Abstract
Background
During development of an A/H1N1 pandemic influenza vaccine, this study was performed to identify the antigen and adjuvant content which would provide optimal antibody response and persistence in adults and the elderly. Dose-sparing strategies, such as inclusion of adjuvants, are critical in ensuring the widest possible population coverage in the event of an influenza pandemic, despite a limited global capacity for vaccine manufacture.
Methods
Healthy subjects aged 18−64 years (n = 1240) and ≥65 years (n = 1352) were vaccinated with 1 of 8 investigational vaccine formulations varying in antigen quantity (3.75 µg to 30 µg of hemagglutinin) and MF59® adjuvant (none, half dose, or full dose). All subjects received 2 vaccine doses administered 3 weeks apart. Antibody response was assessed by hemagglutination inhibition assay 1 and 3 weeks after administration of first and second doses. Antibody persistence was assessed after 6 and 12 mo. Vaccine safety was monitored over 12 mo.
Results
All 8 investigational A/H1N1 vaccine formulations were well tolerated, and rapidly induced high antibody titers which met all of the Center for Biologics Evaluation and Research (CBER) and Committee for Medicinal Products for Human Use (CHMP) licensure criteria 3 weeks after one dose. The highest antibody titers were observed in participants vaccinated with higher quantities of antigen and adjuvant.
Conclusion
A single vaccine dose containing 3.75 µg of A/California/7/2009 (H1N1) antigen with MF59 adjuvant was identified as optimal for young to middle-aged (18−64 years) and older (≥65 years) adult populations.
Introduction
The rapid global spread of the novel A/H1N1 influenza virus, A/California/7/2009, led to the World Health Organization (WHO) declaring the first pandemic of the 21st century in June 2009.Citation1 By mid-February 2010, the 2009 A/H1N1 virus had caused an estimated 59 million cases of influenza disease, 265 000 hospitalizations, and 12 000 deaths in the United States alone.Citation2 In 2009, the continued presence of the pandemic A/H1N1 virus was predicted for future winter influenza seasons in both the northern and southern hemispheres; the WHO recommended that A/California/7/2009 antigen should be included in vaccines for the 2010–2011 through to 2014–2015 influenza seasons accordingly.Citation3-Citation5
Influenza epidemics and pandemics result in substantial socioeconomic damage.Citation6 Mortality and morbidity rates are highest among vulnerable populations such as the elderly, who are especially likely to develop influenza-related complications.Citation7 Among healthy adults, influenza disease is responsible for high rates of outpatient hospital visits, work absenteeism, and general loss of productivity.Citation6,Citation8 Higher hospitalization rates were recorded among older adults during the 2009 pandemic compared with previous influenza seasons, and the highest fatality rates among hospitalized patients were observed in those over 50 y of age.Citation9 Mass vaccination is currently the most effective and economic method of inhibiting viral transmission during the early stages of a pandemic, resulting in much reduced levels of disease and socioeconomic disruption.Citation10
In periods of extremely high demand for vaccine, such as a pandemic, dose-sparing strategies are critical for ensuring that the widest possible population coverage is achieved from a limited supply of existing vaccine, and a limited capacity for further, emergency vaccine production. Decreasing the amount of antigen required per vaccine dose by using an adjuvant is one such strategy. MF59® (Novartis Vaccines), an oil-in-water adjuvant, has a well-established safety profileCitation11-Citation14 and, in addition to enhancing the antibody response to immunization and allowing for a reduction of antigen content per dose,Citation15-Citation22 has been demonstrated to enhance the production of cross-reactive antibodies which can provide the individual with heterologous immunity.Citation19,Citation23-Citation25 The use of adjuvants, such as MF59, in the development of A/H1N1 pandemic vaccines was recommended by the WHO in 2009 to ensure maximal population coverage.Citation26
We report the results of a randomized clinical trial conducted to assess safety, immunogenicity, and long-term persistence of antibodies in response to 8 distinct A/H1N1 vaccine formulations ranging in quantities of antigen and MF59 adjuvant. Antibody responses to vaccination were assessed by US and EU licensure criteria and the optimal antigen and adjuvant content was determined for use in young to middle-aged (18–64 y) and older (≥65 y) adults.Citation27,Citation28
Results
A total of 1240 young to middle-aged adults and 1352 older adult subjects (n = 2592) were included in the analysis. Across vaccine groups, 92–97% of young to middle-aged adult and 93–99% of older adult subjects completed the primary study phase, which was to 3 wk after receipt of the second vaccination (day 43). The main reasons for withdrawal were: subjects being lost to follow-up (2−6% of young to middle-aged adults and <1–2% of older adult subjects across groups) and withdrawal of consent (≤1% of young to middle-aged adults and <1−3% of older adult subjects across groups). Four subjects died during the study, and one subject withdrew following an adverse event (AE); none of these events were considered to be related to the study vaccination following assessment by the investigator. Six mo and 12 mo (days 202 and 387) after the second vaccine dose, 42−68% of enrolled subjects across age and vaccine groups agreed to an optional blood draw for antibody persistence analyses. The young to middle-aged and older adult study population demographics are shown in and , respectively. Within the young to middle-aged and older adult cohorts, these demographics, and the percentage who had received an influenza vaccine prior to the study were similar across the 8 vaccine groups. Immunogenicity was analyzed according to both Center for Biologics Evaluation and Research (CBER) and Committee for Medicinal Products for Human Use (CHMP) licensure criteria (). The full analysis set (FAS) and the per protocol set (PPS) were used for immunogenicity analyses as the difference between FAS and PPS was >10%. Data from the PPS are reported throughout.
