Abstract
HB-AS02 is an investigational adjuvanted hepatitis B virus (HBV) vaccine for potential use in patients with renal insufficiency and other immunocompromized individuals. In this Phase III lot-to-lot consistency study, 450 healthy adult volunteers who had not previously been vaccinated against HBV were randomized to one of three production lots of HB-AS02 at 0 and 1 month and followed until one month after the last vaccine dose. Lot-to-lot consistency was established. High seroprotection rates were already achieved after the first vaccine dose (75.9%). All subjects were seroprotected (anti-HBs antibody concentrations ≥10 mIU/ml) after two doses, with all but one subject achieving anti-HBs antibody concentrations ≥100 mIU/ml (99.7%). Geometric mean anti-HBs antibody concentration was 4594.5 mIU/ml. Local and general symptoms were reported after 80.7% and 45.5% of doses, respectively. However, these were mainly of mild or moderate severity and no subject withdrew from the study due to adverse events.
Introduction
Despite the availability of effective vaccines, hepatitis B virus (HBV) infection remains a major global health problem.Citation1 Worldwide, approximately 2 billion people have been infected with the virus and about 350 million of these have chronic infection.Citation2,Citation3 Approximately one-third of all cases of cirrhosis and half of all cases of hepatocellular carcinoma can be attributed to chronic HBV infection, with HBV infection estimated to be responsible for approximately 600,000 deaths worldwide each year.Citation2,Citation3
HB-AS02 is an investigational adjuvanted HBV vaccine for potential use in patients with renal insufficiency and other immunocompromised individuals who could have a suboptimal response to conventional recombinant HBV vaccines. The prevalence of viral hepatitis is far higher in dialysis patients than in the general population, due to the possibility for exposure during the dialysis procedure.Citation4 In addition, patients with renal insufficiency are known to have an impaired immune response to conventional recombinant HBV vaccines compared to healthy individuals.Citation5–Citation7 HB-AS02 contains recombinant HBV surface antigen (HBsAg) formulated with an oil-in-water emulsion-based Adjuvant System containing monophosphoryl lipid (MPL), a detoxified derivative of the lipopolysaccharide molecule of the bacterial wall of Salmonella minnesota, and QS21, an immunostimulant extracted from the bark of the South American Quillaja saponaria tree.Citation8
A three-dose primary course of HB-AS02 has been shown to induce more rapid, enhanced and persistent protection in predialysis, peritoneal dialysis and haemodialysis patients than a four-dose primary course of HB-AS04 (FENDrix®), the first adjuvanted HBV vaccine licensed in Europe for use in patients with renal insufficiency.Citation9,Citation10 HB-AS02 administered according to a 0-, 1-, 10-month schedule has also been shown to elicit strong and persistent antibody responses against HBsAg in healthy adult volunteers, with all subjects seroprotected after two vaccine doses.Citation11 This lot-to-lot consistency study was undertaken to assess the immunogenicity, safety and reactogenicity of two doses of HB-AS02 in a larger cohort of healthy HBV vaccine-naïve adult volunteers. Three manufacturing lots were used and compared for consistency of immune response.
Results
Study population.
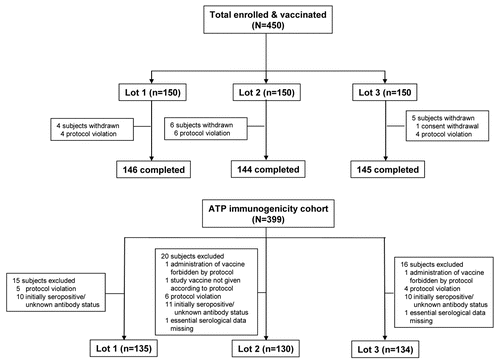
A total of 450 subjects were enrolled in this study (150 in each of the three lot groups). Fifteen subjects were withdrawn from the study (4, 6 and 5 from the three lot groups, respectively). A total of 14 subjects were withdrawn due to protocol violation (prior HBV vaccination in all cases) and 1 subject withdrew consent (not due to an adverse event). In all, 51 subjects were excluded from the according to protocol (ATP) analysis of immunogenicity (15, 20 and 16 subjects from the each of the three lot groups, respectively). Reasons for exclusion from the ATP cohort for immunogenicity are summarized in . The three lot groups were similar in terms of baseline demographic characteristics (). Mean (SD) age of the ATP cohort was 29.9 (6.03) years, 50.4% were female and all were Caucasian.
