Abstract
Owing to their professional antigen-presenting capacity and unique potential to induce tumor antigen-specific T cell immunity, dendritic cells (DCs) have attracted much interest over the past decades for therapeutic vaccination against cancer. Clinical trials have shown that the use of tumor antigen-loaded DCs in cancer patients is safe and that it has the potential to induce anti-tumor immunity which, in some cases, culminates in striking clinical responses. Unfortunately, in a considerable number of patients, DC vaccination is unable to mount effective anti-tumor immune responses and, if it does so, the resultant immunity is often insufficient to translate into tangible clinical benefit. This underscores the necessity to re-design and optimize the current procedures for DC vaccine manufacturing. A new generation of DC vaccines with improved potency has now become available for clinical use as a result of extensive pre-clinical research. One of the promising next-generation DC vaccine candidates are interleukin (IL)-15-differentiated DCs. In this commentary, we will compile the research data that have been obtained by our group and other groups with these so-called IL-15 DCs and summarize the evidence supporting the implementation of IL-15 DCs in DC-based cancer vaccination regimens.
Introduction
Although the idea of vaccinating against cancer has been around for more than two centuries,Citation1 a better understanding of the complex intricacies of the immune system and its role in tumor control has rekindled interest in recent decades in the development of vaccines to treat cancer.Citation2 A major area of investigation in the field of therapeutic cancer vaccination revolves around the use of dendritic cells (DCs).Citation3,Citation4 The concept underlying the use of these specialized immune cells for anti-cancer vaccination is based on two now well-established facts: (1) malignant cells express tumor antigens allowing their recognition and killing by tumor antigen-specific CD8+ cytotoxic T lymphocytes (CTLs); and (2) DCs, whose primary function is the processing and presentation of antigens, are instrumental in the induction of such tumor antigen-specific CTL responses.Citation4
Dendritic cell-based cancer vaccines have moved into human clinical trials in the mid-1990s.Citation5 A large number of clinical trials have been performed since then, most of which have shown that the use of DCs for anti-cancer vaccination is feasible and well tolerated.Citation3 Several of these studies have also provided “proof-of-concept” evidence that DC vaccination can induce tumor antigen-specific CTL immunity in cancer patients.Citation4 Although in some cases spectacular clinical responses have been observed, the overall clinical benefit of DC vaccination is rather limited, indicating that further improvements are needed to enhance the potency and efficacy of the currently used DC vaccine preparations.Citation6,Citation7
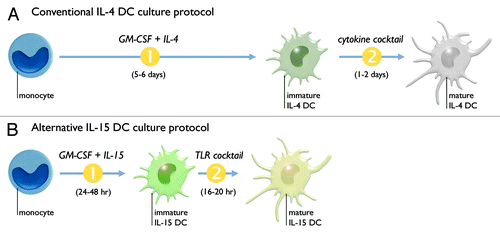
The most widely adopted method for generating DCs for clinical use involves a one-week, two-step in vitro culture procedure. In the first step, autologous peripheral blood monocytes are allowed to differentiate into immature DCs in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin (IL)-4 ().Citation7,Citation8 The second step comprises the last 2 days of the culture and involves the addition of stimuli to induce DC maturation/activation. A mixture of the pro-inflammatory cytokines IL-1β, IL-6, tumor necrosis factor (TNF)-α and prostaglandin E2 (PGE2) is still considered as the gold-standard “cocktail” for inducing monocyte-derived DC maturation ().Citation7,Citation8 It is now well-established that these so-called “IL-4 DCs” result in suboptimal immune activation, which could, at least in part, explain the rather modest clinical efficacy of DC-based anti-cancer vaccination.Citation6 This has led many research teams, including ours, to embark on a search for more potent immunostimulatory DCs. In this regard, weCitation9 and othersCitation10-Citation17 have previously described that IL-4 can be replaced by the far more immunstimulatory cytokine IL-15 during DC differentiation (). The resultant DCs, hereafter called “IL-15 DCs”, display a unique DC immunophenotype characterized by the expression of typical myeloid DC lineage markers (e.g., CD1c, CD11c) in conjunction with the Langerhans cell (LC) marker CD207/Langerin and the prototypic natural killer (NK) cell marker CD56.Citation9,Citation10,Citation18 From a functional point of view, IL-15 DCs are endowed with a range of immune-activating properties, making these cells particularly attractive candidates for DC vaccination. In this paper, we will compile the research data that have been obtained by our group and other groups with this IL-15 DC vaccine system and discuss why IL-15 DCs hold the potential to improve the efficacy of DC-based anti-cancer vaccine strategies.
