Abstract
Antigenic drift, the evolutionary mechanism of influenza viruses, results in an increased susceptibility of vaccinated subjects against circulating viruses. New vaccines able to grant a broader and cross-reactive immune response against drifted influenza variants are needed. Several strategies were explored to enhance the immunogenicity of plain vaccines: adjuvants, carriers and intradermal administration of influenza vaccine emerge as a promising options. To evaluate the ability of a MF59™-adjuvanted and intradermal influenza vaccine to elicit an effective antibody response against circulating viruses presenting antigenic patterns different from those of the vaccine strains, we compared antibody responses elicited by “implemented” vaccines and conventional intramuscular trivalent inactivated vaccine against heterologous circulating influenza A viruses. Different studies, simulating different epidemiological pictures produced by the natural antigenic drift of seasonal influenza viruses, highlighted the superior cross-reactivity of the antibodies elicited by MF59™ and intradermal vaccines, compared with subunit or split vaccine against heterologous viruses.
Impact of Antigenic Drift
Influenza remains a significant problem for global health in terms of mortality and economic burden. Seasonal epidemics affect 5–15% of the world population and cause 3 to 5 million severe cases and more than 500,000 deaths per year.Citation1 The influenza virus is able to elude the immune defenses developed by individuals as a result of previous infections or vaccination due to the continuous accumulation of points mutations on genes encoding the two surface proteins, hemagglutinin (HA) and neuraminidase (NA), through the gradual evolutionary mechanism known as antigenic drift.Citation2,Citation3 Antigenic drift occurs in all human viral types/subtypes, although different types/subtypes show specific pattern. Influenza A(H1N1) and B viruses showed low evolutionary pattern and different lineages tend to co-circulate favoring the re-emergence of previously circulating strains. Influenza A(H3N2) viral subtype undergoes antigenic changes more frequently, allowing the replacement of the old lineages with new drifted variants. Mutations occurs more frequently on 5 antigenic sites (A, B, C, D, E), that have been identified on the main antigenic determinant, the HA1 domain of HA.Citation4-Citation6 Furthermore, children and elderly often do not produce antibodies against all 5 sites, facilitating the emergence of escape mutants.Citation7
It is well documented that the appearance on the epidemiological scene and the circulation of drifted variants, especially of drifted A/H3N2 strains, resulted in increased disease burden.Citation8,Citation9 The circulation of the drift variant A/Sydney/5/97 during 1997–1998 influenza season caused severe outbreaks in Europe and the US.Citation10,Citation11
In the early 2000s, the antigenic evolution of A(H3N2) virus has moved away from A/Sydney/5/1997-like that predominated in 1998 and has gone through two main drift that led to as many epidemics: the spread of influenza A/Fujian/441/2002 in Europe during the 2003–2004 influenza season and the appearance of A/California/7/2004 virus variant that predominated in 2004 and 2005.Citation12,Citation13 More recently, the circulation of two H3N2 drifted variants A/HongKong/2121/2010 and A/Victoria/208/2009, despite the few nucleotide differences from the vaccine strain A/Perth/16/2009, were responsible of several outbreaks in Canada also among vaccinated people. The need for a perfect matching between the virus strains included in the vaccine and the circulating strains together with the great variability of influenza viruses required an active surveillance to continuously monitor the epidemiological picture and update the vaccine composition.Citation14 WHO established the global influenza network in 1952 in order to conduct active surveillance for antigenic characterization of circulating influenza viruses, to monitor antigenic drift and the emergence of new viral strains and to choose the composition of the annual vaccine. The efficacy of the vaccine depends on a good match between vaccine and circulating strains. The occurrence of antigenic mismatch between vaccine and circulating strains may result in a reduction of immunogenicity vs. the circulating strains and of on-field effectiveness elicited by the vaccine. In terms of immunogenicity, drifted strains can compromise vaccine-induced immunity resulting in a reduction of seroprotection rates [assessed as serum haemagglutination-inhibiting (HI) assay titers ≥ 40 IU].Citation15-Citation17
Several observational studies demonstrated a reduction of vaccine effectiveness in seasons in which a mismatch between vaccine and circulating strains occurred. A randomized placebo-controlled trial conducted to evaluate effectiveness and cost benefit of influenza vaccination of healthy working adults showed a strong correlation between antigenic drift and vaccine effectiveness decrease. The study demonstrated that during 1997–1998, when the vaccine virus (A/Wuhan/1995) differed from the predominant circulating viruses (A/Sidney/05/1997), vaccine efficacy against serologically confirmed influenza illness was 50%, while the following influenza season, during which the vaccine and predominant circulating viruses were well matched, vaccine efficacy increased to 86%. These results were further confirmed by data derived from French influenza surveillance network, who reported vaccine effectiveness rates of 70–80% when a good matching exist and of 40% when a drifted strain emerged (i.e., during the 1997–1998 season, the A/H3N2/Sidney/05/1997).Citation18-Citation20 A case control study conducted in Colorado during the 2003–2004 influenza season, when the drifted variant A/Fujian/441/2002 appeared, showed that vaccine efficacy against laboratory confirmed cases was 49,1–55,9% against the expected 70–90% seen in years when a good match between vaccine and circulating strains was observed.Citation21
In a recent study, Skowronski et al. have demonstrated that a reduction in vaccine effectiveness can also occur in the presence of drifted viruses for which an apparent good match to the vaccine strains has been determined by HI test. In fact, such viruses identified as A/HongKong/2121/2010 and A/Victoria/208/2009, circulated during 2010–2011 winter in some regions of Canada giving rise to several outbreaks, despite HI assay had shown a good match with vaccine strain A/Perth/16/2009.Citation14
Several strategies have been proposed to address the need for vaccines able to ensure optimal protection against drifted strains, including the use of adjuvanted vaccines, universal vaccines or alternative modes of delivery such as intradermal (ID) or mucosal administration.Citation22 The introduction of the MF59TM adjuvant proved to be a particularly effective solution to overcome the decrease in immunogenicity and effectiveness observed when a drifted strain circulate. A number of studies have demonstrated that the use of MF59TM-adjuvanted influenza vaccine enhances the immune response inducing higher seroprotection rates especially in elderly who are more susceptible to influenza infection.Citation23,Citation24 Moreover MF59TM-adjuvanted influenza vaccines have also shown to elicit cross-protection against strains not included in vaccine composition and have the potential to confer protection against pandemic strains.Citation25,Citation26,Citation27 Recently, in 2011–2012 influenza season, a novel ID vaccine containing 15ug per strains administered with a microinjection device, has been approved for human use in Europe and was recommended for older than 60. Data about immunogenicity have shown that ID vaccine can elicit a stronger immune response against the vaccine strains respect with that induced by split/subunit intramuscular (IM) vaccines.Citation28 Interesting data on the ability of ID vaccine to induce a broader immune response are now available and will be described in the present review.
