Abstract
An investigational tetravalent vaccine combining pre-pandemic, MF59®-adjuvanted A/H5N1 vaccine with non-adjuvanted, trivalent, seasonal influenza vaccine has been developed, which has the potential to be used for pre-pandemic priming and to improve levels of compliance and coverage. It is important to determine whether the safety and immunogenicity of the combination vaccine is equivalent to that of the two separate vaccines when administered concomitantly. Healthy adults (n = 601) were randomly assigned to three vaccination groups to receive either: (1) tetravalent vaccine and placebo concomitantly (in separate arms) on Day 1, followed by A/H5N1 vaccine on Day 22; (2) A/H5N1 vaccine and placebo concomitantly on Day 1, followed by tetravalent vaccine on Day 22; or (3) A/H5N1 and seasonal vaccines concomitantly on Day 1, followed by A/H5N1 vaccine on Day 22. Antibody responses were measured using single radial hemolysis (SRH), haemagglutination inhibition (HI), and microneutralization (MN) assays on Days 1, 22, and 43. Solicited adverse reactions were recorded for seven days after vaccination. Spontaneous adverse events were recorded throughout the study. The tetravalent vaccine elicited antibody titers equivalent to those for separate A/H5N1 and seasonal vaccines, and sufficient to meet the European licensure criteria against A/H5N1 and all three seasonal strains. Local and systemic reactions were mainly mild to moderate. No vaccine-related serious adverse events occurred. These findings demonstrate that MF59-adjuvanted A/H5N1 and seasonal influenza vaccines had an acceptable safety profile and could be effectively administered as a tetravalent formulation, supporting the possibility of integrating pre-pandemic priming into seasonal influenza vaccination programs.
Introduction
Influenza is characterized by the occurrence of annual (seasonal) epidemics and infrequent worldwide pandemics due to the emergence and spread of novel influenza viruses, as recently illustrated by the A/California/07/2009 (H1N1) pandemic strain.Citation1 The 2009 A/H1N1 virus spread rapidly but was relatively benign, with fatality rates similar to those observed for seasonal influenza.Citation2 However, the highly pathogenic avian influenza virus, A/H5N1, has a different profile, with a case fatality rate of 59%.Citation3 Fortunately, A/H5N1 is not transmitted as easily as A/H1N1 between humans, being restricted to those with direct contact with birds. However, potential mutation to a more readily transmissible, virulent form makes the A/H5N1 virus a serious pandemic threat, which cannot be ignored.Citation4,Citation5
Vaccination is a critical and highly effective intervention during pandemics. However, there are numerous logistical concerns, including the timely production, distribution, and administration of vaccine.Citation6,Citation7 Therefore, strategies to ensure preparedness for a future pandemic include the development of pre-pandemic vaccines able to prime an immunologically naive population for later boosting, should a pandemic occur. In an immunologically naive individual, at least two doses of A/H5N1 vaccine with high antigen content are needed to elicit adequate, seroprotective immune responses.Citation8 However, the use of potent adjuvants, such as the well-established oil-in-water emulsion, MF59® (Novartis Vaccines and Diagnostics), can significantly heighten and enhance the immune response to vaccination, allowing for reduced antigen content per dose; MF59 has also been shown to enhance cross-reactive antibody production, which can protect against mutated viral strains.Citation9-Citation12
The proactive priming of populations with pre-pandemic vaccines requires strategies to facilitate vaccine administration; the incorporation of a potentially pandemic influenza strains into existing trivalent seasonal influenza vaccines—routinely administered on an annual basis—to create a single tetravalent combination vaccine, could improve coverage, compliance, administration logistics, and costs. As such, an investigational tetravalent formulation combining a pre-pandemic, MF59-adjuvanted, A/H5N1 vaccine with a non-adjuvanted, trivalent, seasonal influenza vaccine has been developed, which, as well as being used for pre-pandemic priming, could be beneficial during a pandemic outbreak. It is important to determine whether the safety and immunogenicity of such a combination vaccine is equivalent to that of the individual vaccines when administered concomitantly.