Figure 1. Most clinical DC vaccine studies to date have employed “IL-4 DCs.” These DCs are generated by in vitro differentiation (1) of monocytes for 5–6 d in the presence of granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). These immature IL-4 DCs then undergo maturation (2) for 1–2 d by adding a cocktail of pro-inflammatory cytokines (A). We have previously designed an alternative protocol for the generation of monocyte-derived DCs, termed “IL-15 DCs.” These IL-15 DCs are generated by rapid (24–48 h) differentiation of monocytes using GM-CSF and interleukin-15 (IL-15), followed by maturation induction for 16–20 h through triggering of the Toll-like receptor (TLR)-7/8 signaling pathway (B).
IL-15 DCs Qualify as Immunostimulatory DCs
To be considered as suitable candidates for DC-based anti-cancer vaccination, DCs must meet several requirements: (1) exhibit a mature/activated DC phenotype with high expression of T cell co-stimulatory molecules; (2) possess lymph node-directed migratory capacity; (3) display a cytokine profile that favors a CD4+ T helper type 1 (TH1) immune response; (4) possess strong antigen-presenting capacity.Citation3,Citation9 We have previously devised a shortened and optimized DC culture protocol that allows for the generation of IL-15 DCs that fulfill all of the above requirements.Citation9 This protocol involves the rapid (24–48 h) culture of immature monocyte-derived IL-15 DCs, followed by the overnight (16–20 h) induction of DC maturation through the Toll-like receptor (TLR) signaling pathway.Citation9 Remarkably, we observed that IL-15 DCs are rather unresponsive to the maturation-inducing effects of the pro-inflammatory cytokine cocktail described above.Citation9 This indicates that the conventional maturation procedure used for IL-4 DCs is not suitable for IL-15 DCs and that alternative activation pathways (e.g., TLRs) should be triggered to induce optimal IL-15 DC maturation. Several TLR ligands have been described as potent stimuli for IL-15 DC maturation, including: Pam3Csk4 (TLR2),Citation16 poly(I:C) (TLR3),Citation13,Citation15,Citation16 lipopolysaccharide (LPS; TLR4),Citation10,Citation13,Citation14,Citation16,Citation17 the commercially available Human Papillomavirus vaccine Cervarix® which contains monophosphoryl lipid A (TLR4) (J. Van den Bergh et al., manuscript in preparation), and resiquimod (TLR7/8).Citation9 The latter TLR agonist served as the maturation stimulus in our IL-15 DC culture system () and proved to induce rapid and effective IL-15 DC activation, as evidenced by the prominent expression of CD83 and of the T cell co-stimulatory molecules CD40, CD70, CD80 and CD86 (requirement 1, see above).Citation9,Citation18
Migration of the DCs to the T cell-rich areas of the lymph nodes is considered as another mandatory evaluation criterion of DC vaccines (requirement 2), although this aspect is becoming less important in view of the emerging evidence that the initiation of T cell immunity does not require large numbers of DCs migrating to the lymph nodes.Citation19 Nevertheless, we were able to show that our IL-15 DC culture system gives rise to DCs that are endowed with a potent ability to migrate in the direction of the lymph node chemokine CCL21, a ligand for the chemokine receptor CCR7 which is expressed at high levels on the surface of TLR7/8-activated IL-15 DCs.Citation9
The cytokine milieu provided by DCs is a critical factor in determining the type of adaptive immune response that will be mounted against a particular antigen. In the context of cancer immunotherapy, generation of a TH1 immune response is highly desired as this type of immune response supports the generation of tumor-specific CTLs.Citation6,Citation7 Both murineCitation12,Citation13 and humanCitation16 IL-15 DCs demonstrated a marked ability to drive the differentiation of naïve TH0 cells into TH1 cells (requirement 3). The best understood DC-derived cytokine involved in TH1 polarization is IL-12p70. Although conflicting data exist whether TLR-primed IL-15 DCs are primary producers of IL-12p70,Citation9,Citation12,Citation13,Citation16 a far more relevant question from a biological perspective is whether