serogroup C (12% of isolates) remained an important cause of disease.Citation28 The dominance of serogroup B and serogroup C by the end of the 1970s was similar to the concurrent situation in the USA. However, while serogroup W-135 was not reported as a significant cause of disease in the USA during this period, it was an important cause of IMD in Canada; accounting for 13% of isolates from 1979−1982.Citation28 Similarly, while serogroup Y had emerged as an important cause of IMD in the USA, in Canada serogroup Y accounted for only 2% of isolates from 1979−1982.Citation28
1980s−1990s
By the 1980s, serogroup A had all but disappeared from North America, with serogroup B and serogroup C the dominant causes of disease in the USA.Citation17,Citation29 Surveillance data reported at this time demonstrate that serogroup B (47.9%) and serogroup C (26.2%) were the most frequent causes of IMD from 1978 to 1981, although serogroup Y continued to account for a significant minority of cases (6.6%).Citation17 While serogroup B continued to remain important, the proportion of disease caused by serogroup C subsequently increased dramatically during the 1980s, and was accounting for almost half (~45%) of observed IMD by the late 1980s and early 1990s (between 1989 and 1991, an estimated 2,600 cases of MD occurred annually in the USA).Citation29 Other serogroups were only responsible for a minority of disease at this time (). Similarly, during the 1980s serogroup C rose to become a frequent cause of disease in Canada alongside serogroup B, with the proportion of IMD caused by other serogroups falling over this period (). The proportion of disease caused by serogroup C rose from 12% in 1979–1982 to 26% in 1983–1987 (overall rate of disease in 1987 estimated at 1.2 cases per 100,000 population)Citation18,Citation28,Citation30 and corresponded with the emergence of the virulent ET-15 strain in Canada which represents a subset within the ST-11 complexCitation19.Citation30
Figure 2. Fluctuations over time in the proportion of IMD attributed to different serogroups in the USA.Citation14,Citation16,Citation17,Citation25,Citation29 Footnote: Minor serogroups (serogroups other than serogroups B, C and Y) are grouped; there is a gap in the data available concerning these minor serogroups prior to 1997.
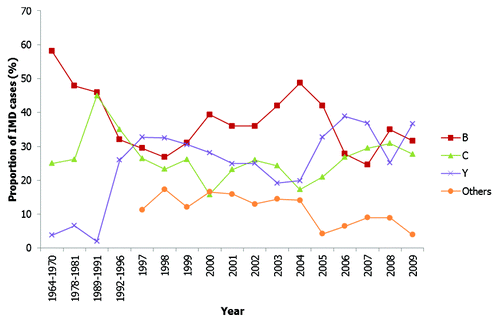
Figure 3. Fluctuations over time in the proportion of IMD attributed to different serogroups in Canada.Citation22,Citation30
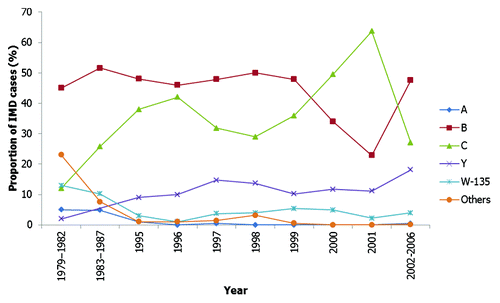
During the 1990s, serogroup B and serogroup C continued to be the most common cause of disease in the USA (32% and 35% respectively from 1992–1996; an estimated 2,454 cases of IMD occurred annually in the USA during this period).Citation16 Notably, much of the serogroup B disease was associated with a strain belonging to the ET-5 (ST-32) complex which spread in the USA.Citation31 However, serogroup Y IMD increased significantly in prevalence from 2% during 1989 through 1991 (of the annual 2,600 estimate cases of IMD) to 37% during 1997 through 2002 (annual estimated case of IMD vary during this period from 2,800 cases in 1997 to 2,200 cases in 2000 and 1,450 cases in 2002).Citation32 An ST-23 clone of serogroup Y accounted for much of serogroup Y disease at this time in both Canada and the USA.Citation33,Citation34 During the 1990s in Canada, the incidence of both serogroup B and serogroup C disease fell (from ~0.4 per 100,000 population and 0.65 per 100,000 population respectively in 1993, to ~0.2 per 100,000 population and 0.1 per 100,000 population respectively in 1998) but remained the two most frequently observed causes of IMD.Citation21 By the end of the 1990s, serogroup C incidence had risen once again (to ~0.4 per 100,000 population in 2000; overall rate of disease was 0.78 per 100,000 in 2000), with the ST-11 complex again responsible for the majority of serogroup C IMD.Citation35 However, the incidence of serogroup Y remained relatively low (~10% of cases) in Canada over this period, in contrast to the dramatic rise seen in the USA during the 1990s.Citation30 Serogroup A and serogroup W-135 only accounted for a minority of disease (< 5%) in both countries during this time.Citation29,Citation30
2000 onwards
The fall in incidence of IMD in the 21st century in the USA was observed across all serogroups.Citation14 Currently, the incidence of serogroup A and serogroup W-135 IMD remains very low in the USA with serogroup B, serogroup Y and serogroup C most commonly observed and accounting for the majority of disease.Citation14,Citation36 Incidence of serogroups B, C and Y disease is very similar with each causing ~0.1 cases per 100,000 population, and each accounting for around one third of the IMD burden in 2009. Serogroup W-135 has remained a very small contributor to meningococcal disease in the USA where it is more frequently associated with foreign travel and less often associated with outbreaks than other serogroups.Citation37 In Canada, a series of serogroup C outbreaks at the beginning of the 21st century led to serogroup C becoming the dominant serogroup observed. However, the incidence of serogroup C disease has since fallen in Canada following the introduction of a mass vaccination program, whereas the incidence of other serogroups has not declined so dramatically. Serogroup C disease (21% of cases), serogroup B (54% of cases) and serogroup Y (13% of cases) were responsible for the majority of disease in Canada in 2006.Citation38
Mortality
The case fatality ratio (CFR) of IMD has remained consistent over time during the post-antibiotic era in the USA, and typically this has been ~10%.Citation14,Citation16,Citation39,Citation40 In Canada, the CFR fell from 28% prior to the 1970s, to 16% across the 1970s and down to approximately 10% throughout the latter 20th century and early 21st century.Citation18,Citation19,Citation22 This fall in CFR has been attributed to an improvement in critical care.Citation18 Disease presentation has an important role in subsequent mortality with septicemia a more common cause of mortality than meningitis. Although meningitis and sepsis accounted for a similar number of inpatient cases in a USA hospital study, 71% of inpatient deaths from meningococcal disease were associated with sepsis.Citation41