Table 1. Study population demographics for young to middle-aged adult subjects (18−64 y)
Table 2. Study population demographics for older adult subjects (≥65 y)
Table 3. CBER (US) and CHMP (European) licensure criteria for hemagglutination inhibition (HI) immunogencity analyses in adult subjects
Immunogenicity—young to middle-aged adults (18–64 y)
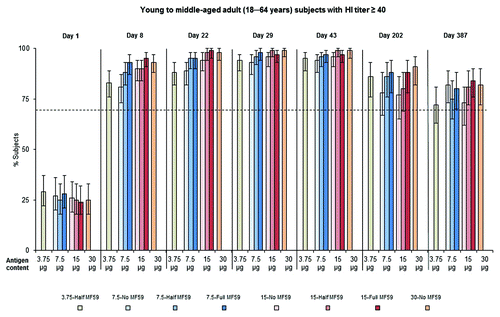
Across vaccine groups, 24−29% of young to middle-aged adult subjects (18–64 y) were seropositive (HI titer ≥ 10) at baseline (day 1). The CBER licensure criteria for seroconversion () and HI titer ≥ 40 () were met by all young to middle-aged adult vaccine groups 1 wk after receiving the first dose (day 8). By days 22 and 43 (3 wk after administration of the first and second vaccine doses, respectively) all groups met the CBER criterion for seroconversion, with percentage of young to middle-aged adults achieving this criterion ranging between 70–91% and 73–93%, respectively. On days 22 and 43, all groups met the CBER criterion for HI titer, with the percentage of young to middle-aged adults achieving this criterion ranging between 88–99% and 94–99%, respectively. Clinically significant long-term antibody persistence was evident in all young to middle-aged adult vaccine groups 6 mo (day 202) and 12 mo (day 387) after vaccination. On day 387, persisting antibody titers remained above levels which would meet the CBER criterion for seroconversion in all young to middle-aged adult vaccine groups (); similarly, percentages of young to middle-aged adults with HI titer ≥ 40 was above CBER criterion levels in all young to middle-aged adult groups apart from 15-No MF59, 3.75-Half MF59, and 7.5-Half MF59 (). All 8 young to middle-aged adult vaccine groups met the CHMP criteria for seroprotection (data not shown), seroconversion (data not shown), and geometric mean ratio (GMR) () at days 22 and 43.
Table 4. Young to middle-aged adult subjects (18−64 y)
Figure 1. Young to middle-aged adult (18−64 y) subjects achieving an HI titer ≥ 40 against the vaccine strain, A/California/7/2009 (H1N1), at baseline (day 1), 1 (day 8), and 3 (day 22) wk after the first vaccine dose, 1 (day 29) and 3 (day 43) wk after the second vaccine dose, and ∼6 mo (day 202) and ∼12 mo (day 387) after immunization. Broken line represents the CBER licensure criterion for HI titer ≥ 40 in adult subjects (70%).
Immunogenicity—older adults (≥65 y)
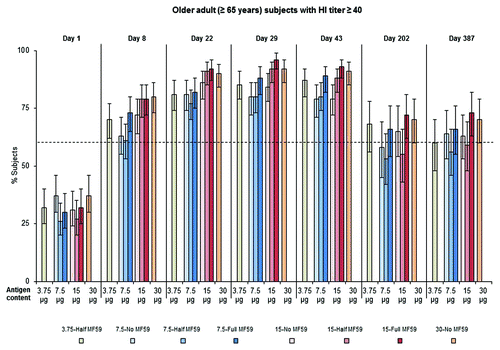
Across vaccine groups, 26−37% of older adult (≥65 y) subjects had an HI titer ≥ 10 at baseline (seropositive). The CBER licensure criteria for seroconversion () and HI titer ≥ 40 () were met by 6 of the 8 older adult vaccine groups 1 wk after receipt of the first dose (day 8); groups 7.5-No MF59 and 7.5-Half MF59 failed to meet the seroconversion and HI titer ≥ 40 criteria at this time point. All older adult vaccine groups met the CBER criteria for seroconversion and HI titer ≥ 40 by day 22. The percentage of older adult achieving seroconversion ranged between 52–72% and 51–73% on days 22 and 43, respectively, and the percentage of older adult with an HI titer ≥ 40 ranged between 77–92% and 79–93%, respectively. Clinically significant long-term antibody persistence was evident in all older adult vaccine groups 6 mo and 12 mo after vaccination. On day 387, persisting antibody titers in group 15-Full MF59 alone were above levels that would meet the CBER criteria for seroconversion () and HI titer ≥ 40 (). All 8 older adult vaccine groups has seroconversion levels that exceeded the CHMP criterion levels(data not shown), seroprotection (data not shown), and GMR () at days 22 and 43.
Table 5. Older adult subjects (≥65 y)
Figure 2. Older adult (≥65 y) subjects achieving an HI titer ≥ 40 against the vaccine strain, A/California/7/2009 (H1N1), at baseline (day 1), 1 (day 8), and 3 (day 22) wk after the first vaccine dose, 1 (day 29) and 3 (day 43) wk after the second vaccine dose, and ∼6 mo (day 202) and ∼12 mo (day 387) after immunization. Broken line represents the CBER licensure criterion for HI titer ≥ 40 in elderly subjects (60%).