they can secrete IL-12p70 following CD40 ligation by T cells. We have shown that CD40/CD40L signaling induces IL-12p70 production by TLR7/8-activated IL-15 DCs, albeit at rather low levels.Citation9 This suggests that mechanisms other than IL-12p70 production may also account for the potent capacity of IL-15 DCs to drive TH1 responses.Citation9,Citation12,Citation13 In this regard, two other TH1-polarizing cytokines have been demonstrated to be important: interferon (IFN)-γ, which is present at high levels in culture supernatants of TLR-primed IL-15 DCs,Citation9,Citation13,Citation15 and IL-15.Citation16 The latter cytokine, which is usually not secreted but expressed as a membrane-bound form,Citation14,Citation16 was recently shown to play a critical role in the TH1-polarizing capacity of IL-15 DCs.Citation16
To determine whether our modified IL-15 DC culture protocol fulfills the requirement for antigen presentation (requirement 4), IL-15 DCs were pulsed with major histocompatibility complex (MHC) class I-restricted peptides derived from frequently occurring viruses (e.g., influenza) and evaluated for their capacity to stimulate autologous, virus-specific CD8+ T cell responses.Citation9 In these experiments, IL-15 DCs proved to be superior antigen-presenting cells as compared with conventional IL-4 DCs, allowing us to conclude that our IL-15 DC system meets all phenotypic and functional criteria for being adopted in DC-based immunotherapy protocols.Citation9
IL-15 DCs and Adaptive Anti-Tumor Immunity: Effect on T Cells
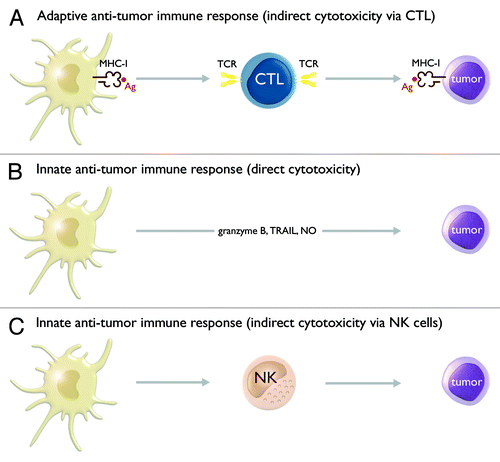
In a next series of experiments, we aimed to determine whether the potent capacity of IL-15 DCs to stimulate viral antigen-specific CD8+ CTLs is also applicable for tumor antigens (). We decided to focus on one particular tumor antigen, the Wilms’ tumor 1 (WT1) protein.Citation20 WT1 is overexpressed in a variety of tumor types, while it is expressed only at very low levels in normal cells or tissues.Citation20 This, together with its favorable immunogenic characteristics which allow it to confer clinically significant anti-tumor immunity, render WT1 an “ideal” target antigen for active specific immunotherapy of cancer.Citation20 Electroporation of WT1 mRNA was selected as the strategy of choice for antigen loading of IL-15 DCs.Citation21 This choice was based, in the first place, on the fact that mRNA electroporation offers some specific advantages over other DC antigen-loading strategies (e.g., presentation of multiple T cell epitopes without the need for prior knowledge of the patient’s HLA type)Citation21 and, in the second place, because of the broad expertise that has been built up within our research group using this particular antigen-loading method.Citation22 We first optimized the parameters for mRNA electroporation of IL-15 DCs. As a consequence of the short-term culture, IL-15 DCs tend to be smaller in size than conventional IL-4 DCs and thus require the application of a more intense electrical field for successful cell membrane permeation.Citation23 Consistent with a previous study by Bürdek et al.,Citation23 we found that short-term cultured monocyte-derived DCs, such as IL-15 DCs, can be efficiently transfected with mRNA using an exponential decay pulse of 300V and 300µF. Electroporation of IL-15 DCs with WT1 mRNA using the above electroporation conditions resulted in high WT1 protein expression levels, as determined by immunocytochemical analysis 24 h post-electroporation. In line with this, we observed that WT1 mRNA-electroporated IL-15 DCs can successfully trigger IFN-γ release from a WT1-specific CD8+ T cell clone.Citation18 These results demonstrate that WT1 mRNA-electroporated IL-15 DCs can translate the electroporated mRNA into protein and subsequently process the translated WT1 antigen for MHC class I presentation. Antigen presentation experiments further determined that WT1 mRNA-electroporated IL-15 DCs and classical IL-4 DCs possess a comparable capacity to stimulate the WT1-specific CD8+ T cell clone (S. Anguille et al., manuscript in preparation). This confirms that mRNA electroporation is an effective strategy for antigen loading of IL-15 DCs.