New Potential Influenza Vaccine Options
In order to overcome the well-documented difficulties of “traditional” plain influenza vaccines to elicit a stronger immune response and a broader protection against heterogeneous variants, in recent years several solutions have been tested, including (1) the use of high-dose vaccines, containing more than 15 µg of viral haemagglutinin, (2) the use of live attenuated influenza vaccines, (3) the DNA vaccines, (4) the recombinant vaccines, presenting the main vaccine antigen in insect cells by a baculovirus, alphavirus, or cell-culture, (5) the use of alternative antigens such as the external domain of the matrix (M2) protein and the nucleoprotein (NP) and (6) the use of universal vaccines. Immunogenicity of most of these new approaches has been only tested against vaccine strains, while a lack of data exists about the cross-protection against drifted viruses offered by the new influenza vaccines.
Data from clinical trials demonstrated that high-dose influenza vaccines elicited a stronger immune response compared with conventional vaccines, especially in population with weaker immune defenses such as persons aged 65 y or older, but no data exists about the ability of high-dose vaccines to elicit a broader immune response against drifted strains.
Experiences have been collected in several years after the introduction of the live attenuated influenza vaccine (LAIV) on the immunogenicity of the product. In animal models, the induction of heterosubtypic immunity was demonstrated after the intranasal administration of live attenuated influenza virus.Citation29-Citation31
Although the availability on the European and US markets from 2010 and 2003, respectively, evaluations of cross-protection provided by LAIV in humans have been only performed in two studies, involving healthy young adults and children.
Ohmit et al. reported that LAIV prevented laboratory confirmed influenza illnesses less efficaciously than inactivated influenza vaccine, in a season in which most circulating viruses were dissimilar to the vaccine strains, in a population of more than 1200 healthy adults.Citation32 Belshe et al. compared LAIV to inactivated influenza vaccine in a randomized controlled multicenter study, demonstrating the superior efficacy of LAIV against both vaccine and drifted stains in the prevention of cultured-confirmed influenza infections.Citation33
New promising approaches to the rapid manufacturing of influenza vaccines providing a strong, long lasting and extensive immune response are offered by DNA and recombinant vaccines.
A number of influenza genes has been assessed as potential DNA vaccine candidates, such as HA, NA, matrix protein (M1), nucleoprotein (NP) or nonstructural protein (NS1).
Vaccine with purified DNA presents several advantages: for example, (1) it obviates the requirement for large-scale production of live, potentially pathogenic influenza viruses, as pandemic strains, and avian viruses which are too contagious to be propagated in eggs, (2) it can be produced, avoiding the substantial contamination hazard due to selected strains with low adaptive capacity to eggs, and removing the need for preservatives containing mercury, (3) it could provide a more rapid immune response to a pandemic threat.Citation34,Citation35 In particular, the DNA vaccine manufacturing process could shorten the period between the recommendations of vaccine strains and the seasonal influenza epidemics, potentially avoiding the occurrence of antigenic mismatch.
The scientific literature accounts that DNA vaccine induces protection in several species, enhances efficacy against both homologous and heterologous challenge -protection will be maximized when the vaccine and challenge viruses are evolutionarily close-, in particular it is clear, from animal studies, that DNA immunization with the NP of influenza A provides heterotypic immunity between different subtypes of influenza A in mice.Citation35-Citation39 In addition, in ferrets immunized with particle-mediated epidermal delivery (PMED) of single HA influenza DNA vaccine, cross-reactive antibodies with drifted viruses have been observed. Some studies demonstrate that matching NA and HA genes in an influenza DNA vaccine can broaden immune responses, conferring better protection against drifted variants than an HA DNA vaccine alone.
To enhance the DNA vaccine effectiveness, in the past 20 y several approaches have been explored, such as codon optimization (H-2Kd-restricted epitope of listeriolisyn 0), targeting technologies, alternative promoters (CMV, SV40), insertion of antigen subcellular targeting systems, plasmid backbone refinements and genetic adjuvants (AdvaxTM, a polysaccharide nanoparticle; CpG oligo; cytokines and chemokines expressed from plasmid DNA; MHC CIITA, regulator of MHC class II expression co-administrated with HPV16 E6 DNA and CIITA DNA; RIG-I, retinoic acid- inducible gene I; MAVS, mitochodrial protein forming prion-like aggregate).Citation40-Citation44
Since the influenza viruses mutate frequently, the virus strains for new vaccine production require continuous manufacturing update, in order to be antigenically well-matched to the circulating strains. For this reason, a vaccine shaped on invariant regions of the virus, providing broadly cross-reactive protection could represent a long-sought goal, particularly for emerging pandemics. Thanks to scientific research developed in recent years, new promises emerged on the world scene, such as the use of universal vaccines that represent good candidates to achieve this result. Data presented by Jimenez et al. showed, in mice, that highly conserved influenza genes NP and M2 inserted in a plasmid DNA vaccine and their combination increased protection against significant viral challenge.Citation45
Matrix protein 2 (M2) represents a possible candidate for universal vaccine, being highly conserved in all human influenza A strains. Although it could be a potential good vaccine target, several study demonstrated its poor antigenicity and immunogenicity, tied to its low molecular-weight (24 amino acids).Citation46 A study conducted by Hikono et al., reported that the recombinant containing an M2 gene derived from an H5N1 avian influenza virus could induce a cross-reactive antibody response to M2e in pigs, furthermore evaluated the protective efficacy of this vaccine in a mouse model after challenge with a heterologous H3N2 influenza virus.Citation47
Recently, Wei et al. reported an high production of neutralizing influenza antibodies directed against the conserved stem region of HA in mice and ferrets primed with a plasmid DNA encoding H1N1 HA vaccine and boosted with seasonal vaccine or with replication-defective adenovirus 5 (rAd5) encoding HA.
Moreover, this approach conferred cross protection against H1N1 drifted strains in non-human primates providing the basis for the future development of an universal vaccine for human.Citation48
The literature shows a low attention for universal influenza B vaccine because an highly conserved domain of M2 protein does not exist. About it a preclinical animal model conducted by Bianchi et al. demonstrated that using HA0 precursor of the viral HA, epitope widely conserved, as vaccine antigen, high immunogenicity and reduction in viral replications in the lower respiratory tract can be obtained.Citation49
Alternative strategies demonstrating to improve immune response against influenza drifted strains are represented by adjuvanted vaccines and ID vaccines.