Immunogenicity.
Anti-HBs seroprotection rates, the proportion of subjects with anti-HBs antibody concentrations ≥100 mIU/ml and anti-HBs geometric mean antibody concentration (GMC) before vaccination and after each vaccine dose in each of the three lot groups are shown in . For each pair of lots, the two-sided asymptotic standardised 95% confidence intervals (95% CI) on the difference between lots in terms of anti-HBs seroprotection rates at Month 2 was within the pre-defined range for equivalence of [−10%, 10%] (difference between groups: 0.00% [95% CI: −2.88, 2.88] for all lot comparisons). Similarly, for each pair of lots, the two-sided 95% CI on the anti-HBs geometric mean antibody concentration (GMC) ratio between the groups at Month 2 was within the pre-defined interval of [0.5, 2.0] (GMC ratios: 1.01 [95% CI: 0.80, 1.28] for lot 1/lot 2; 1.08 [95% CI: 0.86, 1.37] for lot 1/lot 3; 1.07 [95% CI: 0.84, 1.35] for lot 2/lot 3). Thus, lot-to-lot consistency among the three lots of HB-AS02 vaccine was established and data are presented for the pooled vaccine lot groups (). Most subjects were seropositive for anti-HBs antibodies one month after the first vaccine dose (94.7%), and all were seropositive one month after the second vaccine dose. Seroprotection rates were already high one month after the first vaccine dose (75.9%). All subjects were seroprotected one month after the second vaccine dose, with all but one subject (99.7%) having anti-HBs antibody concentrations ≥100 mIU/ml at this time. Anti-HBs antibody GMC was 4594.5 mIU/ml at Month 2.
Reactogenicity and safety.
Solicited and unsolicited local symptoms were reported after 80.7% of doses and general symptoms after 45.5% of doses. These were of grade 3 severity after 5.2% and 4.1% of doses, respectively. Pain was the most frequently reported solicited local symptom, occurring after 80.0% of doses (). However, this was of grade 3 severity after only 3.7% of doses and no cases required medical attention. Fatigue was the most frequently reported solicited general symptom, occurring after 37.3% of doses. This was of grade 3 severity after 2.1% of doses, necessitating medical attention in 2 cases (0.2%). There was no increase in the incidence of local symptoms and a slight increase in the incidence of all general symptoms after the second vaccine dose (data not shown).
Unsolicited symptoms considered related to vaccination were reported following 5.2% of vaccine doses. The most frequently reported causally related unsolicited symptoms were chills, myalgia, influenza-like illness and arthralgia (all reported following <2% of doses). The great majority of causally related unsolicited symptoms were of mild or moderate intensity, with symptoms of grade 3 intensity reported after only 0.6% of doses. Only 1 serious adverse (SAE) was reported during the study (vomiting and diarrhoea with onset 18 days after the first vaccine dose). This was considered by the investigator to have most probably been caused by a gastrointestinal infection and was not considered to be related to vaccination. No subject withdrew from the study due to adverse events or SAEs.