Figure 2. TLR-matured IL-15 DCs have pleiotropic immunostimulatory properties, which allow them to harness both adaptive and innate anti-tumor immune responses. At the level of the adaptive anti-tumor immune response, IL-15 DCs display a potent capacity to present tumor antigen (Ag) in the context of major histocompatibility complex class I (MHC-I) molecules to CD8+ cytotoxic T lymphocytes (CTLs). These CTLs recognize MHC-I/Ag-expressing tumor cells via their T cell receptor (TCR) and subsequently mediate tumor cell lysis (A). At the level of the innate anti-tumor immune response, IL-15 DCs can mediate tumor cell lysis through a direct cytotoxic action. Several mechanisms have been implicated in IL-15 DC-mediated cytotoxicity, including granzyme B, tumor necrosis factor-α-related apoptosis-inducing ligand (TRAIL) and nitric oxide (NO) (B). In addition, IL-15 DCs can also indirectly contribute to innate tumor destruction via activation of natural killer (NK) cells (C).
How WT1 mRNA-electroporated IL-15 DCs behave compared with the “gold-standard” IL-4 DCs in terms of their capacity to prime autologous CTL responses against WT1 remains to be formally investigated. Nevertheless, a recent study by Romano and colleagues provides some interesting hints that can help to already answer this question.Citation24 Their study showed that WT1 mRNA-electroporated LCs are extremely efficient at stimulating WT1-specific CD8+ T cells in healthy volunteers. Transpresentation of IL-15 via IL-15Rα was identified as the mechanism underlying the potent T cell-priming capacity of WT1 mRNA-electroporated LCs. Interestingly, a similar level of T cell priming could be achieved using conventional WT1 mRNA-electroporated monocyte-derived DCs under the condition that IL-15 was exogenously supplemented.Citation24 With this in mind and taking into account the above-mentioned LC resemblance of IL-15 DCs and their known capacity to transpresent IL-15, it is tempting to speculate that WT1 mRNA-electroporated IL-15 DCs are also endowed with a superior capacity to induce WT1-specific T cell immunity. Further support for this claim comes from two other studies which showed that IL-15 DCs are far more efficient than IL-4 DCs at priming naïve CD8+ T cell responses against a range of melanoma antigens.Citation14,Citation15 Taken together, these data indicate that IL-15 DCs are potent stimulators of adaptive anti-tumor immunity and underscore their suitability for use in DC vaccination protocols ().