In this report, we present the results of a phase II, randomized, controlled, proof-of-concept trial conducted in Germany among healthy adults, to assess the safety and immunogenicity of a pre-formulated, tetravalent, MF59-adjuvanted, A/H5N1-seasonal influenza vaccine, compared with safety and immunogenicity profiles following the concomitant administration of individual A/H5N1 and seasonal influenza vaccines in separate arms.
Results
Demographics
Overall, 601 subjects were enrolled in the study and allocated to vaccination Groups A (n = 199), B (n = 203), and C (n = 199). A total of 22 subjects were excluded from analyses (4 subjects in Group A, 11 subjects in Group B, and 7 subjects in Group C). The reasons for exclusion were: lost to follow up, n = 10; protocol deviation, n = 4; withdrawal of consent, n = 3; adverse events (AE), n = 4; and inappropriate enrollment, n = 1. Vaccine groups were similar with respect to age, ethnicity, and previous influenza vaccination; there were more women than men in all groups ().
Table 1. Demographics of the study population. Group A: Tetravalent vaccine and placebo on Day 1, A/H5N1 vaccine on Day 22; Group B: A/H5N1 vaccine and placebo on Day 1, tetravalent vaccine on Day 22; Group C: A/H5N1 and seasonal influenza vaccines on Day 1, A/H5N1 vaccine on Day 22
Immunogenicity
A/H5N1 strain
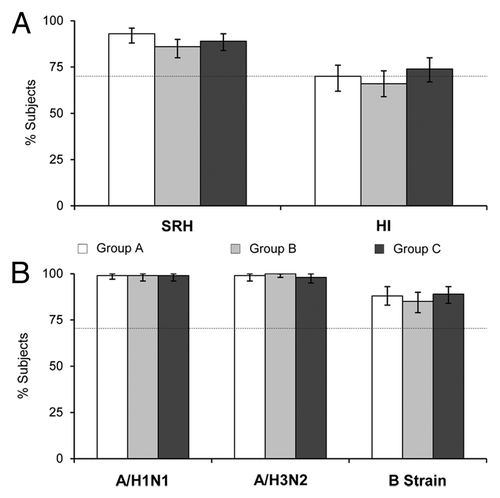
Baseline single radial hemolysis (SRH) geometric mean areas (GMAs) against A/H5N1 were similar in all three groups (4.1–4.61). After one dose, each group displayed a greater than 2-fold increase in GMAs at Day 22 (). Three weeks after the second vaccination, GMAs in all three groups were over 8-fold higher than baseline values, with similar levels in each group. Equivalence of antibody responses was statistically confirmed by showing that the 96.67% confidence intervals (CI) of all three ratios of SRH GMA at Day 43 were within the equivalence range of 0.5–2.0 (). In line with these findings, results from haemagglutination inhibition (HI) and microneutralization (MN) assays show 1.37 to 1.93- and 1.45 to 1.64-fold increases against A/H5N1 in each group after one dose, respectively. Three weeks after the second dose, HI geometric mean antibody titers (GMTs) were 6.07–8.78 times higher compared with baseline values, and 11.00–14.00 times higher by MN assay. Equivalence of antibody responses between groups at Day 43 was confirmed by both assays, except between Groups B and C (GMT ratio 0.69 [0.46–1.03]) for the HI assay (). Seroprotection rates were 93%, 86% and 89% by SRH analyses, and 70%, 66% and 74% by HI analyses in Groups A, B, and C, respectively; all groups met the European Committee for Medicinal Products for Human Use (CHMP) seroprotection criterion of 70%, except for Group B when assessed by HI assay (). Seroprotection rates by MN assay were 90%, 87%, and 89% for Groups A, B, and C, respectively; data not shown in because CHMP licensure criteria do not apply to MN analyses. These rates were achieved against the low baseline values before vaccination, in which only 15 (2.5%) and 6 (1.0%) subjects displayed seroprotective SRH and HI titers against A/H5N1, respectively. In Groups A, B, and C, respective rates of seroconversion or significant increase at Day 43 were 90%, 86%, and 86% for SRH, and 69%, 67%, and 75% for HI; all groups exceeded the CHMP criterion.