Data about the ability of these products to enhance antibody response and improve vaccine effectiveness are reported in several study discussed in the following paragraphs.
MF59TM-Adjuvanted Vaccines
Vaccines with the oil-in water emulsion adjuvant called MF59™ certainly represented a revolution in the field of influenza vaccine when it appeared in 1997.Citation50
The MF59™ adjuvanted vaccine consists of influenza antigens and oil-in-water adjuvant emulsion composed of squalene (5% v/v), polysorbate 80 (TweenTM 80, 0.5% v/v) and sorbitan trioleate (SpanTM 85, 05% v/v), transformed into small uniform droplets after emulsified in a microfluidizer under high pressure conditions.Citation51
As previously pointed out, squalene is a biodegradable and biocompatible compound; in humans, it is precursor of cholesterol and play an important role in the synthesis of steroid hormones and Vitamin D; moreover, it has been demonstrated that the administration of MF59TM-adjuvanted subunit influenza vaccines did not elicit a specific humoral response against squalene.Citation52-Citation54
In 1997, the first MF59™ adjuvanted vaccine has been licensed for human use and registered in Europe for use in adults ≥ 65 y old for seasonal influenza vaccine and furthermore, consistent preclinical, clinical and post-marketing experience on the use of MF59TM adjuvanted pandemic vaccine has been accumulated in the last decade both against avian A(H5N1) and A(H1N1)pdm 09.
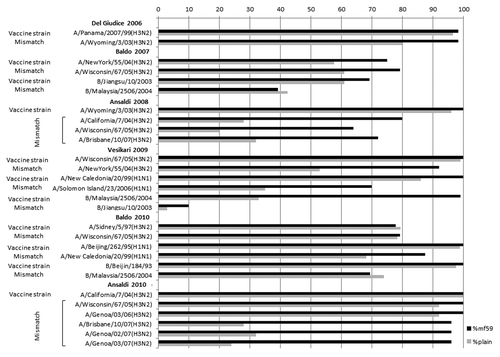
Numerous clinical studies, involving elderly, adults with chronic diseases, immunocompromised patients and, recently, healthy children, demonstrated the advantages offered by MF59™ adjuvanted seasonal vaccine in terms of immunogenicity, compared with conventional not adjuvanted influenza vaccines ().Citation25,Citation51,Citation55-Citation59
Figure 1. Seroprotection rates (%) determined using HI assays after vaccination with MF59-adjuvanted and “plain” vaccines, according to viral strain.
Even though the safety and tolerability of the MF59™-adjuvanted subunit influenza vaccines was repeatedly put on the table due to the “mass mediatic” pressure for the presence of squalene in the vaccine composition, safety profile is acceptable and serious AEs occur with expected frequencies.Citation60
More than 50,000 individuals, including elderly, adults and children, were enrolled in several phase I-IV clinical trials that demonstrated no significant trend for increased reactogenicity; tolerability of MF59TM-adjuvanted subunit influenza vaccines was compared with conventional not-adjuvanted subunit and split vaccines and both local and systemic reactions were taken as mild and transient solving naturally.Citation25,Citation59,Citation61,Citation62
The cross-reactivity against drifted influenza virus strains represents a demonstrated property of MF59™-adjuvanted subunit influenza vaccines, compared with seasonal formulations subunit or split currently available on the market. Recently Khurana et al. reported an improved expansion of protective antibody enhanced by inactivated influenza A(H5N1) vaccine, adjuvanted with MF59™.Citation63
Our research group consistently performed clinical studies demonstrating the higher seroprotection rates offered by MF59™-adjuvanted subunit influenza vaccines against drifted strains not included in the vaccine, than that achieved with not-adjuvanted subunit and split vaccines.
In particular, between 2004 and 2008, we evaluated the performance of MF59™-adjuvanted subunit influenza vaccine against drifted A(H3N2) variants, representing vaccine composition changes for A(H3N2) during the last decade. The studies involved healthy elderly subjects randomly assigned to receive either a single dose of MF59TM-adjuvanted subunit influenza vaccine or a not adjuvanted subunit influenza vaccine.
As shown in during the last decade different studies demonstrated the higher immunogenicity of MF59™-adjuvanted vaccines against both circulating and vaccine strains.
Our results are consistent with other findings reported by other authors during the last years.Citation17,Citation60,Citation64-Citation67
The advantage offered by MF59™, in terms of higher immunogenicity, was seen against viruses showing antigenic and molecular pattern that are indistinguishable from the vaccine strain, but it became even more evident when the antigenic and molecular distance between vaccine and circulating strains increased.
MF59™ emerges as a valid approach to adequately respond to the pandemic threat, thanks to its demonstrated abilities to elicit a strong and persistent immunological memory, to stimulate H5N1 cross-clade neutralizing antibodies and to promote cell-mediated immune responses that can be boosted at least 6 y following priming with a pre-pandemic strain.Citation68 It has been demonstrated that subjects who initially received a MF59™-adjuvanted pre-pandemic vaccine developed higher cross-reactive immune responses, compared with the non-adjuvanted vaccine. A strategy that involves a priming with MF59™-adjuvanted H5 antigen would result in a long-lasting immune memory that can be rapidly mobilized by a booster dose of a distinct H5 vaccine, in order to provide broad heterologous cross-protection.Citation26,Citation27,Citation69,Citation70
Despite the excellent results obtained evaluating the immunogenicity, we have only few data demonstrating the improved effectiveness for influenza prevention and its complications provided by MF59™-adjuvanted vaccine in elderly. Recently, Mannino et al., in a prospective, observational, population-based cohort study, throughout 3 vaccination seasons (2006–2007, 2007–2008 and 2008–2009), demonstrated that vaccination with MF59™-adjuvanted trivalent inactivated vaccine reduced the risk of hospitalization for influenza or pneumonia (without positive laboratory confirmation of influenza virus) in the elderly, during the peak of influenza season by 25% relative to vaccination with plain trivalent inactivated vaccine.Citation71 The epidemiological picture observed during the study period was characterized by both good and partial mismatch, although Mannino et al. did not evaluate the effectiveness according to the pattern of circulating viruses.
AS03-Adjuvanted Vaccines
AS03 is a novel adjuvant system consisting of a 10% (by volume) oil-in-water based emulsion and containing 5% dl-α-tocopherol and 95% squalene, and the aqueous phase with 2% of the non-ionic detergent polysorbate 80 (TweenTM 80). This emulsion-based adjuvant system has been used for the development of a candidate A(H5N1) pre-pandemic influenza vaccine containing 3.75 µg HA of the strain A/Vietnam/1194/2004 NIBRG-14, recommended as a prototype pandemic influenza vaccine strain.Citation72
AS03 adjuvant has been recently adopted in the licensed formulation of a A(H1N1)pdm 09 vaccine, using a 3.75 µg antigen dosage.Citation73 Different studies showed a cross-reactive, persistent antibody response against heterologous viral strains elicited by pre-pandemic vaccine adjuvanted with AS03 system,Citation74-Citation78 but a complete evaluation of this formulation is required in young children, elderly and individuals with chronic disease.