IL-15 DCs and Innate Anti-Tumor Immunity: Direct Effect on Tumor Cells
As mentioned above, weCitation9 and othersCitation15 found that a subpopulation of IL-15 DCs expresses CD56, a finding that is rather intriguing given the fact that CD56 is the archetypal surface marker of NK cells.Citation25 This prompted us to examine the possibility that CD56+ IL-15 DCs may be related to NK cells. Detailed immunophenotypic characterization, however, showed that IL-15 DCs exhibit a “genuine” myeloid DC phenotype and that they, apart from CD56, lack expression of other NK cell-associated markers (e.g., CD7, NKp46, etc.).Citation18 This, together with their demonstrated capacity to stimulate allogeneic T cells and their potent antigen presentation properties, confirms that IL-15 DCs, despite positivity for CD56, are bona fide DCs and that they are not affiliated to the NK cell lineage.Citation18 The finding that CD56 can be expressed on human DC subsets, such as IL-15 DCs, corroborates accumulating evidence that this surface marker is not exclusive to NK cells and that it can also be present on other immune cells with cytotoxic effector function (e.g., NKT cells, γδ T cells, activated αβ T cells).Citation25 In view of this, we hypothesized that CD56+ IL-15 DCs are also equipped with cytotoxic activity that would enable them to directly contribute to tumor cell eradication (). To test this hypothesis, we performed cytotoxicity assays in which CD56+/−-separated IL-15 DC subpopulations were examined for lytic activity against the human tumor cell lines K562 (MHC class I-negative) and U937 (MHC class I-positive).Citation18 While no overt cytotoxicity could be observed toward the U937 cell line, IL-15 DCs, in particular the CD56+ subset, were found to induce apoptotic cell death of the K562 cell line. Conventionally generated IL-4 DCs, which served as controls, failed to induce K562 cell death.Citation18 Importantly, the cytotoxic activity of CD56+ IL-15 DCs appeared to be tumor-specific, since no cytotoxicity was detected against normal, tumor antigen-specific T cells. Further mechanistic studies revealed that granzyme B, but not perforin, and, to a lesser extent, TNF-α-related apoptosis-inducing ligand (TRAIL) were involved in the cytotoxicity of CD56+ IL-15 DCs against K562.Citation18 A recent study found that production of nitric oxide (NO), which in another study was shown to be released at relatively high levels by IL-15 DCs,Citation13 underlied the tumoricidal activity of murine, TLR4-primed IL-15 DCs,Citation17 indicating that different mechanisms can be implicated in IL-15 DC-mediated cytotoxicity ().
Although the first description of “killer DCs” already dates back to the mid-1990s, the concept that DCs can also directly contribute to anti-tumor immunity through a cytotoxic effector function has only recently started to gain acceptance among immunologists and cancer researchers.Citation25 Our work on IL-15 DCs provides further evidence that human DCs can combine both tumor antigen presentation function with direct tumoricidal activity. There are several possibilities to embrace this less conventional aspect of DC biology in future DC-based cancer immunotherapy applications. Ex vivo generated killer DCs could be administered directly into the tumor tissue to mediate tumor cell lysis in collaboration with the “classical” cytotoxic effector cells, such as CTLs and NK cells.Citation26 Another strategy to augment the effector phase of the anti-tumor immune response is to harness the “killer” potential of DCs in vivo (e.g., through administration of IL-15 or TLR ligands). This seems especially relevant considering the fact that in the tumor bed DCs often outweigh other infiltrating immune cells. Finally, the cytotoxic function of DCs could also be exploited to potentiate the anti-tumoral CTL response. It is indeed conceivable that killer DCs, through their tumoricidal action, can supply themselves with a range of (patient-specific) tumor-derived antigens for T cell presentation, allowing them to generate a more diversified repertoire of tumor antigen-specific CD8+ CTLs.Citation26 Direct evidence supporting this hypothesis has been obtained in recently published work from the group of Nicolas Larmonier at the University of Arizona (Tucson, AZ).Citation17 Using the B16-OVA mouse melanoma model, the authors elegantly showed that murine killer IL-15 DCs, after mediating tumor cell lysis, are capable of cross-presenting tumor-derived antigens to T cells in vitro as well as in vivo.Citation17 These data indicate that the “killer” function of DCs may be relevant to the in vivo situation and further reinforce the potential utility of IL-15 DCs for cancer immunotherapy.