Table 2. Immunogenicity analyses against the pre-pandemic vaccine strain, A/Vietnam/1194/2004 (H5N1)
Figure 1. (A) Seroprotection rates (95% CI) against the pre-pandemic vaccine strain, A/Vietnam/1194/2004 (H5N1), after two vaccine doses (Day 43) as measured by SRH and HI assays; (B) seroprotection rates (95% CI) against the seasonal vaccine strains, A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 (B strain) after two vaccine doses (Day 43) as measured by HI assay. Dotted lines represent the CHMP licensure criterion for seroprotection (70%). Group A: Tetravalent vaccine and placebo on Day 1, A/H5N1 vaccine on Day 22; Group B: A/H5N1 vaccine and placebo on Day 1, tetravalent vaccine on Day 22; Group C: A/H5N1 and seasonal influenza vaccines on Day, A/H5N1 vaccine on Day 22.
Seasonal influenza strains
Immune responses based on HI assay against seasonal influenza strains A/H1N1, A/H3N2, and B are presented in and . Pre-vaccination HI GMTs were similar across vaccine groups and ranged from 38 to 54 for A/H1N1, 37 to 45 for A/H3N2, and 11 to 13 for the B strain. Three weeks after receiving a single dose of the seasonal vaccine (Day 22 for Groups A and C, and Day 43 for Group B), similar levels of GMTs were observed in the three groups. For Groups A, B, and C respectively: GMTs against A/H1N1 were 541, 687, and 618; GMTs against A/H3N2 were 414, 451, and 368; and GMTs against the B strain were 98, 113, and 104 (). All three immunization schedules induced antibody responses against the seasonal influenza strains, which fulfilled all three CHMP criteria against all three strains at Day 43. On Day 43, seroprotection was achieved by 99% of subjects against A/H1N1, 98–100% of subjects against A/H3N2, and 85–89% of subjects against the B strain, with no considerable differences between the groups (). HI analysis found all groups to met the geometric mean ratio (GMR) criterion, with ranges of 9.9–16 for A/H1N1, 6.1–11 for A/H3N2, and 7.9–9.9 for the B strain. The seroconverion or significant increase criterion was also met by all groups on HI analysis, with rates of 72–80% for A/H1N1, 71–80% for A/H3N2, and 71–81% for the B strain ().
Table 3. Immunogenicity analysis by HI assay (95% CI) against the seasonal vaccine strains, A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004 (B strain)
Safety and reactogenicity
A total of 600 out of 601 subjects were included in the safety analyses (one subject was excluded due to not receiving the correct vaccine). All vaccines were generally well tolerated, no serious adverse events (SAEs) were related to vaccination, and the local and systemic reactions were mainly mild to moderate in severity and resolved within days. Rates of local and systemic reactions were fewer after the second vaccination compared with the first vaccination. Pain was the most commonly reported solicited local reaction across vaccine groups, followed by induration (). After the first vaccination, pain was reported most frequently (84%, Group A) in the arm receiving the tetravalent vaccine (). Percentages of subjects reporting pain in the arm receiving A/H5N1 vaccine in Groups B and C were lower, ranging from 66% to 69%. Pain after the seasonal vaccine was reported in 41% of subjects (Group C), and the percentages of subjects reporting pain after the placebo ranged from 15% in Group A to 16% in Group B. After the second vaccination, pain was reported in 71% of subjects after the tetravalent vaccine (71%, Group B); pain after the A/H5N1 vaccine in Group A and Group C ranged from 47% to 52% (). Reports of severe pain were rare, occurring in five subjects after tetravalent vaccine (<2%), two subjects after A/H5N1 vaccine (<1%), and one subject after seasonal influenza vaccine (<1%); all resolved within 7 days of vaccination. Other local reactions were reported less frequently (). Rates of solicited systemic reactions are presented in . The most frequently reported systemic reactions across groups and vaccinations were fatigue (18–41%), headache (14–36%), and myalgia (14–35%); the majority of reactions being mild to moderate in severity and transient in nature. Severe systemic reactions were relatively infrequent and were reported by <4% of subjects across groups after the first vaccination and <3% of subjects across groups after the second vaccination. A trend toward more solicited reactions after the tetravalent vaccine was observed, reflected in increased rates of analgesic use: 14% compared with 10% in Group B and 8% in Group C after the first vaccine dose, and 8% compared with 3% (A/H5N1 vaccine) after the second dose. There were no reports of severe fever (>40 °C). Temperatures >38.5 °C were reported in <4% of subjects across vaccine groups. Any spontaneously reported AEs occurred in 34–44% of subjects across groups (23–27% of which were at least possibly vaccine-related) after the first dose, and 24–25% (12–15% at least possibly vaccine-related) after the second dose. The most commonly reported AEs across groups were nasopharyngitis (7–14% after first dose; 5–6% after second dose), and headache (4–5% after first dose; 4% after second dose). Overall, a total of 15 SAEs were reported by 9 subjects (Group A, n = 2; Group B, n = 3; Group C, n = 4), none of which were considered to be vaccine-related. Five subjects were withdrawn from the study due to an AE (Group A, n = 2; Group B, n = 1; Group C, n = 2), one of which was judged to be possibly related to the study vaccine (influenza-like-illness starting with onset of bronchitis three days after the first vaccination; Group A subject). No deaths occurred during this study.