Differences in CFR have been noted by serogroup and by particular disease clones. In the USA, serogroup C and serogroup W-135 have been associated with higher CFR than other serogroups, with CFRs of ~15%−20% compared with a CFR of ~10% for serogroup B and serogroup Y infections.Citation11,Citation16 Similarly, serogroup C is associated with a higher CFR (15%−20%) than other major serogroups in Canada.Citation21,Citation22 The ST-11 clone of Neisseria meningitidis has been identified as particularly lethal, although the increased CFR identified may be an artifact of improved reporting of this clone following its emergence.Citation19
Burden of disease by age-group
The distribution of IMD by age is similar in both the USA and Canada. By far the highest incidence of disease occurs in young infantsCitation11,Citation13,Citation16,Citation42 () but incidence of IMD is also high in young children aged between 1−4 y of age. A second, smaller incidence peak occurs in adolescents aged between 10 y and 19 y. In particular, an increased incidence of disease in adolescents occurred in Canada during a period of serogroup C outbreaks at the beginning of the 21st century.Citation13
Figure 4. Incidence of IMD by age group in Canada.Citation13 Footnote: The introduction of routine vaccination programs is indicated by the dashed vertical lines; however, some provinces introduced routine vaccination after 2002.
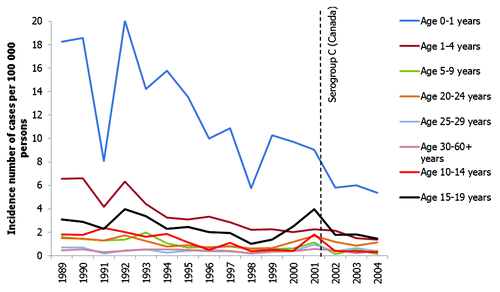
Serogroup B infection is proportionally more common in infants than other meningococcal serogroups (). In the USA, serogroup B is responsible for over 50% of cases in infants aged < 2 y.Citation11 Similarly, serogroup B is responsible for the majority of IMD in those aged under 5 y in Canada.Citation19,Citation43 In older children, adolescents and young adults, serogroup C is proportionally more frequent.Citation14,Citation16,Citation43 The median age of serogroup C infection is 15–20 y in both Canada and the USA.Citation16,Citation43 In contrast, serogroup Y is proportionally more frequent in adults aged > 65 y in the USA () and Canada, accounting for over 50% of IMD in this age group.Citation11,Citation43 However, despite a majority of cases of serogroup C and Y disease affecting adolescents and older persons in the USA, both these serogroups still cause a significant number of cases of disease in the youngest age groups; 7.6% of serogroup C and 13.8% of serogroup Y cases occur in infants aged < 2 y.Citation11
Figure 5. IMD case fatality rate and serogroup specific incidence in different age groups in the USA.Citation11
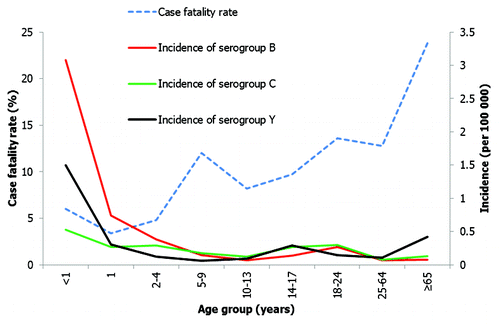
Recent USA data indicate that the observed CFR of 6% among infants aged < 1 y is lower than the CFR observed for older children, adolescents and adults.Citation11 In contrast, CFR has been consistently high in elderly age groups (age > 65 y), with the most recent USA estimates reporting it at over 20%.Citation11,Citation18 () CFR was highest among cases caused by serogroup W-135 (16.3%), and serogroup Y caused most of the meningococcal pneumonia in older adults (median age 53 y), though it causes disease in all ages. Patients with meningococcal bacteremia and pneumonia were more likely to die than those with meningococcal meningitis (CFR 13.2% and 15.9% respectively). While there is a clear trend of CFR increasing with age, multivariable analyses do not support an association with serogroup, isolate source or race.Citation11 Reasons for the observed increased CFR in older age groups are yet to be determined and remain speculative.Citation9,Citation16
Outbreaks
Most cases of meningococcal disease occur as isolated cases. However, occasionally outbreaks of IMD can occur within communities or institutions or across localized regions, where transmission of the virulent agent leads to an increased frequency of cases and may not always reflect the serogroup distribution of endemic IMD. While these outbreaks are important due to the particularly serious sequelae and rapid onset of meningococcal disease, they tend to involve relatively small numbers of cases and generally cause a relatively minor proportion of the total IMD burden; for example, in the US meningococcal outbreaks account for less than 5% of reported cases.Citation32 The majority of IMD outbreaks in the USA and Canada over the past 20 y have been associated with serogroup C, specifically the ST-11 clone.Citation35,Citation44,Citation45 Outbreaks associated with other serogroups occur less frequently. Serogroup A outbreaks are considered historical and confined to reports from the 1970s among USA deprived “skid row” communities.Citation46 A small proportion of more recent USA outbreaks are due to serogroup Y (13% of cases from 1994−2002).Citation44
The emergence of the serogroup C ST-11 clone was associated with an increase in localized outbreaks (1989–1993) in Canada.Citation47 Outbreaks of serogroup C disease remained common at the end of the 20th century; between 1999 and 2001, 8 were reported in Canada.Citation48 An increasing frequency of serogroup C outbreaks was also recognized in the 1990s in the USA; a review of data on all known serogroup C outbreaks in the USA concerning the period 1980−1993 identified 21 outbreaks, of which 8 occurred between January 1992 and June 1993.Citation45 Serogroup C continued to be the most common cause of outbreaks in the USA during the period 1994–2002 with a further review identifying 43 serogroup C outbreaks (62% of all outbreaks) which occurred in the USA during this period.Citation44
Serogroup C outbreaks are often associated with schools or higher education centers, and as such older children, adolescents and young adult populations may be particularly affected.Citation45 However, outbreaks can also affect other age groups; a particularly large serogroup C outbreak (61 cases) occurred in Alberta Canada in 1999–2001, with an ET-15 serotype 2a strain belonging to the ST-11 complex responsible.Citation49 The highest incidence (37 cases per 100,000 population) was observed in those aged < 1 y, but incidence was also high in the 15−24 y olds (26.8 per 100,000 population in those aged 15–19 y and 8.0 per 100,000 population in those aged 20–24 y).