IL-15 DCs and Innate Anti-Tumor Immunity: Effect on NK Cells
Since the basic concept of DC-based cancer vaccination lies in exploiting the antigen-presenting function of DCs to induce tumor antigen-specific T cell responses, the prime focus in the evaluation of novel DC vaccine candidates has been on their capacity to elicit adaptive anti-tumor immunity. The ability of DCs to stimulate innate anti-tumor immune responses is an aspect of evaluation that has been largely neglected so far. This is somewhat surprising given the fact that innate immune effector cells, most notably NK cells, are known to play a key role in anti-tumor defense and considering the plethora of pre-clinical data showing that DCs can interact with NK cells and potentiate their anti-tumor activities.Citation27,Citation28 Based on the limited number of clinical DC vaccine trials in which NK cell immunomonitoring was performed, DCs also appear to be capable of mediating NK cell activation in vivo.Citation27 Both the cytotoxic effector function and the cytokine-secreting capacity (e.g., IFN-γ) of NK cells can be augmented after DC vaccination. These favorable changes in the NK cell compartment have been correlated with clinical outcome in DC vaccination trials, confirming that the efficacy of DC-based immunotherapy strategies relies, in part, on the capacity of the administered DCs to harness the innate arm of the immune system.Citation27
These data underscore the importance of examining the NK cell-stimulatory potential of novel DC vaccine candidates, in addition to their effects on T cell immunity. We have recently initiated a series of experiments to determine whether IL-15 DCs possess NK cell-stimulatory activity (S. Anguille et al., manuscript in preparation). The results from these experiments showed that NK cells acquire an activated phenotype following co-culture with autologous TLR-activated IL-15 DCs, but not with conventional IL-4 DCs. This was paralleled by an enhanced cytotoxic activity of IL-15 DC-activated NK cells toward tumor cells, confirming that IL-15 DCs contribute to innate anti-tumor immunity not only through a direct tumoricidal action but also indirectly via activation of NK cells ().
While we were able to show that IL-15 DCs can activate NK cells, a recent study by Hardy et al.Citation15 uncovered that NK cells can reciprocally influence the T cell-stimulatory function of IL-15 DCs. In that study, NK cells were observed to support the ability of IL-15 DCs to induce a potent CTL response against the melanoma tumor antigen MART-1, thereby providing an elegant example of the so-called “helper” function of NK cells.Citation27 The same authors also found preliminary evidence that IL-15 was important in this process, as blocking of IL-15 resulted in a significant reduction of the observed CTL response.Citation29 These findings, along with our work, collectively show that IL-15 DCs and NK cells can engage in a mutual activating interaction, which presumably involves membrane-bound IL-15, and that this bidirectional “crosstalk” can result in improved innate and adaptive anti-tumor immune responses.
Conclusions and Future Considerations
During the past decade, researchers involved in the field of DC-based immunotherapy have intensively focused on developing novel, optimized protocols for the ex vivo generation of DC vaccines. Although these efforts have resulted in the identification of new DC vaccine candidates with a favorable pre-clinical profile, most clinical trials continue to rely on first-generation IL-4 DC vaccines.Citation8 One of the “next-generation” DC vaccine products that has attracted considerable interest in recent years are IL-15 DCs.Citation9 The data summarized in this commentary indicate that TLR-primed IL-15 DCs may indeed be particularly useful for DC-based cancer immunotherapy, not only because of their potent T cell-stimulatory capacity () but also because they - in contrast to conventional cytokine cocktail-matured IL-4 DCs - possess direct tumoricidal activity () as well as the capacity to harness NK cell effector function against tumors (). Based on this, we think the time has come to abandon the use of IL-4 DCs and to adopt IL-15 DCs in clinical trial protocols. Both IL-15 and a variety of TLR ligands have recently become available as GMP-compatible reagents, which has cleared the path to the immediate clinical application of IL-15 DC-based cancer vaccines. However, considering the myriad of mechanisms by which cancer cells can suppress or evade DC vaccine-induced immunity,Citation7,Citation28 it is conceivable that DC vaccination on its own, even using these next-generation DC vaccines with optimized immunostimulatory activity, may not be sufficient to tilt the balance toward clinically significant anti-tumor immunity. Therefore, we strongly believe that future research efforts should be fully devoted to explore combinations of these next-generation DC vaccine products, such as IL-15 DCs, with other anti-cancer therapies that allow for harnessing the effector arms of the immune system and for overcoming the various immune suppression and escape mechanisms used by tumor cells.Citation7