Virosomal Vaccines
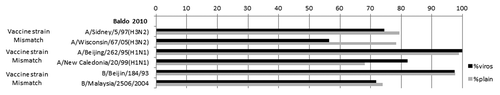
Virosomal-adjuvanted influenza vaccines were introduced into the European market in 1997 and licensed for all subjects aged 6 mo and older; more than 41 million doses were sold and the safety and systemic tolerability profiles were excellent.Citation79 Virosome adjuvant system consists of an influenza virus envelope devoid of inner core and genetic information, with the influenza virus surface antigens neuraminidase and hemagglutinin integrated into phosphatidylcholine bilayer liposomes.Citation80 Virosomal influenza vaccines demonstrated to induce antibody titers comparable to the not-adjuvanted, IM, split/subunit vaccines, against vaccine homologous strains. Only one study evaluated the performances of virosomal influenza vaccine against circulating viruses that did not have a good match with vaccine strains, due to antigenic drift. Baldo et al. assessed the immunogenicity of three inactivated influenza vaccines, a MF59™-adjuvanted subunit vaccine, a virosomal vaccine and a split vaccine, against homologous and heterologous strains of elderly nursing home residents with chronic underlying conditions. Results showed that similar seroprotection rates against the B drifted strain were induced by the three vaccine groups, but virosomal vaccine elicited lower seroprotection rates against both A strains, as compared with MF59™-adjuvanted vaccine.Citation81 ()
Intradermal Vaccines
Adjuvants remain one of the most widely used strategies to enhance the immune response of influenza vaccines, but alternative routes of administration, such as ID, have been deeply studied, in order to meet the need for more immunogenic and effective vaccines.
Vaccination via ID route involves the administration of the antigen into the dermal layer of the skin. Because of the high concentration of specialized immune cells in this skin layer and their ability to effectively stimulate an immune response, ID vaccination provides direct and efficient access to the immune system.Citation82,Citation83 The principal immune target of ID vaccination is the dermal population of specialized dendritic cells, expressing high levels of class II MHC and CD1 molecules, such as Langerhans cells and macrophages infiltrating dermis tissue, resident or recruited from circulating blood.
The availability of a microinjection system (BD Soluvia TM, Becton, Dickinson and Co.) allows the ID vaccine administration, combining simplicity, safety and ease of use and supplying direct and efficient access to the immune system.
Three ID vaccine formulations with two different antigen contents were marketed: IntanzaTM 9 mg and FluzoneTM ID, approved for adults 18 through 59 y in Europe and 18 through 64 y in the USA, respectively, and IntanzaTM 15 μg approved for elderly in Europe and Canada.Citation84,Citation85 In routine clinical practice the ID influenza vaccine showed a good safety and acceptance profile and an optimal compliance to its use was registered among vaccine prescribers.Citation86 During the last years the ID route of administration has been extensively studied in order to assess immunogenicity of different influenza vaccine formulations and in different population targets.
Several studies in the last decade compared ID formulation vs. IM vaccine, in terms of immunogenicity.
Holland et al. asserted a superior immunogenicity of a seasonal influenza vaccine administered by the ID route among healthy subjects aged ≥ 60 y when compared with a similar antigen dose of IM vaccine.Citation82
Arnou et al. in the registrative, randomized, controlled, open-label phase III trial confirmed the higher antibody response elicited by ID 15 μg vaccine respect to IM 15 μg vaccine in the elderly, fulfilling the European Medicine Agency (EMA) criteria.Citation87
In the registrative trial for FluzoneTM ID, a lower antigen dose (9 μg) of ID seasonal influenza vaccine administered using the same microinjection system elicited similar responses when compared with those observed after a 15 μg dose given IM among healthy subjects aged 18–64 y.Citation88
ID immunization may be also considered as a potential antigen-sparing approach for the prevention of influenza A(H5N1) infections. Some studies compared A(H5N1) influenza vaccine administrated by ID route and IM route. Authors reported that the ID vaccination is not inferior to IM, showing comparable post-vaccination antibody levels.Citation83,Citation90
The ID vaccination can offer a promising option to a broader antibody response when an antigenic drift occurs and the mismatch between vaccine and circulating strains may result in a decrease of protective antibody levels conferred by conventional vaccination.Citation18,Citation28,Citation91
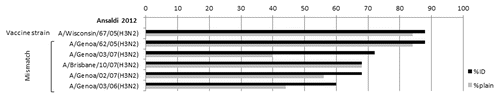
The ability of ID vaccination to arouse a more effective antibody response against circulating viruses that have a different antigenic pattern respect with vaccine strain has been first demonstrated by our research group. In this study, we compared the ability of an ID vaccine (IntanzaTM 15 ug) and a conventional IM inactivated vaccine, both containing the A/Wisconsin/67/05(H3N2) strain, to confer cross-protection against heterologous H3N2 circulating viruses in adults 60 y and older during 2006–2007 influenza season. Post-vaccination HI titer, seroconversion and seroprotection rates were higher against vaccine and circulating heterologous viruses for the IntanzaTM ID vaccine, than the IM vaccine, but statistical difference emerged by using neutralization assays (). In fact, subjects immunized with IntanzaTM 15 μg vaccine showed post-immunization neutralizing antibody titers higher than those vaccinated with standard IM vaccine against five out of six virus strains tested.Citation28
Figure 3. Seroprotection rates (%) determined using HI assays after vaccination with intradermal and “plain” vaccines, according to viral strain.
Recently, our research group first compared the immunogenicity against vaccine strains and heterologous circulating A(H1N1)pdm09 viruses elicited by ID IntanzaTM 15 μg and a virosomal adjuvanted influenza vaccine (InflexalTMV), intramuscularly administered. Preliminary data showed that the immune response conferred by IntanzaTM 15 µg was superior to InflexalTMV when it was evaluated against circulating strains.
The superior cross-protection ability by IntanzaTM 15 µg respect with split influenza vaccine emerged by our experience, but more extensive studies are needed to compare the potential of ID influenza vaccine to elicit an antibody response against drifted variants respect with that of adjuvanted vaccines.