There have also been reports of serogroup B outbreaks in the USA since the 1990s with 17 occurring (25% of all outbreaks) between 1994 and 2002.Citation44 Similar to serogroup C outbreaks, serogroup B outbreaks may be related to emergence of a particular meningococcal clone, and may affect particular age groups. In 1994 the incidence of IMD in Oregon increased to four times the national rate (4.5 cases per 100,000 population), primarily due to a significant rise in serogroup B disease associated with a clonal group of ET-5 strains that are part of the ST-32 complex.Citation31,Citation50 Outbreaks of serogroup B IMD in school and community clusters within Washington and the surrounding states were observed during 1995 and 1996, with the incidence among 15–19 y olds increasing 13-fold from the pre-epidemic period.Citation50 A serogroup B outbreak caused by a different strain was also reported in 2006 in Quebec, Canada, with an incidence of 0.74 cases per 100,000 population observed compared with the national average of 0.34 cases per 100,000 population.Citation38 This outbreak particularly affected adolescents and young adults (45% of cases were aged 10–19 y, 26% of cases aged 20–39 y), with strain B:17:P1.19 ST-269 considered responsible.Citation51
The use of vaccination
Vaccination became available for combating IMD outbreaks in both the USA and Canada in the early 1980s and 1990s.Citation35,Citation45 Initially, only polysaccharide vaccines were available but these have now been superseded by conjugate vaccines. However, they may still retain a useful role for controlling IMD outbreaks.Citation35,Citation52 A primary advantage of conjugate vaccines relates to the longer duration of protection provided; protection generated by polysaccharide vaccines is more transient and so these are not as suitable for use in routine vaccination programs.Citation47,Citation53,Citation54 Additionally, polysaccharide vaccines are considered poorly immunogenic among those aged under 2 y whereas conjugate vaccines are appropriate for use in this age group.Citation55,Citation56
The target groups for vaccination have often been school children and/or undergraduate students, since schools and colleges are frequently sites of IMD outbreaks. Of the USA serogroup C outbreaks described by Jackson and colleagues in 1995, 19 of 21 outbreaks identified were targeted with a vaccination response.Citation45 Of these, additional cases occurred post-vaccination in only 5 outbreaks suggesting vaccination was successful in controlling the outbreak. Similarly, vaccination was considered an effective response to outbreaks in Canada; in Quebec the incidence of serogroup C decreased markedly after a mass immunization campaign with polysaccharide vaccine (incidence decreased from 1.4 cases per 100,000 population in 1990–1992 to 0.3 cases per 100,000 population during 1993–1998).Citation57 In response to the serogroup C outbreak of 1999–2001 in Alberta, a polysaccharide quadrivalent vaccine was estimated to be 84% effective.Citation49 An emerging serogroup C epidemic in Quebec commencing in 2001 was controlled by use mostly of serogroup C conjugate vaccine;Citation58 vaccine effectiveness was estimated at 96.8%. In the age-group targeted, the incidence dropped from 2.1 cases per 100,000 population in 2001 to 0.3 cases per 100,000 population in 2002.
Both the USA and Canada have implemented routine vaccination in the 21st century, though they employ different strategies. In the USA in June 2005, the Advisory Committee on Immunization Practices (ACIP) recommended the newly licensed quadrivalent meningococcal conjugate vaccine for routine use among all those aged 11 y; this was updated in 2007 to include the 11–18 y age-group as part of a catch up vaccination campaign.Citation53,Citation56 More recently in 2010, ACIP recommended a booster dose (at age 16 y) given to those vaccinated originally at age 11 y.Citation54 Based on data indicating that immunity waned after 5 y instead of 10 y as was originally assumed, a booster dose was recommended in order to provide immunity across the whole of adolescence, as was the original goal of the program. The USA approach of routinely vaccinating adolescents is intended to provide direct protection for those age groups particularly associated with outbreaks and where, in terms of total numbers of cases, the burden of meningococcal disease is also considered high. Since the advent of conjugate vaccines, a quadrivalent ACYW-135 conjugate vaccine has been preferred for routine use to confer the broadest possible protection as serogroup Y in addition to serogroup C contributes significantly to the disease burden in the USA. This strategy does not directly protect the youngest age groups where a significant number of cases of disease due to serogroups C and Y also occur.Citation11 However, this strategy does have the potential to result in indirect protection by generating herd protection which may be extended to younger age groups as the approach targets an age group where carriage prevalence is often characteristically high. There is compelling evidence that meningococcal serogroup C conjugate vaccines have the ability to reduce carriage and interrupt transmission among populations with a high prevalence of carriage (such as adolescents) in industrialized countries and it is anticipated that an ability to protect against carriage and thus induce herd protection is likely to be a property of all meningococcal conjugate vaccines.Citation4,Citation59 However, appropriate evidence to support a high carriage prevalence in the USA adolescent population, and demonstrate the subsequent generation of any herd protection that benefits the youngest age groups as a result of routine vaccination, has yet to become available in the USA.