Acknowledgments
This work was supported in part by research grants of the Research Foundation Flanders (FWO Vlaanderen), the Belgian Foundation against Cancer (Stichting tegen Kanker), the Methusalem program of the Flemish Government attributed to Prof Herman Goossens (University of Antwerp, Belgium), the Interuniversity Attraction Pole program (IAP #P6/41) of the Belgian Government and the Belgian Hercules Foundation. SA is a former PhD fellow of the Research Foundation Flanders and currently holds an Emmanuel van der Schueren Fellowship of the Flemish League against Cancer (Vlaamse Liga tegen Kanker). SA also received financial support from the Belgian Foundation against Cancer and the Belgian public utility foundation VOCATIO. YW is supported by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology (IWT). ELS is a post-doctoral fellow of the Research Foundation Flanders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Shurin MR. Cancer as an immune-mediated disease. ImmunoTargets and Therapy 2012; 1:1 - 6; http://dx.doi.org/10.2147/ITT.S29834
- Gilboa E. The promise of cancer vaccines. Nat Rev Cancer 2004; 4:401 - 11; http://dx.doi.org/10.1038/nrc1359; PMID: 15122211
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475 - 80; http://dx.doi.org/10.1038/nm1039; PMID: 15122249
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265 - 77; http://dx.doi.org/10.1038/nrc3258; PMID: 22437871
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2:52 - 8; http://dx.doi.org/10.1038/nm0196-52; PMID: 8564842
- Anguille S, Lion E, Smits E, Berneman ZN, van Tendeloo VF. Dendritic cell vaccine therapy for acute myeloid leukemia: questions and answers. Hum Vaccin 2011; 7:579 - 84; http://dx.doi.org/10.4161/hv.7.5.14652; PMID: 21422813
- Anguille S, Willemen Y, Lion E, Smits EL, Berneman ZN. Dendritic cell vaccination in acute myeloid leukemia. Cytotherapy 2012; 14:647 - 56; PMID: 22686130
- Tuyaerts S, Aerts JL, Corthals J, Neyns B, Heirman C, Breckpot K, et al. Current approaches in dendritic cell generation and future implications for cancer immunotherapy. Cancer Immunol Immunother 2007; 56:1513 - 37; http://dx.doi.org/10.1007/s00262-007-0334-z; PMID: 17503040
- Anguille S, Smits EL, Cools N, Goossens H, Berneman ZN, Van Tendeloo VF. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J Transl Med 2009; 7:109; http://dx.doi.org/10.1186/1479-5876-7-109; PMID: 20021667
- Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med 2001; 194:1013 - 20; http://dx.doi.org/10.1084/jem.194.7.1013; PMID: 11581322
- Saikh KU, Khan AS, Kissner T, Ulrich RG. IL-15-induced conversion of monocytes to mature dendritic cells. Clin Exp Immunol 2001; 126:447 - 55; http://dx.doi.org/10.1046/j.1365-2249.2001.01672.x; PMID: 11737061
- Pulendran B, Dillon S, Joseph C, Curiel T, Banchereau J, Mohamadzadeh M. Dendritic cells generated in the presence of GM-CSF plus IL-15 prime potent CD8+ Tc1 responses in vivo. Eur J Immunol 2004; 34:66 - 73; http://dx.doi.org/10.1002/eji.200324567; PMID: 14971031
- Feili-Hariri M, Falkner DH, Morel PA. Polarization of naive T cells into Th1 or Th2 by distinct cytokine-driven murine dendritic cell populations: implications for immunotherapy. J Leukoc Biol 2005; 78:656 - 64; http://dx.doi.org/10.1189/jlb.1104631; PMID: 15961574
- Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur J Immunol 2007; 37:1678 - 90; http://dx.doi.org/10.1002/eji.200636329; PMID: 17492620
- Hardy MY, Kassianos AJ, Vulink A, Wilkinson R, Jongbloed SL, Hart DN, et al. NK cells enhance the induction of CTL responses by IL-15 monocyte-derived dendritic cells. Immunol Cell Biol 2009; 87:606 - 14; http://dx.doi.org/10.1038/icb.2009.44; PMID: 19546878
- Harris KM. Monocytes differentiated with GM-CSF and IL-15 initiate Th17 and Th1 responses that are contact-dependent and mediated by IL-15. J Leukoc Biol 2011; 90:727 - 34; http://dx.doi.org/10.1189/jlb.0311132; PMID: 21724805
- Hanke NT. Molecular Regulation of the Tumor Killing Activity of Dendritic Cells. PhD dissertation 2012; The University of Arizona, Tucson, AZ, USA.