Discussion
HB-AS02 is an investigational adjuvanted HBV vaccine for potential use in patients with renal insufficiency who require dialysis and other immunocompromised individuals who could have a suboptimal response to conventional recombinant HBV vaccines. A first adjuvanted HBV vaccine HB-AS04 (FENDrix®) was licensed in Europe in 2005 for active immunization against HBV for patients with renal insufficiency (including pre-dialysis and haemodialysis patients) aged over 15 years.Citation9 In a previous study, a three-dose primary course of HB-AS02 at 0, 1 and 6 months was found to induce more rapid, enhanced and persistent protection in pre-dialysis, peritoneal dialysis and haemodialysis patients than a four-dose primary course of HB-AS04 at 0, 1, 2 and 6 months.Citation10
The present study demonstrated lot-to-lot consistency of the HB-AS02 vaccine in terms of immunological response in healthy HBV vaccine-naive adult volunteers one month after completion of a two-dose vaccination course. All subjects were found to be seroprotected one month after two vaccine doses, with all but one subject achieving anti-HBs antibody concentration ≥100 mIU/ml. Studies in hemodialysis patients have shown anti-HBs antibody concentrations ≥100 mIU/ml following vaccination to be predictive of long-term persistence of circulating antibodies.Citation12–Citation14 Indeed, this cut-off is considered to be the best surrogate marker of long-term seroprotection in immunocompromised patients by a number of vaccination advisory bodies.Citation15,Citation16 Results of an earlier study of the HB-AS02 vaccine in a smaller group of healthy volunteers also found all subjects to be seroprotected after two vaccine doses.Citation11 This compares with seroprotection rates of 50–95% in healthy adults one month after two doses of a nonadjuvanted HBV vaccine based on the same recombinant HBsAg as HB-AS02 (Engerix-B®).Citation17
Of note, 75% of subjects were already seroprotected after just one dose of HB-AS02 in the present study. Similarly, in the previous study in healthy volunteers, 85.7% of subjects were found to be seroprotected after the first vaccine dose.Citation11 In contrast, only 5.4–20.4% of healthy adults are seroprotected after just one dose of non-adjuvanted HBV vaccine.Citation17 Likewise, in the previous comparative study in patients with renal insufficiency, 77.0% of subjects were found to be seroprotected after just one dose of HB-AS02 compared with only 39.0% of those who received HB-AS04.Citation10 The seroprotection rates achieved in healthy volunteers vaccinated with HB-AS02 in this study are similar to those seen with another adjuvanted HBV vaccine in clinical development (HBV-ISS; Heplisav™).Citation18 HBV-ISS consists of HBsAg with an immunostimulatory DNA sequence containing cytosine phosphoguanine motifs that stimulate immune response.Citation18 In a Phase II study, 79% of healthy volunteers achieved seroprotective anti-HBs antibody levels after one dose of HBV-ISS compared with only 12% of those who received a conventional non-adjuvanted recombinant HBV vaccine.Citation19
Persistence of anti-HBs is known to be closely related to the peak antibody concentration obtained post-vaccination.Citation20–Citation22 Two doses of the HB-AS02 vaccine induced GMCs of 4594.5 mIU/ml in healthy volunteers in this study and 8,000 mIU/ml in a previous trial.Citation11 This compares with anti-HBs GMCs of 18–65 mIU/ml in healthy adults one month after two doses of non-adjuvanted HBV vaccine,Citation17 and 1,603 mIU/ml in healthy volunteers after two doses of HBV-ISS.Citation19 In the comparative study in patients with renal insufficiency, anti-HBs GMCs were 83 mIU/ml after two doses of HB-AS02 compared with only 7.3 mIU/ml after two doses of HB-AS04.Citation10 Peak anti-HBs antibody concentrations achieved one month after completion of the respective three- and four-dose vaccination courses were 10401.9 and 66.8 mIU/ml, respectively.Citation10 Twelve months after the first vaccine dose, 94% of subjects who received HB-AS02 remained seroprotected compared with 79% of those who received HB-AS04.Citation10
The enhanced immunogenicity of HB-AS02 compared with conventional recombinant HBV vaccines and HB-AS04 is most likely linked to the adjuvant used. Adjuvants have long been used to enhance the immune response to vaccine antigens.Citation23 HB-AS04 consists of yeast-derived recombinant HBsAg formulated with MPL and aluminum phosphate. Results of clinical studies show HB-AS04 to elicit higher and more persistent levels of anti-HBs antibodies than double doses of a conventional recombinant HBV vaccine.Citation24–Citation27 The non-aluminum based AS02 adjuvant system combines MPL and QS21 in an oil-in-water emulsion with synergistic immunostimulant properties.Citation8 The AS02 adjuvant system has previously been shown to elicit stronger and more persistent antibody responses against HBsAg both in healthy adult volunteers and in patients with renal insufficiency than adjuvant systems containing MPL alone.Citation10,Citation11 As well as inducing high levels of antibodies, HB-AS02 also induced a strong and persistent CD4+ T-cell response in healthy volunteers, characterized by T cells with high lymphoproliferative capacity and a Th1 profile.Citation11 The AS02 adjuvant has also been used in other candidate vaccines against complex pathogens, including Plasmodium falciparum.Citation8,Citation28