Safety
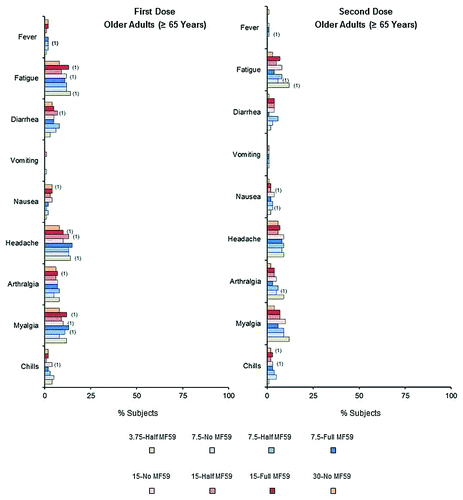
In young to middle-aged and older adult subjects, reporting of local reactions was slightly higher in subjects who were administered the MF59-adjuvanted vaccine, whereas reporting of systemic reactions was similar across all vaccine groups. There were generally fewer local and systemic reactions following the second dose than following the first dose. The incidences of any local reactions across vaccine groups after first and second doses were 31−61% and 26−46% for young to middle-aged adults, and 12−33% and 10−24% for the older adults, respectively. The incidences of any systemic reactions across vaccine groups after first and second doses were 33−44% and 19−29% for young to middle-aged adults, and 23−30% and 11−24% for the older adults, respectively. Injection site tenderness and pain were the most frequently reported local reactions, across all groups (). Most local reactions were mild to moderate in severity. Less than 1% of subjects reported severe local reactions and these only occurred following the first vaccine dose. One young to middle-aged adult subject experienced severe tenderness (3.75-Half MF59 group). One case of severe erythema (>10 cm) was reported in the older adult 15-Full MF59 group, which lasted for 2 days. Headache was the most commonly reported systemic reaction in both the young to middle-aged and older adult groups, reported by 19–24% and 11−21% of young to middle-aged adult subjects (), and 8−15% and 6−9% of older adult subjects () following the first and second doses, respectively. Across vaccine groups, severe systemic reactions were reported by ≤3% of young to middle-aged adults and ≤1% of older adult subjects. Less than 2% of subjects reported fever (≥38 °C) across all age and vaccine groups. One older adult subject who received the 7.5-Half MF59 vaccine reported high fever (≥40 °C) after both vaccine doses. Clinical safety assessments identified 13 young to middle-aged adult subjects with either decreased levels of hemoglobin, or increased levels of aspartate aminotransferase (AST) or alanine aminotransferase (ALT) (toxicity grades 3−4). Nineteen older adult subjects had either decreased levels of hemoglobin or increased levels of ALT, AST, or creatinine (toxicity grades 3−4). However, 72−93% of these abnormal findings were present on baseline analyses, and were therefore, not vaccine-related. The subjects with abnormal findings not present at baseline were spread across the vaccine groups.
Figure 3. Percentages of young to middle-aged (18−64 y) and older adult (≥65 y) subjects experiencing solicited adverse local reactions within 1 wk of receiving first and second vaccine doses. Numbers in parentheses show percentages of subjects experiencing severe local reactions.
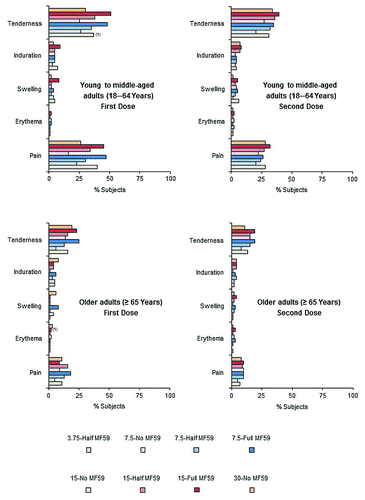
Figure 4. Percentages of young to middle-aged adult (18−64 y) subjects experiencing solicited adverse systemic reactions within 1 wk of receiving first and second vaccine doses. Numbers in parentheses show percentages of adult subjects experiencing severe systemic reactions.
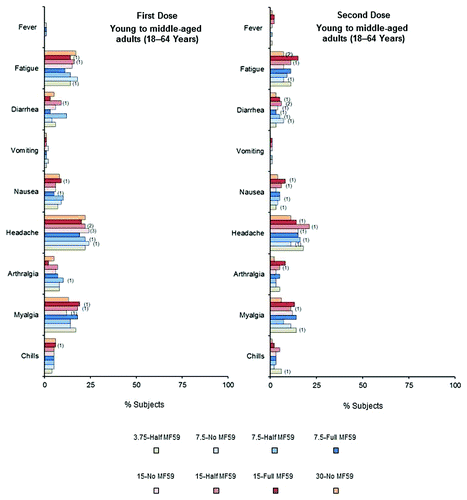
Figure 5. Percentages of older adult (≥65 y) subjects experiencing solicited adverse systemic reactions within 1 wk of receiving first and second vaccine doses. Numbers in parentheses show percentages of elderly subjects experiencing severe systemic reactions.