- Anguille S, Lion E, Tel J, de Vries IJ, Couderé K, Fromm PD, et al. Interleukin-15-induced CD56(+) myeloid dendritic cells combine potent tumor antigen presentation with direct tumoricidal potential. PLoS One 2012; 7:e51851; http://dx.doi.org/10.1371/journal.pone.0051851; PMID: 23284789
- Celli S, Day M, Müller AJ, Molina-Paris C, Lythe G, Bousso P. How many dendritic cells are required to initiate a T-cell response?. Blood 2012; 120:3945 - 8; http://dx.doi.org/10.1182/blood-2012-01-408260; PMID: 22995897
- Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia 2012; 26:2186 - 96; http://dx.doi.org/10.1038/leu.2012.145; PMID: 22652755
- Smits EL, Anguille S, Cools N, Berneman ZN, Van Tendeloo VF. Dendritic cell-based cancer gene therapy. Hum Gene Ther 2009; 20:1106 - 18; http://dx.doi.org/10.1089/hum.2009.145; PMID: 19656053
- Van Tendeloo VF, Van de Velde A, Van Driessche A, Cools N, Anguille S, Ladell K, et al. Induction of complete and molecular remissions in acute myeloid leukemia by Wilms’ tumor 1 antigen-targeted dendritic cell vaccination. Proc Natl Acad Sci U S A 2010; 107:13824 - 9; http://dx.doi.org/10.1073/pnas.1008051107; PMID: 20631300
- Bürdek M, Spranger S, Wilde S, Frankenberger B, Schendel DJ, Geiger C. Three-day dendritic cells for vaccine development: antigen uptake, processing and presentation. J Transl Med 2010; 8:90; http://dx.doi.org/10.1186/1479-5876-8-90; PMID: 20920165
- Romano E, Cotari JW, Barreira da Silva R, Betts BC, Chung DJ, Avogadri F, et al. Human Langerhans cells use an IL-15R-α/IL-15/pSTAT5-dependent mechanism to break T-cell tolerance against the self-differentiation tumor antigen WT1. Blood 2012; 119:5182 - 90; http://dx.doi.org/10.1182/blood-2011-09-382200; PMID: 22510877
- Roothans D, Smits E, Lion E, Tel J, Anguille S. CD56 marks human dendritic cell subsets with cytotoxic potential. Oncoimmunology 2013; 2:e23037; http://dx.doi.org/10.4161/onci.23037; PMID: 23524451
- Larmonier N, Fraszczak J, Lakomy D, Bonnotte B, Katsanis E. Killer dendritic cells and their potential for cancer immunotherapy. Cancer Immunol Immunother 2010; 59:1 - 11; http://dx.doi.org/10.1007/s00262-009-0736-1; PMID: 19618185
- Lion E, Smits EL, Berneman ZN, Van Tendeloo VF. NK cells: key to success of DC-based cancer vaccines?. Oncologist 2012; 17:1256 - 70; http://dx.doi.org/10.1634/theoncologist.2011-0122; PMID: 22907975
- Lion E, Willemen Y, Berneman ZN, Van Tendeloo VF, Smits EL. Natural killer cell immune escape in acute myeloid leukemia. Leukemia 2012; 26:2019 - 26; http://dx.doi.org/10.1038/leu.2012.87; PMID: 22446501
- Hardy MY. Manipulation of human dendritic cell subsets and the design of optimal preparations for tumour immunotherapy. PhD dissertation 2008; The University of Queensland, QLD, Australia.