The higher immunogenicity profile against both homologous and heterologous strains, the excellent safety results and the good tolerability suggest that ID vaccination may be an appropriate strategy to deal with the annual antigenic drift of influenza viruses and the decrease of immune responses in elderly people.
Conclusions
The need for influenza vaccines that provide an enhanced profile of immunogenicity in older people and against drifted viruses led to the development and approval of vaccines with adjuvants, carriers and a higher antigen content and to the use of routes of administration other than IM.
Higher and broader antibody responses to drifted influenza viruses can be offered by MF59™-adjuvanted vaccines, making them a strong candidate for seasonal influenza vaccination programs in elderly and high risk populations.
Based on reported pre- and clinical experience, ID vaccines can be considered safe and immunogenic, being a valid alternative to MF59™-adjuvanted vaccines for the active immune-prevention of seasonal influenza, showing higher immune response than plain vaccines against vaccine strain and when the epidemiological picture is characterized by a wide and heterogeneous circulation of influenza variants.
Further studies are in progress to evaluate the potential of the virosomal vaccine to elicit a broader antibody response.
| Abbreviations: | ||
| AE | = | adverse events |
| CHMP | = | Committee for Medicinal Products for Human Use |
| GMT | = | geometric mean titer |
| HI | = | hemagglutination inhibition |
| ID | = | intradermal |
| IM | = | intramuscular |
| MFI | = | mean-fold increase |
| NT | = | neutralization |
| SAE | = | serious adverse event |
Disclosure of Potential Conflicts of Interest
F.A., G.I. and P.D. have previously participated at speaker’s bureaus and advisory board meetings sponsored by GSK, Novartis, Pfizer and Sanofi Pasteur and have received research funding as principal investigators or co-investigators from Crucell Berna, Novartis, GSK, Pfizer and Sanofi Pasteur. A.O., D.D.F., A.C., V.P., P.C. and M.C. have no conflict of interest. No other relationships/conditions/circumstances that present a potential conflict of interest exist.
References
- Stöhr K. Influenza--WHO cares. Lancet Infect Dis 2002; 2:517; http://dx.doi.org/10.1016/S1473-3099(02)00366-3; PMID: 12206966
- Cox NJ, Subbarao K. Global epidemiology of influenza: past and present. Annu Rev Med 2000; 51:407 - 21; http://dx.doi.org/10.1146/annurev.med.51.1.407; PMID: 10774473
- Both GW, Sleigh MJ, Cox NJ, Kendal AP. Antigenic drift in influenza virus H3 hemagglutinin from 1968 to 1980: multiple evolutionary pathways and sequential amino acid changes at key antigenic sites. J Virol 1983; 48:52 - 60; PMID: 6193288
- Caton AJ, Brownlee GG, Yewdell JW, Gerhard W. The antigenic structure of the influenza virus A/PR/8/34 hemagglutinin (H1 subtype). Cell 1982; 31:417 - 27; http://dx.doi.org/10.1016/0092-8674(82)90135-0; PMID: 6186384
- Cox NJ, Black RA, Kendal AP. Pathways of evolution of influenza A (H1N1) viruses from 1977 to 1986 as determined by oligonucleotide mapping and sequencing studies. J Gen Virol 1989; 70:299 - 313; http://dx.doi.org/10.1099/0022-1317-70-2-299; PMID: 2732691
- Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature 1981; 289:373 - 8; http://dx.doi.org/10.1038/289373a0; PMID: 6162101
- Wang ML, Skehel JJ, Wiley DC. Comparative analyses of the specificities of anti-influenza hemagglutinin antibodies in human sera. J Virol 1986; 57:124 - 8; PMID: 3941436
- Treanor J. Influenza vaccine--outmaneuvering antigenic shift and drift. N Engl J Med 2004; 350:218 - 20; http://dx.doi.org/10.1056/NEJMp038238; PMID: 14724300
- Koelle K, Cobey S, Grenfell B, Pascual M. Epochal evolution shapes the phylodynamics of interpandemic influenza A (H3N2) in humans. Science 2006; 314:1898 - 903; http://dx.doi.org/10.1126/science.1132745; PMID: 17185596
- Aymard M, Valette M, Lina B, Thouvenot D. Surveillance and impact of influenza in Europe. Groupe Régional d’Observation de la Grippe and European Influenza Surveillance Scheme. Vaccine 1999; 17:Suppl 1 S30 - 41; http://dx.doi.org/10.1016/S0264-410X(99)00103-6; PMID: 10471178
- Klimov A, Simonsen L, Fukuda K, Cox N. Surveillance and impact of influenza in the United States. Vaccine 1999; 17:Suppl 1 S42 - 6; http://dx.doi.org/10.1016/S0264-410X(99)00104-8; PMID: 10471179
- Ansaldi F, Icardi G, Gasparini R, Campello C, Puzelli S, Bella A, et al. New A/H3N2 influenza variant: a small genetic evolution but a heavy burden on the Italian population during the 2004-2005 season. J Clin Microbiol 2005; 43:3027 - 9; http://dx.doi.org/10.1128/JCM.43.6.3027-3029.2005; PMID: 15956452
- Paget WJ, Meerhoff TJ, Meijer A, EISS. Epidemiological and virological assessment of influenza activity in Europe during the 2003-2004 season. Euro Surveill 2005; 10:107 - 11; PMID: 15879646
- Skowronski DM, Janjua NZ, De Serres G, Winter AL, Dickinson JA, Gardy JL, et al. A sentinel platform to evaluate influenza vaccine effectiveness and new variant circulation, Canada 2010-2011 season. Clin Infect Dis 2012; 55:332 - 42; http://dx.doi.org/10.1093/cid/cis431; PMID: 22539661
- de Jong JC, Beyer WE, Palache AM, Rimmelzwaan GF, Osterhaus AD. Mismatch between the 1997/1998 influenza vaccine and the major epidemic A(H3N2) virus strain as the cause of an inadequate vaccine-induced antibody response to this strain in the elderly. J Med Virol 2000; 61:94 - 9; http://dx.doi.org/10.1002/(SICI)1096-9071(200005)61:1<94::AID-JMV15>3.0.CO;2-C; PMID: 10745239
- Ansaldi F, Bacilieri S, Banfi F, Durando P, Sticchi L, Icardi G, et al. Neutralizing and hemagglutination-inhibiting activities of antibodies elicited by the 2004-2005 influenza vaccine against drifted viruses. Clin Vaccine Immunol 2006; 13:162 - 4; http://dx.doi.org/10.1128/CVI.13.1.162-164.2006; PMID: 16426017