Table 4. Percentages of subjects experiencing mild to moderate (and severe) solicited local adverse reactions within one week of receiving first (two doses, one in each arm) and second vaccine doses
Table 5. Percentages of subjects experiencing mild to moderate (and severe) solicited systemic reactions within one week of vaccination
Discussion
Published data indicate that the highly pathogenic A/H5N1 virus has significant potential to spread among mammals and cause a human pandemic.Citation5,Citation13 The importance of pre-pandemic preparedness strategies has been highlighted by experiences from the recent A/H1N1 influenza pandemic in 2009. The 2009 A/H1N1 pandemic demonstrated that in a primed population only one vaccine dose was needed to achieve seroprotective antibody concentrations.Citation14,Citation15 Pre-pandemic priming with viral subtypes that do not circulate widely could make a considerable difference to the response to a future pandemic. The low immunogenicity of novel pandemic antigens can be overcome by potent adjuvants such as MF59, which has been shown to enhance the immunogenicity of A/H5N1 vaccine, with the additional benefits of reducing antigen content per vaccine dose, and enhancing cross-protective antibody responses.Citation9,Citation16-Citation18
The logistics of pre-pandemic priming could be simplified if seasonal and pandemic vaccines were administered as a single tetravalent vaccine using the existing infrastructure for routine, annual, seasonal vaccination programs, providing that such a combination has no impact on the immunogenicity and reactogenicity of either individual vaccine. It has previously been shown that non-adjuvanted seasonal vaccine and MF59-adjuvanted A/H1N1 vaccine can be administered sequentially or concomitantly without compromising antibody responses.Citation19,Citation20 A recently study provided the first evidence that seasonal and A/H5N1 influenza vaccines can be given as a mixed injection, or as simultaneous separate injections without affecting immunogenicity or safety.Citation21 Here we report the findings of a proof-of-concept study to assess the safety and immunogenicity of a pre-formulated, tetravalent, MF59-adjuvanted, A/H5N1-seasonal influenza vaccine, as compared with A/H5N1 and seasonal influenza vaccines given concomitantly in separate arms.
The results of this study demonstrate that the tetravalent combination vaccine was highly immunogenic and elicited high antibody titers against A/H5N1, A/H1N1, A/H3N2, and B strain vaccine antigens. Antibody responses to the tetravalent vaccine were equivalent to those after concomitant administration of A/H5N1 and seasonal influenza vaccines; therefore, combining the pandemic and seasonal vaccines did not negatively affect levels of immunogenicity. Three weeks after the second vaccine dose, GMAs against A/H5N1 by SRH were over 8-fold higher compared with baseline values, with similar levels observed in each group. All three immunization schedules met the CHMP criteria against A/H5N1 when assessed by SRH and MN assays; two out of three subject groups also met the CHMP criteria when assessed by HI assay. The antibody responses to the MF59-adjuvanted, tetravalent vaccine compared well with the monovalent A/H5N1 formulation, in this and previous studies,Citation9,Citation21 and compared well with the immunogenicity profiles of other adjuvanted A/H5N1 vaccines.Citation10,Citation15 Strong antibody responses were also observed against the seasonal influenza antigens, fulfilling all CHMP criteria against all three strains. The CHMP criterion for seroprotection was met by 99% of subjects against A/H1N1, 98–100% of subjects against A/H3N2, and 85–89% of subjects against the B strain.
