Abstract
Nimotuzumab is a humanized therapeutic monoclonal antibody against epidermal growth factor receptor (EGFR). Clinical trials are ongoing globally to evaluate nimotuzumab in different indications. Nimotuzumab has been granted approval for use in squamous cell carcinoma of head and neck (SCCHN), glioma and nasopharyngeal cancer in different countries. This review focuses on the unique functional characteristics of nimotuzumab. Also, it discusses the safety and efficacy data obtained from the Phase IIb clinical trial conducted in India in SCCHN. Post Marketing Surveillance data from Cuba for the use of nimotuzumab in pediatric and adult glioma is also discussed. Overall, nimotuzumab has immense therapeutic potential in cancers of epithelial origin.
Introduction
The capacity for autonomous and dysregulated proliferation in cancerous cells happens by uncontrolled enhanced production of growth factors that actively support cell growth or by overexpression of their cognate growth factor receptors on the cell membranes to which these growth factors bind. Both phenomena cause activation of downstream signaling pathways that ultimately lead to the proliferation of cancer cells, induction of angiogenesis and metastasis.Citation1 The majority of human epithelial cancers exhibit marked overexpression of growth factors [e.g., epidermal growth factor (EGF), transforming growth factor α (TGFα)] and receptors of the epidermal growth factor receptor (EGFR) family. Cancer cell proliferation involving EGFR dysregulation can happen by receptor overexpression, growth factor independent dimerization processes, autocrine growth factor loops and deficiency of specific phosphatases. EGFR dimerization induces intracellular tyrosine kinase mediated phosphorylation of the cytoplasmic domain of the receptor, which in turn provides docking sites for adaptor proteins and signaling enzymes. This results in a receptor mediated activation of downstream signaling protein kinases involved in cellular events such as proliferation and survival. This signalling matrix is redundant as a result of the involvement of multiple ligands, receptor homo- or hetero-dimerization and an abundance of intracellular downstream effectors.Citation1,Citation2 New data describes possible mechanisms of innate and acquired resistance to EGFR inhibitors, such as overexpression of insulin-like growth factor, Ras, Braf and PTEN mutations. Combination therapies using EGFR inhibitors along with drugs acting on other key receptors and downstream signaling molecules involved in tumorigenesis could reduce incidence of innate and acquired resistance.Citation2
In more than 20 years of drug development, agents targeting members of the human epidermal growth factor receptor family—EGFR (also known as HER-1, erbB1) and HER-2/neu (also known as erbB2)—have shown encouraging therapeutic efficacy. The first to be approved by the US Food and Drug Administration (FDA) in 1998 was trastuzumab (Herceptin) for the treatment of HER-2 (erbB-2)-positive breast cancer. Over the past few years, four EGFR specific agents have also received regulatory approval. Cetuximab (Erbitux) for metastatic colorectal cancer (mCRC) and squamous cell carcinoma of the head and neck (SCCHN), Gefitinib (Iressa) for advanced or metastatic non-small-cell lung cancer (mNSCLC), Erlotinib (Tarceva) for advanced or metastatic pancreatic cancer and non-small-cell lung cancer (NSCLC), and Panitumumab (Vectibix) for metastatic colorectal cancer (mCRC).Citation1,Citation3
This review presents the current status of Nimotuzumab, a humanized IgG1 monoclonal antibody targeting EGFR. The unique safety properties of Nimotuzumab as compared to the other monoclonals already launched namely Cetuximab and Panitumumab is discussed. The clinical development programs being pursued worldwide including India and Cuba are presented.
Nimotuzumab is presently approved for the following indications—For squamous cell carcinoma in head and neck (SCCHN) in India, Cuba, Argentina, Colombia, Ivory Coast, Gabon, Ukraine, Peru and Sri Lanka; for glioma (pediatric and adult) in Cuba, Argentina, Philippines and Ukraine; for nasopharyngeal cancer in China. It has been granted orphan drug status for glioma in USA and for glioma and pancreatic cancer in Europe.