- Del Giudice G, Hilbert AK, Bugarini R, Minutello A, Popova O, Toneatto D, et al. An MF59-adjuvanted inactivated influenza vaccine containing A/Panama/1999 (H3N2) induced broader serological protection against heterovariant influenza virus strain A/Fujian/2002 than a subunit and a split influenza vaccine. Vaccine 2006; 24:3063 - 5; http://dx.doi.org/10.1016/j.vaccine.2006.01.015; PMID: 16464520
- Kojimahara N, Maeda A, Kase T, Yamaguchi N. Cross-reactivity of influenza A (H3N2) hemagglutination-inhibition antibodies induced by an inactivated influenza vaccine. Vaccine 2006; 24:5966 - 9; http://dx.doi.org/10.1016/j.vaccine.2006.05.009; PMID: 16777274
- Bridges CB, Thompson WW, Meltzer MI, Reeve GR, Talamonti WJ, Cox NJ, et al. Effectiveness and cost-benefit of influenza vaccination of healthy working adults: A randomized controlled trial. JAMA 2000; 284:1655 - 63; http://dx.doi.org/10.1001/jama.284.13.1655; PMID: 11015795
- Legrand J, Vergu E, Flahault A. Real-time monitoring of the influenza vaccine field effectiveness. Vaccine 2006; 24:6605 - 11; http://dx.doi.org/10.1016/j.vaccine.2006.05.063; PMID: 16806607
- Centers for Disease Control and Prevention (CDC). Preliminary assessment of the effectiveness of the 2003-04 inactivated influenza vaccine–Colorado, December 2003. WR Morb Mortal Wkly Rep 2004; 53:8 - 11
- Doherty PC, Kelso A. Toward a broadly protective influenza vaccine. J Clin Invest 2008; 118:3273 - 5; PMID: 18802488
- Auewarakul P, Kositanont U, Sornsathapornkul P, Tothong P, Kanyok R, Thongcharoen P. Antibody responses after dose-sparing intradermal influenza vaccination. Vaccine 2007; 25:659 - 63; http://dx.doi.org/10.1016/j.vaccine.2006.08.026; PMID: 17011678
- Squarcione S, Sgricia S, Biasio LR, Perinetti E. Comparison of the reactogenicity and immunogenicity of a split and a subunit-adjuvanted influenza vaccine in elderly subjects. Vaccine 2003; 21:1268 - 74; http://dx.doi.org/10.1016/S0264-410X(02)00401-2; PMID: 12559808
- Baldo V, Baldovin T, Floreani A, Carraro AM, Trivello R, Family Medicine Group of Pianiga. MF59-adjuvanted influenza vaccine confers superior immunogenicity in adult subjects (18-60 years of age) with chronic diseases who are at risk of post-influenza complications. Vaccine 2007; 25:3955 - 61; http://dx.doi.org/10.1016/j.vaccine.2007.02.045; PMID: 17383057
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937 - 43; http://dx.doi.org/10.1016/S0140-6736(00)05066-2; PMID: 11425416
- Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, Zambon MC, et al. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis 2005; 191:1210 - 5; http://dx.doi.org/10.1086/428948; PMID: 15776364
- Ansaldi F, Canepa P, Ceravolo A, Valle L, de Florentiis D, Oomen R, et al. Intanza(®) 15 mcg intradermal influenza vaccine elicits cross-reactive antibody responses against heterologous A(H3N2) influenza viruses. Vaccine 2012; 30:2908 - 13; http://dx.doi.org/10.1016/j.vaccine.2012.02.003; PMID: 22342501
- Straight TM, Ottolini MG, Prince GA, Eichelberger MC. Evidence of a cross-protective immune response to influenza A in the cotton rat model. Vaccine 2006; 24:6264 - 71; http://dx.doi.org/10.1016/j.vaccine.2006.05.092; PMID: 16860444
- Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, Osterhaus AD, et al. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 2007; 25:612 - 20; http://dx.doi.org/10.1016/j.vaccine.2006.08.036; PMID: 17005299
- Takada A, Matsushita S, Ninomiya A, Kawaoka Y, Kida H. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 2003; 21:3212 - 8; http://dx.doi.org/10.1016/S0264-410X(03)00234-2; PMID: 12804850
- Ohmit SE, Victor JC, Rotthoff JR, Teich ER, Truscon RK, Baum LL, et al. Prevention of antigenically drifted influenza by inactivated and live attenuated vaccines. N Engl J Med 2006; 355:2513 - 22; http://dx.doi.org/10.1056/NEJMoa061850; PMID: 17167134
- Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al, CAIV-T Comparative Efficacy Study Group. Live attenuated versus inactivated influenza vaccine in infants and young children. [Erratum in: N Engl J Med 2007; 356] [12] N Engl J Med 2007; 356:685 - 96; http://dx.doi.org/10.1056/NEJMoa065368; PMID: 17301299
- Drape RJ, Macklin MD, Barr LJ, Jones S, Haynes JR, Dean HJ. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 2006; 24:4475 - 81; http://dx.doi.org/10.1016/j.vaccine.2005.08.012; PMID: 16150518
- Webster RG. Potential advantages of DNA immunization for influenza epidemic and pandemic planning. Clin Infect Dis 1999; 28:225 - 9; http://dx.doi.org/10.1086/515123; PMID: 10064231
- Donnelly JJ, Friedman A, Ulmer JB, Liu MA. Further protection against antigenic drift of influenza virus in a ferret model by DNA vaccination. Vaccine 1997; 15:865 - 8; http://dx.doi.org/10.1016/S0264-410X(96)00268-X; PMID: 9234535
- Macklin MD, McCabe D, McGregor MW, Neumann V, Meyer T, Callan R, et al. Immunization of pigs with a particle-mediated DNA vaccine to influenza A virus protects against challenge with homologous virus. J Virol 1998; 72:1491 - 6; PMID: 9445052
- Justewicz DM, Morin MJ, Robinson HL, Webster RG. Antibody-forming cell response to virus challenge in mice immunized with DNA encoding the influenza virus hemagglutinin. J Virol 1995; 69:7712 - 7; PMID: 7494280
- Donnelly JJ, Friedman A, Martinez D, Montgomery DL, Shiver JW, Motzel SL, et al. Preclinical efficacy of a prototype DNA vaccine: enhanced protection against antigenic drift in influenza virus. Nat Med 1995; 1:583 - 7; http://dx.doi.org/10.1038/nm0695-583; PMID: 7585127
- Cristillo AD, Ferrari MG, Hudacik L, Lewis B, Galmin L, Bowen B, et al. Induction of mucosal and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol 2011; 92:128 - 40; http://dx.doi.org/10.1099/vir.0.023242-0; PMID: 21169215
- Kim D, Hoory T, Monie A, Ting JP, Hung CF, Wu TC. Enhancement of DNA vaccine potency through coadministration of CIITA DNA with DNA vaccines via gene gun. J Immunol 2008; 180:7019 - 27; PMID: 18453624