Vaccination coverage is increasing in the USA, although no conclusive evidence of impact of universal vaccination has been published. MCV4 (quadrivalent meningococcal conjugate vaccine) coverage in adolescents 13 to 17 y of age increased from 11.7% in 2006, to 32.4% in 2007, to 41.8% in 2008 and to 53.6% in 2009.Citation60-Citation62 The most recent vaccine coverage data available (2010) suggest coverage has subsequently improved to ~62% for adolescents who have received at least one dose.Citation62 During 1998–2007, the rate of IMD decreased among the 11–19 y old population, both for serogroups contained in the MCV4 vaccine program (started in 2005) and for serogroup B, which is not covered by the vaccine.Citation11 However, during this period the vaccine coverage was low and the timeframe considered may have been too short for the drop in incidence to be attributed to vaccination with certainty. Probability simulation estimates the vaccine effectiveness at 80%–85%.Citation36 More recent evidence indicates that the incidence of IMD in the younger vaccinated population (aged 11–14 y) has fallen by 74% since introduction of routine vaccination.Citation54 Nevertheless, a peak of disease persists in the at-risk older adolescent age groups; this peak is thought to be due to waning immunity after vaccination, and as a result the booster dose was recommended for 16 y olds in the USA, as described above.Citation54 This improvement in vaccine coverage may also improve the likelihood that possible indirect protection may become manifest.
In Canada, universal infant serogroup C vaccination programs were implemented over a period of several years. The current recommendation is for an initial dose during the first 12 mo of life followed by a booster dose during an infant’s second year.Citation63 Canada employs a single conjugate serogroup C vaccine as serogroup C was causing the majority of observed disease in 2001 when routine vaccination was being considered ().Citation63 More recent recommendations allow for the consideration of quadrivalent vaccine use in Canada as well.Citation64 In some provinces (depending on the prevalence of serogroup A, serogroup Y and serogroup W-135) these are recommended over monovalent serogroup C vaccine for catch up in older children and adolescents.
The coverage of universal vaccination programs has also increased year by year in Canada across the country.Citation22,Citation65 Incidence of serogroup C disease decreased significantly following vaccination from 0.23 cases per 100,000 population in 2002 to 0.08 cases per 100,000 population in 2006.Citation22 Between 2002 and 2006, there was a decrease in serogroup C incidence in provinces adopting the universal vaccination program early (in 2002) but not in those provinces adopting universal vaccination programs later (2005). Bettinger and colleagues also reported that the incidence of serogroups B and Y (which were not included in the vaccination program and may therefore be viewed as reflecting natural trends in disease incidence) remained stable in Canada over this study period. Together these points indicate an effect of the universal vaccination program in reducing serogroup C disease.Citation22,Citation23
There is also evidence that the routine vaccination approach employed in Canada generated some herd protection. The incidence of serogroup C infection significantly decreased in adults older than 30 y of age in early adopting provinces, but did not decrease in adults resident in later adopting provinces. It is unlikely that adults would have been vaccinated as part of the program, but instead indirectly benefited from the vaccination provided to younger residents in the population.Citation22 Incidence has also been shown to decrease in other poorly or non-vaccinated cohorts (those aged over 17 y) which may be attributed to herd protection.Citation23 Further evidence for herd protection may be forthcoming in the longer-term, through continued surveillance and monitoring of incidence in age groups not targeted by vaccination in both Canada and the USA. However, while the strongest evidence for indirect protection is an observed concurrent reduction in cases of disease in age groups not targeted by vaccination, there is also a need for appropriate carriage data in both these countries to demonstrate the ability of the vaccine to reduce carriage prevalence in those age groups most likely to be asymptomatic carriers, illustrating a reduction in transmission and support a herd protection effect.
Conclusions
Both historically and currently, the epidemiology of IMD in the USA and Canada differs. IMD incidence has been steadily declining in recent years, but is still important, particularly in key age groups such as infants, school children and adolescents. The case fatality ratio has remained constant despite the falling incidence, with serogroup C a particularly important contributor to high mortality. Currently serogroups B and C cause a substantial proportion of the disease burden in both the USA and Canada, having risen to prominence in the mid-20th century. However, serogroup Y also now contributes significantly to the disease burden in the USA, whereas the burden of disease caused by serogroup Y in Canada is considerably smaller. The rise in prominence of serogroup Y in the USA took place relatively recently (during the latter half of the 1990s) and unexpectedly when compared with the long-term continued dominance of serogroup B and serogroup C disease. The increase in the prevalence of serogroup Y is attributed to the emergence of an ST-23 clone of serogroup Y which accounts for much of serogroup Y IMD in both Canada and the USA.Citation33,Citation34 While the incidence of all serogroups has fallen in the USA in recent years, serogroup Y continues to be responsible for approximately a third of endemic disease cases. In both the USA and Canada, serogroup A was a major cause of disease historically, but is now rare. In both countries, serogroup W-135 is also rare with the few IMD cases currently caused by this serogroup being mainly associated with foreign travel.Citation37 However, serogroup W-135 has emerged as an important cause of meningococcal disease in the Middle East and African Meningitis belt at the beginning of the 21st century, and there is also some evidence that it is now becoming more frequently observed as a cause of disease in Latin America.Citation66-Citation68 Therefore it may remain important to continue to consider the need to provide protection against W-135 disease.