The reactogenicity profile of the HB-AS02 vaccine was as previously reported.Citation10,Citation11 Although associated with a higher rate of local and general symptoms than seen with conventional recombinant HBV vaccines, these reactions were predominantly mild to moderate in intensity, transient in nature and rarely required medical attention. Furthermore, no subject withdrew from the study due to adverse events and no vaccine-related SAEs were reported. The HB-AS02 vaccine was also found to be more reactogenic than HB-AS04 in patients with renal insufficiency.Citation10 However, as in the present study, local and general reactions were mainly mild to moderate in intensity and transient in nature, with no increase in reactogenicity observed after the third vaccine dose.Citation10 Given the improved immunogenicity profile of HB-AS02, this increase in reactogenicity is likely to be acceptable in patients with renal insufficiency and other patient populations who have a suboptimal response to conventional recombinant HBV vaccines.
The main limitation of this study is that immunogenicity, reactogenicity and safety were assessed after only two vaccine doses. However, the main aim of this study was to demonstrate manufacturing consistency. It was considered that any differences in immunogenicity between vaccine lots would be more likely to be detected in healthy subjects who had not previously been vaccinated against HBV than in the target population of patients with renal insufficiency, who would most likely be more variable both in terms of HBV vaccination history and immune response. In a previous study in healthy volunteers, all subjects who received the investigational HB-AS02 vaccine had been shown to be seroprotected after only two vaccine doses.Citation11 Given that this vaccine is not intended for use in healthy subjects, it was therefore not considered necessary or appropriate to administer additional doses of this vaccine in order to demonstrate lot-to-lot consistency. The immunogenicity, reactogenicity and safety of the full three-dose course of HB-AS02 has been assessed in the target population of patients with renal insufficiency in an international multicenter Phase III trial.Citation10 Results showed the three-dose primary course of HB-AS02 to induce more rapid, enhanced and persistent protection in pre-dialysis, peritoneal dialysis and hemodialysis patients than a four-dose primary course of HB-AS04, an adjuvanted HBV vaccine already licensed in Europe for use in this patient population.Citation10
In summary, the investigational adjuvanted HBV vaccine HB-AS02 was found to be highly immunogenic in healthy adult volunteers, with all subjects seroprotected after just two doses administered 1 month apart. Coupled with clinical experience in patients with renal insufficiency,Citation10 our findings suggest that adjuvanted vaccines such as this may have potential utility in distinct populations who are at risk for contracting HBV infection and have a suboptimal response to conventional recombinant HBV vaccines.
Materials and Methods
Study design and population.
This was a Phase III, randomized, double-blind, single centre study undertaken in the Czech Republic between January–April 2008. Male and female healthy adults aged 18–40 years at the time of the first vaccination who had not previously been vaccinated against HBV were eligible for inclusion. Female subjects of child-bearing potential were required to have a negative pregnancy test and to use adequate contraception from 30 days prior to vaccination to 2 months after completion of the vaccination course. Pregnant and lactating women were excluded from study entry. Other exclusion criteria were: prior HBV vaccination or infection; known exposure to HBV during the previous 6 months; a history of allergic disease likely to be exacerbated by any vaccine component; chronic administration (defined as more than 14 days) of immunosuppressive or immune-modifying therapy within 6 months of study entry; administration of immunoglobulins and/or any blood products within 3 months of the first vaccine dose; use of any investigational or non-registered drug or vaccine within 30 days of the first dose of study vaccine or planned use during the study period; and use of any other registered vaccine within 14 (for inactivated/non-live vaccines) or 30 (for attenuated/live vaccines) days of the first vaccine dose.