These data demonstrate the potential benefits of combining MF59-adjuvanted A/H5N1 and non-adjuvanted seasonal influenza vaccines with regard to pre-pandemic priming or emergency use during a pandemic outbreak. The vaccine formulations in the present study were generally well tolerated, with AEs of expected frequency and severity for MF59-adjuvanted vaccine.Citation22,Citation23 Overall, solicited local and systemic reactions were more common after administration of the tetravalent vaccine. Reactions were transient, mainly mild to moderate in severity, and fewer after the second vaccine dose. The most frequently reported local reaction to either vaccine was pain, and the most frequently reported systemic reactions were fatigue, headache, and myalgia. No vaccine-related SAEs occurred during this study. Previously published data of spontaneously reported AEs from approximately 12 million administered doses of MF59-adjuvanted A/H1N1 vaccine during the 2009 pandemic support the good safety profile of MF59-adjuvanted vaccines.Citation9,Citation24,Citation25
This study provides evidence that MF59-adjuvanted A/H5N1 and non-adjuvanted seasonal influenza vaccines could be safely and effectively administered as a tetravalent formulation, or as separate injections. Using tetravalent formulations to merge seasonal and pre-pandemic vaccination programmesCitation26,Citation27 and thereby decrease the number of injections required by the individual may improve rates of compliance. Further immunogenicity and safety studies of tetravalent A/H5N1-seasonal influenza vaccine in larger populations with wider age ranges are warranted.
Materials and Methods
Study design and objective
This phase II, randomized, controlled, observer blind study was performed across two centers in Germany from November 2007 to November 2008. The protocol was approved by the local ethical committees, and the study was conducted in accordance with the Declaration of Helsinki and German regulatory requirements. All volunteers provided written informed consent before enrolment. The primary objective of this study was to demonstrate the equivalence of (Day 43) A/H5N1-specific antibody responses to each of the three different vaccination groups (Groups A, B, and C). Secondary objectives were to assess immunogenicity against A/H5N1, A/H1N1, A/H3N2, and B strains according to CHMP criteria; solicited local and systemic reactions, spontaneously reported AEs, and SAEs were also evaluated.
Subjects
Subjects (n = 601) were 18- to 60-y-old male and female adults in general good health, as determined by medical history, a physical examination, and the clinical judgment of the investigator. The main exclusion criteria included: prior receipt of 2007–2008 seasonal influenza vaccine; hypersensitivity to vaccine components; administration of another investigational agent within four weeks preceding the study; receipt of another vaccine within three weeks prior to the first visit, or planned vaccination within three weeks following the last study vaccination; acute illness or infection within seven days before enrolment; and fever within three days prior to the first visit. Subjects were randomized to three study groups (Groups A, B, and C) in a 1:1:1 ratio using a randomization schedule provided by the study sponsor. Subjects in Group A received tetravalent vaccine and placebo concomitantly (in separate arms) on Day 1, followed by A/H5N1 vaccine on Day 22. Subjects in Group B received A/H5N1 vaccine and placebo concomitantly (in separate arms) on Day 1, followed by tetravalent vaccine on Day 22. Subjects in Group C received A/H5N1 and seasonal vaccines concomitantly (in separate arms) on Day 1, followed by A/H5N1 vaccine on Day 22. Placebo injections were used to maintain blinding, ensuring that all subjects in Groups A, B, and C received one injection in both arms on Day 1. Blood samples were collected for immunogenicity analyses by HI, MN, and SRH assays on Day 1 (baseline), three weeks after the first vaccine dose (Day 22), and three weeks after the second vaccine dose (Day 43). Blood samples from the first 150 subjects enrolled also underwent clinical chemistry and hematology analyses (Interlab Laboratory). All subjects were monitored by an independent Data Monitoring Committee for local and systemic reactions occurring within seven days of each vaccination, and for any unsolicited AEs for six weeks after the first visit.