No pattern of association was observed between the numbers of spontaneously reported AEs and quantities of antigen or MF59 adjuvant. From vaccination to day 43, AEs were reported by 28–41% of young to middle-aged adults and 28−34% of older adult subjects; 3−8% of these cases were considered to be at least possibly related to vaccination. The most frequently reported AEs in the young to middle-aged adult vaccine groups were headache (2−8%), oropharyngeal pain (3–6%), and cough (2−6%). Among older adult vaccine groups, these were headache (1−4%), nasopharyngitis (1−4%), and cough (1−3%). During the 12-mo study period, 44 young to middle-aged adult subjects reported serious adverse events (SAEs), 2 cases of which were considered to be at least possibly related to receipt of the study vaccine. A 42-y-old African-American male in the 15-Half MF59 group experienced Bell’s palsy, which began 11 d after the second vaccine dose and had a duration of 33 d. Another case of Bell’s palsy was reported by a 20-y-old Caucasian female in the 7.5-Full MF59 group, with onset 9 d after the first vaccination and a duration of 2 d. Neither case of Bell’s palsy resulted in hospitalization nor was considered to be life threatening, and both subjects recovered completely by the end of the study period. A total of 64 young to middle-aged adult subjects experienced the onset of a new chronic disease, and the incidence was balanced across vaccine groups. The most commonly reported new onset of chronic disease included hypertension (14 subjects) and attention deficit disorder/hyperactivity and depression (5 subjects each). One case of hypertension was considered to be possibly vaccine-related; this was in a 48-y-old Caucasian male, with an onset of 51 d after the second dose (15-Full MF59 group). Two subjects experienced AEs which lead to their withdrawal from the study; none of these AEs were considered to be related to the vaccine. Across vaccine groups, medically attended visits ranged from 15−25% (primary study phase; day 1−43) and 42–50% (follow-up study phase; day 44−387). Two deaths occurred within the young to middle-aged adult cohort, neither was vaccine-related (cancer of the gall bladder, 7.5-Full MF59 group; cardiac arrest, 15-Full MF59 group).
In the older adult cohort, 112 subjects reported SAEs and 3 subjects withdrew from the study following an AE; none these events were considered to be related to the study vaccine. A total of 141 subjects experienced the onset of a new chronic disease, and the incidence was balanced across vaccine groups. The most commonly reported new onset of chronic disease included hypertension (15 subjects) and hypothyroidism (11 subjects). One case of idiopathic sleep disorder in a 74-y-old Caucasian male was considered to be possibly related to the study vaccine (7.5-No MF59 group, onset 52 d after first vaccination). Across vaccine groups, medically attended visits ranged from 29−41% during the primary study phase, and 70–82% during the follow-up study phase. Two deaths occurred within the older adult cohort, neither of which was vaccine-related (cardiac arrest, 7.5-No MF59 group; myocardial infarction, 7.5-Half MF59 group).
Discussion
The data from this study identified the investigational vaccine formulation containing 3.75 µg A/California/7/2009 (H1N1) influenza antigen together with MF59 adjuvant as optimal for young to middle-aged adult (18−64 y) and older adult (≥65 y) populations, based on the non-inferiority of the formulation containing the lowest antigen concentration, and the benefit of antigen-sparing in pandemic influenza outbreaks. This low-dose vaccine would be appropriate for pandemic use, ensuring maximal population coverage despite limited existing vaccine supplies and a limited global capacity for further production. The vaccine formulation which contained 15 µg antigen without adjuvant was also found to be highly immunogenic and provided acceptable levels of seroprotection in young to middle-aged and older adults; these data support the decisions of various health authorities to select the non-adjuvanted 15 µg vaccine for use during the 2009 A/H1N1 influenza pandemic. All 8 investigational vaccine formulations induced antibody responses at a level which met all CBER and CHMP licensure criteria by day 22 following vaccination.
The rapidity and strength of antibody responses which were seen following administration of the MF59-adjuvanted vaccine are similar to the results of other clinical trials conducted to evaluate the immunogenicity of MF59-adjuvanted vaccine in young to middle-aged and older adult populations.Citation29-Citation31 A recent meta-analysis of 18 randomized vaccine trials involving over 16 000 subjects that assessed the safety and immunogenicity of various A/H1N1 pandemic vaccines ranging in antigen and adjuvant contentCitation32 generally found a single dose of A/H1N1 vaccine to be adequate for adults and adolescents, whereas sometimes 2 vaccine doses were needed to provide an adequate level of seroprotection in older adults and children. Recent studies of H1N1 influenza vaccine have demonstrated that a single vaccine dose containing 3.75 µg of A/California/07/2009 antigen and the oil-in-water adjuvant AS03 is sufficiently immunogenic in young to middle-aged and older adult subjects.Citation33-Citation36 These data support our finding that one 3.75 µg dose of MF59-adjuvanted vaccine is optimal for these age groups. As this study was conducted during the A/H1N1 pandemic, it could be expected that the immunological response observed may have been in part due to prior exposure to the pandemic virus. However, in this study we excluded any subjects with laboratory-confirmed or suspected influenza disease within 6 mo prior to enrolment. Any sub-clinically infected subjects were not excluded but randomization would lead to these subjects not biasing the study results. Any effect on immunogenicity due to post-vaccination exposure to the pandemic virus would also have been identifiable, due to the reporting of any influenza-like symptoms as AEs during the safety follow-up.