Functional Attributes of Nimotuzumab
Nimotuzumab (also known by the lab code h-R3) is a humanized IgG1 isotype monoclonal antibody. It was obtained by transplanting the complementarity determining regions (CDR) of the murine IgG2a monoclonal ior egf/r3, to a human framework assisted by computer modeling.Citation5 The parental murine monoclonal ior egf/r3 was generated by fusing the murine myeloma cells SP2/Ag14 with splenocytes from Balb/c mice immunized with a purified human placenta fraction enriched in EGFR and not with EGFR purified from cultured cells.Citation6 Studies have shown nimotuzumab mediates anti-tumor effects by its capacity to inhibit proliferation, survival and angiogenesis. The in vitro antiproliferative activity of nimotuzumab was tested in both two and three dimensional A431 squamous cell carcinoma cultures.Citation7 There was a dose dependent inhibition of vascular endothelial growth factor (VEGF) expression in A431 monolayer cultures. In studies with A431 subcutaneous tumor xenografts, northern blot analysis of the tumors confirmed VEGF inhibition by nimotuzumab. In addition, treated tumors were characterized by a pronounced decrease in vascularity in terms of both microvascular density and the diameter of vascular channels. Also Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining revealed a five-fold increase in the apoptotic index of the nimotuzumab treated tumors. Nimotuzumab could also be cytolytic on target tumors by its capacity to cause Antibody dependent cell mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC).Citation8
Nimotuzumab has demonstrated a unique clinical profile, where anti-tumor activity was observed in absence of severe skin, renal, gastrointestinal mucosa toxicities commonly associated with EGFR-targeting antibodies, Cetuximab and Panitumumab. gives comparison of the affinity constants of Nimotuzumab and Cetuximab (ImClone Systems) to EGFR expressed in placenta of humans and green monkeys. provides Kd of the different anti-EGFR monoclonals and the capacity of them to cause acneiform rash.Citation9 Cetuximab shows higher binding affinities than Nimotuzumab in human and in green monkey placentas. These results could be correlated with the dose-dependent skin toxicity effects observed at all dose levels with Cetuximab in a 39-week repeated-dose study in Cynomolgus monkeys.Citation10 It is hypothesized that a strong correlation could exist between the affinity constant and tumor distribution for these different therapeutic monoclonals.Citation11 EGFR is considered to play a role in follicular physiology and in cancer patients treated with Cetuximab and Panitumumab, significant cutaneous toxic effects has been observed. Nimotuzumab has not demonstrated any toxic cutaneous effect in numerous clinical trials, even though its clinical benefit was equivalent or superior to other similar anti epidermal growth factor monoclonal antibodies.Citation12 It has been hypothesized that higher binding and internalization of monoclonal antibodies in the tumor together with a lower level of internalization in non-cancerous tissues is obtained with intermediate Kd values between 10−9 M to 10−8 M.Citation13 Crombet et al.Citation11 developed a mathematical model composed of four differential equations, reflecting the behavior of a monoclonal antibody in four compartments (plasma, tumor, liver and skin). The model predicts that the maximum difference between the area under the curve (AUC) for the tumor (high tumor uptake) and the normal tissues (low uptake) is reached when the antibody has intermediate affinity (10−8 to 10−9 M).
Recent experimental observations demonstrate that in contrast to other anti-EGFR antibodies, the intrinsic properties of Nimotuzumab requires bivalent binding (i.e., binding with both antibody arms to two targets simultaneously) for stable attachment to cellular surface, which leads to Nimotuzumab selectively binding to cells that express moderate to high EGFR levels.Citation14 When EGFR expression is low as on healthy tissues, Cetuximab and Panitumumab still has high avidity target binding because of their higher affinity constants. In contrast, since Nimotuzumab has lesser affinity and hence binds with less avidity, the target binding interaction is transient. Thus it spares healthy tissues and avoids the severe dose limiting toxicities. When EGFR expression is moderate to high, all antibodies can bind with similar higher avidity. At the moment, there is no clinical evidence from studies with Panitumumab and Cetuximab that suggests higher affinity leads to greater efficacy, though stronger binding clearly leads to higher toxicities. The binding properties of Nimotuzumab may lead to improved in vivo targeting of EGFR-overexpressing tumors, as compared with the other antibodies.
Phase IIB Clinical Trial in Indian Patients with Squamous Cell Carcinoma of Head and Neck (SCCHN)
Objective.