Vaccines
One 0.5 mL dose of the MF59-adjuvanted (9.75 mg squalene), pre-pandemic influenza vaccine (Aflunov®, Novartis Vaccines and Diagnostics) contained 7.5 µg of A/Vietnam/1194/2004 (H5N1; clade 1) hemagglutinin (HA) surface antigen. One 0.5 mL dose of the licensed, non-adjuvanted, trivalent, seasonal influenza vaccine (Agrippal®, Novartis Vaccines and Diagnostics) contained 15 µg HA from each of the three World Health Organization reference strains for the 2007–2008 influenza season in the northern hemisphere: A/Solomon Islands/3/2006 (H1N1); A/Wisconsin/67/2005 (H3N2); and B/Malaysia/2506/2004 (total 45 µg HA). One 0.5 mL dose of the MF59-adjuvanted, investigational tetravalent vaccine contained 7.5 µg of A/Vietnam/1194/2004 (H5N1) HA, 15 µg A/Solomon Islands/3/2006 (H1N1) HA, 15 µg A/Wisconsin/67/2005 (H3N2) HA, and 15 µg B/Malaysia/2506/2004 HA (total 52.5 µg HA). Placebo injections consisted of 0.5 mL saline. All vaccines were administered in the deltoid muscles of the arms. The randomization process for vaccine administration included the allocation of specific doses to specific arms of the subjects, to ensure that any solicited local reactions occurring at the sites of injection could be associated with the correct vaccine/dose. All vaccinations were performed by nurses who maintained blinding from the study participants, monitors, and investigators.
Immunogenicity analyses
Serum samples obtained on Days 1, 22, and 43 were kept at a temperature of −18°C or below and shipped to the Novartis Vaccines Clinical Serology Laboratory for further analyses. HI titers were expressed as the reciprocal of the highest dilution at which hemagglutination was totally inhibited. For MN assays, serial dilutions of serum started at 1:20, and the reciprocals of 2-fold dilutions that achieved ≥50% neutralization of viral growth were considered to be a positive result. Seroconversion in the individual was defined as (1) a negative pre-vaccination antibody titer of <10 to a positive post-vaccination titer of ≥ 40 by HI assay, and (2) a pre-vaccination area ≤4 mm2 to a post-vaccination area ≥25 mm2 by SRH assay. A significant increase in antibody titer was defined as a ≥4-fold increase for HI, and as a ≥50% increase in area for SRH. HI titers below the detection limit of 1:10 were assigned to half that limit for the purpose of analysis. SRH areas below the lower limit of detection were set to 4 mm2 for analysis. Antibody responses were assessed by SRH assay (for A/H5N1 only), HI assay (for A/H5N1, A/H1N1, A/H3N2, and B strains), and MN assay (for A/H5N1 only).
Safety analyses
Subjects were observed for 30 min after each immunization to monitor for immediate adverse reactions. Participants were provided with study diaries and the frequency and severity of a predefined set of solicited local and systemic reactions were recorded for seven days immediately after vaccination. Solicited local reactions (recorded for both injection sites when two vaccine doses were given concomitantly in separate arms) were pain at the injection site, erythema, ecchymosis, induration, and swelling. Solicited systemic reactions were chills, malaise, myalgia, arthralgia, headache, nausea, sweating, coughing, and fever (axillary temperature of ≥38.0 °C; high fever ≥40.0 °C). Other recorded indicators of reactogenicity were the use of analgesic or antipyretic medication, and events causing subjects to stay at home. The diameter of local reactions were categorised as either none, 1 to ≤10 mm, 11 to ≤25 mm, 26 to ≤50 mm, 51 to ≤100 mm, or >100 mm (severe). Reports of any AEs were recorded from Day 1 to Day 43. SAEs and AEs leading to study withdrawal were recorded throughout the entire study period. The severity of AEs were categorized as either mild, moderate, or severe, if they resulted in no limitation of, some limitation of, or an inability to perform normal daily activities, respectively. Assessments of the causal relationship between unsolicited AEs and vaccination were classified by the investigator as either not related, possibly related, or probably related.