The enhancement of long-term antibody persistence is an important requirement of any pandemic influenza vaccine. This study found that all 8 of the tested formulations lead to clinically significant long-term antibody responses which persisted up to 12 mo after administration. The ability of MF59-adjuvanted A/H1N1 vaccine to enhance seroprotective long-term antibody persistence compared with non-adjuvanted vaccine is well documented.Citation37,Citation38 The long-term antibody persistence seen during our study resulted from 2 primary vaccine doses. Further studies are required to assess whether these antibody titers significantly decrease 1 y after the single dose administration schedule, which has been identified as optimal. All the participants in the present study were judged at enrollment to be healthy, therefore, the results of this trial should not be extended to immunocompromised individuals. All investigational vaccine formulations were, in general, well tolerated, and showed safety profiles which were in agreement with previous data.Citation20
In this study, we did not include testing of the non-adjuvanted formulation with the lowest antigen content (3.75-No MF59) or the adjuvanted formulation with the highest antigen content (30-Full MF59), based on information on the immunogenicity of pandemic vaccines available at the time. Previous studies on A/H5N1 suggested that, without adjuvant, only the higher antigen doses could generate an acceptable antibody response,Citation39,Citation40 and given the A/H1N1 pandemic was occurring at the time of the study, it was considered unethical to expose subjects with a formulation that was expected to generate a very low antibody response (3.75-No MF59). Similarly, no adjuvanted formulation of the 30μg dose was tested, as previous studies had suggested that adjuvanted lower antigen content doses were sufficient in inducing an adequate antibody response, in line with dose-sparing objectives.
In summary, this study shows that the MF59-adjuvanted, A/H1N1 pandemic influenza vaccine was well tolerated and highly immunogenic. A single dose of MF59-adjuvanted vaccine containing 3.75 µg of hemagglutinin surface antigen was found to be adequate for young to middle-aged and older adult populations, leading to seroprotective antibody titers against A/H1N1 influenza disease which persisted for up to 12 mo after vaccination.
Methods
Study design and objectives
This phase II/III, randomized, multicenter, single-blind study was conducted across 21 sites in the US between September 2009 and October 2010. Prior to enrollment, written informed consent was obtained from all subjects. The study protocol was approved by a central Institutional Review Board for all study centers, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. The primary objective was to assess the immunogenicity of 8 distinct A/H1N1 vaccine formulations in healthy young to middle-aged and older adult subjects according to US licensure criteria (CBER).Citation27 Immunogenicity also was assessed according to CHMP criteria.Citation28 Safety and reactogenicity profiles were evaluated for each vaccine formulation. The long-term persistence of antibody responses was analyzed 6 and 12 mo after vaccination.
Subjects
In total, 1240 healthy young to middle-aged adult (18−64 y) and 1352 healthy older adult (≥65 y) subjects (n = 2592) were included in the study. Data obtained from an additional 127 subjects at one site were excluded from all analyses as the site failed a GCP audit. Individuals could not participate in the study if they did not agree to the retention of serum samples after completion of the study, could not follow the study procedures, or had a cognitive or behavioral impairment that may adversely affect their ability to participate. Additional exclusion criteria were: any history of or ongoing serious condition, anaphylaxis, or adverse reactions to any vaccine component; an altered or impaired immune system; laboratory-confirmed or suspected influenza disease up to 6 mo prior to enrollment; receipt of any inactivated vaccine 2 wk before enrollment or any live vaccine 4 wk before enrollment; receipt of an influenza vaccine within 1 wk before enrollment; receipt of any investigational agent within 30 d before enrollment; acute illness or fever within 3 days prior to enrollment; pregnancy or currently breast-feeding; and receipt of any antipyretic/analgesic medication within 24 h prior to enrollment. In addition, family or household members of study staff were excluded.
Study procedures
Subjects were assigned randomly, using an interactive, web-based, randomization system (Cenduit, Inc.), to 1 of 8 vaccine groups which differed in quantity of antigen and adjuvant content. Subjects were administered 2 doses of a vaccine containing either: 3.75 µg of antigen and half the standard quantity of MF59 adjuvant per dose (3.75-Half MF59); 7.5 µg of antigen with no MF59 (7.5-No MF59), half MF59 (7.5-Half MF59), or full MF59 (7.5-Full MF59) content; 15 µg of antigen with no MF59 (15-No MF59), half MF59 (15-Half MF59), or full MF59 (15-Full MF59) content; or 30 µg of antigen with no MF59 (30-No MF59). The vaccine doses were administered 3 wk apart, preferably into the deltoid muscle of the non-dominant arm. For immunogenicity analyses, blood samples (10 mL) were obtained at baseline (day 1) and at 1 (day 8) and 3 wk (day 22) after receipt of the first vaccine dose; 1 (day 29) and 3 wk (day 43) after receipt of the second vaccine dose; and 6 (day 202) and 12 (day 387) mo after the first vaccination. Additional blood samples were obtained on days 1, 8, and 22 for white blood cell counts and assessment of hemoglobin, ALT, AST, and creatine levels. Prior history of influenza vaccination was reviewed and recorded by the investigator at screening.
Vaccines
The vaccine used was an investigational, monovalent, pandemic, A/H1N1 influenza vaccine (Novartis Vaccines), which contained hemagglutinin and neuraminidase surface antigens that had been harvested following egg-based cultivation of the influenza strain A/California/7/2009 (H1N1). The seed virus was prepared from the reassortant virus NYMC X-179A (New York Medical College, New York) generated from the A/California/7/2009 strain, as recommended by the WHO.Citation41 The oil-in-water emulsion, MF59, is an adjuvant which contains naturally-occurring squalene. A full dose of MF59 contains 9.75 mg squalene. The investigational vaccine was manufactured using the same platform as the seasonal influenza vaccine, Fluvirin® (Novartis Vaccines), which is approved for persons 4 y of age and older, and is well established in the US market. The vaccine was supplied as monodose, pre-filled vials (non-adjuvanted vaccine) or syringes (MF59-adjuvanted vaccine). Adjuvanted and non-adjuvanted stock vaccines were mixed immediately before administration to generate the vaccine formulations containing 7.5 µg and 15 µg of antigen with half a dose of MF59. As this was a single-blind study, investigators were aware of which vaccine was being administered but mixing of the formulations was performed away from the subjects, to ensure subject blinding. Each vaccine dose received by subjects in the 3.75-Half MF59 vaccine group was administered in a volume of 0.25 mL; all other vaccine formulations were administered in a volume of 0.5 mL.