Strategies used to control meningococcal disease also differ between the USA and Canada. In Canada, a particular focus on combating serogroup C disease has resulted from the large number of outbreaks that have occurred in both countries over the past 20 y. While both the USA and Canada combated outbreaks using targeted vaccination of at-risk populations, more recently both countries have initiated universal vaccination programs for young people although the precise age groups targeted differ between the two countries. Additionally, the approach taken for universal vaccination has been different in each country, reflecting differences in the epidemiology of IMD with respect to the prevalence of serogroups responsible, and the availability of licensed vaccines (there are no serogroup C monovalent vaccines licensed for routine vaccination use in the USA).
Canada has instigated routine vaccination in infants using a monovalent serogroup C specific conjugate vaccine. Although this has been successful in controlling serogroup C disease with some evidence of a herd protection effect that benefits age groups not eligible for routine vaccination, it does not provide protection against disease caused by other serogroups. In contrast the USA authorities recommend routine vaccination using a quadrivalent conjugate vaccine in adolescent age groups, recently reinforced with the addition of a booster dose. This approach provides broader serogroup protection, including against those rarely causing disease. It also has the potential to maximize any herd protection by targeting an age group where carriage prevalence is likely to be at its highest. However, the success of this vaccination program in reducing the incidence of disease is as yet inconclusive, with no evidence currently available to demonstrate an impact that can be attributed to either direct or indirect protection. Furthermore, a decrease in the incidence of IMD started prior to the introduction of routine vaccination and has continued throughout the vaccination period, making the impact of the vaccination program difficult to evaluate in the context of other changing environmental factors.
Despite this lack of clarity surrounding the absolute impact of the adolescent vaccination program, recent long-term data presented at the October 2010 ACIP meeting suggested that this vaccination program has been beneficial in reducing IMD incidence, albeit mainly in younger adolescents. Waning vaccine induced immunity is believed to be responsible for the continued peak in older adolescents.Citation69 The most recent data indicate that vaccine coverage is now improving following the introduction of a booster dose for adolescents which may further impact the epidemiology of meningococcal disease in the USA as a result of both increased direct protection and an increased likelihood of indirect protection. However, in both the USA and Canada there is a need to generate contemporary carriage data across the age range to investigate the extent of any potential herd protection effects and improve understanding of the epidemiology and transmission patterns of the meningococcus in this continent. Carriage data are also essential to facilitate bespoke mathematical modeling to explore the longer-term impact of various vaccination strategy options in the USA and Canada and would be valuable to assist policy makers in both these countries.
Neisseria meningitidis epidemiology has an unpredictable nature; new strains can emerge and rise to prominence quickly and unexpectedly. This may necessitate periodic reconsideration of vaccination strategies and available vaccines to continually provide optimal protection against this ever-changing threat to public health in North America. At present, the burden of serogroup B IMD suggests an important role for vaccination against serogroup B, for which vaccines are in development (although none providing broad protection are currently licensed). In particular, the high incidence of serogroup B disease in infants makes it likely that use of these vaccines will be important to protect this youngest age group. However, there may additionally be merit in reviewing the need to provide routine protection against other specific serogroups that currently also cause a burden of disease in younger age groups in the USA and Canada.
Methods
This literature review targeted citations describing the epidemiology (incidence or mortality rate), of meningococcal disease and meningococcal vaccination coverage in the USA and Canada during the period 1945 to 2010. Index term and keyword searches were conducted in major literature databases and online sources (in particular national health agencies). Bibliographies of included studies were also screened for relevant references.
The literature databases Medline and Embase were searched using a combined search interface (Embase.com) in order to retrieve published data concerning IMD epidemiology. Searches were targeted using the following index terms or keyword terms to identify citations that reported “epidemiological,” “surveillance,” “incidence,” “prevalence,” “morbidity,” “mortality,” “outbreak” or “fatality” data alongside “meningitis,” “meningococcal disease” or “Neisseria meningitidis.” The search was restricted to identify citations concerning data from the USA or Canada. Reviews and the discussion sections of included studies were examined in order to identify further published literature or gray literature containing relevant information.
Further searches for information relevant to the study objective were conducted across sources of published data outside of indexed journals. The Public Health Agency of Canada (PHAC) website (http://www.phac-aspc.gc.ca) and the Active Bacterial Core surveillance (ABCs) website of the USA Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov/abcs/index.html) were searched using disease/causal agent keywords (e.g., meningococcal) and by following relevant index links in order to identify data concerning the epidemiology of IMD in Canada and the USA.
Inclusion in the review was reliant on the presence of incidence, mortality and or vaccination coverage data, for the USA and Canada between the time periods of 1945 and 2010 in English language publication; sources were excluded from the review if these conditions were not met.
| Abbreviations: | ||
| ACIP | = | Advisory Committee on Immunization Practices |
| CFR | = | case fatality ratio |
| IMD | = | invasive meningococcal disease |
| MCV4 | = | quadrivalent meningococcal conjugate vaccine |
| PHAC | = | Public Health Agency of Canada |
| ABCs | = | Active Bacterial Core surveillance |
Acknowledgments
The authors thank Jacqueline Miller, MD (GSK Vaccines) for input in the manuscript and Wouter Houthoofd (XPE Pharma and Science, on behalf of GlaxoSmithKline Vaccines) for editorial assistance in co-ordination and submission of the manuscript
Disclosure of Potential Conflicts of Interest and Source of Funding
GlaxoSmithKline (GSK) Biologicals SA paid for all costs associated with development and publication of the present manuscript. GSK Biologicals SA has meningococcal conjugate vaccines licensed for use. A.T. and H.W. are employees of Heron Evidence Development Ltd which received funding from GSK Biologicals SA to conduct research for this study and to provide writing assistance for this manuscript. C.B. and A.V. are employees of GSK Group of companies.