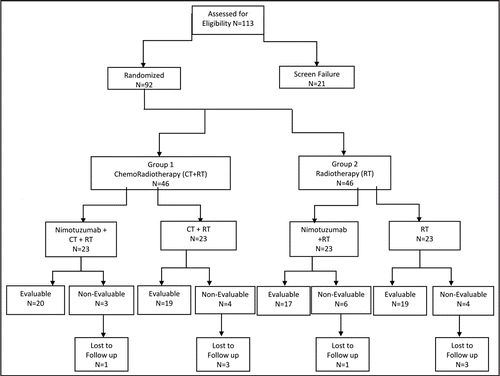
An open-label, randomized, multicentric study to assess the safety and efficacy of Nimotuzumab in combination with chemotherapy and radiotherapy or radiotherapy alone in patients with advanced (stage III or IVA) histologically documented squamous cell carcinoma of head and neck (SCCHN) was conducted in three cancer specialty centers in India. Study was designed to assess the safety and efficacy of Nimotuzumab in combination with chemotherapy (CT) and radiotherapy (RT) (Group I) or standard radiotherapy (RT) alone (Group II) in patients with advanced (stage III or IVA), histologically documented SCCHN. The primary objective was to evaluate the overall response rate and the secondary objectives included evaluation for survival rate including progression free survival (PFS), disease free survival (DFS) and overall survival (OS) in the two study groups. A tertiary objective of the study was to determine the level of EGFR expression in SCCHN at screening. and provides information on EGFR expression levels in all the patients randomized into the trial. EGFR expression was determined by ImmunoHistoChemistry. The study was initiated in September 2004 and currently is in follow up phase. An interim analysis of the study was done in July 2008.
Asian patients were included in the trial. Patients who met all the eligibility criteria were considered for either radiotherapy (Group I) or chemoradiotherapy (Group II) based on their suitability to receive chemotherapy. In each group the patients were randomized in equal proportion based on the randomization codes generated by the statistical group. A total of 92 patients were recruited from September 2004 to July 2005, 23 in each arm (). Group I consisted of two arms where Nimotuzumab was added to cisplatin based chemoradiotherapy in the study drug arm (Nimotuzumab + CT + RT) and chemoradiotherapy alone in the control arm (CT + RT). Similarly, in Group II, Nimotuzumab was added to radiotherapy in study drug arm (Nimotuzumab + RT) and radiotherapy alone in control arm (RT).
Patient characteristics.
Patients between 18 to 70 years of age with advanced unresectable SCCHN of oral cavity, oropharynx or larynx, Stage III to IVA, who were suitable candidates for concurrent chemoradiotherapy or radiotherapy alone, were included in the study. Other key eligibility criteria were: Karnofsky performance status (KPS) ≥60, a life expectancy of greater than six months, leukocyte count ≥4,000/µL, absolute neutrophil count ≥1,500/µL, serum creatinine <1.4 mg/dl, total bilirubin ≤1.2 mg/dL and platelets ≥100,000/µL. Patients with history of prior malignancy, prior chemotherapy, immunotherapy, radiotherapy, evidence of distant metastasis or a concurrent secondary malignancy, pregnancy or lactation were excluded from the study.
Dosing schedule.
Each patient in study drug arms of both the groups received 200 mg of Nimotuzumab infusion intravenously over 60 minutes once weekly for six weeks. Radiotherapy was given once daily through conventional comprehensive radiotherapy technique and the regimen consisted of a total dose of 60–66 Gy over 6–6.5 weeks. Chemotherapy regimen was cisplatin based and patient received 50 mg of Cisplatin infusion intravenously over two hours once a week for six weeks. After the initial 6 to 6.5 weeks of concomitant phase, patients' follow up is ongoing. The safety and efficacy data discussed in this review is up to 30 months from inclusion of subjects into the study. An event of disease progression, withdrawal of informed consent or a grade 3 or 4 toxicity as per National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) led to the withdrawal of patients from the study.
Evaluation of efficacy.
Subjects who received all six doses of Nimotuzumab and had at least one MRI done for tumor response evaluation, either at 12th week or 24th week were considered evaluable for efficacy analysis.
A total of 76 evaluable subjects in both groups were reviewed for the overall tumor response at 24 weeks and survival rates including progression free survival (PFS), disease free survival (DFS) and overall survival (OS) at 30 months.