Subjects were randomly assigned in a 1:1:1 ratio to receive one of three production lots of the HB-AS02 vaccine at 0 and 1 month as an intramuscular injection in the deltoid region of the arm. Subjects were followed until one month after the last vaccine dose (i.e., Month 2).
This study was sponsored by Henogen. The study was approved by the ethics committee of the University Hospital, Hradec Králové, Czech Republic and was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects provided written informed consent to participate.
Study vaccine.
The HB-AS02V vaccine was manufactured by GlaxoSmithKline Biologicals (Rixensart, Belgium). Each 0.5 ml dose of the HB-AS02V vaccine contained AS02V (oil-in-water emulsion-based Adjuvant System [50 µg MPL + 50 µg QS21]) and 20 µg recombinant HBsAg produced in Saccharomyces cerevisiae. Three consecutive production lots were used (DHBVA015A/DA2VA008A, DHBVA016A/DA2VA009A and DHBVA017A/DA2VA010A).
Assessment of immunogenicity.
Blood samples were obtained at months 0, 1 and 2 and assayed for the presence of anti-HBs antibodies using a commercial enzyme immunoassay kit (AxSYM; Abbott Laboratories, Chicago, USA). Subjects with anti-HBs antibody concentrations greater than or equal to the assay cut-off (2 mIU/ml) were considered seropositive. Anti-HBs antibody concentrations ≥10 mIU/ml were considered seroprotective.Citation29,Citation30 The proportion of subjects with anti-HBs antibody concentrations ≥100 mIU/ml was also assessed.
Assessment of reactogenicity and safety.
Subjects were provided with diary cards to record the occurrence of solicited local (pain, redness and swelling at the injection site) and general (fatigue, fever, gastrointestinal symptoms [nausea, vomiting, diarrhea and/or abdominal pain] and headache) symptoms on the day of vaccination and for 3 subsequent days. Fever was defined as axillary or oral temperature ≥37.5°C. The intensity of solicited symptoms was rated on a scale of 0.3, with grade 3 symptoms defined as redness or swelling >50 mm in diameter, fever >39.5°C or as preventing normal daily activities for all other symptoms. Unsolicited symptoms and SAEs were recorded up to 30 days after each vaccine dose.
Statistical analysis.
Anti-HBs seropositivity and seroprotection rates, the percentage of subjects with anti-HBs antibody concentrations ≥100 mIU/ml and anti-HBs antibody GMCs were calculated with exact 95% CI in each group at Months 0, 1 and 2. GMC calculations were performed by taking the antilog of the mean of the log transformations of antibody concentrations. Antibody concentrations below the cut-off of the assay were given an arbitrary value of half the cut-off value (i.e., 1.0 mIU/ml) for the purpose of this analysis.
The primary objective of this study was to demonstrate the lotto- lot consistency of 3 manufacturing lots of HB-AS02 in terms of anti-HBs seroprotection rate (i.e., the percentage of subjects with anti-HBs antibody concentrations ≥10 mIU/ml) at Month 2. The primary analysis was performed on the ATP cohort for immunogenicity. The target sample size was 405 subjects to be evaluated for immunogenicity (135 subjects in each vaccine lot group), allowing the study at least 95% power to conclude on the primary objective. Allowing for approximately 10% of subjects not to be evaluable for the analysis of immunogenicity, 150 subjects per group (a total of 450 subjects) were planned to be enrolled. For each pair of lots, the two-sided asymptotic standardized 95% CI on the difference between lots in terms of anti- HBs seroprotection rate at Month 2 was computed. Lot-to-lot consistency was demonstrated if 95% CI were within the range [−10%, 10%] for all three vaccine lot pairs. For each pair of lots at Month 2, the two sided 95% CI on the anti-HBs GMC ratio was also computed using an analysis of variance (ANOVA) model on the log10 transformation of the concentrations. The ANOVA model included the vaccine group as the only fixed effect. Lot-to-lot consistency was considered observed if 95% CI were within [0.5, 2.0] for all three vaccine lot pairs.