References
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351:1849 - 59; http://dx.doi.org/10.1056/NEJMoa040845; PMID: 15509818
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases; 4th edition. Centers for Disease Control and Prevention. 2012.
- World Health Organisation. Meningococcal meningitis Fact sheet N°141. <http://www.who.int/mediacentre/factsheets/fs141/en/index.html>
- Christensen H, May M, Bowen L, Hickman M, Trotter CL. Meningococcal carriage by age: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:853 - 61; http://dx.doi.org/10.1016/S1473-3099(10)70251-6; PMID: 21075057
- Soriano-Gabarró M, Wolter J, Hogea C, Vyse A. Carriage of Neisseria meningitidis in Europe: a review of studies undertaken in the region. Expert Rev Anti Infect Ther 2011; 9:761 - 74; http://dx.doi.org/10.1586/eri.11.89; PMID: 21905785
- Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev 2006; 19:142 - 64; http://dx.doi.org/10.1128/CMR.19.1.142-164.2006; PMID: 16418528
- Donnelly J, Medini D, Boccadifuoco G, Biolchi A, Ward J, Frasch C, et al. Qualitative and quantitative assessment of meningococcal antigens to evaluate the potential strain coverage of protein-based vaccines. Proc Natl Acad Sci USA 2010; 107:19490 - 5; http://dx.doi.org/10.1073/pnas.1013758107; PMID: 20962280
- Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine 2009; 27:Suppl 2 B51 - 63; http://dx.doi.org/10.1016/j.vaccine.2009.04.063; PMID: 19477562
- Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines 2010; 9:285 - 98; http://dx.doi.org/10.1586/erv.10.3; PMID: 20218857
- Racloz VN, Luiz SJ. The elusive meningococcal meningitis serogroup: a systematic review of serogroup B epidemiology. BMC Infect Dis 2010; 10:175; http://dx.doi.org/10.1186/1471-2334-10-175; PMID: 20565757
- Cohn AC, MacNeil JR, Harrison LH, Hatcher C, Theodore J, Schmidt M, et al. Changes in Neisseria meningitidis disease epidemiology in the United States, 1998-2007: implications for prevention of meningococcal disease. Clin Infect Dis 2010; 50:184 - 91; http://dx.doi.org/10.1086/649209; PMID: 20001736
- Hershey JH, Hitchcock W. Epidemiology and meningococcal serogroup distribution in the United States. Clin Pediatr (Phila) 2010; 49:519 - 24; http://dx.doi.org/10.1177/0009922809347797; PMID: 20507868
- PHAC. Notifiable diseases online. <http://dsol-smed.phac-aspc.gc.ca/dsol-smed/ndis/index-eng.php> Accessed Apr. 2010.
- CDC. ABC surveillance reports. <http://www.cdc.gov/abcs/reports-findings/surv-reports.html> Accessed Oct. 2011.
- Bennet JV, Young LS. Trends in meningococcal disease. J Infect Dis 1969; 120:634 - 5; PMID: 5346539
- Rosenstein NE, Perkins BA, Stephens DS, Lefkowitz L, Cartter ML, Danila R, et al. The changing epidemiology of meningococcal disease in the United States, 1992-1996. J Infect Dis 1999; 180:1894 - 901; http://dx.doi.org/10.1086/315158; PMID: 10558946
- Schlech WF 3rd, Ward JI, Band JD, Hightower A, Fraser DW, Broome CV. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA 1985; 253:1749 - 54; http://dx.doi.org/10.1001/jama.1985.03350360075022; PMID: 3871869
- Varughese PV. Meningococcal disease in Canada: surveillance summary to 1987. CMAJ 1989; 141:567 - 9; PMID: 2505917
- Whalen CM, Hockin JC, Ryan A, Ashton F. The changing epidemiology of invasive meningococcal disease in Canada, 1985 through 1992. Emergence of a virulent clone of Neisseria meningitidis. JAMA 1995; 273:390 - 4; http://dx.doi.org/10.1001/jama.1995.03520290042027; PMID: 7823384
- Harrison LH. Epidemiological profile of meningococcal disease in the United States. Clin Infect Dis 2010; 50:Suppl 2 S37 - 44; http://dx.doi.org/10.1086/648963; PMID: 20144015
- PHAC. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 2002, through 31 December, 2003. Can Commun Dis Rep 2006; 32:97 - 107; PMID: 16685767
- Bettinger JA, Scheifele DW, Le Saux N, Halperin SA, Vaudry W, Tsang R, Canadian Immunization Monitoring Program, Active (IMPACT). The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J 2009; 28:220 - 4; http://dx.doi.org/10.1097/INF.0b013e31819040e7; PMID: 19209096
- De Wals P, Deceuninck G, Lefebvre B, Boulianne N, De Serres G. Effectiveness of serogroup C meningococcal conjugate vaccine: a 7-year follow-up in Quebec, Canada. Pediatr Infect Dis J 2011; 30:566 - 9; http://dx.doi.org/10.1097/INF.0b013e31820e8638; PMID: 21326136
- World Health Organisation. Weekly Epidemiological Record Year 20. <http://www.who.int/wer/archives/en/> Accessed May 2011.