Immunogenicity assessment
Blood samples were centrifuged immediately after collection; sera were stored at –18 °C or below and transported to the Novartis Vaccines Clinical Serology for analysis. Antibody responses were measured by hemaglutination inhibition (HI) assay, following standard methodology.Citation42 HI titer was expressed as the reciprocal of the highest dilution at which hemagglutination was totally inhibited (e.g., a titer of 40 corresponds to a dilution of 1:40). The lower limit of detection for HI titers was 10 and therefore titers <10 were arbitrarily assigned as being half the limit (i.e., titer of 5) for the purposes of analysis. HI antibody responses were expressed as geometric mean titers (GMTs) and GMRs of post- to pre-vaccination titers. The US CBER and European CHMP licensure criteria defined seroconversion in individual young to middle-aged and older adult vaccinees as a pre-vaccination HI titer < 10 (seronegative at baseline) to a post-vaccination HI titer ≥ 40, or a ≥4-fold increase in HI titer for subjects with a pre-vaccination titer ≥ 10 (seropositive at baseline). All immunogenicity assays were performed using A/California/7/2009 (H1N1), the vaccine antigen strain.
Safety assessment
Subjects were observed for the first 30 min following each vaccination for any immediate adverse reactions. Subjects were given diary cards and the frequency and severity of predefined, solicited local and systemic reactions were recorded for 7 d after each vaccination. Solicited local reactions were pain, erythema, induration, swelling, and tenderness. Solicited systemic reactions were chills, myalgia, arthralgia, headache, nausea, vomiting, diarrhea, fatigue, and fever (≥38 °C). Other indicators of reactogenicity included use of analgesic or antipyretic medication, and events which caused the subject to remain at home. Local and systemic reactions were evaluated in accordance with CBER-defined toxicity grading scales: grade 1 (mild); grade 2 (moderate); grade 3 (severe); and grade 4 (potentially life threatening).Citation43 Unsolicited AEs were recorded from day 1 to day 43. SAEs, the onset of new chronic diseases, medically attended visits, and AEs leading to study withdrawal were recorded up to day 387 (the entire study period). Any SAEs were reported to the study sponsor within 24 h. The severity of AEs and causal relationships of AEs to the study vaccination were classified by the investigator. Severity was defined as mild, moderate, or severe, if they resulted in no limitation of, some limitation of, or an inability to perform normal daily activities, respectively.
Statistical analyses
Immunogenicity analyses are presented for the PPS, which included all enrolled subjects with no major protocol deviations, who correctly received the vaccine and provided evaluable serum samples at the relevant time points. The FAS included all enrolled subjects who received at least one dose of the study vaccine, and provided baseline and at least one post-baseline evaluable serum sample. As the FAS and the PPS differed by more than 10%, the immunogenicity analyses also were performed on the FAS (data not shown). Safety was analyzed for all vaccinated subjects. There were no formal statistical hypotheses tested for either the immunogenicity endpoints or the safety objectives. Group sample sizes were chosen to provide adequate estimates for immunogenicity endpoints based on CBER licensure criteria. The US CBER licensure criterion for group seroconversion was defined as: the lower bound of the two-sided 95% CI for the percentage of subjects achieving seroconversion (defined above) for HI antibody must be ≥40% and ≥30% for adult (<65 y) and elderly (≥65 y) subjects, respectively (). The CBER criterion for HI titer≥ 40 was defined as: the lower bound of the two-sided 95% CI for the percentage of subjects individually achieving an HI antibody titer ≥ 40 must be ≥70% and ≥60% for adult and elderly subjects, respectively. The European CHMP licensure criterion for group seroconversion was defined as: the percentage of subjects achieving seroconversion or significant increase (defined above) for HI antibody must be >40% and >30% for adult (18−60 y) and elderly (>60 y) subjects, respectively (). The CHMP criterion for group seroprotection was defined as: the percentage of subjects individually achieving an HI antibody titer ≥ 40 must be >70% and >60% for adult and elderly subjects, respectively. The CHMP criterion for group GMR was defined as: GMR must be >2.5 and >2.0 for adults and elderly subjects, respectively. Immunogenicity was analyzed to reflect the above endpoints, with corresponding two-sided 95% CIs calculated for each vaccine group. Safety data were evaluated descriptively. All statistical analyses were performed by Novartis Vaccines using SAS 9.1® software (SAS Institute).
| Abbreviations: | ||
| AE | = | adverse event |
| BMI | = | body mass index |
| CBER | = | Center for Biologics Evaluation and Research |
| CHMP | = | Committee for Medicinal Products for Human Use |
| FAS | = | full analysis set |
| GMR | = | geometric mean ratio |
| GMT | = | geometric mean titer |
| HI | = | hemagglutination inhibition |
| PPS | = | per protocol set |
| SAE | = | serious adverse event |
Disclosure of Potential Conflicts of Interest
A.A., P.P., and M.L. are permanent employees of Novartis Vaccines. S.H. was a permanent employee of Novartis Vaccines at the time of the study.
Funding Statement
This project has been funded in whole or in part with federal funds from the US Office of Public Health Emergency Preparedness, Office of Research and Development Coordination, under contract number HHSO100200700030C.