In the Group I, at the end of 24 weeks of treatment, twenty subjects (100%) in Nimotuzumab + CT + RT arm showed an overall response as compared to fourteen subjects (70%) in the control arm. The difference between the groups was statistically significant (p = 0.02) ().
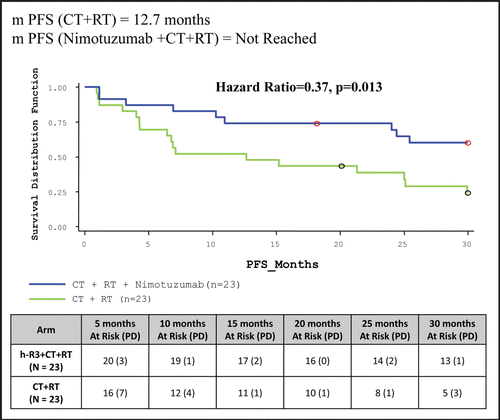
At the end of 30 months, PFS for Intent to Treat (ITT) population was significantly longer when Nimotuzumab was added to CT + RT when compared to control arm (Median duration yet to be achieved Vs. 12.66 months). Thirteen patients (56.52%) were surviving without disease progression in Nimotuzumab + CT + RT arm compared to five patients (21.74%) in control arm which was statistically significant (p < 0.02) (, ).
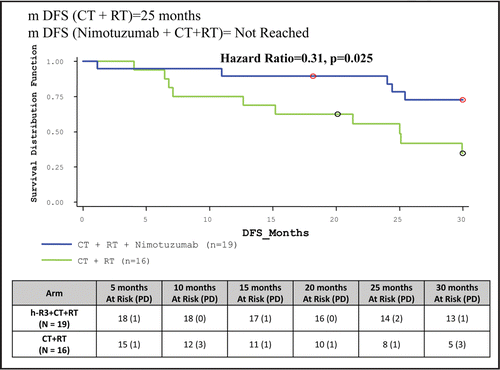
DFS for ITT population was significantly improved when Nimotuzumab was added to chemoradiotherapy (Median duration yet to be achieved vs. 25 months). Thirteen (56.52%) patients in study drug arm were surviving with complete disappearance of tumor compared to five (21.74%) in control arm (, ).
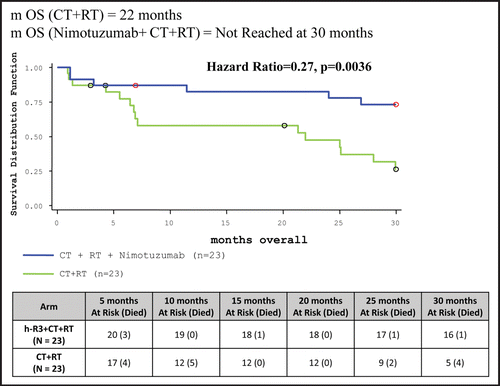
Median OS duration for ITT population has not yet been achieved in Nimotuzumab added to chemoradiotherapy arm as compared to 21.96 months in the chemoradiotherapy arm. Sixteen (69.57%) patients in study drug arm as compared to five (21.74%) patients in control arm were alive at the end of 30 months (, ).
In group II, at the end of 24 weeks of treatment the overall response in the study drug arm (Nimotuzumab + RT) stood at 76% (thirteen subjects) compared to 40% (seven subjects) in control arm (RT alone). The improvement was statistically significant (p = 0.023) ().
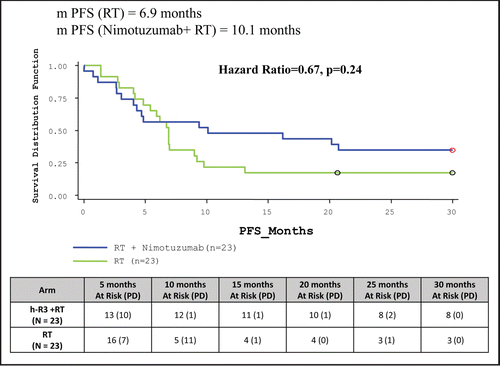
At the end of 30 months PFS was significantly longer when Nimotuzumab was added to RT. The median duration of PFS in study arm for ITT population was 10.1 months compared to 6.9 months in control arm. Eight (34.78%) patients were surviving without disease progression in study arm compared to three (13.04%) in control arm (, ).