Statistical analyses
Immunogenicity analyses were run on the per-protocol set (PPS), which consisted of subjects who received all the relevant vaccine doses correctly, provided at least one evaluable serum sample at the relevant time points, and had no major protocol violations. Safety was analyzed for all subjects who received study vaccine. A sample size of 200 subjects per group (600 subjects in total) was estimated to provide adequate power to assess the primary study objective (equivalence of Day 43 A/H5N1-specific antibody responses in Groups A, B, and C). No assumptions and power considerations were made for the secondary study objectives. Antibody responses were considered equivalent if the two-sided confidence interval (CI) of the GMA ratio was within the equivalence range of 0.5−2.0. As three hypotheses were tested, α = 0.0167 (= 0.05/3) was chosen to account for multiplicity, leading to a two-sided 96.67% CI. Log10-transformed antibody responses were modeled using analysis of variance (ANOVA) with vaccine groups and center as qualitative factors, and GMTs, GMRs, and corresponding CI were calculated. Immunogenicity was assessed according to the following European CHMP licensure criteria: the proportion of subjects achieving seroconversion or significantly increased antibody titers should be >40% (seroconversion criterion); pre- to post-vaccination GMRs should be >2.5 (GMR criterion); and the proportion of subjects achieving an HI titer ≥ 40 or SRH titer > 25 mm2 should be >70% (seroprotection criterion).
| Abbreviations: | ||
| AE | = | adverse event |
| BMI | = | body mass index |
| CHMP | = | Committee for Medicinal Products for Human Use |
| GMA | = | geometric mean area |
| GMR | = | geometric mean ratio |
| GMT | = | geometric mean titre |
| HI | = | hemagglutination inhibition |
| MN | = | microneutralization |
| PPS | = | per protocol set |
| SAE | = | serious adverse event |
| SD | = | standard deviation |
| SRH | = | single radial hemolysis |
Conflicts of interest
Fragapane E and Nicolay U are permanent employees of the study sponsor, Novartis Vaccines and Diagnostics. Borkowski A and Clemens R are former employees of Novartis Vaccines and Diagnostics. All other authors have no financial interest to declare, other than being reimbursed for any expenses incurred in performing the study.
Funding statement
The study was fully funded by the sponsor, Novartis Vaccines and Diagnostics.
The authors are grateful to all the volunteers who participated in this trial. The authors would also like to thank Jamie Stirling and Shivani Vadapalli (both Novartis Vaccines and Diagnostics), and Patricia de Groot (CHC Europe) for providing editorial assistance in the preparation of this manuscript.
Acknowledgments
MF59 is the licensed trademark of Novartis AG. Clinical Trials Registration: NCT00620815
References
- Belshe RB. Implications of the emergence of a novel H1 influenza virus. N Engl J Med 2009; 360:2667 - 8; http://dx.doi.org/10.1056/NEJMe0903995; PMID: 19423870
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, et al, Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708 - 19; http://dx.doi.org/10.1056/NEJMra1000449; PMID: 20445182
- World Health Organization. Influenza. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. 29 August 2013: http://www.who.int/influenza/human_animal_interface/EN_GIP_20130829CumulativeNumberH5N1cases.pdf.
- Neumann G, Chen H, Gao GF, Shu Y, Kawaoka Y. H5N1 influenza viruses: outbreaks and biological properties. Cell Res 2010; 20:51 - 61; http://dx.doi.org/10.1038/cr.2009.124; PMID: 19884910
- Russell CA, Fonville JM, Brown AE, Burke DF, Smith DL, James SL, Herfst S, van Boheemen S, Linster M, Schrauwen EJ, et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 2012; 336:1541 - 7; http://dx.doi.org/10.1126/science.1222526; PMID: 22723414
- Jennings LC, Monto AS, Chan PK, Szucs TD, Nicholson KG. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis 2008; 8:650 - 8; http://dx.doi.org/10.1016/S1473-3099(08)70232-9; PMID: 18922487
- Rebmann T, Zelicoff A. Vaccination against influenza: role and limitations in pandemic intervention plans. Expert Rev Vaccines 2012; 11:1009 - 19; http://dx.doi.org/10.1586/erv.12.63; PMID: 23002981
- Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med 2006; 354:1343 - 51; http://dx.doi.org/10.1056/NEJMoa055778; PMID: 16571878
- Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009; 4:e4384; http://dx.doi.org/10.1371/journal.pone.0004384; PMID: 19197383
- Leroux-Roels I, Borkowski A, Vanwolleghem T, Dramé M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet 2007; 370:580 - 9; http://dx.doi.org/10.1016/S0140-6736(07)61297-5; PMID: 17707753
- Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, Zambon MC. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet 2001; 357:1937 - 43; http://dx.doi.org/10.1016/S0140-6736(00)05066-2; PMID: 11425416
- Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, Zambon M. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine 2003; 21:1687 - 93; http://dx.doi.org/10.1016/S0264-410X(02)00632-1; PMID: 12639491
- Herfst S, Schrauwen EJ, Linster M, Chutinimitkul S, de Wit E, Munster VJ, Sorrell EM, Bestebroer TM, Burke DF, Smith DJ, et al. Airborne transmission of influenza A/H5N1 virus between ferrets. Science 2012; 336:1534 - 41; http://dx.doi.org/10.1126/science.1213362; PMID: 22723413
- Kelly H, Barr I. Large trials confirm immunogenicity of H1N1 vaccines. Lancet 2010; 375:6 - 9; http://dx.doi.org/10.1016/S0140-6736(09)62132-2; PMID: 20018366
- Manzoli L, De Vito C, Salanti G, D’Addario M, Villari P, Ioannidis JP. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One 2011; 6:e24384; http://dx.doi.org/10.1371/journal.pone.0024384; PMID: 21915319
- Fragapane E, Gasparini R, Schioppa F, Laghi-Pasini F, Montomoli E, Banzhoff A. A heterologous MF59-adjuvanted H5N1 prepandemic influenza booster vaccine induces a robust, cross-reactive immune response in adults and the elderly. Clin Vaccine Immunol 2010; 17:1817 - 9; http://dx.doi.org/10.1128/CVI.00461-09; PMID: 20810680
- Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med 2010; 2:ra5; http://dx.doi.org/10.1126/scitranslmed.3000624; PMID: 20371470
- Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:85ra48; http://dx.doi.org/10.1126/scitranslmed.3002336; PMID: 21632986
- Gasparini R, Schioppa F, Lattanzi M, Barone M, Casula D, Pellegrini M, Veitch K, Gaitatzis N. Impact of prior or concomitant seasonal influenza vaccination on MF59-adjuvanted H1N1v vaccine (Focetria) in adult and elderly subjects. Int J Clin Pract 2010; 64:432 - 8; http://dx.doi.org/10.1111/j.1742-1241.2009.02309.x; PMID: 20039974
- Vajo Z, Tamas F, Sinka L, Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009-10 influenza season: a multicentre, randomised controlled trial. Lancet 2010; 375:49 - 55; http://dx.doi.org/10.1016/S0140-6736(09)62039-0; PMID: 20018367
- Lopez P, Caicedo Y, Sierra A, Tilman S, Banzhoff A, Clemens R. Combined, concurrent, and sequential administration of seasonal influenza and MF59-adjuvanted A/H5N1 vaccines: a phase II randomized, controlled trial of immunogenicity and safety in healthy adults. J Infect Dis 2011; 203:1719 - 28; http://dx.doi.org/10.1093/infdis/jir191; PMID: 21606530
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959 - 65; http://dx.doi.org/10.1016/j.vaccine.2009.08.101; PMID: 19751689
- Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine 2008; 26:3209 - 22; http://dx.doi.org/10.1016/j.vaccine.2008.03.093; PMID: 18462843
- Crucitti A, Tsai TF. Explorations of clinical trials and pharmacovigilance databases of MF59®-adjuvanted influenza vaccines for associated cases of narcolepsy: a six-month update. Scand J Infect Dis 2011; 43:993; http://dx.doi.org/10.3109/00365548.2011.608714; PMID: 21851329
- Tsai TF, Crucitti A, Nacci P, Nicolay U, Della Cioppa G, Ferguson J, Clemens R. Explorations of clinical trials and pharmacovigilance databases of MF59®-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand J Infect Dis 2011; 43:702 - 6; PMID: 21534891
- Stöhr K. Vaccinate before the next pandemic?. Nature 2010; 465:161; http://dx.doi.org/10.1038/465161a; PMID: 20463719
- Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448 - 52; http://dx.doi.org/10.1038/nature04795; PMID: 16642006