The authors wish to thank: all members of the clinical teams, including Meriza Viray, Charlys Trevino, and Michelle Kramer; Nicolaos Gaitzatis for serological/laboratory analyses; Ragini Khedoe, Michel Rehatta, and Kenneth Hansen for data management; Sandrine Tilman for statistical support, Kelly Lindert for study design (all Novartis Vaccines), and Jamie Stirling (Novartis Vaccines), Jennifer Howie (Novartis Vaccines), and Patricia de Groot (CtrlP) for providing editorial assistance in the preparation of this manuscript.
Author Contributions
All authors participated in the conception, design, and implementation of the trial. All authors were involved in the interpretation of analyzed data and the decision to submit for publication.
References
- World Health Organization. Director-General statement following the meeting of the Emergeny Committee. 2009: http://www.who.int/csr/disease/swineflu/4th_meeting_ihr/en/index.html.
- U.S. Centers for Disease Control and Prevention (CDC). Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009–February 13, 2010: http://www.cdc.gov/H1N1flu/estimates/April_February_13.htm.
- World Health Organization. Recommended viruses for influenza vaccines for use in the 2010-2011 northern hemisphere influenza season: http://www.who.int/influenza/vaccines/virus/recommendations/recommendations2010_11north/en/index.html.
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2012-2013 northern hemisphere influenza season. 2012: http://www.who.int/influenza/vaccines/virus/recommendations/2012_13_north/en/index.html
- World Health Organization. Recommended composition of influenza virus vaccines for use in the 2011-2012 northern hemisphere influenza season: http://www.who.int/influenza/vaccines/virus/2011_12north/en/index.html.
- Gasparini R, Amicizia D, Lai PL, Panatto D. Clinical and socioeconomic impact of seasonal and pandemic influenza in adults and the elderly. Hum Vaccin Immunother 2012; 8:21 - 8; http://dx.doi.org/10.4161/hv.8.1.17622; PMID: 22252007
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179 - 86; http://dx.doi.org/10.1001/jama.289.2.179; PMID: 12517228
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, Thompson WW, Wortley PM, Weintraub E, Bridges CB. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine 2007; 25:5086 - 96; http://dx.doi.org/10.1016/j.vaccine.2007.03.046; PMID: 17544181
- Bautista E, Chotpitayasunondh T, Gao Z, Harper SA, Shaw M, Uyeki TM, Zaki SR, Hayden FG, Hui DS, Kettner JD, et al, Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza. Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med 2010; 362:1708 - 19; http://dx.doi.org/10.1056/NEJMra1000449; PMID: 20445182
- Ferguson NM, Cummings DA, Fraser C, Cajka JC, Cooley PC, Burke DS. Strategies for mitigating an influenza pandemic. Nature 2006; 442:448 - 52; http://dx.doi.org/10.1038/nature04795; PMID: 16642006
- Banzhoff A, Haertel S, Praus M. Passive surveillance of adverse events of an MF59-adjuvanted H1N1v vaccine during the pandemic mass vaccinations. Hum Vaccin 2011; 7:539 - 48; http://dx.doi.org/10.4161/hv.7.5.14821; PMID: 21422814
- Crucitti A, Tsai TF. Explorations of clinical trials and pharmacovigilance databases of MF59®-adjuvanted influenza vaccines for associated cases of narcolepsy: a six-month update. Scand J Infect Dis 2011; 43:993; http://dx.doi.org/10.3109/00365548.2011.608714; PMID: 21851329
- Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine 2008; 26:3209 - 22; http://dx.doi.org/10.1016/j.vaccine.2008.03.093; PMID: 18462843
- Tsai TF, Crucitti A, Nacci P, Nicolay U, Della Cioppa G, Ferguson J, Clemens R. Explorations of clinical trials and pharmacovigilance databases of MF59®-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand J Infect Dis 2011; 43:702 - 6; PMID: 21534891
- Arguedas A, Soley C, Abdelnour A, Sales V, Lindert K, Della Cioppa G, Clemens R, Costa Rican H1N1 Vaccine Study Group. Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. Hum Vaccin 2011; 7:58 - 66; http://dx.doi.org/10.4161/hv.7.1.13411; PMID: 21285531
- Banzhoff A, Nacci P, Podda A. A new MF59-adjuvanted influenza vaccine enhances the immune response in the elderly with chronic diseases: results from an immunogenicity meta-analysis. Gerontology 2003; 49:177 - 84; http://dx.doi.org/10.1159/000069172; PMID: 12679609
- Camilloni B, Neri M, Lepri E, Iorio AM. Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. Vaccine 2009; 27:4099 - 103; http://dx.doi.org/10.1016/j.vaccine.2009.04.078; PMID: 19410623
- Durando P, Icardi G, Ansaldi F. MF59-adjuvanted vaccine: a safe and useful tool to enhance and broaden protection against seasonal influenza viruses in subjects at risk. Expert Opin Biol Ther 2010; 10:639 - 51; http://dx.doi.org/10.1517/14712591003724662; PMID: 20218923
- Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med 2011; 3:85ra48; http://dx.doi.org/10.1126/scitranslmed.3002336; PMID: 21632986
- Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine 2009; 27:6959 - 65; http://dx.doi.org/10.1016/j.vaccine.2009.08.101; PMID: 19751689
- Vesikari T, Karvonen A, Tilman S, Borkowski A, Montomoli E, Banzhoff A, Clemens R. Immunogenicity and safety of MF59-adjuvanted H5N1 influenza vaccine from infancy to adolescence. Pediatrics 2010; 126:e762 - 70; http://dx.doi.org/10.1542/peds.2009-2628; PMID: 20819892
- Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med 2011; 365:1406 - 16; http://dx.doi.org/10.1056/NEJMoa1010331; PMID: 21995388
- Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One 2009; 4:e4384; http://dx.doi.org/10.1371/journal.pone.0004384; PMID: 19197383
- Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A 2009; 106:7962 - 7; http://dx.doi.org/10.1073/pnas.0903181106; PMID: 19416838
- Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, et al. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc Natl Acad Sci U S A 2009; 106:3877 - 82; http://dx.doi.org/10.1073/pnas.0813390106; PMID: 19237568
- World Health Organization. Global alert and response: Recommendations on pandemic (H1N1) 2009 vaccines. 2009: http://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/index.html.