Analysis of reactogenicity was performed on the total vaccinated cohort. The percentage of doses followed by any solicited or unsolicited symptoms was calculated with exact 95% CI in each group.
Conflict of interest
Authors acknowledge the following potential conflicts of interest: Ms. Sherine Kuriyakose and Dr. Maarten Leyssen are employed by GlaxoSmithKline Biologicals; Dr. Murielle Surquin was employed by Henogen at the time of this trial and currently working at CHU-Brugmann; Dr. Sophie Houard was employed by Henogen at the time of this trial and currently working at European Vaccine Initiative; Dr. Jirí Beran, Dr. Lenka Hobzová and Dr. Veronika Wertzová declare no conflict of interest.
FENDrix and Engerix-B are registered trademarks of the GlaxoSmithKline group of companies; Heplisav is a trademark of Dynavax Technologies Corp.
ClinicalTrials.gov Identifier: NCT00480116.
Abbreviations
| HBV | = | hepatitis B virus |
| HBsAg | = | hepatitis B virus surface antigen |
| MPL | = | monophosphoryl lipid |
| SD | = | standard deviation |
| GMC | = | geometric mean antibody concentration |
| 95% CI | = | 95% confidence interval |
| SAE | = | serious adverse event |
Figures and Tables
Figure 2 Anti-HBs seroprotection rates, proportion of subjects with antibody concentrations ≥100 mIU/ml and GMCs for the three vaccine lots (ATP cohort for immunogenicity). Footnote: Error bars indicate 95% CIs
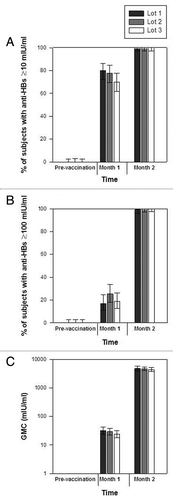
Table 1 Demographic characteristics of the three HB-AS 02 vaccine groups (ATP cohort for immunogenicity)
Table 2 Anti-HBs seropositivity rates, seroprotection rates, proportion of subjects with antibody concentrations .100 mIU/ml and GMCs (ATP cohort for immunogenicity)
Table 3 Percentage of vaccine doses followed by solicited local and general symptoms (Total vaccinated cohort)
Acknowledgements
The authors wish to thank all members of the local study teams, particularly Martina Voborníková and Alena Žáková. The authors also thank Jennifer Coward (Independent Medical Writer, Bollington, UK) for writing and editorial assistance in the preparation of this manuscript, Christina Caporaso, Krystel Carlier, Edwin Kolp and Xavier Barthelemy (Henogen SA, Gosselies, Belgium) for their advice and technical assistance and Manjula K. for publication management (GlaxoSmithKline Biologicals).