- Wiggins GL, Hollis DG, Weaver R. Prevalence of serogroups and sulfonamide resistance of meningococci from the civilian population in the United States, 1964-1970. Am J Public Health 1973; 63:59 - 65; http://dx.doi.org/10.2105/AJPH.63.1.59; PMID: 4630078
- Meningococcal Disease Surveillance Group. Analysis of endemic meningococcal disease by serogroup and evaluation of chemoprophylaxis. J Infect Dis 1976; 134:201 - 4; http://dx.doi.org/10.1093/infdis/134.2.201; PMID: 823273
- Band JD, Chamberland ME, Platt T, Weaver RE, Thornsberry C, Fraser DW. Trends in meningococcal disease in the United States, 1975-1980. J Infect Dis 1983; 148:754 - 8; http://dx.doi.org/10.1093/infdis/148.4.754; PMID: 6313816
- Varughese PV, Carter AO. Meningococcal disease in Canada and serogroup distribution. Can Dis Wkly Rep 1983; 9:177 - 80
- Jackson LA, Wenger JD. Laboratory-based surveillance for meningococcal disease in selected areas, United States, 1989-1991. MMWR CDC Surveill Summ 1993; 42:21 - 30; PMID: 8510639
- Tsang RSW, Squires SG, Zollinger WD, Ashton FE. Distribution of serogroups of Neisseria meningitidis and antigenic characterization of serogroup Y meningococci in Canada, January 1, 1999 to June 30, 2001. Can J Infect Dis 2002; 13:391 - 6; PMID: 18159416
- Fischer M, Perkins B. Neisseria meningitidis serogroup B: Emergence of the ET-5 complex. Semin Pediatr Infect Dis 1997; 8:50 - 6; http://dx.doi.org/10.1016/S1045-1870(97)80009-X
- Atkinson W, Wolfe S, Hamborsky J. Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12 ed. Washington DC: Public Health Foundation. 2011.
- Tsang RSW, Henderson AM, Cameron ML, Tyler SD, Tyson S, Law DKS, et al. Genetic and antigenic analysis of invasive serogroup Y Neisseria meningitidis isolates collected from 1999 to 2003 in Canada. J Clin Microbiol 2007; 45:1753 - 8; http://dx.doi.org/10.1128/JCM.02134-06; PMID: 17442798
- Whitney AM, Coulson GB, von Gottberg A, Block C, Keller N, Mayer LW, et al. Genotypic comparison of invasive Neisseria meningitidis serogroup Y isolates from the United States, South Africa, and Israel, isolated from 1999 through 2002. J Clin Microbiol 2009; 47:2787 - 93; http://dx.doi.org/10.1128/JCM.00091-09; PMID: 19571028
- Advisory Committee Statement. An Advisory Committee Statement (ACS). Statement on recommended use of meningococcal vaccines. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/04vol30/dr3003a-eng.php. Can Commun Dis Rep 2001; 27: 2-36.
- Macneil JR, Cohn AC, Zell ER, Schmink S, Miller E, Clark T, et al, Active Bacterial Core surveillance (ABCs) Team and MeningNet Surveillance Partners. Early estimate of the effectiveness of quadrivalent meningococcal conjugate vaccine. Pediatr Infect Dis J 2011; 30:451 - 5; PMID: 21206392
- Doyle TJ, Mejia-Echeverry A, Fiorella P, Leguen F, Livengood J, Kay R, et al. Cluster of serogroup W135 meningococci, southeastern Florida, 2008-2009. Emerg Infect Dis 2010; 16:113 - 5; http://dx.doi.org/10.3201/eid1601.091026; PMID: 20031054
- Advisory Committee Statement. An update on the invasive meningococcal disease and meningococcal vaccine conjugate recommendations. An Advisory Committee Statement (ACS). http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/04vol30/dr3003a-eng.php. Can Commun Dis Rep 2009; 35: 1-40.
- Havens PL, Garland JS, Brook MM, Dewitz BA, Stremski ES, Troshynski TJ. Trends in mortality in children hospitalized with meningococcal infections, 1957 to 1987. Pediatr Infect Dis J 1989; 8:8 - 11; http://dx.doi.org/10.1097/00006454-198901000-00003; PMID: 2922245
- Feldman RA, Koehler RE, Fraser DW. Race-specific differences in bacterial meningitis deaths in the United States, 1962-1968. Am J Public Health 1976; 66:392 - 6; http://dx.doi.org/10.2105/AJPH.66.4.392; PMID: 1267089
- O’Brien JA, Caro JJ, Getsios D. Managing meningococcal disease in the United States: Hospital case characteristics and costs by age. Value Health 2006; 9:236 - 43; http://dx.doi.org/10.1111/j.1524-4733.2006.00113.x; PMID: 16903993
- Shepard CW, Rosenstein NE, Fischer M, Active Bacterial Core Surveillance Team. Neonatal meningococcal disease in the United States, 1990 to 1999. Pediatr Infect Dis J 2003; 22:418 - 22; http://dx.doi.org/10.1097/01.inf.0000066876.77453.04; PMID: 12792381
- Squires SG, Pelletier L, Mungai M, Tsang R, Collins F, Stoltz J. Invasive meningococcal disease in Canada, 1 January 1997 to 31 December 1998. Can Commun Dis Rep 2000; 26:177 - 82; PMID: 11107657
- Brooks R, Woods CW, Benjamin DK Jr., Rosenstein NE. Increased case-fatality rate associated with outbreaks of Neisseria meningitidis infection, compared with sporadic meningococcal disease, in the United States, 1994-2002. Clin Infect Dis 2006; 43:49 - 54; http://dx.doi.org/10.1086/504804; PMID: 16758417
- Jackson LA, Schuchat A, Reeves MW, Wenger JD. Serogroup C meningococcal outbreaks in the United States. An emerging threat. JAMA 1995; 273:383 - 9; http://dx.doi.org/10.1001/jama.1995.03520290035026; PMID: 7823383