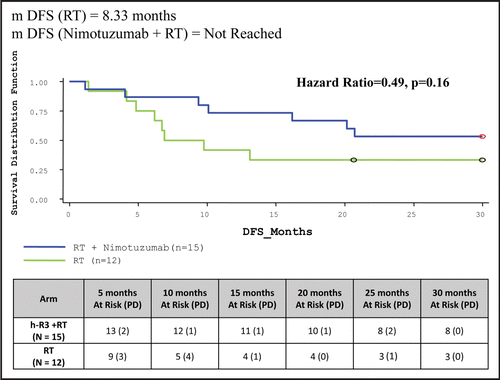
Median DFS duration for ITT population in study arm has not been achieved yet as compared to 8.33 months in control arm. Eight (34.78%) patients were surviving with complete disappearance of tumor in study arm compared to three (13.04%) patients in control arm (, ).
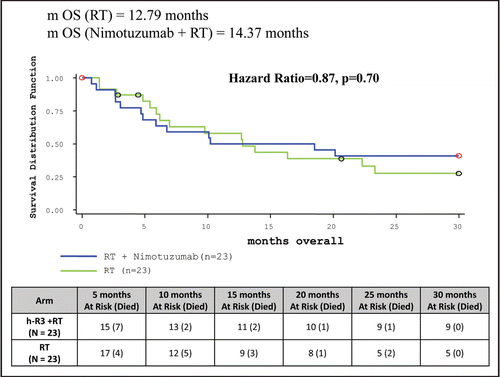
Median OS duration for ITT population was 14.37 months when Nimotuzumab was added to radiotherapy arm as compared to 12.79 months in the control arm. Nine (39.13%) patients in the study arm compared to five (21.74%) patients in control arm were still alive at the end of 30 months (, ).
Evaluation of safety.
The Nimotuzumab related adverse events (AEs) in group I were asthenia, dizziness, microscopic hematuria, vomiting and loose stools. The Nimotuzumab related AEs in group II were fever, chills, pruritus, rash, headache, hypertension and fluctuation in blood pressure. They were considered mild to moderate in severity, self-limiting, reversible. There were only four cases of skin reactions including rash, urticaria and pruritus in subjects exposed to Nimotuzumab. One subject had an infusion reaction at the first infusion of Nimotuzumab and was the only Nimotuzumab related Serious Adverse Event (SAE) reported in the study. Nimotuzumab was found to be safe and well tolerated with few mild to moderate self limiting adverse events.
Discussion
The results of the clinical trial, the first of its kind in an Indian population cohort clearly indicated that Nimotuzumab exhibited a very safe profile and it can be a promising add-on therapy to the existing standard of care in patients with advanced SCCHN. The Bonner StudyCitation19 compared radiotherapy plus Cetuximab versus radiotherapy in SCCHN. The radiotherapy regimen in this study included concomitant boost radiotherapy (selected most frequently; 56 percent), once-daily fractionation (26 percent) and twice-daily fractionation (18 percent) with each regimen having a total radiation dose of ≥70 Gy. The use of once daily regimen (55 patients in the radiotherapy arm and 50 patients in the radiotherapy + Cetuximab arm) resulted in a median overall survival—15.3 months in the RT arm vs. 18.9 months in the RT + Cetuximab arm with a Hazard Ratio of 1.01. Addition of Cetuximab to Radiotherapy gave a 36 month improvement in locoregional control of 13% which translates to an overall survival benefit of 10% at three years. In the case of Nimotuzumab, the progression free survival rate in the Nimotuzumab + radiotherapy arm at 30 months was 34.78% as compared to 13.04% in the radiotherapy arm (p = 0.0839, not statistically significant). The overall survival did not achieve statistical significance in this group. Interestingly, when we compare the Progression Free Survival in the Nimotuzumab + chemoradiotherapy vs. chemoradiotherapy arm, addition of Nimotuzumab to chemoradiotherapy gave a 30 month improvement in Progression Free Survival of 35% (p = 0.0157) which translates to an overall survival benefit of 48% at 30 months (p = 0.0011). The sample size used in the study, as well as the fact that once daily conventional radiotherapy regimen (instead of accelerated boost) was used, could have contributed to not achieving statistical significance in the median overall survival where Nimotuzumab added to radiotherapy was compared to the radiotherapy regimen. On the other hand, achieving a statistically significant improvement in median overall survival in the Nimotuzumab + chemoradiotherapy as compared to chemoradiotherapy, albeit a small sample size, strongly favors the role of Nimotuzumab as an add-on therapy to standard of care of chemoradiotherapy in India. Schedule Y (the guideline in India to be followed for approval to market drugs or to undertake clinical trials) accepts abbreviated clinical data requirements for drugs indicated in life threatening diseases. Based on the data of this study, the Drug Controller Government of India (DCGI) gave marketing approval for Nimotuzumab for use in patients with SCCHN. provides information on the different clinical trials with Nimotuzumab conducted globally and the ongoing clinical trials in different disease indications. Biocon is currently evaluating the post marketing surveillance data of Nimotuzumab use in SCCHN.