References
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: A historical overview. Vaccine 2008; 26:6266 - 6273
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 2004; 11:97 - 107
- World Health Organization (WHO). Hepatitis B. Fact sheet N°204 2010 Revised August 2008. Available at: http://www.who.int/mediacentre/factsheets/fs204/en/
- Wong PN, Fung TT, Mak SK, Lo KY, Tong GM, Wong Y, et al. Hepatitis B virus infection in dialysis patients. J Gastroenterol Hepatol 2005; 20:1641 - 1651
- Peces R, Laurés AS. Persistence of immunologic memory in long-term hemodialysis patients and healthcare workers given hepatitis B vaccine: role of a booster dose on antibody response. Nephron 2001; 89:172 - 176
- Bel'eed K, Wright M, Eadington D, Farr M, Sellars L. Vaccination against hepatitis B infection in patients with end stage renal disease. Postgrad Med J 2002; 78:538 - 540
- DaRoza G, Loewen A, Djurdjev O, Love J, Kempston C, Burnett S, et al. Stage of chronic kidney disease predicts seroconversion after hepatitis B immunization: earlier is better. Am J Kid Dis 2003; 42:1184 - 1192
- Garçon N, Chomez P, Van Mechelen M. GlaxoSmithKline Adjuvant Systems in vaccines: concepts, achievements and perspectives. Expert Rev Vaccines 2007; 6:723 - 739
- European Medicines Agency (EMEA). European Public Assessment Reports (EPARs) for authorized medicinal products for human use. Fendrix. EPAR H-C-50 2010 Available at: http://www.emea.europa.eu/humandocs/Humans/EPAR/fendrix/fendrix.htm
- Surquin M, Tielemans C, Kulcsár I, Ryba M, Vörös P, Mat O, et al. Rapid, enhanced and persistent protection of patients with renal insufficiency by AS02V-adjuvanted hepatitis B vaccine. Kidney Int 2010; 77:247 - 255
- Vandepapelière P, Horsmans Y, Moris P, Van Mechelen M, Janssens M, Koutsoukos M, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine 2008; 26:1375 - 1386
- Sezer S, Ozdemir FN, Güz G, Arat Z, Colak T, Sengul S, et al. Factors influencing response to hepatitis B virus vaccination in hemodialysis patients. Transplant Proc 2000; 32:607 - 608
- Ramezani A, Velayati AA, Eslamifar A, Banifazl M, Ahmadi F, Maziar S, et al. Persistence of hepatitis B vaccine immunity in hemodialysis patients. Ther Apher Dial 2008; 12:143 - 146
- Ramezani A, Eslamifar A, Banifazl M, Ahmadi F, Maziar S, Razeghi E, et al. Efficacy and long-term immunogencitiy of hepatitis B vaccine in haemodialysis patients. Int J Clin Pract 2009; 63:394 - 397
- Salisbury DM, Begg NT. Immunisation against Infectious Diseases 1996; London HM Stationary Office 95 - 108
- Impfempfehlungen der ständigen impfkommission (STIKO) an RKI. Epidemiogisches Bulletin No. 2 2000; Berlin Robert Koch Institut
- Keating GM, Noble S. Recombinant hepatitis B vaccine (Engerix-B): a review of its immunogenicity and protective efficacy against hepatitis B. Drugs 2003; 63:1021 - 1051
- Barry M, Cooper C. Review of hepatitis B surface antigen-1018 ISS adjuvant-containing vaccine safety and efficacy. Expert Opin Biol Ther 2007; 7:1731 - 1737
- Halperin SA, Dobson S, McNeil S, Langley JM, Smith B, McCall-Sani R, et al. Comparison of the safety and immunogenicity of hepatitis B virus surface antigen co-administered with an immunostimulatory phosphorothioate oligonucleotide and a licensed hepatitis B vaccine in healthy young adults. Vaccine 2006; 24:20 - 26
- Jilg W, Schmidt M, Deinhardt F, Zachoval R. Hepatitis B vaccination: how long does protection last?. Lancet 1984; 458
- Jilg W, Schmidt M, Deinhardt F. Persistence of specific antibodies after hepatitis B vaccination. J Hepatol 1988; 6:201 - 207
- European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity?. Lancet 2000; 355:561 - 565
- Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med 2005; 11:63 - 68
- Kong NCT, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int 2005; 68:2298 - 2303
- Kong NCT, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, et al. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int 2008; 73:856 - 862
- Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines 2007; 6:133 - 140
- Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-hemodialysis and hemodialysis patients. Expert Opin Biol Ther 2008; 8:235 - 247
- Bojang KA. RTS,S/AS02A for malaria. Expert Rev Vaccines 2006; 5:611 - 615
- Progress in the control of viral hepatitis: memorandum from a WHO meeting. Bull World Health Organ 1988; 66:443 - 455
- Hepatitis B virus: A comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee. MMWR 1991; 40:1 - 25