- Filice GA, Englender SJ, Jacobson JA, Jourden JL, Burns DA, Gregory D, et al. Group A meningococcal disease in skid rows: epidemiology and implications for control. Am J Public Health 1984; 74:253 - 4; http://dx.doi.org/10.2105/AJPH.74.3.253; PMID: 6198932
- National Advisory Committee on Immunisation (NACI). An Advisory Committee Statement (ACS). Statement on recommended use of meningococcal vaccines. Can Commun Dis Rep 2001; 27:2 - 36; PMID: 11706686
- Squires SG, Deeks SL, Tsang RS. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 1999, through 31 December, 2001. Can Commun Dis Rep 2004; 30:17 - 28; PMID: 14971276
- Tyrrell GJ, Chui L, Johnson M, Chang N, Rennie RP, Talbot JA, Edmonton Meningococcal Study Group. Outbreak of Neisseria meningitidis, Edmonton, Alberta, Canada. Emerg Infect Dis 2002; 8:519 - 21; http://dx.doi.org/10.3201/eid0805.010337; PMID: 11996690
- Diermayer M, Hedberg K, Hoesly F, Fischer M, Perkins B, Reeves M, et al. Epidemic serogroup B meningococcal disease in Oregon: the evolving epidemiology of the ET-5 strain. JAMA 1999; 281:1493 - 7; http://dx.doi.org/10.1001/jama.281.16.1493; PMID: 10227318
- Law DKS, Lorange M, Ringuette L, Dion R, Giguère M, Henderson AM, et al. Invasive meningococcal disease in Quebec, Canada, due to an emerging clone of ST-269 serogroup B meningococci with serotype antigen 17 and serosubtype antigen P1.19 (B:17:P1.19). J Clin Microbiol 2006; 44:2743 - 9; http://dx.doi.org/10.1128/JCM.00601-06; PMID: 16891487
- Advisory Committee on Immunization Practices. Control and prevention of serogroup C meningococcal disease: evaluation and management of suspected outbreaks: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1997; 46:RR-5 13 - 21; PMID: 9048847
- Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices. Revised recommendations of the Advisory Committee on Immunization Practices to Vaccinate all Persons Aged 11-18 Years with Meningococcal Conjugate Vaccine. MMWR Morb Mortal Wkly Rep 2007; 56:794 - 5; PMID: 17694617
- Centers for Disease Control and Prevention (CDC). Updated recommendations for use of meningococcal conjugate vaccines --- Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep 2011; 60:72 - 6; PMID: 21270745
- Addendum. Update: guidelines for the prevention and control of meningococcal disease. Can Commun Dis Rep 2006; 32:270 - 2; PMID: 17115511
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention and control of meningococcal disease: recommendations for use of meningococcal vaccines in pediatric patients. Pediatrics 2005; 116:496 - 505; http://dx.doi.org/10.1542/peds.2005-1314; PMID: 15995007
- De Wals P, De Serres G, Niyonsenga T. Effectiveness of a mass immunization campaign against serogroup C meningococcal disease in Quebec. JAMA 2001; 285:177 - 81; http://dx.doi.org/10.1001/jama.285.2.177; PMID: 11176810
- De Wals P, Deceuninck G, Boulianne N, De Serres G. Effectiveness of a mass immunization campaign using serogroup C meningococcal conjugate vaccine. JAMA 2004; 292:2491 - 4; http://dx.doi.org/10.1001/jama.292.20.2491; PMID: 15562128
- Maiden MC, Ibarz-Pavón AB, Urwin R, Gray SJ, Andrews NJ, Clarke SC, et al. Impact of meningococcal serogroup C conjugate vaccines on carriage and herd immunity. J Infect Dis 2008; 197:737 - 43; http://dx.doi.org/10.1086/527401; PMID: 18271745
- CDC. MCV4 1 or more doses. <http://www.cdc.gov/vaccines/stats-surv/nisteen/figures/2008_map.htm> Accessed Apr. 2010.
- Centers for Disease Control and Prevention (CDC). Vaccination coverage among adolescents aged 13-17 years - United States, 2007. MMWR Morb Mortal Wkly Rep 2008; 57:1100 - 3; PMID: 18846032
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117 - 23; PMID: 21866084
- National Advisory Committee on Immunization (NACI). Meningococcal C conjugate vaccination recommendations for infants. An Advisory Committee Statement (ACS). Can Commun Dis Rep 2007; 33:ACS-11 1 - 12; PMID: 18064747
- PHAC. Advice for consideration of quadrivalent (A, C, Y, W135) meningococcal conjugate vaccine, for use by provinces and territories. Can Commun Dis Rep 2010; 36
- Siu T, Tang W, Dawar M, Patrick DM. Impact of routine immunization using meningococcal C conjugate vaccine on invasive meningococcal disease in British Columbia. Can J Public Health 2008; 99:380 - 2; PMID: 19009920
- Lingappa JR, Al-Rabeah AM, Hajjeh R, Mustafa T, Fatani A, Al-Bassam T, et al. Serogroup W-135 meningococcal disease during the Hajj, 2000. Emerg Infect Dis 2003; 9:665 - 71; http://dx.doi.org/10.3201/eid0906.020565; PMID: 12781005
- Mueller JE, Borrow R, Gessner BD. Meningococcal serogroup W135 in the African meningitis belt: epidemiology, immunity and vaccines. Expert Rev Vaccines 2006; 5:319 - 36; http://dx.doi.org/10.1586/14760584.5.3.319; PMID: 16827617
- Efron AM, Sorhouet C, Salcedo C, Abad R, Regueira M, Vázquez JA. W135 invasive meningococcal strains spreading in South America: significant increase in incidence rate in Argentina. J Clin Microbiol 2009; 47:1979 - 80; http://dx.doi.org/10.1128/JCM.02390-08; PMID: 19357205
- Cohn A. Optimizing the adolescent meningococcal vaccination program. <http://www.cdc.gov/vaccines/recs/acip/slides-oct10.htm#mening> Accessed Feb. 2011.