In Cuba, where Nimotuzumab is approved for adult and pediatric glioma, post marketing surveillance data is available. Patients with histological or imaging diagnosis of brain tumors were eligible as part of Nimotuzumab Expanded Access Program. Inclusion criteria were life expectancy within four weeks and Karnofsky performance status higher than 50%. The induction phase involved weekly infusions of Nimotuzumab (100 mg for pediatric patients and 200 mg for adult patients), by intravenous route, during the first six weeks. This was followed by maintenance therapy every two weeks with the same dose (100 mg for pediatric patients and 200 mg for adult patients), until disease progression evaluated by CT/MRI or worsening status performance. Different treatment modalities were allowed, monotherapy, combination with chemotherapy (Cisplatin, Cyclophosphamide, Procarbazine) or radiotherapy (54–60 Gy) or with concurrent chemotherapy and radiotherapy. The evaluation criteria were monitoring of safety and symptoms, response evaluation using CT/MRI and survival follow up. Between July 2005 to June 2007, 99 patients were enrolled, 58 of them were evaluable for one year follow up: 28 pediatrics and 30 adults. At the inclusion the patient's diagnosis were, among the pediatric: 13 brain stem tumors, 11 high grade glioma (HGG), 3 ependymoblastoma and one patient with low grade glioma. and among adult patients: 27 HGG, 2 meningioma and one patient with germinoma. Nimotuzumab was very well tolerated in all treatment modalities, even after more than one year of treatment, A small proportion of patients had related adverse events, all of them grade 1/2 according to CTCAE: chills, fever, nauseas, vomiting, mucositis, hyperpigmentation, pruritus were the main AEs. Control of disease in pediatric patients was achieved in 89% [25/28 patients: 11 complete response (CR), 4 partial response (PR) and 10 stable disease (SD)]. Overall survival rate was 78%, 22/28 patients are still alive. In adults patients control disease rate was 60% (18/30 patients: 8 CR, 1 PR, 9 SD). Overall survival rate was 63%, 19/30 patients are still alive. In adult patients with worst prognosis of glioblastoma multiforme, the median survival was 14.53 months, whereas the median survival for Anaplastic Astrocytoma patients has not been reached. Nimotuzumab was very well tolerated in all patients, without increased toxicity in concurrent combination with chemotherapy or radiotherapy. There was a substantial improvement of clinical outcome, long lasting objective responses and disease control in paediatric and adult patients.
In conclusion, Nimotuzumab has unique functional properties unlike other anti-EGFR antibodies. These properties along with an encouraging safety profile allow Nimotuzumab to be explored for maintenance dosing schedules unlike other anti-EGFR monoclonals. The role of Nimotuzumab as a therapeutic opportunity in cancers of epithelial origin has been substantiated from the approvals obtained in different countries.
Figures and Tables
Table 1 Values of Kd for human recombinant EGF, cetuximab and nimotuzumab in EGF receptors from human and green monkey placentas
Table 2 Relationship between receptor affinities and incidence of acneiform rash for anti-EGFR monoclonal antibodies
Table 3 EGFR expression across treatment arms for nimotuzumab + RT and RT arm
Table 4 EGFR expression across treatment arms for nimotuzumab + CT + RT and CT + RT arm
Table 5 Clinical study results: summary
Table 6 Completed and ongoing clinical trials with nimotuzumab
Acknowledgements
We would like to acknowledge the immense contributions from the investigators who conducted the Indian Clinical Trial with Nizotuzumab, Doctors L. Viswanath, M.S.K. Reddy, R. Vidyasagar, A. Koteshwar, N. Shenoy, G. Thimmaiah, Govind Babu, G. Nanjundappa, B. Joseph, R. Bonnathiya and P.P. Bapsy.