- U.S. Food and Drug Administration. Vaccines, Blood & Biologics. Guidance for Industry: Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines. 2007: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074786.htm.
- European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP). Guideline on dossier structure and content for pandemic influenza vaccine marketing authorisation application. 2008: http://www.ema.europa.eu/ema/.
- Fukase H, Furuie H, Yasuda Y, Komatsu R, Matsushita K, Minami T, Suehiro Y, Yotsuyanagi H, Kusadokoro H, Sawata H, et al. Assessment of the immunogenicity and safety of varying doses of an MF59®-adjuvanted cell culture-derived A/H1N1 pandemic influenza vaccine in Japanese paediatric, adult and elderly subjects. Vaccine 2012; 30:5030 - 7; http://dx.doi.org/10.1016/j.vaccine.2012.03.053; PMID: 22472791
- Hatz C, Cramer JP, Vertruyen A, Schwarz TF, von Sonnenburg F, Borkowski A, Lattanzi M, Hilbert AK, Cioppa GD, Leroux-Roels G. A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. Vaccine 2012; 30:4820 - 7; http://dx.doi.org/10.1016/j.vaccine.2012.05.013; PMID: 22626675
- Hatz C, von Sonnenburg F, Casula D, Lattanzi M, Leroux-Roels G. A randomized clinical trial to identify the optimal antigen and MF59(®) adjuvant dose of a monovalent A/H1N1 pandemic influenza vaccine in healthy adult and elderly subjects. Vaccine 2012; 30:3470 - 7; http://dx.doi.org/10.1016/j.vaccine.2012.03.017; PMID: 22446638
- Manzoli L, De Vito C, Salanti G, D’Addario M, Villari P, Ioannidis JP. Meta-analysis of the immunogenicity and tolerability of pandemic influenza A 2009 (H1N1) vaccines. PLoS One 2011; 6:e24384; http://dx.doi.org/10.1371/journal.pone.0024384; PMID: 21915319
- Ferguson M, Risi G, Davis M, Sheldon E, Baron M, Li P, Madariaga M, Fries L, Godeaux O, Vaughn D. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. J Infect Dis 2012; 205:733 - 44; http://dx.doi.org/10.1093/infdis/jir641; PMID: 22315336
- Ikematsu H, Tenjinbaru K, Li P, Madan A, Vaughn D. Evaluation of immune response following one dose of an AS03A-adjuvanted H1N1 2009 pandemic influenza vaccine in Japanese adults 65 y of age or older. Hum Vaccin Immunother 2012; 8:1119 - 25; http://dx.doi.org/10.4161/hv.21081
- Roman F, Clément F, Dewé W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol 2011; 18:835 - 43; http://dx.doi.org/10.1128/CVI.00480-10; PMID: 21450978
- Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis 2010; 51:668 - 77; http://dx.doi.org/10.1086/655830; PMID: 20687838
- Block SL, Ruiz-Palacios GM, Guerrero ML, Beygo J, Sales V, Holmes SJ. Dose-range study of MF59-adjuvanted versus nonadjuvanted monovalent A/H1N1 pandemic influenza vaccine in six- to less than thirty-six-month-old children. Pediatr Infect Dis J 2012; 31:e92 - 8; http://dx.doi.org/10.1097/INF.0b013e318257644f; PMID: 22481427
- Nassim C, Christensen S, Henry D, Holmes S, Hohenboken M, Kanesa-Thasan N. Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to 1 year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to < 9 years of age. Pediatr Infect Dis J 2012; 31:e59 - 65; http://dx.doi.org/10.1097/INF.0b013e31824b9545; PMID: 22418661
- Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, Noah DL, He F, Hill H. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 2008; 197:667 - 75; http://dx.doi.org/10.1086/527489; PMID: 18260764
- Banzhoff A, Pellegrini M, Del Giudice G, Fragapane E, Groth N, Podda A. MF59-adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza Other Respir Viruses 2008; 2:243 - 9; http://dx.doi.org/10.1111/j.1750-2659.2008.00059.x; PMID: 19453401
- World Health Organization. Global Alert and Response. Availability of a candidate reassortant vaccine virus for the novel influenza A (H1N1) vaccine development X-179A. 2009: http://www.who.int/csr/resources/publications/swineflu/candidates_X-179a/en/.
- Stephenson I, Nicholson KG, Wood JM, Zambon MC, Katz JM. Confronting the avian influenza threat: vaccine development for a potential pandemic. Lancet Infect Dis 2004; 4:499 - 509; http://dx.doi.org/10.1016/S1473-3099(04)01105-3; PMID: 15288823
- U.S. Food and Drug Administration. Vaccines, Blood & Biologics. Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. 2007: http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074775.htm.