- Luke JM, Simon GG, Söderholm J, Errett JS, August JT, Gale M Jr., et al. Coexpressed RIG-I agonist enhances humoral immune response to influenza virus DNA vaccine. J Virol 2011; 85:1370 - 83; http://dx.doi.org/10.1128/JVI.01250-10; PMID: 21106745
- Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 2011; 146:448 - 61; http://dx.doi.org/10.1016/j.cell.2011.06.041; PMID: 21782231
- Li L, Saade F, Petrovsky N. The future of human DNA vaccines. J Biotechnol 2012; 162:171 - 82; http://dx.doi.org/10.1016/j.jbiotec.2012.08.012; PMID: 22981627
- Jimenez GS, Planchon R, Wei Q, Rusalov D, Geall A, Enas J, et al. Vaxfectin-formulated influenza DNA vaccines encoding NP and M2 viral proteins protect mice against lethal viral challenge. Hum Vaccin 2007; 3:157 - 64; http://dx.doi.org/10.4161/hv.3.5.4175; PMID: 17637571
- Liu X, Guo J, Han S, Yao L, Chen A, Yang Q, et al. Enhanced immune response induced by a potential influenza A vaccine based on branched M2e polypeptides linked to tuftsin. Vaccine 2012; 30:6527 - 33; http://dx.doi.org/10.1016/j.vaccine.2012.08.054; PMID: 22959982
- Hikono H, Miyazaki A, Mase M, Inoue M, Hasegawa M, Saito T. Induction of a cross-reactive antibody response to influenza virus M2 antigen in pigs by using a Sendai virus vector. Vet Immunol Immunopathol 2012; 146:92 - 6; http://dx.doi.org/10.1016/j.vetimm.2012.01.017; PMID: 22336036
- Wei CJ, Boyington JC, McTamney PM, Kong WP, Pearce MB, Xu L, et al. Induction of broadly neutralizing H1N1 influenza antibodies by vaccination. Science 2010; 329:1060 - 4; http://dx.doi.org/10.1126/science.1192517; PMID: 20647428
- Bianchi E, Liang X, Ingallinella P, Finotto M, Chastain MA, Fan J, et al. Universal influenza B vaccine based on the maturational cleavage site of the hemagglutinin precursor. J Virol 2005; 79:7380 - 8; http://dx.doi.org/10.1128/JVI.79.12.7380-7388.2005; PMID: 15919893
- Bruzzone B, Crovari P, Durando P, Setti M. [Influenza vaccination: advantages of new generation carriers]. Ann Ig 2002; 14:Suppl 3 11 - 8; PMID: 12389406
- Podda A. The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. Vaccine 2001; 19:2673 - 80; http://dx.doi.org/10.1016/S0264-410X(00)00499-0; PMID: 11257408
- Podda A, Del Giudice G. MF59-adjuvanted vaccines: increased immunogenicity with an optimal safety profile. Expert Rev Vaccines 2003; 2:197 - 203; http://dx.doi.org/10.1586/14760584.2.2.197; PMID: 12899571
- Bloch KE. Sterol structure and membrane function. CRC Crit Rev Biochem 1983; 14:47 - 92; http://dx.doi.org/10.3109/10409238309102790; PMID: 6340956
- Del Giudice G, Fragapane E, Bugarini R, Hora M, Henriksson T, Palla E, et al. Vaccines with the MF59 adjuvant do not stimulate antibody responses against squalene. Clin Vaccine Immunol 2006; 13:1010 - 3; http://dx.doi.org/10.1128/CVI.00191-06; PMID: 16960112
- Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine 2008; 26:3209 - 22; http://dx.doi.org/10.1016/j.vaccine.2008.03.093; PMID: 18462843
- Durando P, Fenoglio D, Boschini A, Ansaldi F, Icardi G, Sticchi L, et al. Safety and immunogenicity of two influenza virus subunit vaccines, with or without MF59 adjuvant, administered to human immunodeficiency virus type 1-seropositive and -seronegative adults. Clin Vaccine Immunol 2008; 15:253 - 9; http://dx.doi.org/10.1128/CVI.00316-07; PMID: 18003811
- Baldo V, Menegon T, Bonello C, Floreani A, Trivello R, Collaborative Group. Comparison of three different influenza vaccines in institutionalised elderly. Vaccine 2001; 19:3472 - 5; http://dx.doi.org/10.1016/S0264-410X(01)00060-3; PMID: 11348713
- Vesikari T, Groth N, Karvonen A, Borkowski A, Pellegrini M. MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. Vaccine 2009; 27:6291 - 5; http://dx.doi.org/10.1016/j.vaccine.2009.02.004; PMID: 19840662
- Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O’Hagan DT, et al. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatr Infect Dis J 2009; 28:563 - 71; http://dx.doi.org/10.1097/INF.0b013e31819d6394; PMID: 19561422
- Durando P, Icardi G, Ansaldi F. MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin Biol Ther 2010; 10:639 - 51; http://dx.doi.org/10.1517/14712591003724662; PMID: 20218923
- Novartis. Vaccine and diagnostics. Data on file; 2007.
- Frey S, Poland G, Percell S, Podda A. Comparison of the safety, tolerability, and immunogenicity of a MF59-adjuvanted influenza vaccine and a non-adjuvanted influenza vaccine in non-elderly adults. Vaccine 2003; 21:4234 - 7; http://dx.doi.org/10.1016/S0264-410X(03)00456-0; PMID: 14505903
- Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med 2010; 2:ra5; http://dx.doi.org/10.1126/scitranslmed.3000624; PMID: 20371470
- Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133 - 8; http://dx.doi.org/10.1016/j.virusres.2004.02.025; PMID: 15163501
- Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, et al. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine 2008; 26:1525 - 9; http://dx.doi.org/10.1016/j.vaccine.2008.01.019; PMID: 18294741
- Ansaldi F, Canepa P, Parodi V, Bacilieri S, Orsi A, Compagnino F, et al. Adjuvanted seasonal influenza vaccines and perpetual viral metamorphosis: the importance of cross-protection. Vaccine 2009; 27:3345 - 8; http://dx.doi.org/10.1016/j.vaccine.2009.01.081; PMID: 19200846
- Baldo V, Baldovin T, Floreani A, Fragapane E, Trivello R, Family Medicine Group. Response of influenza vaccines against heterovariant influenza virus strains in adults with chronic diseases. J Clin Immunol 2007; 27:542 - 7; http://dx.doi.org/10.1007/s10875-007-9100-4; PMID: 17514499
- Bridges CB, Lim W, Hu-Primmer J, Sims L, Fukuda K, Mak KH, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997-1998. J Infect Dis 2002; 185:1005 - 10; http://dx.doi.org/10.1086/340044; PMID: 11930308
- Brauer V, Laghi-Pasini F, Capecchi PL, et al. Priming for pandemic influenza: Antigen-sparing MF59-adjuvanted A / H5N1 vaccine induces immunological memory and shows cross-reactive potential in adults including the elderly. 13th International Congress on Infectious Diseases 2008, Kuala Lumpur, Malaysia, 19–22 June 2008.
- Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 2003; 21:1687 - 93; http://dx.doi.org/10.1016/S0264-410X(02)00632-1; PMID: 12639491
- Mannino S, Villa M, Apolone G, Weiss NS, Groth N, Aquino I, et al. Effectiveness of adjuvanted influenza vaccination in elderly subjects in northern Italy. Am J Epidemiol 2012; 176:527 - 33; http://dx.doi.org/10.1093/aje/kws313; PMID: 22940713
- Chu DW, Hwang SJ, Lim FS, Oh HM, Thongcharoen P, Yang PC, et al, H5N1 Flu Study Group for Hong Kong, Singapore, Taiwan and Thailand. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine 2009; 27:7428 - 35; http://dx.doi.org/10.1016/j.vaccine.2009.07.102; PMID: 19683087
- Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine 2010; 28:1740 - 5; http://dx.doi.org/10.1016/j.vaccine.2009.12.014; PMID: 20034605
- Leroux-Roels I, Roman F, Forgus S, Maes C, De Boever F, Dramé M, et al. Priming with AS03 A-adjuvanted H5N1 influenza vaccine improves the kinetics, magnitude and durability of the immune response after a heterologous booster vaccination: an open non-randomised extension of a double-blind randomised primary study. Vaccine 2010; 28:849 - 57; http://dx.doi.org/10.1016/j.vaccine.2009.10.017; PMID: 19835828
- Leroux-Roels I, Bernhard R, Gérard P, Dramé M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One 2008; 3:e1665; http://dx.doi.org/10.1371/journal.pone.0001665; PMID: 18301743
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Dramé M, Clement F, Hons E, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580 - 9; http://dx.doi.org/10.1016/S0140-6736(07)61297-5; PMID: 17707753
- Schwarz TF, Horacek T, Knuf M, Damman HG, Roman F, Dramé M, et al. Single dose vaccination with AS03-adjuvanted H5N1 vaccines in a randomized trial induces strong and broad immune responsiveness to booster vaccination in adults. Vaccine 2009; 27:6284 - 90; http://dx.doi.org/10.1016/j.vaccine.2009.01.040; PMID: 19856521
- Rümke HC, Bayas JM, de Juanes JR, Caso C, Richardus JH, Campins M, et al. Safety and reactogenicity profile of an adjuvanted H5N1 pandemic candidate vaccine in adults within a phase III safety trial. Vaccine 2008; 26:2378 - 88; http://dx.doi.org/10.1016/j.vaccine.2008.02.068; PMID: 18407382
- Herzog C, Hartmann K, Künzi V, Kürsteiner O, Mischler R, Lazar H, et al. Eleven years of Inflexal V-a virosomal adjuvanted influenza vaccine. Vaccine 2009; 27:4381 - 7; http://dx.doi.org/10.1016/j.vaccine.2009.05.029; PMID: 19450630
- Mischler R, Metcalfe IC. Inflexal V a trivalent virosome subunit influenza vaccine: production. Vaccine 2002; 20:Suppl 5 B17 - 23; http://dx.doi.org/10.1016/S0264-410X(02)00512-1; PMID: 12477413
- Baldo V, Baldovin T, Pellegrini M, Angiolelli G, Majori S, Floreani A, et al. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol 2010; 2010:517198; http://dx.doi.org/10.1155/2010/517198; PMID: 20369059
- Holland D, Booy R, De Looze F, Eizenberg P, McDonald J, Karrasch J, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis 2008; 198:650 - 8; http://dx.doi.org/10.1086/590434; PMID: 18652550
- Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines 2008; 7:1201 - 14; http://dx.doi.org/10.1586/14760584.7.8.1201; PMID: 18844594
- Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia™ licensed micro injection system. Hum Vaccin Immunother 2012; 8:67 - 75; PMID: 22293531
- Ansaldi F, de Florentiis D, Durando P, Icardi G. Fluzone(®) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines 2012; 11:17 - 25; http://dx.doi.org/10.1586/erv.11.154; PMID: 22149703
- Durando P, Alicino C, Alberti M, Sticchi L, Turello V, Marensi L, et al, Italian Intradermal Influenza Vaccine Working Group. Acceptance and safety of the intradermal influenza vaccine among the elderly in Italy: an on-field national study. Adv Ther 2012; 29:312 - 26; http://dx.doi.org/10.1007/s12325-012-0012-1; PMID: 22529024
- Arnou R, Eavis P, Pardo JR, Ambrozaitis A, Kazek MP, Weber F. Immunogenicity, large scale safety and lot consistency of an intradermal influenza vaccine in adults aged 18-60 years: Randomized, controlled, phase III trial. Hum Vaccin 2010; 6:346 - 54; http://dx.doi.org/10.4161/hv.6.4.10961; PMID: 20372053
- Frenck RW Jr., Belshe R, Brady RC, Winokur PL, Campbell JD, Treanor J, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone®) administered by intradermal and intramuscular route in healthy adults. Vaccine 2011; 29:5666 - 74; http://dx.doi.org/10.1016/j.vaccine.2011.06.010; PMID: 21699951
- Ledgerwood JE, Hu Z, Gordon IJ, Yamshchikov G, Enama ME, Plummer S, et al, VRC 304 and VRC 305 Study Teams. Influenza virus h5 DNA vaccination is immunogenic by intramuscular and intradermal routes in humans. Clin Vaccine Immunol 2012; 19:1792 - 7; http://dx.doi.org/10.1128/CVI.05663-11; PMID: 22956656
- Patel SM, Atmar RL, El Sahly HM, Guo K, Hill H, Keitel WA. Direct comparison of an inactivated subvirion influenza A virus subtype H5N1 vaccine administered by the intradermal and intramuscular routes. J Infect Dis 2012; 206:1069 - 77; http://dx.doi.org/10.1093/infdis/jis402; PMID: 22891287
- Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, et al. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine 2010; 28:4123 - 9; http://dx.doi.org/10.1016/j.vaccine.2010.04.030; PMID: 20433807