References
- Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. New Engl J Med 2008; 358:1160 - 1174
- Speake G, Holloway B, Costello G. Recent developments related to the EGFR as a target for cancer chemotherapy. Curr Opin Pharmacol 2005; 5:343 - 349
- Hynes NE, Lane HA. Errb receptors and cancer: The complexity of targeted inhibitors. Nat Rev Cancer 2005; 5:341 - 354
- Thomas SM, Grandis JR. Pharmacokinetic and pharmacodynamic properties of EGFR inhibitors under clinical investigation. Cancer Treat Rev 2004; 30:255 - 268
- Mateo C, Moreno E, Amour K, Lombardero J, Harris W, Perez R. Humanization of a mouse monoclonal antibody that blocks the epidermal growth factor receptor: recovery of antagonistic activity. Immunotechnology 1997; 3:71 - 78
- Fernandez A, Spitzer E, Perez R, Boehmer FD, Eckert K, Zschiesche W, et al. A new monoclonal antibody for the detection of EGF-R in Western blot and paraffin embedded tissue sections. J Cell Biochem 1992; 49:157 - 165
- Crombet-Ramos T, Rak J, Perez R, Viloria-Petit A. Antiproliferative, antiangiogenic and proapoptotic activity of h-r3: a humanized anti-egfr antibody. Int J Cancer 2002; 101:567 - 575
- Miqueli AD, Blanco R, Garcia B, Badia T, Batista AE, Alonso R, et al. Biological activity in vitro of anti-Epidermal Growth Factor Receptor monoclonal antibodies with different affinities. Hybridoma 2007; 26:423 - 431
- Arteaga ME, Ledón N, Casacó A, Pardo B, García M, Boleda M, et al. Systemic and skin toxicity in Cercopithecus aethiops sabaeus monkeys treated during 26 Weeks with a high intravenous dose of the anti-Epidermal Growth Factor Receptor monoclonal antibody nimotuzumab. Cancer Biol Ther 2007; 6:1390 - 1395
- 2007 Available at http://www.emea.europa.eu/humandocs/PDFs/EPAR/erbitux/089404en6.pdf
- Crombet T, Osorio M, Cruz T, Roca C, del Castillo R, Mon R, et al. Use of the humanized anti-Epidermal Growth Factor Receptor monoclonal antibody h-R3 in combination with radiotherapy in the treatment of locally advanced head and neck cancer patients. J Clin Oncol 2004; 22:1646 - 1654
- Allan D. Nimotuzumab: Evidence of clinical benefit without rash. Oncologist 2005; 10:760 - 761
- Shockley TR, Lin K, Sung C, Nagy JA, Tompkins RG, Dedrick RL, et al. A quantitative analysis of tumor specific monoclonal antibody uptake by human melanoma xenografts: Effects of antibody immunological properties and tumor antigen expression levels. Cancer Res 1992; 52:357 - 366
- Tikhomirov IA, Hidalgo GG, Yang E, Sherman I, Rodriguez RP. Bivalent binding properties of Epidermal Growth Factor Receptor (EGFR) targeted monoclonal antibodies: factors contributing to differences in observed clinical profiles 2008. AACR Meeting on Cancer Clinical Trials and Personalized Medicine
- Agero AL, Dusza SW, Benvenuto-Andrade C, Busam KJ, Myskowski P, Halpern AC. Dermatologic side effects associated with the epidermal growth factor receptor inhibitors. J Am Acad Dermatol 2006; 55:657 - 670
- Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anti-Cancer Drug 2007; 18:7 - 15
- Vanhoefer U, Tewes M, Rojo F, Dirsch O, Schleucher N, Rosen O, et al. J Phase I study of the humanized anti-epidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumors that express the epidermal growth factor receptor. J Clin Oncol 2004; 22:175 - 184
- Saif MW, Kim R. Incidence and management of cutaneous toxicities associated with cetuximab. Expert Opin Drug Saf 2007; 6:175 - 182
- Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus Cetuximab for squamous—cell carcinoma of the head and neck. New Engl J Med 2006; 354:567 - 578